Abstract
Pulmonary arterial hypertension (PAH) contributes to morbidity and mortality of patients with lung and heart diseases. We demonstrated that hypoxia induced PAH and increased pulmonary arterial wall thickness in wild-type mice. Mice deficient in toll-like receptor 4 (TLR4−/−) spontaneously developed PAH, which was not further enhanced by hypoxia. Echocardiography determined right ventricular hypertrophy and decreased pulmonary arterial acceleration time were associated with the development of PAH in TLR4−/− mice. In pulmonary arterial smooth muscle cells (PASMC), hypoxia decreased TLR4 expression and induced reactive oxygen species (ROS) and Nox1/Nox4. Inhibition of NADPH oxidase decreased hypoxia-induced proliferation of wild-type PASMC. PASMC derived from TLR4−/− mice exhibited increased ROS and Nox4/Nox1 expression. Our studies demonstrate an important role of TLR4 in maintaining normal pulmonary vasculature and in hypoxia-induced PAH. Inhibition of TLR4, by genetic ablation or hypoxia, increases the expression of Nox1/Nox4 and induces PASMC proliferation and vascular remodeling. These results support a novel function of TLR4 in regulating the development of PAH and reveal a new regulatory axis contributing to TLR4 deficiency-induced vascular hypertrophy and remodeling.
Keywords: Pulmonary Smooth Muscle Cells, Proliferation, Remodeling, Toll-Like Receptor, Oxidative Stress Signals
2. INTRODUCTION
Pulmonary arterial hypertension (PAH) is a disease characterized by vascular proliferation, hypertrophy, and fibrosis, leading to right ventricular hypertrophy, which contributes to the morbidity and mortality of patients with lung and heart diseases (1;2). All forms of pulmonary hypertension have similar clinical presentations, functional derangements and pathologic features regardless of their etiology. The pathogenesis of PAH includes structural remodeling of pulmonary arteries and sustained pulmonary vasoconstriction (2). Vascular remodeling represents a key step in the progression of PAH, which involves medial hypertrophy, as well as intimal and adventitial thickening. These changes are believed to result from an imbalance between proliferation and apoptosis of the various cell types forming the vascular walls (1). Hypertrophy and hyperplasia of smooth muscle cells (SMC) are critical cellular events associated with vascular remodeling of the media. Hypoxia-induced PAH is a common experimental model for PAH. Chronic hypoxia induces vascular remodeling with medial hypertrophy leading to the development of pulmonary hypertension.
Chronic hypoxia-induced PAH is mediated by increased production of reactive oxygen species (ROS). Hypoxia-induced ROS generation has been well documented (3;4). The Nox family of NADPH oxidases is the primary enzyme that transports electrons across the plasma membrane to generate superoxide and other downstream ROS in the vasculature (4). NADPH oxidases play a major role in the regulation of physiological and pathophysiological processes and contribute to a variety of vascular diseases, including hypertension (5), aortic media hypertrophy (6;7), atherosclerosis (5) and pulmonary hypertension (8;9). Increase in NADPH oxidase expression or activity has been demonstrated in hypoxic pulmonary hypertension (9;10). Nox-derived ROS have also been shown to promote cell survival/proliferation (11–15) and inhibit cell death (16). Therefore, ROS-induced increased proliferation and decreased apoptosis may contribute to the abnormal accumulation of pulmonary arterial SMC (PASMC), and thus vascular remodeling in the pathogenesis of PAH.
