Abstract
There is considerable interest in the role of sleep in weight regulation, yet few studies have examined this relationship in overweight/obese (OW/OB) adults. Using a within-subject, counterbalanced design, 12 OW/OB women were studied in lab with two nights of short (5 hours time in bed [TIB]) and two nights of long (9 hours TIB) sleep. Hunger, consumption at a buffet, and fasting hormone levels were obtained. Significant polysomnographic differences occurred between conditions in total sleep time and sleep architecture (p's < .001). Percent energy from protein at the buffet increased following short sleep. No differences were observed for total energy intake or measured hormones. Further research is needed to determine how lengthening sleep impacts weight regulation in OW/OB adults.
Introduction
Epidemiological studies have documented an association between short sleep and obesity risk (Cappuccio et al., 2008). Experimental studies with normal weight adults have suggested that changes in hormones associated with eating behaviors and caloric intake may play a role in the association between sleep and obesity risk.
Several studies have demonstrated decreases in the satiety-signaling hormone leptin (Mullington et al., 2008; Spiegel, Leproult et al., 2004; Spiegel, Tasali, Penev & van Cauter, 2004) and increases in the hunger-signaling hormone ghrelin (Benedict et al., 2011; Schmid et al., 2008; Spiegel, Tasali et al., 2004) under conditions of sleep restriction when caloric intake is carefully controlled and weight maintained. However, findings are not always consistent. In part this may be due to differences in experimental designs such as ad libitum access to food during laboratory stays (Nedeltcheva et al., 2009) or periods of time spent in free living conditions during which energy intake was not monitored (Schmid et al., 2009)-both of which may influence overall energy balance and thus levels of leptin and ghrelin. It is important to note, though, other studies that either carefully controlled energy intake or had participants fast during the overnight sleep period have not found changes in leptin (Benedict et al., 2011; Schmid et al., 2008; St-Onge et al., 2012) or ghrelin (e.g., in female participants; St-Onge et al., 2012) in association with changes in sleep duration.
Results of recent studies evaluating the effect of sleep restriction on eating behaviors in healthy, normal weight participants have also been mixed. For example, one experimental study in normal weight men found that participants increased their observed ad libitum food intake by 559 kcal (across breakfast, lunch, snack, and dinner) following one night of 4 hours time in bed (TIB) compared to one night of 8 hours TIB (Brondel, Romer, Nouges, Touyarou, & Davenne, 2010). Two additional studies with normal weight adults found similar albeit more moderate increases in energy intake following four to five nights of sleep restriction (i.e., 4-5 hours/night) compared to four to five nights of sleep extension (i.e., 9 hours/night) (St-Onge et al., 2011; Markwald et al., 2013). In contrast, two additional studies demonstrated no differences in energy intake in short versus long sleep conditions. The first was conducted with a sample of normal weight men and compared energy intake following two nights of approximately 4 hours TIB to two nights of approximately 8 hours TIB (Schmid et al., 2009). Although there were differences in percent of calories consumed from fat between the two conditions, there were no differences in overall caloric intake or reported hunger and appetite. The second study was also conducted with normal weight men and compared energy intake following one night of total sleep deprivation and one night of 8 hour TIB (Benedict et al., 2011). Despite reported increases in hunger following sleep deprivation, there were no observed differences in caloric or macronutrient intake at a buffet when comparing sleep deprivation to the rested condition.
A number of differences in study designs may account for differences in findings to date. These include sex of the participants, timing and presentation of food (e.g., buffet or fixed meal, continuous availability of food upon request), and variability in how sleep restriction was executed (e.g., circadian timing of sleep, difference in sleep length between conditions) (Schmid et al., 2009; St-Onge, 2013). Given marked variability in study designs it is difficult to identify key factors that could account for differences in findings. Despite this variability, findings from these studies have been used to argue that increasing sleep duration should be promoted for weight loss (Ayas, 2010; Sivak, 2006). To our knowledge, only two studies of the effects of sleep duration on eating behaviors have included overweight/obese participants (Bosy-Westphal et al., 2008; Nedeltcheva et al., 2009). Bosy-Westphal and colleagues (2008) found that, as a whole, normal weight and overweight/obese participants gained weight and reported increased food intake during a decreasing TIB period. However, interpretation of these findings is limited due to lack of randomization. The second study with overweight participants found increased consumption of energy under conditions of sleep restriction (Nedeltcheva et al., 2009); however, once participants’ baseline weight and treatment period were controlled for in analyses, this effect was no longer significant.
