Abstract
Cry1Ac protoxin has potent mucosal and systemic adjuvant effects on antibody responses to proteins or polysaccharides. In this work, we examined whether Cry1Ac increased protective immunity against fatal Naegleria fowleri infection in mice, which resembles human primary amoebic meningoencephalitis. Higher immunoglobulin G (IgG) than IgA anti-N. fowleri responses were elicited in the serum and tracheopulmonary fluids of mice immunized by the intranasal or intraperitoneal route with N. fowleri lysates either alone or with Cry1Ac or cholera toxin. Superior protection against a lethal challenge with 5 × 104 live N. fowleri trophozoites was achieved for immunization by the intranasal route. Intranasal immunization of N. fowleri lysates coadministered with Cry1Ac increased survival to 100%; interestingly, immunization with Cry1Ac alone conferred similar protection to that achieved with amoebal lysates alone (60%). When mice intranasally immunized with Cry1Ac plus lysates were challenged with amoebae, both IgG and IgA mucosal responses were rapidly increased, but only the increased IgG response persisted until day 60 in surviving mice. The brief rise in the level of specific mucosal IgA does not exclude the role that this isotype may play in the early defense against this parasite, since higher IgA responses were detected in nasal fluids of mice intranasally immunized with lysates plus either Cry1Ac or cholera toxin, which, indeed, were the treatments that provided the major protection levels. In contrast, serum antibody responses do not seem to be related to the protection level achieved. Both acquired and innate immune systems seem to play a role in host defense against N. fowleri infection, but further studies are required to elucidate the mechanisms involved in protective effects conferred by Cry1Ac, which may be a valuable tool to improve mucosal vaccines.
Our group has reported that the Cry1Ac protoxin of Bacillus thuringiensis is highly immunogenic (25, 33) and has mucosal and systemic adjuvant effects, since it increases specific antibody responses to proteins such as bovine serum albumin and the hepatitis B surface antigen (34), and to polysaccharides such as the type 6 capsular pneumococcal polysaccharide and a polyvalent pneumococcal polysaccharide vaccine (26). Cry1Ac has additional advantages over other mucosal adjuvants (16, 30): it is nontoxic to vertebrates, and its production costs are low (13, 14). Cry1Ac may be an attractive candidate to improve the efficacy of vaccines against mucosal infections; however, it had not been evaluated for the ability to confer protective immunity.
The model we selected to test if Cry1Ac increased protective immunity is the experimental mouse model of Naegleria fowleri meningoencephalitis, a fatal acute infectious disease initiated at the nasal mucosa (18, 21). N. fowleri is a free-living, ubiquitous, amphizoic protozoon (28). Human infection by N. fowleri causes a fatal disease of the central nervous system called primary amoebic meningoencephalitis that leads to death 3 to 7 days after exposure. Victims of this fatal disease are usually healthy young individuals with a history of recent swimming in freshwater or warm heated pools (4, 35).
Several attempts have been made to induce protective immunity against experimental N. fowleri in mice, but the study of immunity in experimental and natural infections has been limited to the analysis of serum antibody responses (3, 9, 10, 19, 32). The immunization protocols used to induce protection against intranasal challenge with N. fowleri amoebae in mice, including different antigens— such as N. fowleri amoebal lysates, live and fixed amoebae, and culture medium—administered by the intranasal (i.n.), intravenous, and intraperitoneal (i.p.) routes, have led to variable results with partial protective immunity (3, 9, 10, 19, 32). Therefore, additional strategies are clearly needed to achieve total protective immunity against experimental primary amoebic meningoencephalitis.
To analyze protective immunity against N fowleri infection, we evaluated the survival to the i.n. lethal challenge with live N. fowleri amoebae in mice that previously had been immunized by the i.n. or the i.p. routes with amoebal lysates either alone or coadministered with two potent mucosal adjuvants: either Cry1Ac or cholera toxin (CT). Anti-N. fowleri antibody responses in serum and tracheopulmonary and nasopharyngeal fluids were also analyzed.
Our results show that the adjuvant effect of Cry1Ac is protective per se and increases protective immunity against experimental N. fowleri meningoencephalitis. Mucosal but not serum antibody levels seem to be related to protection against N. fowleri infection. Interestingly, i.n. immunization with Cry1Ac or CT alone confers similar protection to immunization with amoebal lysates. Our data support the notion that both the innate and acquired immune systems play a role in host defense against N. fowleri infection. However, further studies are needed to elucidate the mechanisms of the protective adjuvant effect conferred by Cry1Ac. Additionally, this work supports the potential utility of Cry1Ac in the development of vaccines against amoebae and other microbial pathogens entering through the nasal mucosa.
MATERIALS AND METHODS
N. fowleri cultures and maintenance of amoebal virulence.
N. fowleri ATCC 30808 (American Type Culture Collection, Manassas, Va.) was cultured axenically at 37°C in Bacto-Casitone broth (Difco, Le Pont de Claix, France) supplemented with 10% bovine serum (GIBCO, Grand Island, N.Y.). The virulence of N. fowleri was reactivated by serial passage in mice. The amoebae were intranasally inoculated into male BALB/c mice, and after 4 days, samples of brain from infected mice were cultured axenically at 37°C for no more than 1 month before another mouse passage. Only freshly recovered virulent amoebae that were passaged through mice a minimum of six times were used to infect control and immunized mice and evaluate protection. The virulence of the amoebae was verified by analyzing their lethality. All animals were handled in accordance with Mexican federal regulations for animal experimentation and care (NOM-062-ZOO-1999, Ministry of Agriculture, Mexico City Mexico) and approved by the Institutional Animal Care and Use Committee.
Lethal dose of N. fowleri amoebae.
