Abstract
A model of pneumococcal meningitis in young adult rats receiving antibiotics once the infection was established was developed. The intent was to mimic clinical and histopathological features of pneumococcal meningitis in humans. The primary aim of the present study was to evaluate whether medical boosting of the peripheral neutrophil count affected the outcome of the meningitis. The risk of terminal illness over the first 7 days after infection was significantly reduced for rats who had elevated peripheral white blood cell counts after receiving granulocyte-colony-stimulating factor (G-CSF) prior to the infection compared to that for untreated rats (P = 0.039 by the log rank test). The improved outcome was associated with reduced signs of cerebral cortical damage (P = 0.008). Furthermore, the beneficial effects of G-CSF were associated with reduced bacterial loads in the cerebrospinal fluid (median, 1.1 × 105 versus 2.9 × 105 CFU/ml; P = 0.023) and in blood (median, 2.9 × 102 versus 6.3 × 102 CFU/ml; P = 0.024), as well as attenuated pleocytosis (median, 800 × 106 versus 1,231 × 106 cells/liter; P = 0.025), 24 h after the infection. Conversely, initiation of G-CSF therapy 28 h postinfection did not alter the clinical or histological outcome relative to that for non-G-CSF-treated rats. The magnitude of bacteremia and pretreatment with G-CSF were found to be prognostic factors for both outcome and brain damage. In summary, elevated neutrophil levels prior to the development of meningitis result in reduced risks of death and brain damage. This beneficial effect is most likely achieved through improved control of the systemic disease.
Streptococcus pneumoniae meningitis remains a life-threatening disease that may lead to severe neurological sequelae in survivors. Timely administration of antibiotic therapy soon after symptoms emerge is essential for a favorable outcome, although peri-infection mortality and postinfection morbidity remain high despite access to such therapy. Recent studies indicate that adjunctive steroids further improve prognosis, although the outcome remains poor (5). There is, therefore, a need to better understand the mechanisms that determine the outcome of pneumococcal meningitis.
Histopathological findings in the brains of patients with bacterial meningitis include cerebral arteritis, venous thrombosis, cortical necrosis, and neuronal apoptosis in the hippocampus (3, 20, 25). The mechanisms responsible for these pathophysiological changes are not fully understood, but involvement of host inflammatory reactions to the invading pathogen, characterized by neutrophil accumulation in the central nervous system, has been proposed. Conversely, neutrophils ordinarily serve an important antibacterial role for the host, although this has yet to be demonstrated in meningitis.
Previous studies have addressed the potential negative aspects of the host inflammatory response in bacterial meningitis by investigating various types of anti-inflammatory treatment (13, 16, 31). Østergaard et al. have previously studied an alternative approach in a rabbit meningitis model where the availability of circulating neutrophils to penetrate into cerebrospinal fluid was increased by pretreatment with granulocyte-colony-stimulating factor (G-CSF) (22). Surprisingly, the elevation of peripheral neutrophil levels was associated with an attenuation of both white blood cell (WBC) counts and cytokine levels in cerebrospinal fluid.
However, to what extent the G-CSF pretreatment-induced modulation of the inflammatory response influences mortality or the development of brain damage remains to be determined. The aim of the present study was to address this issue in an experimental rat model of meningitis developed to mimic the outcome of, and brain damage from, pneumococcal meningitis in humans.
MATERIALS AND METHODS
Bacterial strain.
An S. pneumoniae type 3 strain (strain 68034; Statens Serum Institut, Copenhagen, Denmark) was used for the experiments. After several passages in rat cerebrospinal fluid, bacteria were cultured on 5% blood agar plates (State Serum Institute), suspended in sterile beef broth, and stored frozen at −80°C. Frozen bacterial stocks were thawed and grown on 5% blood agar plates for 18 h, suspended in beef broth, and grown for 4 h, 15 min, to ensure mid-log-phase. After centrifugation (at 3,500 × g and 4°C), the bacteria were washed, centrifuged twice in cold sterile saline, and subsequently diluted in cold saline to achieve a final concentration of 1 × 105 to 2 × 105 CFU/ml, as confirmed by quantitative cultures.
Experimental pneumococcal meningitis.
The study was based on a previously described model of bacterial meningitis in rats (13, 29). The experimental protocol was approved by the Danish Animal Inspectorate (Dyreforsoegstilsynet). Young adult male Wistar rats (weight, approximately 200 g) were used for the experiments. Normal day/night cycles and free access to food and water were provided for the animals.
Experimental procedures and analysis.
On the day of infection, rats were anesthetized with a mixture of Hypnorm (0.315 mg of fentanyl/ml and 10 mg of fluanisone/ml) and Dormicum (5 mg of midazolam/ml), diluted 1:1:2 in sterile water, at a dose of 1.3 ml/kg of body weight, injected subcutaneously (s.c.). Animals were infected by intracisternal injection of 30 μl of the bacterial suspension by using a 25-gauge butterfly needle.
Twenty-four hours after infection, a cerebrospinal fluid and blood tap was performed to verify meningitis. By using 10 μl of cerebrospinal fluid or blood, cerebrospinal fluid pleocytosis and blood WBC levels were determined on an automatic cell counter (Swelab Autocounter AC 920; Swelab Instruments, Stockholm, Sweden). Concentrations of bacteria in cerebrospinal fluid were determined by plating 10-fold serial dilutions of 10 μl of cerebrospinal fluid. In addition, 50 μl of undiluted blood and a 20× dilution were also plated.