Before prescribing increased sleep duration to promote weight loss in overweight/obese individuals, it is critical to show that changes in sleep length affect eating-related behaviors and appetite-regulating hormones within this population. We therefore performed a randomized study to examine whether changes in sleep are associated with eating pathways within overweight and obese women in a controlled experimental setting. We hypothesized that participants would report greater hunger and consume more total energy and a greater percent of energy from fat at a buffet meal following two nights of sleep restriction compared to two nights of extended sleep. Our secondary hypotheses were that sleep restriction would result in lower fasting levels of leptin and higher fasting ghrelin, glucose, insulin, and homeostatic model assessment of insulin resistance (HOMA-IR).
Methods
Participants
Participants had to be 25-55 years old and have a BMI between 25 and 40 kg/m2. Additional eligibility criteria were consistent with previous experimental studies (Markwald et al., 2013; Nedeltcheva et al., 2009; Schmid et al., 2009; St-Onge et al., 2011). Participants had to report sleeping 6-8 hours per night on ≥ 4 nights per week (with ≤ 3 hour difference on all other nights), take <1 nap per week, and report a “typical” nocturnal sleep-wake schedule (i.e., no shift work). In addition, participants were required to be healthy and report no chronic health problems, severe psychiatric conditions, or acute illnesses. Participants needed to report liking foods used in the study and have no dietary restrictions or allergies that would preclude participation. Individuals with narcolepsy, chronic insomnia (Pittsburgh Sleep Quality Index [PSQI] > 5), excessive daytime sleepiness (Epworth Sleepiness Scale > 10), symptoms of obstructive sleep apnea or current use of continuous positive airway pressure treatment, or use of medications that could affect sleep were excluded. Additional exclusions included excessive alcohol (> 7 drinks per week for women or > 14 drinks per week for men) or caffeine (> 360 mg of caffeine per day) intake, significant weight gain or loss within the previous month (> 10 lbs), and prolonged travel beyond two time zones in the month prior to participation.
One hundred seventy participants were screened to identify 21 (13%) eligible participants. Primary reasons for ineligibility included BMI being outside of the eligible range (22%), reported sleep being outside of the eligible range (12%), and use of medications affecting sleep (12%). Four participants were found to be ineligible post consent leaving seventeen participants who were randomized. Of these 17 participants, two (12%) participants dropped out due to scheduling conflicts, and one (6%) participant was withdrawn from the study for not adhering to the schedule the week before an overnight stay. Fourteen participants (12 female; 2 male) completed the study; because of the small sample of men and inability to stratify by sex, our primary analyses focus on the women only. On average, these 12 women were 41.7 ± 10.3 years with a mean BMI of 31.0 ± 4.2 kg/m2 (range = 25.8-38.4); 92% were non-Hispanic White.
Procedures
All study procedures were approved by the Institutional Review Board at Lifespan. Participants were recruited between August and December 2010 through advertisements in local newspapers and the internet, and with posted flyers. Written informed consent was obtained, and participants were paid for participation. To habituate individuals to the experimental setting and to confirm that participants did not evidence obstructive sleep apnea, all participants completed an adaptation night in the laboratory prior to commencing the study. TIB during the adaptation night was consistent with participants’ pre-study self-reported TIB. A within-subject design was used, in which all subjects completed two nights of long sleep (9 hours TIB) and two nights of short sleep (5 hours TIB; for two participants it was 4 hours TIB) in counterbalanced order at the sleep laboratory with a minimum of seven days between conditions. This created a 4 to 5 hour TIB difference between conditions. To approximate “real world” changes in sleep and control for the influence of wake time on measured hormones, changes in TIB were made by changing bedtimes; wake times remained consistent, and were based on each participant's reported free-living wake time. To control for any potential influence of extreme changes in sleep prior to each experimental condition on study outcomes, participants were asked to maintain a fixed sleep schedule that was consistent with their self-reported sleep schedule (7.5-8 hour time in bed per night). This was confirmed by having participants wear an Ambulatory Monitoring, Inc (AMI) Motionlogger® on their non-dominant wrist, complete bedtime and rise time call-ins to a time stamped answering machine, and complete sleep diaries for the five nights preceding each condition. Activity data were collected in 1-minute epochs, and were downloaded and scored using the Sadeh algorithm (Sadeh, Sharkey, & Carskadon, 1994) in Action W version 2.6.9905 (AMI, Ardsley, NY).