To determine the lethality of the N. fowleri amoebal strain and choose the lethal dose for the rest of the work (i.e., the minimal amount of i.n. instilled amoebae required to kill all infected animals before day 10), four groups of 10 mice were intranasally challenged with 2.5 × 104, 5 × 104, 7.5 × 104, or 1 × 105 virulent live amoebae and the mortality rate was determined after the infected animals were monitored for up to 60 days. The lethal dose selected was 5 × 104 amoebae, since this dose killed all infected animals by day 9 after inoculation.
Amoebal antigens. (i) Fixed amoebae.
Trophozoites were chilled and harvested during the logarithmic phase of growth (72 h); after centrifugation at 1,500 × g for 10 min, the amoebae were washed three times with phosphate-buffered saline (0.14 M NaCl, 2.7 mM KCl, 1.5 mM KH2PO4; 8.1 mM Na2HPO4 [pH 7.2]) (PBS), the supernatants were discarded, the trophozoites were adjusted to 106 per ml and fixed with 0.25% glutaraldehyde in PBS for 1 h, and fixed amoebae were washed with PBS and suspended at a density of 106 in 25 μl of PBS for immunization. Fixed amoebae were dead since they did not show evidence of movement and did not replicate after culture.
(ii) Amoebal lysates.
Amoebae were harvested as described above; the supernatants were discarded, and the cellular pellets were washed three times with PBS. To prevent proteolysis, pellets containing 5 × 107 trophozoites were suspended in 3 ml of PBS containing 5 mM p-hydroxymercuribenzoic acid (Sigma Chemical Co., St. Louis, Mo.). Amoebae were disrupted by sonication with one 10-s pulse at 100 W of amplitude (Fisher Sonic Dismembrator model 300) and immersed in boiling water for 10 min. The resulting lysates were stored at −70°C and examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The protein concentration was determined by the Bradford method (2).
Recombinant Cry1Ac.
Escherichia coli JM103(pOS9300) was kindly donated by D. Dean, Ohio State University. The recombinant strain was grown in Luria-Bertani medium containing 50 μg of ampicillin per ml, and Cry1Ac production was induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) (13). Recombinant Cry1Ac was purified from IPTG-induced E. coli JM103(pOS9300) cultures as follows. Cell pellets harvested by centrifugation were suspended in 50 mM Tris-HCl-50 mM EDTA (pH 8) (TE buffer) and sonicated (Fisher Sonic Dismembrator Model 300) three times for 5 min in ice; inclusion bodies were collected by centrifugation at 10,000 × g for 10 min; and pellets were washed twice with TE buffer, twice with 0.5 M NaCl, once with 0.5 M NaCl-1% Triton X-100, once with 0.5 M NaCl, and once with cold distilled water and finally solubilized in CBP buffer (0.1 M Na2CO3 1% 2-mercaptoethanol [pH 9.6]). Particulate material was discarded by centrifugation at 10,000 × g for 10 min, and the purified solubilized protoxin was stored at 4°C and examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The protein concentration was determined by the Bradford method (2).
Immunization.
In all experiments, 8- to 10-week-old male BALB/c mice were used. Antigens were administered by the i.n. or i.p. route. For i.n. immunization, mice were lightly anesthetized with ethyl ether and the antigen (in 30 μl of PBS) was delivered into the nostrils. Each experimental group contained 20 animals, to which four antigen doses were applied on days 1, 7, 14, and 21 by the i.n. or i.p. route. The antigens applied to each group were (i) 106 fixed amoebae, (ii) amoebal lysate (100 μg of protein), (iii) amoebal lysate plus 50 μg of Cry1Ac, (iv) amoebal lysate plus 1 μg of CT (Sigma Chemical Co.), (v) 50 μg of Cry1Ac, and (vi) 1 μg of CT. Control mice received 30 μl of PBS. Ten mice from each group were sacrificed on day 28, and serum and mucosal samples were collected from them. The 10 mice remaining in each group were challenged with live virulent amoebae.
i.n. challenge with live amoebae.
Ten mice from each group were lightly anesthetized with ethyl ether, and 5 × 104 virulent N. fowleri amoebae in 30 μl of PBS were delivered into the nostrils of each mouse. The mice were examined daily for at least 60 days. Death from meningoencephalitis was confirmed by autopsy and recovery of amoebae in axenic culture, and the survival rate for each experimental group was estimated.
Sample collection.
Serum samples were obtained from blood extracted by cardiac puncture from ether-anesthetized mice. Tracheopulmonary fluid was collected as described previously (26); the trachea and lungs were excised and washed thoroughly with cold RPMI medium to eliminate contaminating blood; 1 ml of cold medium was then flushed through the trachea until it transudated through the lungs and was collected in a 5-ml petri dish. Washes were centrifuged at 4°C for 10 min at 8,000 × g, and their supernatants were frozen immediately and stored at −70°C. Nasal fluids were also collected from some mice. Exsanguinated mice were decapited, the heads were rinsed with ice-cold RPMI medium to remove blood, a polyethylene tube was inserted via the oropharynx into the nasopharyngeal cavity, and contents of the nasal passages from individual mice were washed out of the nares with 1 ml of ice-cold RPMI. Samples that became contaminated with blood during collection were discarded. At least five nasal samples per treatment were obtained.
Anti-N. fowleri antibodies.
Anti-N. fowleri antibody levels in serum and mucosal samples were determined for nonimmunized mice and in the following experimental groups: (i) mice that were immunized with fixed amoebae, with lysates alone, or with Cry1Ac or CT as described above and were sacrificed 7 days after the last immunization; (ii) surviving mice that had been immunized i.n. with lysates plus Cry1Ac, CT alone, or Cry1Ac alone and that were sacrificed 60 days after a lethal challenge with N. fowleri; and (iii) mice that were immunized i.n. with lysates plus Cry1Ac and were sacrificed at certain times (1, 2, 3, and 4 days) after i.n. lethal challenge with N. fowleri.