Twenty-eight hours after infection, antibiotic therapy with ceftriaxone (Rocephalin; F. Hoffman-La Roche, Ltd., Basel, Switzerland), administered every 24 h s.c. at a dose of 125 mg/kg, was initiated and continued for a total of 6 days. Saline (2.5 ml given three times daily) was injected s.c. during the period of severe illness (24 h to 72 h) to reduce dehydration.
Experimental study design.
Two days before the intracisternal bacterial inoculation, rats were randomly allocated to receive G-CSF (Neupogen; Amgen, Hellerup, Denmark) as pretreatment (10 μg/kg s.c. every 12 h for a total of 5 days), late G-CSF treatment (10 μg/kg s.c. every 12 h, initiated 28 h postinfection for a total of 2 days), or no G-CSF treatment (control group). Data were collected from three homogenous trials with equal distribution of controls. Two rats were died in the G-CSF pretreatment group: one died during anesthesia, and one survivor was erased from data due to signs of subarachnoidal hemorrhage and brain stem lesions from the spinal tap. Two animals were lost in the control group: one died during anesthesia, and one died shortly after a traumatic cerebrospinal fluid tap. Data from 82 animals are therefore presented. A trial including 28 animals (control group and G-CSF pretreatment group) was not included due to lower bacterial concentrations in both blood and cerebrospinal fluid after 24 h and absence of terminal illness in the control group.
Assessment of clinical outcome.
After the bacterial inoculation, animals were clinically and neurologically assessed every 8 h over the first 3 days and then every 24 h over the rest of the experimental period. The final clinical outcome was classified as either (i) terminal illness, for animals developing cyanosis, breathing difficulty, and coma, or opisthotonus or seizures (time of terminal illness was registered as time of death), (ii) motor sequelae, for animals with complete or partial limb paralysis or spastic paresis, or circling when walking, or (iii) normal outcome, for animals with no signs of motor sequelae.
To verify terminal illness, animals were observed continuously from 24 to 72 h postinfection and were euthanized immediately with Hypnorm-Dormicum and pentobarbital (200 mg/ml) if any of the terminal illness criteria were fulfilled. Survivors were observed for a total of 7 days to ensure recovery and, at the end of this period, were assessed for signs of motor sequelae. Animals with motor sequelae and severe paralysis were euthanized before day 8 for ethical reasons. Brains were harvested after perfusion with 1.5% paraformaldehyde through the left ventricle of the heart. Randomization of treatment was blinded to the evaluators of clinical and neurological status.
Histopathology.
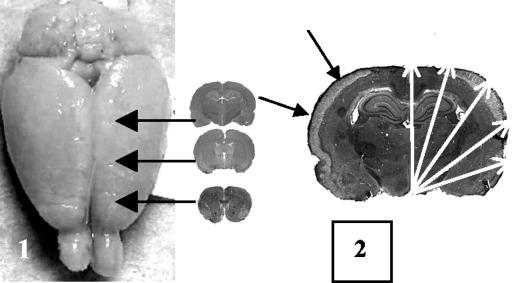
Brains were stored in paraformaldehyde for a week, transferred to 30% sucrose for 6 days, and then frozen in n-hexane for cryosectioning. Six 45-μm-thick coronal cryosections from three parts of the brain (18 sections in total) were stained with hematoxylin and eosin, and brain damage was graded as shown in Fig. 1 (17).
FIG. 1.
Quantification of brain damage. (Left) Histological specimens were obtained from the frontal cortex, mid-frontal cortex, and midcortex. (Right) Division of each cerebral hemisphere into five segments for quantification of brain damage. The band of necrosis in the outer cortex layers is indicated by solid arrows.
Each brain slice was divided into 10 segments, and if one or more signs of damage in a segment were noted, then the segment was given a score of 1 point. Hence, the maximum “brain tissue damage score” per animal, if damage was present in all segments of all sections, was 180 points. This semiquantitative histopathological scoring, used due to the observation of large variations in both damage type and size, was performed by investigators blinded to the treatment groups.
Statistical analysis.
All data are presented as medians. Comparisons between groups were performed using the Mann-Whitney U test and the Kruskal-Wallis test. Fisher's exact test and logistic regression analysis were used for comparison between categorical data. Transformation of continuous data into categorical data for logistic regression analysis was performed with 33-percentile cutoff values. Correlation analysis was performed using the Spearman rank test. The log rank test was applied for survival analysis. A P value of <0.05 was considered significant for the Mann-Whitney U test, Fisher's exact test, logistic regression analysis, and the log rank test, whereas a P value of <0.01 was considered significant for the Spearman rank test.
RESULTS
Effect of G-CSFs treatment on fatal outcome and motor sequelae.