Across both conditions, participants were placed on fixed schedules with all study procedures being conducted at identical times post-wake. The only timing that differed between conditions was the prescribed bedtime. Participants arrived at the laboratory on Friday evening and remained through Sunday at noon. They were supervised throughout their stay and were not permitted to leave the facility nor engage in any structured physical activity. Participants were allowed to self-select meals/snacks during their first laboratory stay, and were given identical foods and portions during their second stay. Specifically, participants were provided with sandwich options on Friday evening, utilized meal replacement bars and shakes on Saturday, and then fasted for approximately 12 hours until the buffet meal on Sunday. Participants reported their subjective feelings of hunger during the first night at the laboratory, upon waking each morning, and every two hours post wake using 100 mm visual analogue scales (Spiegel et al., 2004). After the second night of sleep, participants had fasting blood samples drawn approximately 15 minutes after waking, were provided an opportunity to shower and dress, completed study questionnaires and measures, and were presented with a buffet meal approximately three hours after waking. All outcomes (with the exception of hunger ratings) were measured by staff blinded to treatment condition.
Measures
Anthropometrics
Prior to participation in the experimental sleep conditions, height and weight were measured by trained staff using a balance beam scale and wall-mounted stadiometer. Participants were measured in street clothes and without shoes. Body mass index (BMI) was calculated as kg/m2.
Sleep
Sleep was continuously recorded by polysomnography (PSG) in the laboratory during the scheduled sleep episodes and monitored by trained technicians. PSG included central, occipital and frontal referential electroencephalogram (EEG) derivations (C3/A2 and C4/A1; O1/A2 and O2/A1; Fp1/A2 and Fp2/A1), along with right and left electrooculogram (EOG), electromyogram (EMG; mentalis, submentalis), and electrocardiogram (modified lead IV). EEG electrode placements were measured using the international 10-20 system (Jasper, 1958). Breathing variables (pressure transducer airflow, thoracic and abdominal respiration, saturation of peripheral oxygen, end tidal carbon dioxide, plethsymography, thermistor) and leg EMG were recorded to screen for sleep disordered breathing or periodic limb movement disorder.
The TWin system (Astromed, Grass, West Warwick, RI) was used for the digital PSG acquisition; signals were collected unfiltered with TWin AS40 bedside amplifiers and filtered off-line (high-pass EEG filter 0.3 Hz; low-pass filter 35 Hz; notch filter 60 Hz). These signals were collected digitally with a sampling resolution of 400 Hz. Sleep stages were scored visually from digital records off-line in 30-second epochs using C3/A2, EOG, and EMG tracings according to the criteria of Rechtschaffen and Kales (1968). The following measures were analyzed for this report: total sleep time (TST; minutes of sleep scored within the scheduled sleep episode), sleep onset latency (SOL; minutes from lights out to the first of 3 consecutive epochs of sleep), wake after sleep onset (WASO; minutes of wake after falling asleep and before final arousal), minutes of non-rapid eye movement (NREM) Stages 2, and SWS (stages 3 + 4), and minutes of rapid eye movement (REM) sleep. Sleep efficiency (TST/Time in Bed) and percent time spent in each sleep stage were also calculated.
Assays
Blood samples were collected approximately 15 minutes after waking on the last day of each condition following an overnight fast; timing from the last meal remained constant across conditions. EDTA plasma was stored at −80C until the end of the study. 4-(2-Aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF: Sigma Aldrich, St. Louis, MO) was added to the blood collection tubes to protect acylated ghrelin from degradation. All samples were run in duplicate in the same assay. Leptin was measured by radioimmunoassay (Millipore/Linco Research, Inc. St Charles, MO), which has a within assay coefficient of variation (CV) of 4.7% at a leptin concentration of 15.7 ng/mL. Acylated ghrelin was measured by double antibody sandwich ELISA (Millipore/Linco Research, Inc. St Charles, MO) which has a within assay CV of 3.2% at 397 pg/mL. Glucose was measured using the glucose oxidase method (Roche COBAS MIRA Clinical Analyzer) and insulin quantitated by radioimmunoassay (Millipore/Linco Research, Inc. St Charles, MO). HOMA-IR was calculated as fasting insulin (μU/ml) X fasting glucose (mg/dL) divided by 405, according to standard procedures.