Antibody levels in sera and mucosal samples were determined by an indirect enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well plates were coated with 100 μl of N. fowleri lysate (10 μg of protein/ml) in carbonate bicarbonate buffer (15 mM Na2CO3, 35 mM NaHCO3 [pH 9.6]). The plates were incubated for 2 h at 37°C and washed three times with 0.05% Tween-20 in PBS (PBST). Blocking was performed by treatment with PBST plus 6% fat-free milk and by further washing with PBST. Each sample was tested in duplicate. Serum samples were diluted 1:1,000 in 1% fat-free milk dissolved in PBST. Tracheopulmonary and nasal fluid samples were not diluted. The plates were incubated overnight at 4°C and washed with PBST; then 100 μl of goat anti-mouse anti-immunoglobulin G (IgG), anti-IgM (Pierce, Rockford, Ill.), anti-IgA (Zymed Laboratories, San Francisco, Calif.), or horseradish peroxidase-labeled or biotinylated goat anti-mouse IgG1 or IgG2a secondary antibodies (Zymed Laboratories) were added per well, and the plates were incubated for 2 h at room temperature. Plates incubated with biotinylated antibodies for IgG subclass analysis were washed with PBST, and then 100 μl of conjugated horseradish peroxidase-streptavidin was added per well and the plates were incubated for 2 h at room temperature. The plates were washed with PBST, and the enzymatic reactions were started by adding substrate solution (0.5 mg of o-phenylenediamine per ml− plus 0.01% H2O2 in 0.05 M citrate buffer [pH 5.2]). After 15 min, the reactions were stopped with 25 μl of 2.5 M H2SO4 and the absorbance at 492 nm (A492) was measured in a Multiscan Ascent (Thermo Labsystems) microplate reader.
Calculations and statistics.
In the figures, bars represent mean A492 values for antibody levels from each experimental group, along with standard deviations (SD). Significant differences in antibody levels between pairs of groups were determined using two-way analysis of variance followed by a Tukey test, or a log-rank-test for survival assays with the PRISM computer program (GraphPad, San Diego, Calif.) P < 0.05 was considered statistically significant.
RESULTS
Protection and survival.
To analyze protective immunity in experimental N. fowleri infection, we evaluated survival to the i.n. lethal challenge with 5 × 104 live N. fowleri amoebae in immunized mice.
The survival of control and immunized mice that first received four i.n. or i.p. doses of fixed amoebae, amoebal lysates alone, lysates plus Cry1Ac, or lysates plus CT and then were challenged i.n. with a lethal dose of virulent live amoebae is shown in Fig. 1. All unimmunized mice challenged with live amoebae died within 9 days. Different survival rates were achieved with each treatment. For i.n. immunization, mice immunized i.n. with lysates plus Cry1Ac had the highest protection percentage (100%), which was significantly higher than those achieved in mice immunized with lysate alone (60%) or fixed amoebae (40%) or in control mice (P < 0.05) but was not statistically different from the group immunized with lysate plus CT (80%) (P > 0.05). For i.p. immunization, the highest protection percentage was achieved in mice immunized i.p. with lysates plus Cry1Ac or CT (80%), and this percentage was significantly higher than those achieved in mice immunized with fixed amoebae (40%) or in control mice (P < 0.05) but were not different from the group immunized with lysate alone (60%) (P > 0.05). Surprisingly, mice immunized by the i.n. route with Cry1Ac or CT alone (Fig. 2) had the same survival rate (60%) as those immunized with lysates alone (Fig. 1). All experimental groups were repeated twice with identical results.
FIG. 1.
Survival of mice immunized with amoebal lysate alone or coadministered with an adjuvant after a lethal i.n. challenge with live N. fowleri amoebae. Four weekly doses of 106 fixed amoebae (•), amoebal lysate (100 μg of protein) alone (•), amoebal lysate plus 50 μg of Cry1Ac (▾), or amoebal lysate plus 1 μg of CT (▪) were administered to male BALB/c mice by the intranasal (i.n.) or intraperitoneal (i.p.) route. A lethal challenge of 5 × 104 amoebae was applied i.n. to groups of 10 immunized or control (▿) mice 7 days after the last antigen dose.
FIG. 2.
Survival of mice immunized with only Cry1Ac or CT after a lethal i.n. challenge with live N. fowleri amoebae. Four weekly doses of 50 μg of Cry1Ac (•) or 1 μg of CT (○) were administered to male BALB/c mice by the i.n. or i.p. route. A lethal challenge with 5 × 104 amoebae was applied i.n. to groups of 10 immunized and control mice (▾) 7 days after the last antigen dose.
Anti-N. fowleri serum antibody responses in immunized mice.
In general, mice immunized with fixed amoebae or amoebal lysates either alone or coadministered with Cry1Ac or CT by the i.n. or i.p. route elicited high serum IgG and IgM anti-N. fowleri responses (P < 0.001). In contrast, IgA responses were low with all the immunization protocols by both routes (Fig. 3). The magnitude of the antibody responses varied with the route and treatment used. When the i.p. route was used, coadministration of Cry1Ac or CT with lysates had any adjuvant effect on the antibody response of the IgG and IgM isotypes; in fact, the IgG responses elicited by these treatments were significantly lower than those achieved with lysates alone. CT had a slight adjuvant effect on serum IgA response when it was administered by the i.p. route.
FIG. 3.
Anti-N. fowleri antibody responses in serum and tracheopulmonary fluid. Four weekly doses of 106 fixed amoebae, amoebal lysate (100 μg of protein), amoebal lysate plus 50 μg of Cry1Ac, or amoebal lysate plus 1 μg of CT were administered to male BALB/c mice by the i.n. (filled bars) or i.p. (open bars) route. Anti-N. fowleri IgA, IgG, and IgM antibody levels were determined by ELISA in serum samples diluted 1:1,000 and undiluted tracheopulmonary fluid samples. Individual samples were run in duplicate. Mean A492 values ± SD, from each experimental group (n = 10) are shown. Significant differences (P < 0.05) in the levels of each antibody isotype between groups are indicated as follows: •, versus control group; +, versus group immunized with lysate alone; *, versus immunization route.