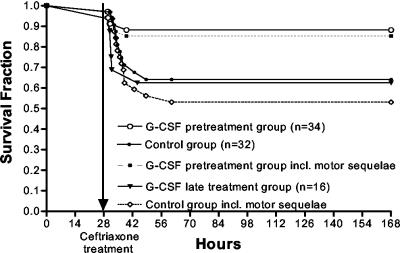
The risk of terminal illness was attenuated in the G-CSF pretreatment group compared to the control group (Fig. 2) (P = 0.039 by the log rank test), whereas the risk was comparable between animals started on G-CSF treatment 28 h after infection and controls (Fig. 2) (P > 0.1 by the log rank test). If the clinical outcome considered was the composite of terminal illness or motor sequelae among surviving animals, the difference between the G-CSF-pretreated group and the control group became slightly larger (Fig. 2) (P = 0.014 by the log rank test). Surviving control animals tended to experience motor sequelae more frequently than the two G-CSF treatment groups, although no significant differences were observed (Table 1).
FIG. 2.
Kaplan-Meier analysis of the G-CSF pretreatment and late-treatment groups compared to the control group (all shown with solid lines). Survival was significantly improved in the G-CSF pretreatment group compared to the control group by the log rank test (P = 0.039). Late G-CSF treatment did not change survival compared to that of the control group. Dashed lines shown for the G-CSF pretreatment group and the control group include both lethal infection and survival with motor sequelae.
TABLE 1.
Effects of G-CSF treatment on clinical outcome and brain damagea
| Group (n) | Median % (no. with adverse outcome/total no.) with:
|
Median brain damage score (interquartile range) | |
|---|---|---|---|
| Terminal illness | Motor sequelae | ||
| Control (32) | 34.4 (11/32)* | 19.0 (4/21) | 1.53 (0 to 5.22)** |
| G-CSF pretreatment (34) | 11.8 (4/34)* | 3.3 (1/30) | 0 (0 to 1.05)** |
| G-CSF late treatment (16) | 37.5 (6/16) | 10.0 (1/10) | 1.58 (0 to 8.55) |
Asterisks indicate statistically significant differences. *, P = 0.039, **, P = 0.008.
Effect of G-CSF pretreatment on WBC counts in blood and cerebrospinal fluid in the first 24 h postinfection.
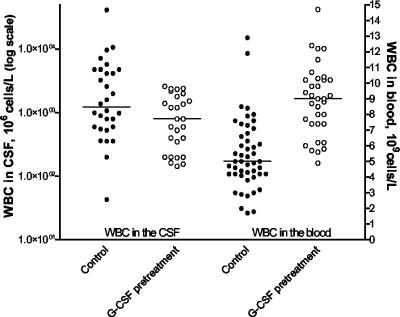
For comparisons of WBC and bacterial concentrations in cerebrospinal fluid and blood between the G-CSF pretreatment group and the control group, data from the late G-CSF treatment group were pooled with those from the control group (samples were obtained from the late-treatment group before G-CSF administration). WBC counts in the blood increased over the first 24 h of infection but remained higher in animals treated with G-CSF prior to the time of infection (Fig. 3) (median WBC counts were 5.7 × 109 versus 3.2 × 109/liter [P = 0.0007] at 0 h and 9.0 × 109 versus 5.0 × 109/liter [P = 0.0001] at 24 h), whereas WBC counts in cerebrospinal fluid were significantly attenuated 24 h postinfection in the group receiving G-CSF pretreatment (Fig. 3; median WBC counts, 800 × 106 versus 1,231 × 106/liter [P = 0.025]).
FIG. 3.
Comparison between the control group and the G-CSF pretreatment group of WBC counts in cerebrospinal fluid (CSF) and blood. Solid circles, control group; open circles, G-CSF pretreatment group. G-CSF pretreatment increased peripheral-blood WBC counts (P < 0.0001), whereas WBC counts in cerebrospinal fluid were significantly attenuated (P = 0.025) compared to those for the control group.
Effects of G-CSF pretreatment on bacterial concentrations in blood and cerebrospinal fluid 24 h postinfection.
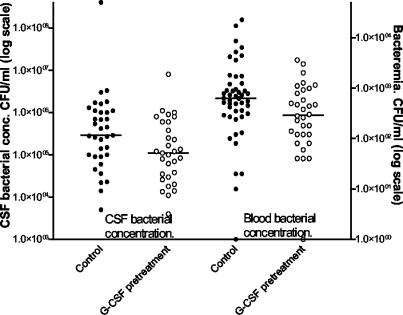
Again, data from the control group and late G-CSF treatment group were pooled for comparisons with the G-CSF pretreatment group. At 24 h postinfection, bacterial concentrations in both blood and cerebrospinal fluid were significantly attenuated in rats pretreated with G-CSF (Fig. 4; median concentrations in blood, 2.9 × 102 versus 6.3 × 102 CFU/ml [P = 0.024]; median concentrations in cerebrospinal fluid, 1.1 × 105 versus 2.9 ×105 CFU/ml [P = 0.023]).
FIG. 4.
Comparison between the control group and the G-CSF pretreatment group of bacterial concentrations in cerebrospinal fluid (CSF) and blood. Solid circles, control group; open circles, G-CSF pretreatment group. Pretreatment with G-CSF significantly attenuated the bacterial loads in cerebrospinal fluid (P = 0.023) and blood (P = 0.024) compared to those for controls.
Correlations between paraclinical (microbiological and cytochemical) data 24 h postinfection.