Hunger
Hunger was assessed on the first night in the laboratory, upon waking each morning, and approximately every two hours thereafter on each day in the lab. Following the procedure used by Spiegel and colleagues (2004) subjects were asked “How hungry do you feel right now?” and indicated their response on a 100-mm visual analog scale (anchored with “not at all hungry” and “extremely hungry”).
Food Intake
Participants were served a buffet meal approximately three hours after waking on the last day of each condition after completion of scheduled lab activities. The buffet included a large selection of food items, including eggs, breakfast meats, muffins/danish, fruit, yogurt, cheese, and beverages with each item served in large quantities and pre-weighed to the nearest gram. Participants were seated in front of the buffet and allowed a minimum of 20 minutes to eat, with participants indicating after that time when they were finished. At the end of the meal, all items were re-weighed to the nearest gram and the pre-post changes were used to determine consumption. This measure has been used in previous trials and shown to be reliable (Arvaniti et al., 2000; Druce et al., 2006; Klausen et al., 1999).
Statistical analysis
Hypotheses were tested with a general linear mixed effect modeling framework, adjusting for clustering within person over the repeated measurement occasions. Models and parameter estimates were obtained with Stata software (version 13, College Station, Texas). Specifically, we regressed aspects of sleep (Table 1), total energy intake, participant weight, and macronutrient composition at the morning buffet (Table 2), and metabolic variables (Table 3) on a dummy indicator reflecting whether the outcome followed a night of 9 (the reference condition) or 5 hours of sleep. For hunger, we employed a multilevel, random effects regression model in which the outcome of hunger was nested within condition, which was nested within subject. After obtaining estimates for the total effect of study condition, we repeated the models introducing fixed effects for order (i.e., whether the participant slept 9 then 5 or 5 then 9 hours), retest or practice (to control for differences due to the second measurement occasion), and age.. Because significant differences were observed for participant weight across the two conditions, body mass index (BMI) was treated as a time varying covariate. These analyses were run both with and without the two male participants. With only two men we cannot report on findings stratified by sex. Thus to eliminate the possible confound of sex on study outcomes, main findings are presented for women only.
Table 1.
Unadjusted means (Standard Deviations) and associated regressions of sleep variables on study condition controlling for order, retest/practice, participant age, and participant BMI (N = 12).
| Short Sleep1 (5 hr TIB) | Long Sleep1 (9hr TIB) | Coef2 | p-value | |
|---|---|---|---|---|
| Sleep Period | 283 ± 23 | 518 ± 14 | −234.97 | < .001 |
| Sleep Onset Latency (SOL) | 8 ± 7 | 24 ±14 | −15.75 | < .001 |
| Total Sleep Time | 261 ± 26 | 446 ± 31 | −186.16 | < .001 |
| Wake After Sleep Onset (WASO) | 23 ± 20 | 74 ± 31 | −51.03 | < .001 |
| Stage 1 | 15 ± 11 | 34 ± 19 | −19.06 | < .001 |
| Stage 2 | 140 ± 32 | 265 ± 33 | −125.37 | < .001 |
| Slow Wave Sleep (SWS) | 59 ± 34 | 64 ± 46 | −5.48 | 0.37 |
| REM | 47 ± 16 | 84 ± 21 | −36.10 | < .001 |
| Sleep Efficiency (%) | 90 ± 7 | 82 ± 6 | 0.07 | .002 |
| Percent Time Stage 13 | 6 ± 4 | 8 ± 5 | −0.02 | 0.02 |
| Percent Time Stage 23 | 53 ± 10 | 59 ± 7 | −0.06 | 0.02 |
| Percent Time SWS3 | 22 ± 13 | 14 ± 9 | 0.08 | < .001 |
| Percent Time REM3 | 18 ± 7 | 19 ± 5 | 0.00 | 0.94 |
All values are unadjusted Means ± SD
Reported coefficients and associated p-values control for order, retest, participant age, and participant BMI.
Percentage values for sleep stages are based on TST.
Table 2.
Unadjusted means (Standard Deviations) and associated regressions of blood chemistry variables controlling for order, retest/practice, participant age, and BMI on study condition (N = 12).
| Short Sleep1 (5 hr TIB) | Long Sleep1 (9 hr TIB) | Coeff2 | p-value | |
|---|---|---|---|---|
| Leptin (ng/ml) | 36.2 ± 13.1 | 35.9 ± 12.3 | −0.43 | 0.73 |
| Ghrelin (pg/ml) | 379.4 ± 103.0 | 404.7 ± 140.6 | −23.29 | 0.09 |
| Insulin (uU/ml) | 26.5 ± 16.2 | 27.6 ± 15.0 | −1.89 | 0.06 |
| Glucose (mg/dl) | 92.7 ± 9.4 | 90.9 ± 9.4 | 1.35 | 0.10 |
| HOMA-IR | 6.3 ± 4.4 | 6.4 ± 4.0 | −0.33 | 0.24 |
All values are unadjusted means ± SDs.