Cry1Ac coadministration via the i.n. route had a small adjuvant effect on the IgG and IgA antibody responses, which were significantly higher than those achieved by i.n. immunization with lysates (P < 0.001). In contrast, CT did not have any adjuvant effect on serum IgM and IgG antibody responses. Mice immunized i.n. with fixed amoebae had the lowest IgG responses (P < 0.001).
Anti-N. fowleri antibody responses in tracheopulmonary fluids.
All immunization protocols induced significant tracheopulmonary IgG and IgM but not IgA anti-N. fowleri antibody responses (P < 0.001); only very low IgA responses were induced by i.n. immunization with lysates plus Cry1Ac (Fig. 3). In general, as found for serum samples, the magnitude of the tracheopulmonary response of the antibody isotypes analyzed also depended on the treatment and route used. Similar high levels of specific IgG responses were induced in mice immunized with either amoebal lysate or fixed amoebae by the i.p. or i.n. route. Cry1Ac had a clear adjuvant effect on the specific IgG antibody response in tracheopulmonary fluid samples after administration by the i.p. or i.n. route (P < 0.001). In contrast, CT coadministration did not have any adjuvant effect on the IgG response.
Similar high IgM responses in tracheopulmonary fluid were induced by i.p. immunization with fixed amoebae, amoebal lysates alone, or lysates plus Cry1Ac. When the i.n. route was used, the highest IgM responses were elicited with lysates alone.
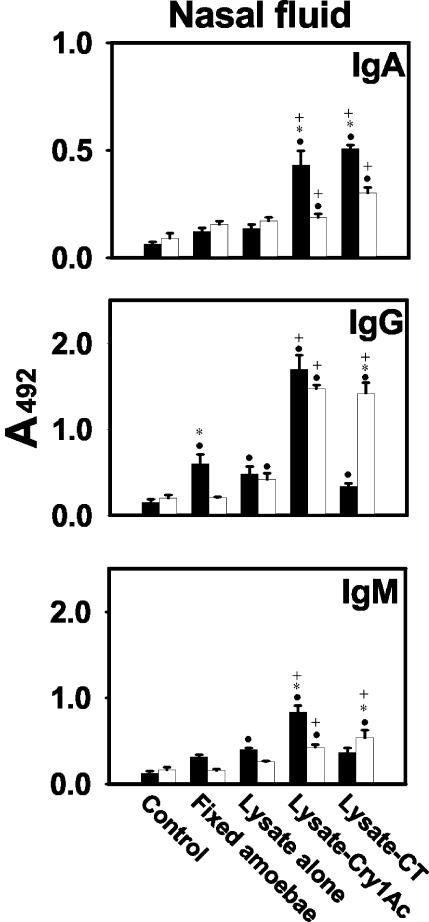
Anti-N. fowleri antibody response in nasal fluids.
Both Cry1Ac and CT had adjuvant effects on IgA responses when administered by both routes (P < 0.001). When administered via the i.p. route, neither lysates alone nor fixed amoebae elicited significant antibody responses, but when the lysates were coadministered with Cry1Ac or CT, the IgG responses were significantly increased (P < 0.001). Also, higher IgG than IgA responses were detected in nasal fluid samples (Fig. 4). Although IgA responses in nasal fluids from mice immunized with lysates plus either Cry1Ac or CT by both routes were moderate, they were higher than those found in tracheopulmonary fluids (P < 0.001). Nasal samples were collected in a more diluted state than were tracheopulmonary fluid samples since we collected the same volume of both fluids and the nasopharyngeal tract is smaller.
FIG. 4.
Anti-N. fowleri antibody responses in nasopharyngeal fluid. Four weekly doses of 106 fixed amoebae, amoebal lysate (100 μg protein), amoebal lysate plus 50 μg of Cry1Ac, or amoebal lysate plus 1 μg of CT were administered to male BALB/c mice by the i.n. (solid bars) or i.p. (open bars) route. Anti-N. fowleri IgA, IgG, and IgM antibody levels were determined by ELISA in undiluted nasopharyngeal samples. Individual samples were run in duplicate. Mean A492 values ± SD from each experimental group (n = 5) are shown. Significant differences (P < 0.05) in the levels of each antibody isotype between groups are indicated as follows: •, versus control group; +, versus group immunized with lysate alone; *, versus immunization route.
IgG subclasses in serum.
All i.n. and i.p. immunizations induced higher IgG1 than IgG2a responses in both serum and tracheopulmonary fluid samples (P < 0.001) (Fig. 5). All i.p. immunizations elicited high IgG1 and moderate IgG2a responses in serum; these responses were higher than those achieved via i.n. immunization (P < 0.001), except when mice were immunized with lysates plus Cry1Ac, which elicited similar high IgG1 responses by both routes. Neither Cry1Ac nor CT coadministration had any adjuvant effect on serum IgG1 and IgG2a responses for i.p. immunization. However, Cry1Ac increased the IgG1 antibody responses compared to lysate alone when administered by the i.n. route. Only very low serum IgG2a responses were induced with lysate alone or coadministered with Cry1Ac via the i.n. route.
FIG. 5.