Significant correlations between bacterial concentrations in cerebrospinal fluid and WBC counts in cerebrospinal fluid at 24 h postinfection were found in both the control group (P < 0.0001; ρ = 0.65) and the G-CSF pretreatment group (P = 0.0016; ρ = 0.57). Furthermore, a significant negative correlation was found between WBC counts in peripheral blood and bacterial concentrations in cerebrospinal fluid in the G-CSF pretreatment group (P = 0.0014; ρ = −0.54). The corresponding ρ value for the control group was 0.08 (P = 0.64). No correlations were found between WBC counts in the cerebrospinal fluid and WBC counts in peripheral blood at 24 h postinfection either overall, in the G-CSF pretreatment group, or in all other groups together.
Effect of G-CSF treatment on brain damage.
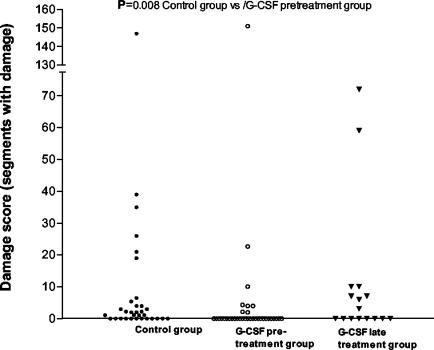
The proportion of animals in the G-CSF pretreatment group with histological evidence of tissue damage in at least one segment of the brain was significantly lower (8 of 34 [24%]) than that in the control group (20 of 32 [63%]; P = 0.0025 by Fisher's exact test) but not significantly different from that in the group treated with G-CSF postinfection (8 of 16 [50%]; P > 0.05). As a consequence, the brain tissue injury score was significantly lower in the G-CSF pretreatment group than in the control group (Table 1 and Fig. 5; P = 0.008 by the Mann-Whitney U test), whereas the G-CSF late-treatment group was not found to be different from the control group (Table 1 and Fig. 5; P > 0.05). In animals with detectable injury, brain tissue damage injury scores were comparable for the three groups (Fig. 5). When only animals that did not develop terminal illness were included, the brain tissue injury score diminished, as expected. However, the difference between the G-CSF pretreatment group and the control group remained (the proportions with a score of above 0 were 4 of 30 [13%] versus 9 of 21 [43%], respectively [P = 0.025]; the median [interquartile range] scores were 0 [0 to 0] and 0 [0 to 2], respectively [P = 0.089 by the Mann-Whitney U test]). Details of the histological changes observed are described further below.
FIG. 5.
Brain damage. Brain damage was detected in 20 out of 32 rats in the control group (solid circles) compared to 8 out of 34 in the G-CSF pretreatment group (open circles) and 8 out of 16 in the G-CSF late-treatment group (solid triangles). G-CSF pretreatment significantly reduced brain damage relative to that in the control group (P = 0.008). G-CSF as a late treatment did not reduce brain damage (P = 0.92).
Prognostic factors for brain damage and adverse outcomes.
By using a single cutoff value (above versus below the 33rd percentile; the model did not allow us to construct three groups of variables based on the predefined 33rd and 66th percentile cutoffs) for WBC and bacterial concentrations in cerebrospinal fluid and blood obtained 24 h after bacterial inoculation, a blood bacterial concentration of >284 CFU/ml was the only parameter significantly associated with terminal illness, adverse clinical outcome, and brain damage (a univariate logistic regression analysis gave odds ratios [OR] [95% confidence intervals {95% CI}] of 10.0 [1.2 to 82.0] [P = 0.03],16.5 [2.0 to 134] [P = 0.009], and 8.0 [2.0 to 31] [P = 0.003], respectively; data not shown for other parameters). The OR (95% CI) for terminal illness, adverse clinical outcome, or brain damage with G-CSF pretreatment was 0.26 (0.07 to 0.9) (P = 0.04), 0.02 (0.06 to 0.6) (P = 0.007), or 0.16 (0.05 to 0.47) (P = 0.001), respectively, in a univariate logistic regression analysis. In a multivariate logistic regression analysis, including the prognostic factors for outcome in the univariate analysis, again G-CSF pretreatment and blood bacterial concentrations of >284 CFU/ml were independently associated with outcome (OR [95% CI] for terminal illness, 0.40 [0.10 to 1.5] [P = 0.18] and 7.3 [0.9 to 63] [P = 0.070], respectively; OR [95% CI] for clinical outcome, 0.31 [0.09 to 1.1] [P = 0.07] and 11.7 [1.4 to 99] [P = 0.02], respectively; OR [95% CI] for brain damage, 0.21 [0.07 to 0.7] [P = 0.01] and 5.4 [1.3 to 23] [P = 0.02], respectively).
Brain histopathology.