Reported coefficients and associated p-values control for order, retest, participant age, and participant BMI.
Table 3.
Unadjusted means (Standard Deviations) and associated regressions of total energy and macronutrient variables controlling for order, retest/practice, participant age, and BMI on study condition (N = 12).
| Short Sleep (5 hr TIB) | Long Sleep (9 hr TIB) | Coeff | p- value | |
|---|---|---|---|---|
| Energy (kcal) | 1051 ± 393 | 1119 ± 350 | −57.53 | .44 |
| Percent kcal from Fat | 41 ± 7 | 43 ± 7 | −0.02 | .06 |
| Percent kcal from Carbohydrates | 45 ± 7 | 44 ± 8 | 0.00 | .68 |
| Percent kcal from Protein | 15 ± 3 | 13 ± 3 | 0.02 | .01 |
1 All values are unadjusted means ± SDs.
2 Reported coefficients and associated p-values control for order, retest, participant age, and participant BMI.
Results
Given that findings were consistent across models, findings from models controlling for all potential confounders are presented below and in associated tables.
Sleep Variables
Mean bedtimes for the two nights of sleep in the laboratory were 2155 (range: 2030-2315) during the long condition and 0156 (range: 0100-0300) during the short condition. Mean rise times throughout the study were 0647 (range: 0530-0730) during the long condition and 0640 (range: 0530-0730) during the short condition. Table 1 presents data on observed mean differences in sleep measures during the short and long sleep conditions, and associated coefficients from regression analyses controlling for order, retest/practice, participant age and BMI. As expected, mean TST was shorter during short than the long sleep condition (p < .001) as were WASO (p < .001), time spent in Stage 2 (p < .001), and time spent in REM sleep (p < .001). Mean SOL also differed between the short and long conditions with shorter SOL during the short condition (p < .001). There were no differences in SWS (p = 0.43). Findings were consistent when the two male participants were included.
Blood Chemistries
Effects of sleep pattern on biochemical measures are shown in Table 2. We found no significant difference in blood chemistries between the short and long conditions (p's > .05). When the two male participants were included in analyses, findings were largely consistent. However, inclusion of the two males resulted in significant differences in insulin with slight elevations of mean (SD) insulin during the long (35.0 [26.2]) compared to the short sleep condition (32.4 [24.5]) (p = .03).
Self-Reported Hunger and Measured Food Intake
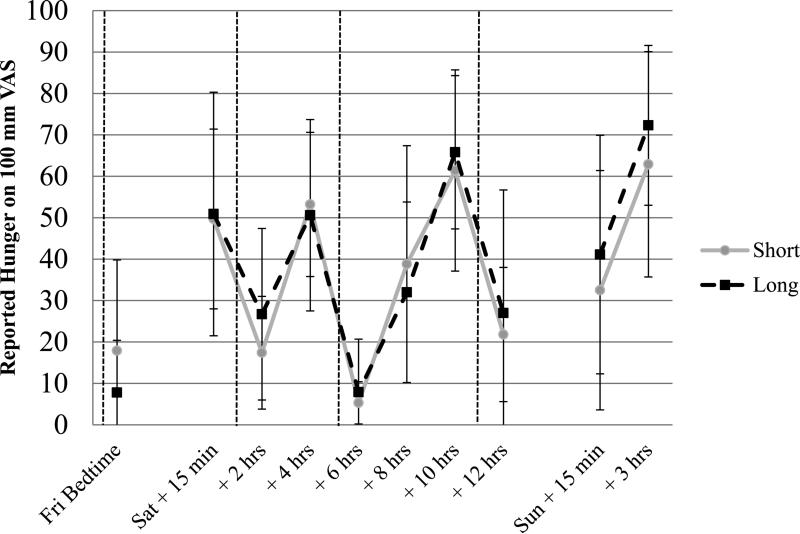
Figure 1 illustrates self-reported hunger ratings. There was a significant main effect for time of day on hunger ratings, as would be expected, with increasing hunger before meals and decreasing hunger after meals, χ2 = 203.4, p < .001. However, hunger ratings showed neither a main effect of sleep condition nor a sleep condition by time interaction with and without controlling for order, retest/practice, age, and BMI.