IgG subclasses of anti-N. fowleri antibodies from serum and tracheopulmonary fluid. Four weekly doses of 106 fixed amoebae, amoebal lysate (100 μg of protein), amoebal lysate plus 50 μg of Cry1Ac, or amoebal lysate plus 1 μg of CT were administered to male BALB/c mice by the i.n. (solid bars) or i.p. (open bars) route. Anti-N. fowleri IgG1 and IgG2a antibody levels were determined by ELISA in serum samples diluted 1:1,000 and undiluted tracheopulmonary fluid samples. Individual samples were run in duplicate. Mean A492 values ± SD from each experimental group (n = 10) are shown. Significant differences (P < 0.05) in the levels of each antibody isotype between groups are indicated as follows: •, versus control group; +, versus group immunized with lysate alone; *, versus immunization route.
IgG subclasses in tracheopulmonary fluids.
Both routes elicited significant IgG1 and IgG2a anti-N. fowleri responses in tracheopulmonary fluids (P < 0.001). Higher IgG1 and IgG2a antibody responses were elicited via the i.p. than the i.n. route, except when mice were immunized with lysates alone. Both Cry1Ac and CT exhibited adjuvant effects on the IgG1 and IgG2a anti-N. fowleri antibody responses when administered via the i.p. route. However, the highest IgG1 response was achieved with lysates alone administered via the i.n. route and with fixed amoebae administered via the i.p. route (Fig. 5).
Anti-N. fowleri antibody response in surviving mice.
Mice immunized i.n. with amoebic lysates plus Cry1Ac and that survived the lethal challenge with amoebae and were sacrificed 60 days after challenge showed similar low IgA antibody responses in both serum and tracheopulmonary fluids, to those found in mice that were only immunized and sacrificed 7 days after the last immunization (P < 0.001) (Fig. 6). IgG responses were higher in both serum and tracheopulmonary fluids of surviving mice than in the same samples from mice that were only immunized (P < 0.001). The IgM response in the serum of surviving mice was higher than in the serum mice that were not challenged (P < 0.001).
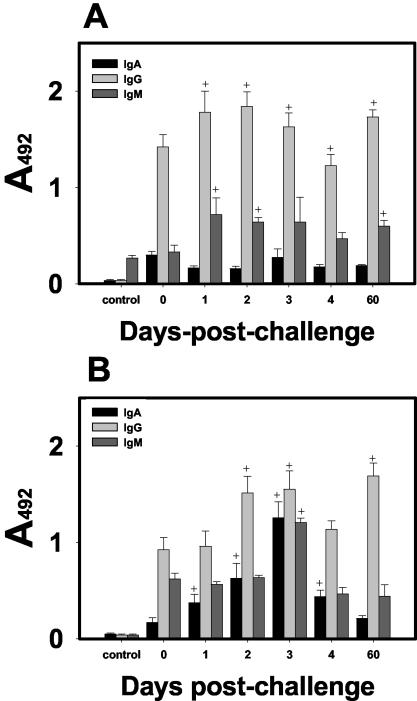
FIG. 6.
Anti-N. fowleri antibodies in serum and tracheopulmonary fluid of mice immunized and sacrificed 7 days after the last immunization or at different times after the lethal challenge. Four weekly doses of amoebal lysate (100 μg of protein) plus 50 μg of Cry1Ac were administered to mice by the i.n. route, and they were (i) sacrificed 7 days after the last immunization (bar 0), (ii) challenged in with 5 × 104 virulent amoebae and sacrificed at different early days (bars 1 to 4), or (iii) sacrificed 60 days later (bar 60). Anti-N. fowleri IgA, IgG, and IgM antibody levels were determined by ELISA in serum samples diluted 1:1,000 (A) and undiluted tracheopulmonary fluid samples (B). Control mice were not immunized or challenged. Individual samples were run in duplicate. Each data point shows the man A492 value mean ± SD (n = 5). Significant differences (P < 0.05) in the levels of each antibody isotype between groups are indicated as follows: +, versus group immunized and sacrificed 7 days after the last immunization.
Interestingly, mice that were immunized with either Cry1Ac or CT alone and survived a lethal challenge with amoebae did not elicit serum antibodies to N. fowleri antigens (data not shown).
Antibody response in immunized mice at early times after lethal challenge with N. fowleri.
The antibody levels in serum and tracheopulmonary fluid samples recorded at certain early days after i.n. challenge with N. fowleri in mice that have been immunized i.n. with lysates plus Cry1Ac, are shown in Fig. 6. In tracheopulmonary fluids, IgA antibody levels were increased during a short period (1 to 3 days) after inoculation of amoebae. The maximal IgA response was detected on day 3 but declined by day 4, suggesting that this isotype may participate in the early defense against this parasite. In contrast, the IgA response in serum remained low at all times tested. In serum and tracheopulmonary fluids, IgG was the highest isotype response detected at all analyzed times (except at 3 days, when the levels of the three analyzed isotypes were similar in tracheopulmonary fluids). IgG mucosal levels increased rapidly since day 1 after challenge and continued to be high until day 60. In serum, IgM antibody levels were slightly increased and maintained at similar moderate levels. However, in tracheopulmonary fluids, the IgM response was increased only at 3 days postchallenge.
DISCUSSION
Our group has found that the Cry1Ac protoxin from B. thuringiensis is highly immunogenic and has mucosal and systemic adjuvant effects when administered to mice by systemic and mucosal routes (25, 26, 33, 34). However, the protoxin had not been tested to determine whether coadministration of Cry1Ac with antigens could increase protective immunity in a mucosal infection model. The major finding of the present work is the proof that Cry1Ac increases protective immunity against experimental N. fowleri meningoencephalitis in mice. The i.n. coadministration of Cry1Ac with amoebal lysates significantly increased survival to 100% in mice challenged with a lethal dose of amoebae, whereas immunization with amoebal lysates alone provided 60% protection. Interestingly, administration of either Cry1Ac or CT alone also had protective effects against N. fowleri infection, since both adjuvants increased survival as did immunization with amoebal lysates alone.