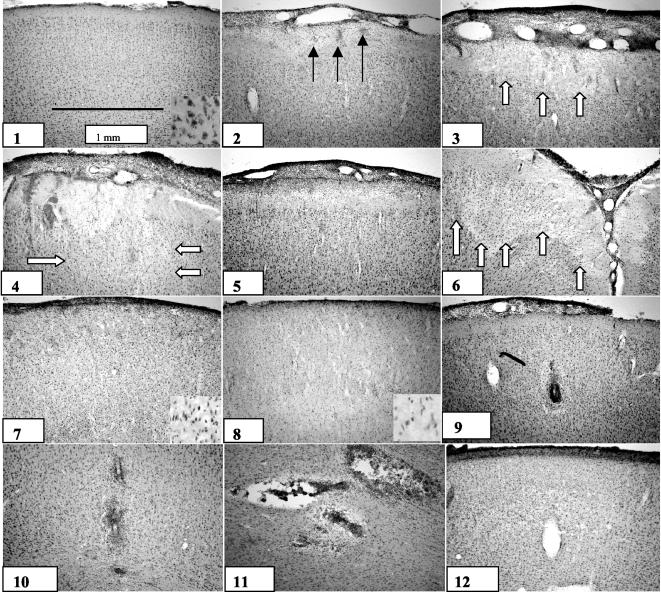
Large variations in the size and type of cerebral cortical damage were observed (Fig. 6), e.g., necrosis, abscess, hemorrhage, and reorganization. The largest lesions presented were cortical necrosis with a very regular distribution in the cortex. The smallest lesions were due to vasculitic obstructed vessels surrounded by a halo of edema and ballooning neurons.
FIG. 6.
Histopathological changes in the cerebral cortices of rats with pneumococcal meningitis. (1) Normal rat brain cortex; (2) prenecrotic lesion with areas of peripheral bleeding; (3 and 4) early necrotic lesions with neuronal loss; (5) necrotic lesion with neutrophil invasion of outer cortex layer; (6) wedge-shaped necrotic lesion; (7) broad band of necrosis; (8) liquefied cortex—total necrosis; (9) vasculitis with halo of edema; (10 and 11) abscess formation; (12) cortical reorganization. Solid arrows, hemorrhages; open arrows, necrotic areas.
The following histopathological findings were typically observed in the cortex. (i) The spectrum of necrotic lesions included punctuate hemorrhages and eosinophilia in the outer cortex layer but no obvious neuronal loss; early necrotic lesions with hemorrhage into the neuronal layers, eosinophilia, and shrunken neurons; neutrophil granulocyte invasion of the outer cortex layer and neuronal loss in the cortex; late necrotic lesions with a regular distributed necrotic band involving the outer cortical neuronal layers down to layer 3 to 4, or wedge-shaped necrotic lesions as described by others (17); and areas of cortical cellular infiltration that were, unlike the lesions described above, not macroscopically visible. The latter changes were labeled “tissue reorganization” and were most likely to occur following necrotic lesions, since such infiltration was observed only in rats surviving to day 7. (ii) Neutrophil accumulation in the cortex could manifest as severely vasculitic vessels obstructed by neutrophil granulocytes but without signs of neuronal loss in the cortex; multiple vessels surrounded by neutrophil granulocytes, resembling small abscesses, with a halo of edema and ballooning neurons surrounding the lesion; or small abscesses with massive neutrophil accumulation and local necrosis.
Hippocampal apoptosis was not evaluated, because apoptotic neurons appearing in the acute stages of the disease are expected to have disappeared in animals surviving to day 8.
DISCUSSION
The present study has, for the first time in a meningitis model, demonstrated that a significant increase in peripheral WBC counts due to treatment with G-CSF prior to infection significantly reduces the risk of fatality and protects from cerebral damage in experimental pneumococcal meningitis. In contrast, treatment with G-CSF initiated 28 h postinfection did not affect the risk of death or the degree of brain damage. A study by Dallaire et al. (4) in a mouse model of pneumococcal pneumonia published survival curves very similar to those presented here, showing the beneficial effects of G-CSF initiated prior to infection (with an infectious inoculum similar to ours), but not after infection had been established.
The beneficial effects of an increase in peripheral WBC counts due to G-CSF pretreatment on survival and the extent of brain damage were associated with attenuation of the WBC count in cerebrospinal fluid and attenuation of bacterial loads in cerebrospinal fluid and blood, parameters that may be associated with a more favorable clinical outcome and a lower degree of brain damage. We address each of these issues below.
Effect of G-CSF pretreatment on systemic infection.
The most evident effect of G-CSF pretreatment is the significant increase in both the number of peripheral neutrophils and their phagocytic activity. This increase presumably leads to improved control of the infection in the systemic compartment by reducing the number of bacteria (1, 4, 6, 12, 13, 19, 21, 22, 24, 26), as also seen in the present study. In pneumococcal meningitis, approximately 70% of patients become bacteremic, and bacteremia has been associated with an increased risk of mortality (5, 10, 11, 25; D. van de Beek and J. de Gans, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. L-615, p. 413, 2003). The magnitude of bacteremia in patients with sepsis has also been reported to be a prognostic indicator (32).
In the present study, the magnitude of bacteremia was an independent prognostic factor for clinical outcome and brain damage and was more closely related to outcome measures than G-CSF pretreatment. It therefore appears that control of the systemic infection may be crucial in determining the outcome in pneumococcal meningitis.
Effect of G-CSF on meningeal inflammation.
The attenuation of pleocytosis in cerebrospinal fluid confirms previous findings obtained in a rabbit meningitis model (22). The host inflammatory response to an invading pathogen has been proposed to participate in the severe course of bacterial meningitis. Various anti-inflammatory modes of treatment (e.g., blockage of leukocyte entry, dexamethasone, matrix metalloproteinase inhibitors) have been shown to reduce mortality and brain damage in animal models (13, 16, 31).