Figure 1.
Unadjusted means (Standard Deviations) of subjective hunger ratings during short and long sleep conditions (N = 12).
Note. All ratings were obtained at identical times during each condition, and were specific to individual participant's prescribed schedules. The first ratings were obtained at the participant's prescribed bedtime on Friday. Saturday reports were obtained 15 minutes after waking, and then every two hours thereafter; Sunday ratings were obtained 15 minutes after waking and then right before consuming the buffet meal (approximately 3 hours post-wake). Dotted vertical lines indicate when meals were consumed. Breaks in the data indicate when the sleep period occurred. Because hunger values cannot be negative, lower limits of standard deviations were truncated at zero.
Short = Short Condition; Long = Long Condition; Fri=Friday; Sat=Saturday; Sun=Sunday; Bed=Bedtime.
Macronutrient intake during the buffet meal is shown in Table 3. There were no significant differences between the short and long sleep conditions for total energy intake with participants consuming over 1,000 kcal at the buffet during each condition. There were also no differences in percent of energy consumed from fat or carbohydrates, but a greater percentage of energy consumed from protein during the long than the short sleep condition (p = 0.01). All findings were consistent across unadjusted and adjusted models. There was considerable variability in change in energy intake across participants with five participants consuming fewer calories, three participants consuming approximately the same, and four participants consuming more calories following the short versus the long sleep conditions. Hunger and food intake findings were consistent when the two male participants were included in analyses.
Discussion
The present study is one of the first to examine the effect of experimental manipulations of sleep duration on hunger, food intake, and appetite regulating hormones in a sample of overweight/obese women. Although PSG data documented marked differences in TST and sleep architecture, we found few significant effects of study condition on other measures. There were no significant effects of sleep condition on metabolic outcomes. There was an effect of condition on the percent of calories from protein, which was greater after short sleep than long. However, we found no differences in hunger or overall energy consumption at a buffet meal.
Although we observed overall signs of insulin resistance (ADA, 2010) as would be expected for an overweight/obese sample, we did not find a significant effect of the mean 181 minute difference in sleep duration across condition on fasting insulin, glucose or HOMA. Although previous studies that carefully controlled energy intake have found differences in leptin and ghrelin upon sleep restriction (Mullington et al., 2008; Spiegel, Leproult et al., 2004; Spiegel, Tasali, Penev & van Cauter, 2004; Benedict et al., 2011; Schmid et al., 2008; Spiegel, Tasali et al., 2004), similar to the present study, others that have likewise controlled energy balance or had participants fast during the overnight sleep period did not find consistent differences (Benedict et al., 2011; Schmid et al., 2008; St-Onge et al., 2012). It is also important to note that relatively small sample sizes as well as differences in sampling rates and timing of blood chemistry, and/or variability in how sleep restriction was executed (e.g., circadian timing) may contribute to variability in findings to date (Schmid et al., 2009).
We observed expected changes in hunger ratings over the course of the day, but no difference in hunger ratings between conditions. Similarly, although we found increased percentage of calories from protein following sleep restriction, there was no difference in overall caloric intake at the buffet meal. It is important to note that the buffet included large quantities and variety of food, which may have led to excessive intake (greater than 1,000 kcal on average) and overwhelmed the effect of sleep on food intake. Brondel and colleagues (2010) compared a 4 hour TIB to an 8 hour TIB condition and found no observed differences in food intake at a lunch buffet with participants consuming comparably large quantities of food (i.e., > 1,200 kcal). However, significant differences in food intake in that study were observed for breakfast and dinner, which were not served in a buffet style. Thus, it is possible that focusing on a single buffet meal in the present study precluded our ability to detect changes in energy intake across multiple meals. Yet although three previous studies found significant differences in food intake across multiple eating bouts when comparing partial sleep restriction to a rested condition (Brondel et al., 2010; Markwald et al., 2013; St-Onge et al., 2011) two others found no significant effects (Benedict et al., 2011; Schmid et al., 2009). In addition to the influence of the buffet meal itself, similar to its influence on metabolic measures, we cannot rule out an influence of circadian timing on our null findings (i.e., that the buffet meal may have occurred at different circadian phases). Moreover, there are a number of differences in design across studies, including how food intake was measured and the timing of food intake measurement, which make it difficult to identify any given factor as the reason for variability in findings to date.