The protection attained by Cry1Ac coadministration with amoebal lysates was significant with respect to that provided by immunization with lysates alone but not statistically different from that achieved by CT coadministration (80%), which is considered the most potent mucosal adjuvant (16, 30). Moreover, the use of Cry1Ac as adjuvant has two additional advantages over CT: it is nontoxic for vertebrates, and its production cost is lower (13, 14).
Several aspects of the innate immune system, such as complement (15, 27, 29, 36), phagocytic cells such as macrophages (5, 6) or neutrophils (8, 9), and cytokines (8, 12, 24), appear to play an important role in resistance to Naegleria infection. The high protection rates achieved by administration of Cry1Ac or CT alone (60%), as well as the fact that mice which survived N. fowleri infection and which had been treated with one these proteins did not elicit significant antibody responses to N. fowleri (data not shown), suggest that both mucosal adjuvants can stimulate innate immune mechanisms. Accordingly, it has been reported that administration of CT alone confers a protective adjuvant effect against influenza (22), supporting the notion that CT can stimulate innate immunity.
A number of attempts have been made to immunize mice against infection by N. fowleri. In general, total protective immunity was not achieved in mice except when five doses of a fraction of N. fowleri culture fluid were injected i.p. (32). Even if supernatants of N. fowleri culture medium used as immunogen achieve similar protection to that attained with lysates plus Cry1Ac, the procedure is much more laborious and the amount of antigen recovered is much smaller than that obtained from total amoebal lysates. On the other hand, the protection rates obtained with supernatants appear to be highly variable, since the five fractions tested yielded a 31% protection average (range, 18 to 57%) (32).
The quality and quantity of amoebal lysate used to immunize mice may also influence the protection induced. We obtained 60% protection by both i.n. and i.p. routes in mice receiving four doses of amoebal lysates, but other groups have obtained lower rates (30%) after five i.p. immunizations with higher doses of amoebal lysate protein (0.5 mg) than the one used in this work (32).
Inoculations with live N. fowleri have afforded highly variable protection against infection (3, 9, 19). These variations may be attributed to differences in the virulence of the amoebae used (which, in several studies, is not considered) and to the difficulty in evaluating protection against an acute fatal infection induced by inoculating live microorganisms (19). To control these variables, we first reactivated the virulence of amoebae, then established the lethal dose of virulent amoebae needed to challenge the mice, and finally used amoebal lysates to immunize the mice.
In the studies in which mice have been immunized to achieve protection against N. fowleri infection, serum antibodies but not mucosal immune responses were analyzed (3, 9, 10, 19, 32). In this work, we found that mice immunized by the i.n. or i.p. routes with N. fowleri lysates alone or coadministered with either Cry1Ac or CT elicited significant antibody responses in both serum and mucosal samples. Notably, in view of the infection route of this parasite, i.n. coadministration of either Cry1Ac or CT, which were the treatments that conferred the highest protection, had adjuvant effects on anti-N. fowleri antibody responses elicited in fluids of the nasal mucosa.
While inoculation of Cry1Ac by the i.n. route had only a small adjuvant effect on the serum and the tracheopulmonary antibody responses and inoculation via the i.p. route increased only the tracheopulmonary IgG responses, inoculation of CT did not increase the serum or tracheopulmonary antibody responses. The lack of adjuvanticity of CT on antibody responses has been previously reported (1, 17). It has been proposed that this reduction might be characteristic of whole-cell vaccines or other particulate antigens, possibly because CT induces tolerance to the immunogen or redirects the immune response toward itself (1).
It has been suggested that protective immunity to N. fowleri is mediated mainly by serum IgG, since protection against N. fowleri is associated with increasing specific IgG antibody titers and can be transferred by immune serum but not by spleen cells (10). We found that higher IgG than IgA antibody responses were induced in both serum and mucosal samples of immunized mice. However, our data suggest that serum IgG antibody responses did not correlate with protection against N. fowleri infection, since the highest protective effect was achieved by i.n. immunization but the highest serum IgG antibody responses were elicited by the i.p. route. In contrast, mucosal antibody responses may participate in the host defense against N. fowleri infection. In mice immunized i.n. with Cry1Ac plus lysates, both IgG and IgA responses in mucosal tracheopulmonary fluid were rapidly increased when the mice were challenged with amoebae. Although the rise in specific mucosal IgA response was brief compared to the mucosal IgG response, which persisted at a high level until day 60 in surviving mice, this does not exclude a role for this isotype in the initial defense against this parasite, since N. fowleri infection is rapidly established (18, 21). We have observed that some amoebae invade the nasal epithelium as early as 24 h following i.n. inoculation and that numerous amoebae are found in the olfactory bulb, where tissue damage is severe, after 4 days (21; S. Rojas-Hernandez, unpublished data). Moreover, significantly higher IgA responses were elicited exclusively in nasal fluids of mice immunized i.n. with lysates plus either Cry1Ac or CT, which indeed were the treatments that provided the major protection levels.
The role of antibodies in protection against N. fowleri infection is controversial, since it has been observed in vitro that when trophozoites interact with IgG or IgA antibody, amoebae can recover from antibody by capping, internalizing, and shedding the antibody (11, 31). Nevertheless, it has been suggested that IgA may participate in the resistance to amoebic invasion by inhibiting the adherence of the trophozoites to nasal mucosa, since IgA antibodies that recognize N. fowleri surface proteins are able to inhibit the adhesion of trophozoites to collagen (31).
Induction of mucosal IgA responses has been correlated with protection against several viral, bacterial, protozoan, and helmintic pathogens (20). However, a protective role of mucosal IgG antibodies against mucosal infections cannot be excluded. Indeed, protective mucosal immunity against influenza, in an IgA knockout mouse model, seems to be mediated by IgG and IgM antibodies (23). Accordingly, our data showing that IgA and IgG antibody responses were significantly increased in mucosal samples of mice immunized i.n. with lysates plus Cry1Ac suggest that induction of both antibody isotypes in the nasal mucosa may be related to the high rates of protection against N. fowleri infection in this experimental group.