The role of leukocytes in the outcome of bacterial meningitis has been investigated by Tuomanen et al. An improvement in survival in a rabbit model of pneumococcal meningitis was found by use of a monoclonal antibody to CD11b/CD18 that blocked the entry of leukocytes to the cerebrospinal fluid (31). However, blocking of neutrophil entry by the selectin blocker fucoidin resulted in increased mortality in our rat model of meningitis (C. Brandt, J. D. Lundgren, S. P. Lund, N. Frimodt-Møller, T. Christensen, T. Benfield, F. Espersen, D. Hougaard, and C. Østergaard, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. B-1513, p. 62, 2003). Differences in animal models may well explain these conflicting findings, since we used low concentrations of living bacteria as the inoculum, whereas Tuomanen et al. used serial injections with high concentrations of heat-killed bacteria, which induce a massive meningeal inflammatory response but no secondary bacteremia. Interestingly, the beneficial effect of dexamethasone therapy in human pneumococal meningitis (5) seems to be due to an effect on systemic infection rather than neurological complications (van de Beek and de Gans, 43rd ICAAC).
Effect of G-CSF pretreatment on the bacterial load in cerebrospinal fluid.
In the present study, we found a small but significant attenuation of the bacterial load in the cerebrospinal fluid of G-CSF-pretreated rats, a result that was not observed in a previous study with a rabbit model (22). This discrepancy is most likely due to differences in the sampling time (0 to 16 h in the rabbit model and 24 h in the rat model) but could also be due to differences in the inoculum size and/or animal model used. High bacterial or pneumococcal concentrations have been correlated with a poor outcome in patients with bacterial meningitis (8, 9, 28). It has been shown experimentally by Kim et al. (13) that the dose of bacterial inoculum in an infant rat model of pneumococcal meningitis is an important factor influencing the extent of brain damage and the number of animals with seizures. Thus, the beneficial effect of G-CSF pretreatment could be due to a reduced bacterial load in cerebrospinal fluid. However, in the present study, the bacterial load in cerebrospinal fluid was not significantly associated with outcome.
Significant correlations were found between the WBC count and the bacterial load in the cerebrospinal fluid 24 h postinfection for both the control group and the G-CSF pretreatment group. Also, a correlation between a high peripheral WBC count and an attenuated bacterial load in cerebrospinal fluid was found in the G-CSF pretreatment group but not in the control group. This suggests that the ability of G-CSF to boost the peripheral neutrophil level also resulted in antibacterial effects within the central nervous system. It is difficult to explain why the bacterial load was reduced in animals pretreated with G-CSF despite the fact that these animals had a lower WBC count in cerebrospinal fluid. One possible explanation is the development of an equilibrium between bacterial concentrations in cerebrospinal fluid and blood, as has been shown in a hematogenous model of pneumococcal meningitis (30). A similar correlation between bacterial loads in blood and cerebrospinal fluid has also been reported for the human disease (15). These findings support the hypothesis that controlling and reducing the bacterial load in one compartment contributes to bacterial reductions in other compartments. Since bacteremia following intracisternal infection inevitably develops in the model used here (13), the hypothesis of bacterial circulation between compartments could also be applied in the present study. The increases in the peripheral WBC count and phagocytotic capacity in the G-CSF pretreatment group could therefore be directly responsible for the lower bacterial load in cerebrospinal fluid by reducing the reentry of bacteria from the blood into cerebrospinal fluid. In support of this hypothesis, the WBC count in cerebrospinal fluid per se has not been shown consistently to influence the bacterial load in cerebrospinal fluid (7, 23, 27).
The observations presented here were made with a model of pneumococcal meningitis in young adult rats that was developed with the intention of mimicking the disease severity (5) and histopathological findings (3, 25) observed for the human disease. In the present model, the histopathological findings were diverse and included various degrees of peripheral multiple cortical hemorrhages, single or band-like necrotic lesions, vasculitis in cortical vessels, abscess formation, and cortical reorganization with cellular infiltration.
While injury to the cerebral cortex in models of meningitis in adult rats has not recently been reported to reach the level of severity presented in this study (14, 29), extensive damage has been reported for a meningitis model in neonate rats (17, 18). The mechanisms underlying either direct or indirect brain tissue damage induced by the bacterial pathogen remain unknown. We believe that the virulence of the bacteria is a crucial factor, since large areas of cerebral cortical necrosis were observed only when a bacterial strain isolated from rats with severe illness was used. It is likely that the strain used in these experiments has the ability to change its virulence characteristics. We have seen that the brain-damaging capacity of a strain can disappear following a number of passages in cerebrospinal fluid (personal observation).
Some of the observed injuries to the cerebral cortex may be caused by septic shock and a resultant depressed systemic and cerebral perfusion. The histological examples characterized as exhibiting a regular distributed band of necrosis also suggest a vascular component in the pathology that could be attributed to global hypoperfusion. Macroscopically, petechial hemorrhages were observed on the surfaces of brains. The prognostic significance of the severity of bacteremia for brain damage supports this view. Furthermore, a study of pigs with septic shock produced histopathological findings for the brains similar, in part, to the histopathological changes presented here (2).
In conclusion, a causal sequence of events is postulated based on the antibacterial rather than anti-inflammatory properties of G-CSF. We found that G-CSF-induced attenuation of bacterial concentrations in blood was a significant prognostic factor for both clinical outcome and brain damage, suggesting that future improvements in the treatment of bacterial meningitis could be achieved through management of septicemia. This study, however, underlines the need for further research investigating the importance of the systemic and meningeal infection, thereby improving our understanding of this disease.