An important strength of the present study was that it used a well-controlled, experimental design in which sleep was effectively manipulated. In addition, objective assessment of food intake was obtained. The novel focus on overweight/obese adults is also a significant strength. However, our findings must be interpreted within the context of the experimental design that was used, and different design decisions may well have led to different outcomes. Limitations include a small number of participants who were studied; however the observed data provide no evidence to suggest that a larger sample might have changed conclusions. We also focused on women, whereas other studies only focused on men, and we did not control for phase of menstrual cycle. Due to the study design, which focused on eating pathways linking sleep and obesity risk as well as limited opportunity to engage in physical activity, we did not assess changes in physical activity in the present study. Furthermore, hormone measures as well as measures of food intake were each assessed at a single time point early in the day. Mounting evidence suggests that differences in food intake based on changes in sleep length may arise in the evening hours (Nedeltcheva et al., 2009; Markwald et al., 2013; Hart et al., 2013). Thus future studies should consider aligning the timing of assessments of food intake to include the full 24-hour period or to focus on later in the day, which may be a period of higher risk. Lastly, we focused on the effect of acute changes in sleep on study outcomes. It is possible that longer exposure to different sleep lengths may be needed to demonstrate hypothesized differences in measured hormones and eating behaviors.
In conclusion, acute changes in sleep duration resulted in increased percent of calories from protein at a buffet meal. However, these acute changes were not associated with differences in metabolic outcomes, reported hunger, or overall energy intake in a sample of adults who are overweight or obese. When considered in light of inconsistent findings with normal weight adults, further study is needed to clarify the extent of the effect of sleep duration on these weight and eating-related variables.
Acknowledgements
We thank all of the staff for their help in conducting the study and collecting data: Jessica Lawton, Jennifer Trautvetter, Rachel Ogilvie, Christopher Davila, Sara Cournoyer, Stephen Godbout, Alyssa Cairns, Deborah Ranslow-Robles, Jose DaCruz, Will Coon, Ellyn Ferriter, Jon Lassonde, Katie Esterline, David Bushnell, Margaret Gordon-Fogelson, Jena Burgner, Erin Campopiano, Gretchen Surhoff, Tifenn Raffray, Caroline Gredvig-Ardito, and Brown University students. We thank Joseph L. Fava, Ph.D. and Douglas Tommet, Ph.D. for assistance in some of the statistical analysis. We also thank the participants for their invaluable participation in the study.
Drs. Wing, Carskadon, Hart, Sharkey conceived of the study and its design. Drs. Raynor and Considine assisted with the study design. Drs. Demos, Van Reen, Carskadon and Sharkey helped execute the study. Dr. Considine developed procedures for analyzing blood chemistries and performed analyses. Dr. Jones conceived of and performed data analysis. All authors were involved in writing the paper and had final approval of the submitted version.
Competing Interests: This study was funded by the National Cancer Institute grant U01 CA150387-01 (RRW). The funding agency played no role in the design and conduct of the study. The authors declare no conflicts of interest immediately related to this article.
References
- American Diabetes Association Introduction: The American Diabetes Association's (ADA) evidence-based practice guidelines, standards, and related recommendations and documents for diabetes care. Diabetes Care. 2012;35:S1–S2. doi: 10.2337/dc12-s001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvaniti K, Richard D, Tremblay A. Reproducibility of energy and macronutrient intake and related substrate oxidation rates in a buffet-type meal. British Journal of Nutrition. 2000;83:489–495. [PubMed] [Google Scholar]
- Ayas NT. If you weigh too much, maybe you should try sleeping more. Sleep. 2010;33:143–4. doi: 10.1093/sleep/33.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict C, Hallschmid M, Lassen A, Mahnke C, Schultes B, Schiöth HB, Lange T. Acute sleep deprivation reduces energy expenditure in healthy men. American Journal of Clinical Nutrition. 2011;93:1229–1236. doi: 10.3945/ajcn.110.006460. [DOI] [PubMed] [Google Scholar]