On the other hand, our results suggest that immunization with amoebal lysates either alone or coadministered with Cry1Ac or CT may elicit mainly Th2-type responses, since higher IgG1 than IgG2a responses were elicited by all immunization treatments in serum and mucosal samples by both routes. In several studies in which CT has been used as a mucosal adjuvant to enhance the responses to coadministered protein antigens (7, 30), it has been found that this protein induces Th2-type cytokines to itself and enhances Th2-type responses to coadministered proteins (16, 30, 37, 38).
Finally, although several pieces of evidence suggest that both innate and adaptive immune mechanisms participate in host defense against N. fowleri infection, further studies are required to elucidate the mechanisms involved in protective effects conferred by Cry1Ac against N. fowleri infection.
In summary, the present study demonstrates that Cry1Ac is an effective mucosal adjuvant to improve protective immunity to N. fowleri infection. The data presented also support the potential utility of this protoxin to improve vaccination against pathogens invading the nasal mucosa.
Acknowledgments
We thank Javier Varona Santos for reviewing the manuscript.
This work was supported by CONACyT 34834 and UNAM DGAPA PAPIIT IN207800 and IN213903 grants.
Editor: J. D. Clements
REFERENCES
- 1.Berstad, A. K. H., J. Holst, B. Mogster, I. L. Haugen, and B. Heneberg. 1997. A nasal whole-cell pertussis vaccine can induce strong systemic and mucosal antibody responses which are not enhanced by cholera toxin. Vaccine 15:1473-1478. [DOI] [PubMed] [Google Scholar]
- 2.Bradford, M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein, utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 3.Bush, L. E., and D. T. John. 1988. Intranasal immunization of mice against Naegleria fowleri. J. Protozool. 35:172-176. [DOI] [PubMed] [Google Scholar]
- 4.Carter, R. F. 1970. Description of a Naegleria sp. isolated from two cases of primary amebal meningo-encephalitis, and of the experimental pathological changes induced by it. J. Pathol. 100:217-244. [DOI] [PubMed] [Google Scholar]
- 5.Cleary, S. F., and F. Marciano-Cabral. 1986. Activated macrophages demonstrate direct cytotoxicity, antibody-dependent cellular cytotoxicity, and enhanced binding of Naegleria fowleri amoebae. Cell Immunol. 98:125-136. [DOI] [PubMed] [Google Scholar]
- 6.Cleary, S. F., and F. Marciano-Cabral. 1986. Soluble amebicidal factors mediate cytolysis of Naegleria fowleri by activated macrophages. Cell. Immunol. 101:62-71. [DOI] [PubMed] [Google Scholar]
- 7.Elson, C. O., and M. T. Dertzbaugh. 1994. Mucosal adjuvants, p. 391-401. In P. L. Ogra, J. Mestecky, M. E. Lamm, W. Strober, J. R. McGhee, and J. Bienenstock (ed.), Handbook of mucosal immunology, Academic Press, Inc., San Diego, Calif.
- 8.Ferrante, A. 1989. Augmentation of the neutrophil response to Naegleria fowleri by tumor necrosis factor alpha. Infect. Immun. 57:3110-3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrante, A. 1991. Free-living amoebae: pathogenecity and immunity. Parasite Immunol. 13:31-47. [DOI] [PubMed] [Google Scholar]
- 10.Ferrante, A., and B. Rowan-Kelly. 1988. The role of antibody in immunity against experimental Naegleria meningoencephalitis (“amoebic meningitis”). Immunology 64:241-244. [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrante, A., and Y. H. Thong. 1979. Antibody induced capping and endocytosis of surface antigens in Naegleria fowleri. Int. J. Parasitol. 9:599-601. [DOI] [PubMed] [Google Scholar]
- 12.Fischer-Stenger, K., G. A. Cabral, and F. Marciano-Cabral. 1992. Separation of soluble amoebicidal and tumorocidal activity of activated macrophages. J. Protozool. 39:235-241. [DOI] [PubMed] [Google Scholar]
- 13.Ge, A. Z., R. M. Pfister, and D. H. Dean. 1990. Hyperexpression of a Bacillus thuringiensis delta-endotoxin-encoding gene in Escherichia coli: properties of the product. Gene 93:49-54. [DOI] [PubMed] [Google Scholar]
- 14.Höfte, H., and H. Whiteley. 1989. Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol. Rev. 53:242-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holbrook, T. W., R. J. Boackle, B. W. Parker, and J. Vesely. 1980. Activation of the alternative complement pathway by Naegleria fowleri. Infect. Immun. 30:58-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmgren, J., C. Czerkinsky, K. Eriksson, and A. Mharandi. 2003. Mucosal immunisation and adjuvants: a brief overview of recent advances and challenges. Vaccine 21:89-95. [DOI] [PubMed] [Google Scholar]
- 17.Hordnes, K., T. Tynning, T. A. Brown, B. Haneberg, and R. Jonsson. 1997. Nasal immunization with group B streptococci can induce high levels of specific IgA antibodies in cervicovaginal secretions of mice. Vaccine 15:1244-1251. [DOI] [PubMed] [Google Scholar]
- 18.Jarolim, K. L., J. K. McCosh, M. J. Howard, and D. T. John. 2000. A light microscopy study of the migration of Naegleria fowleri from the nasal submucosa to the central nervous system during the early stage of primary amebic meningoencephalitis in mice. J. Parasitol. 86:50-55. [DOI] [PubMed] [Google Scholar]
- 19.John, D. T. 1993. Opportunistically pathogenic free-living amebae, p. 204-209. In J. P. Kreier and J. R. Baker (ed.), Parasitic protozoa, 2nd ed. Academic Press, Inc., San Diego, Calif.