Acknowledgments
This work was supported in part by the following foundations: Den Lægevidenskabelige Forskningsfond for Storkøbenhavn, Færøerne og Greenland; Eivind Eckbos dansk-norske Legat; Lily Benthine Lunds Fond; Christian Larsen og Dommer Ellen Larsens Legat; Direktør Jacob Madsen og Hustru Olga Madsens Fond; Fonden til Lægevidenskabens Fremme; and H:S Fondet.
We thank Stephen Leib for invaluable help with the rat model and useful scientific discussions. We also thank Jesper Madsen for statistical support, Ian Rowland for help with preparing the manuscript, and Jacob Vang, Bente Scherfig, Gitte Kristiansen, and Frank Hansen for expert technical assistance.
Editor: J. N. Weiser
REFERENCES
- 1.Attalah, H. L., E. Azoulay, K. Yang, C. Lasclos, H. Jouault, C. J. Soussy, T. Guillot, L. Brochard, C. Brun-Buisson, A. Harf, and C. Delclaux. 2002. Granulocyte colony-stimulating factor enhances host defenses against bacterial pneumonia following peritonitis in nonneutropenic rats. Crit. Care Med. 30:2107-2114. [DOI] [PubMed] [Google Scholar]
- 2.Bogdanski, R., M. Blobner, I. Becker, F. Hanel, H. Fink, and E. Kochs. 2000. Cerebral histopathology following portal venous infusion of bacteria in a chronic porcine model. Anesthesiology 93:793-804. [DOI] [PubMed] [Google Scholar]
- 3.Cairns, H., and D. Russel. 1946. Cerebral arteritis and phlebitis in pneumococcal meningitis. J. Pathol. Bacteriol. 58:649-665. [DOI] [PubMed] [Google Scholar]
- 4.Dallaire, F., N. Ouellet, M. Simard, Y. Bergeron, and M. G. Bergeron. 2001. Efficacy of recombinant human granulocyte colony-stimulating factor in a murine model of pneumococcal pneumonia: effects of lung inflammation and timing of treatment. J. Infect. Dis. 183:70-77. [DOI] [PubMed] [Google Scholar]
- 5.de Gans, J., and D. van de Beek. 2002. Dexamethasone in adults with bacterial meningitis. N. Engl. J. Med. 347:1549-1556. [DOI] [PubMed] [Google Scholar]
- 6.Eaves-Pyles, T., and J. W. Alexander. 1996. Granulocyte colony-stimulating factor enhances killing of translocated bacteria but does not affect barrier function in a burn mouse model. J. Trauma 41:1013-1017. [DOI] [PubMed] [Google Scholar]
- 7.Ernst, J. D., J. M. Decazes, and M. A. Sande. 1983. Experimental pneumococcal meningitis: role of leukocytes in pathogenesis. Infect. Immun. 41:275-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldman, W. E. 1977. Relation of concentrations of bacteria and bacterial antigen in cerebrospinal fluid to prognosis in patients with bacterial meningitis. N. Engl. J. Med. 296:433-435. [DOI] [PubMed] [Google Scholar]
- 9.Feldman, W. E., C. M. Ginsburg, G. H. McCracken, Jr., D. Allen, P. Ahmann, J. Graham, and L. Graham. 1982. Relation of concentrations of Haemophilus influenzae type b in cerebrospinal fluid to late sequelae of patients with meningitis. J. Pediatr. 100:209-212. [DOI] [PubMed] [Google Scholar]
- 10.Hodges, G. R., and R. L. Perkins. 1975. Acute bacterial meningitis: an analysis of factors influencing prognosis. Am. J. Med. Sci. 270:427-440. [DOI] [PubMed] [Google Scholar]
- 11.Hoen, B., J. F. Viel, A. Gerard, J. B. Dureux, and P. Canton. 1993. Mortality in pneumococcal meningitis: a multivariate analysis of prognostic factors. Eur. J. Med. 2:28-32. [PubMed] [Google Scholar]
- 12.Kaposzta, R., and L. Marodi. 1995. Chronic neutropenia and defect in superoxide generation of granulocytes in two patients: enhancement of bactericidal capacity and respiratory burst activity by treatment with recombinant human granulocyte colony-stimulating factor. Pediatr. Res. 37:50-55. [DOI] [PubMed] [Google Scholar]
- 13.Kim, Y. S., R. A. Sheldon, B. R. Elliott, Q. Liu, D. M. Ferriero, and M. G. Tauber. 1995. Brain injury in experimental neonatal meningitis due to group B streptococci. J. Neuropathol. Exp. Neurol. 54:531-539. [DOI] [PubMed] [Google Scholar]
- 14.Koedel, U., W. M. Scheld, and H. W. Pfister. 2002. Pathogenesis and pathophysiology of pneumococcal meningitis. Lancet Infect. Dis. 2:721-736. [DOI] [PubMed] [Google Scholar]
- 15.La Scolea, L. J., Jr., and D. Dryja. 1984. Quantitation of bacteria in cerebrospinal fluid and blood of children with meningitis and its diagnostic significance. J. Clin. Microbiol. 19:187-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leib, S. L., J. M. Clements, R. L. Lindberg, C. Heimgartner, J. M. Loeffler, L. A. Pfister, M. G. Tauber, and D. Leppert. 2001. Inhibition of matrix metalloproteinases and tumour necrosis factor alpha converting enzyme as adjuvant therapy in pneumococcal meningitis. Brain 124:1734-1742. [DOI] [PubMed] [Google Scholar]