- Bosy-Westphal A, Hinrichs S, Jauch-Chara K, Hitze B, Later W, Wilms B, Muller MJ. Influence of partial sleep deprivation on energy balance and insulin sensitivity in healthy women. Obesity Facts. 2008;1:266–273. doi: 10.1159/000158874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men. American Journal of Clinical Nutrition. 2010;91:1550–1559. doi: 10.3945/ajcn.2009.28523. [DOI] [PubMed] [Google Scholar]
- Cappuccio FP, Taggart FM, Kandala NB, Currie A, Peile E, Stranges S, Miller MA. Meta-analysis of short sleep duration and obesity in children and adults. Sleep. 2008;31:619–626. doi: 10.1093/sleep/31.5.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Caro JF. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. New England Journal of Medicine. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- Druce MR, Neary NM, Small CJ, Milton J, Monteiro M, Patterson M, Bloom SR. Subcutaneous administration of ghrelin stimulates energy intake in healthy lean human volunteers. International Journal of Obesity. 2006;30:293–296. doi: 10.1038/sj.ijo.0803158. [DOI] [PubMed] [Google Scholar]
- Hart CN, Carskadon MA, Considine R, Fava JL, Lawton J, Raynor HA, Wing RR. Changes in children's sleep duration on food intake, weight, and leptin. Pediatrics. 2013;132:e1473–80. doi: 10.1542/peds.2013-1274. doi: 10.1542/peds.2013- 1274. [DOI] [PubMed] [Google Scholar]
- Jasper HH. The ten-twenty electrode system of the International Federation. Electroencephalography and Clinical Neurophysiology. 1958;10:371–375. [PubMed] [Google Scholar]
- Klausen B, Toubro S, Ranneries C, Rehfeld JF, Holst JJ, Christensen NJ, Astrup A. Increased intensity of a single exercise bout stimulates subsequent fat intake. International Journal of Obesity & Related Metabolic Disorders. 1999;23:282–287. doi: 10.1038/sj.ijo.0801074. [DOI] [PubMed] [Google Scholar]
- Klingenberg L, Chaput JP, Holmback U, Jennum P, Astrup A, Sjodin A. Sleep restriction is not associated with a positive energy balance in adolescent boys. American Journal of Clinical Nutrition. 2012;96:240–248. doi: 10.3945/ajcn.112.038638. [DOI] [PubMed] [Google Scholar]
- Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, Wright KP., Jr. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proceedings of the National Academies of Sciences of the United States of America. 2013;110:5695–5700. doi: 10.1073/pnas.1216951110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullington JM, Chan JL, Van Dongen HP, Szuba MP, Samaras J, Price NJ, Mantzoros CS. Sleep loss reduces diurnal rhythm amplitude of leptin in healthy men. Journal of Neuroendocrinology. 2003;15:851–4. doi: 10.1046/j.1365-2826.2003.01069.x. [DOI] [PubMed] [Google Scholar]
- Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. American Journal of Clinical Nutrition. 2009;89:126–133. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. A manual of standardized terminology, techniques, and scoring system for sleep stage of human subjects. 1968 doi: 10.1046/j.1440-1819.2001.00810.x. [DOI] [PubMed] [Google Scholar]
- Schmid SM, Hallschmid M, Jauch-Chara K, Born J, Schultes B. A single night of sleep deprivation increases ghrelin levels and feelings of hunger in normal-weight healthy men. Journal of Sleep Research. 2008;17:331–334. doi: 10.1111/j.1365-2869.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- Schmid SM, Hallschmid M, Jauch-Chara K, Wilms B, Benedict C, Lehnert H, Schultes B. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. American Journal of Clinical Nutrition. 2009;90:1476–1482. doi: 10.3945/ajcn.2009.27984. [DOI] [PubMed] [Google Scholar]
- Sivak M. Sleeping more as a way to lose weight. Obesity Reviews. 2006;7:295–296. doi: 10.1111/j.1467-789X.2006.00262.x. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Leproult R, L'Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. Journal of Clinical Endocrinology and Metabolism. 2004;89:5762–5771. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- Spiegel K, Tasali E, Penev P, Van Cauter E. Brief communication: Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels, and increased hunger and appetite. Annals of Internal Medicine. 2004;141:846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- St-Onge MP, O'Keefe M, Roberts AL, RoyChoudhury A, Laferrere B. Short sleep duration, glucose dysregulation and hormonal regulation of appetite in men and women. Sleep. 2012;35:1503–1510. doi: 10.5665/sleep.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Onge MP, Roberts AL, Chen J, Kelleman M, O'Keeffe M, RoyChoudhury A, Jones PJ. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. American Journal of Clinical Nutrition. 2011;94:410–416. doi: 10.3945/ajcn.111.013904. [DOI] [PMC free article] [PubMed] [Google Scholar]