- 20.Lamm, M. E. 1997. Interaction of antigens and antibodies at mucosal surfaces. Annu. Rev. Microbiol. 51:311-340. [DOI] [PubMed] [Google Scholar]
- 21.Martínez, A. J., R. J. Duma, E. C. Nelson, and F. L. Moretta. 1973. Experimental Naegleria meningoencephalitis in mice. Penetration of the olfactory mucosal epithelium by Naegleria and pathologic changes produced: a light and electron microscopy study. Lab. Investig. 29:121-133. [PubMed] [Google Scholar]
- 22.Matsuo, K., T. Yoshikawa, H. Asanuma, T. Iwasaki, Y. Hagiwara, Z. Chen, S. E. Kadowaki, H. Tsujimoto, T. Kurata, and S. I. Tamura. 2000. Induction of innate immunity by nasal influenza vaccine administered in combination with an adjuvant (cholera toxin). Vaccine 18:2713-2722. [DOI] [PubMed] [Google Scholar]
- 23.Mbawuike, I. N., S. Pacheco, C. L. Acuna, K. C. Switzer, Y. Zhang, and G. R. Harriman. 1999. Mucosal immunity to influenza without IgA: an IgA knockout mouse model. J. Immunol. 162:2530-2537. [PubMed] [Google Scholar]
- 24.Michelson M. K., W. R. Henderson, Jr., E. Y. Chi, T. R. Fritsche, and S. J. Klebanoff. 1990. Ultrastructural studies on the effect of tumor necrosis factor on the interaction of neutrophils and Naegleria fowleri. Am. J. Trop. Med. Hyg. 42:225-233. [DOI] [PubMed] [Google Scholar]
- 25.Moreno-Fierros, L., N. García, R. Gutiérrez, R. López-Revilla, and R. I. Vázquez-Padrón. 2000. Intranasal, rectal and intraperitoneal immuniztation with protoxin Cry1Ac from Bacillus thuringiensis induces compartmentalized serum, intestinal, vaginal and pulmonary immune responses in BALB/c mice. Microb. Infect. 2:885-890. [DOI] [PubMed] [Google Scholar]
- 26.Moreno-Fierros, L., E. J. Ruiz-Medina, R. Esquivel, R. López-Revilla, and S. Piña-Cruz. 2003. Intranasal Cry1Ac protoxin ia an effective mucosal and systemic carrier and adjuvant of Streptococcus pneumoniae polysaccharides in mice. Scand. J. Immunol. 57:45-55. [DOI] [PubMed] [Google Scholar]
- 27.Reilly, M. F., K. L. White., and S. G. Bradley. 1983. Host resistance of mice to Naegleria fowleri infections. Infect. Immun. 42:645-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez-Zaragoza, S. 1994. Ecology of free-living amoebae. Crit. Rev. Microbiol. 20:225-241. [DOI] [PubMed] [Google Scholar]
- 29.Rowan-Kelly, B., A. Ferrante, and Y. H. Thong. 1980. Activation of complement by Naegleria. Trans. R. Soc. Trop. Med. Hyg. 74:333-336. [DOI] [PubMed] [Google Scholar]
- 30.Ryan, E. J., L. M. Daly, and K. H. G. Mills. 2001. Immunomodulators and delivery systems for vaccination by mucosal routes. Trends Biotechnol. 19:293-304. [DOI] [PubMed] [Google Scholar]
- 31.Shibayama, M., J. J. Serrano-Luna, S. Rojas-Hernández, R. Campos-Rodríguez, and V. Tsutsumi. 2003. Interaction of secretory immunoglobulin A antibodies with Naegleria fowleri trophozoites and collagen type I. Can. J. Microbiol. 49:164-170. [DOI] [PubMed] [Google Scholar]
- 32.Thong, Y. H., A. Ferrante, B. Rowan-Kelly, and D. E. O'Keefe. 1980. Immunization with live amoebae, amoebic lysate and culture supernatant in experimental Naegleria meningoencephalitis. Trans. Roy. Soc. Trop. Med. Hyg. 74:570-576. [DOI] [PubMed] [Google Scholar]
- 33.Vázquez-Padrón, R. I., L. Moreno-Fierros, L. Neri-Bazán, G. de la Riva, and R. López-Revilla. 1999. Intragastric and intraperitoneal administration of Cry1Ac protoxin from Bacillus thuringiensis induce systemic and mucosai antibody responses in mice. Life Sci. 64:1897-1912. [DOI] [PubMed] [Google Scholar]
- 34.Vázquez-Padrón, R. I., L. Moreno-Fierros, L. Neri-Bazán, G. de la Riva, and R. López-Revilla. 1999. Bacillus thuringiensis Cry1Ac protoxin is a potent systemic and mucosal adjuvant. Scand. J. Immunol. 49:578-584. [DOI] [PubMed] [Google Scholar]
- 35.Warhurst, D. C. 1985. Pathogenic free-living amoebae. Parasitol. Today 1:24-28. [DOI] [PubMed] [Google Scholar]
- 36.Whiteman, L. Y., and F. Marciano-Cabral. 1989. Resistance of highly pathogenic Naegleria fowleri amoebae to complement-mediated lysis. Infect. Immun. 57:3869-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu-Amano, J. H., H. Kiyono, R. J. Jackson, H. F. Staats, K. Fujihashi, P. D. Burrows, C. O. Elson, S. Pillai and J. R. McGhee. 1993. Helper T cell subsets for immunoglobulin A responses: oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa associated tissues. J. Exp. Med. 178:1309-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu-Amano, J. H., R. J. Jackson, K. Fujihashi, H. Kiyono, H. F. Staats, and J. R. McGhee. 1994. Helper Th1 and Th2 cell responses following mucosal or systemic immunization with cholera toxin. Vaccine 12:903-911. [DOI] [PubMed] [Google Scholar]