- 17.Leib, S. L., Y. S. Kim, D. M. Ferriero, and M. G. Tauber. 1996. Neuroprotective effect of excitatory amino acid antagonist kynurenic acid in experimental bacterial meningitis. J. Infect. Dis. 173:166-171. [DOI] [PubMed] [Google Scholar]
- 18.Leib, S. L., D. Leppert, J. Clements, and M. G. Tauber. 2000. Matrix metalloproteinases contribute to brain damage in experimental pneumococcal meningitis. Infect. Immun. 68:615-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell, G. B., B. N. Albright, and J. L. Caswell. 2003. Effect of interleukin-8 and granulocyte colony-stimulating factor on priming and activation of bovine neutrophils. Infect. Immun. 71:1643-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nau, R., A. Soto, and W. Bruck. 1999. Apoptosis of neurons in the dentate gyrus in humans suffering from bacterial meningitis. J. Neuropathol. Exp. Neurol. 58:265-274. [DOI] [PubMed] [Google Scholar]
- 21.Noursadeghi, M., M. C. Bickerstaff, J. Herbert, D. Moyes, J. Cohen, and M. B. Pepys. 2002. Production of granulocyte colony-stimulating factor in the nonspecific acute phase response enhances host resistance to bacterial infection. J. Immunol. 169:913-919. [DOI] [PubMed] [Google Scholar]
- 22.Ostergaard, C., T. Benfield, B. Gesser, A. Kharazmi, N. Frimodt-Moller, F. Espersen, and J. D. Lundgren. 1999. Pretreatment with granulocyte colony-stimulating factor attenuates the inflammatory response but not the bacterial load in cerebrospinal fluid during experimental pneumococcal meningitis in rabbits. Infect. Immun. 67:3430-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ostergaard, C., R. V. Yieng-Kow, T. Benfield, N. Frimodt-Moller, F. Espersen, and J. D. Lundgren. 2000. Inhibition of leukocyte entry into the brain by the selectin blocker fucoidin decreases interleukin-1 (IL-1) levels but increases IL-8 levels in cerebrospinal fluid during experimental pneumococcal meningitis in rabbits. Infect. Immun. 68:3153-3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pajkrt, D., A. Manten, T. van der Poll, M. M. Tiel-van Buul, J. Jansen, J. Wouter ten Cate, and S. J. van Deventer. 1997. Modulation of cytokine release and neutrophil function by granulocyte colony-stimulating factor during endotoxemia in humans. Blood 90:1415-1424. [PubMed] [Google Scholar]
- 25.Quaade, F., and K. P. Kristensen. 1962. Purulent meningitis. A review of 658 cases. Acta Med. Scand. 171:543-550. [PubMed] [Google Scholar]
- 26.Roilides, E., T. J. Walsh, P. A. Pizzo, and M. Rubin. 1991. Granulocyte colony-stimulating factor enhances the phagocytic and bactericidal activity of normal and defective human neutrophils. J. Infect. Dis. 163:579-583. [DOI] [PubMed] [Google Scholar]
- 27.Saez-Llorens, X., H. S. Jafari, C. Severien, F. Parras, K. D. Olsen, E. J. Hansen, I. I. Singer, and G. H. McCracken, Jr. 1991. Enhanced attenuation of meningeal inflammation and brain edema by concomitant administration of anti-CD18 monoclonal antibodies and dexamethasone in experimental Haemophilus meningitis. J. Clin. Investig. 88:2003-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider, O., U. Michel, G. Zysk, O. Dubuis, and R. Nau. 1999. Clinical outcome in pneumococcal meningitis correlates with CSF lipoteichoic acid concentrations. Neurology 53:1584-1587. [DOI] [PubMed] [Google Scholar]
- 29.Tauber, M. G., S. L. Kennedy, J. H. Tureen, and D. H. Lowenstein. 1992. Experimental pneumococcal meningitis causes central nervous system pathology without inducing the 72-kd heat shock protein. Am. J. Pathol. 141:53-60. [PMC free article] [PubMed] [Google Scholar]
- 30.Tsao, N., W. W. Chang, C. C. Liu, and H. Y. Lei. 2002. Development of hematogenous pneumococcal meningitis in adult mice: the role of TNF-α. FEMS Immunol. Med. Microbiol. 32:133-140. [DOI] [PubMed] [Google Scholar]
- 31.Tuomanen, E. I., K. Saukkonen, S. Sande, C. Cioffe, and S. D. Wright. 1989. Reduction of inflammation, tissue damage, and mortality in bacterial meningitis in rabbits treated with monoclonal antibodies against adhesion-promoting receptors of leukocytes. J. Exp. Med. 170:959-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yagupsky, P., and F. S. Nolte. 1990. Quantitative aspects of septicemia. Clin. Microbiol. Rev. 3:269-279. [DOI] [PMC free article] [PubMed] [Google Scholar]