ROS have been linked to activation of toll-like receptor 4 (TLR4) signaling (17). The toll-like receptors are a group of type I transmembrane proteins that play a central role in specific recognition of pathogen-associated molecular patterns and are critical for the induction of innate immunity and inflammation (18). They can also cause significant immunopathology if over-activated or insufficiently controlled (19). TLRs are mainly expressed on antigen-presenting cells, such as macrophages and dendritic cells. In addition, expression of these receptors has currently been described on a variety of other cells, including the cells of the arterial wall (20;21). Expression of these innate immune receptors, especially TLR4 in healthy and pathological arteries has implicated its role in the homeostasis of vasculature. Hypoxia has been shown to decrease TLR4 expression in endothelial cells via increased ROS (22). On the other hand, TLR4 deficiency increased ROS production in lung endothelial cells (23). However, the function of TLR4 in regulating ROS production and proliferation of PASMC is unknown. Using a hypoxia-induced PAH mouse model, the present studies investigated the role of TLR4 in the pathogenesis of PAH and the underlying mechanisms. We found that TLR4 deficient mice spontaneously developed PAH, which was not enhanced by hypoxia. Our studies suggest that TLR4 plays an important role in maintaining normal pulmonary vasculature, and that hypoxia induces PAH via TLR4. Mechanistic studies further demonstrated increased expression of Nox1 and 4 in primary PASMC from TLR4−/− mice. Furthermore, hypoxia induced the expression of Nox1 and Nox4, whereas inhibition of Nox activity blocked hypoxia-induced proliferation of PASMC. Taken together, these studies support a novel connection between TLR4 innate immune signal and redox signaling in PASMC in regulating vascular remodeling during pathogenesis of PAH.
3. MATERIALS AND METHODS
3.1. Experimental Animals
TLR2 and TLR4 deficient mice, originally provided by S. Akira (24) (Osaka University, Osaka, Japan), are maintained and bred under specific pathogen-free conditions at the animal facility at University of Alabama at Birmingham (UAB). All knockout mice used were back-crossed with wild type C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME) for more than 8 generations. All animal experiments were performed according to protocols approved by UAB Institutional Animal Care and Use Committee.
3.1.1. Hypoxic exposure of animals
Mice at 10 weeks old were exposed to hypoxia (12% oxygen) or normal air (20% oxygen) in a Plexiglas chamber for 6 weeks as previously described (25;26). The O2 concentration (OM-100 oxygen analyzer, Newport Medical Instruments), humidity, temperature, and barometric pressure (Fisherbrand Digital Barometer, Fisher Scientific) within the chamber were monitored continuously. Daily animal maintenance was carried out with exposure of the animals to room air for less than 10 minutes per day. Control animals were caged similarly and exposed to filtered room air for the same periods.
3.1.2. Right ventricular pressure and mass measurement
At the end of six weeks, mice were weighed and anesthetized with a mixture of ketamine (80 mg/kg ip) and xylazine (12 mg/kg ip). Right ventricles (RV) were cannulated in situ by a closed-chest technique for RV pressure (26). After measurement of RV pressure, mice were sacrificed and hearts were isolated for determination of the weights of RV and left ventricle plus septum (LV+S). RV pressure was used as an index of PAH and the development of RV hypertrophy was identified by the ratio of RV/ (LV+S).
3.1.3. Tissue processing and immunohistochemical analysis
For histological and morphometric analysis, the trachea was cannulated using a 24-G angiocath and the lungs were fixed using 10% formalin and then placed in 4% paraformaldehyde for 24 hours before being placed in a tissue cassette for paraffin embedding (25;26). Hematoxylin and eosin (H&E) staining was used for histological analysis. Quantitative morphometric analysis of pulmonary vessels was performed with serial five-micron lung sections by computer assisted image analysis (Bioquant Image Analysis software, R & M Biometrics). The pulmonary artery wall thickness (WT) and vessel diameter (D) were determined along two axes perpendicular to each other in at least 20 consecutive pulmonary arteries cut transversely (longer axis <50% greater than shorter axis). Pulmonary arteries are defined as vessels that accompanied airways (veins are interlobular). Vessels <25 μm in external diameter were not considered for analysis, as wall thickness is not uniform in these vessels. External vessel diameter (distance within external elastic lamella) and medial thickness (distance between external and internal elastic lamellae) were measured; and the wall thickness index of the pulmonary arteries was determined by the percentage of wall thickness to the vessel diameter (2*WT/D) (25). Morphometric analysis was carried out by two independent examiners who were blinded with respect to the treatment assignment of the tissue samples examined.
3.1.4. Doppler echocardiography analysis of cardiac function
Echocardiography was performed with a Vevo770 High-Resolution In vivo Micro-System (VISUALSONICS, Toronto, Ontario, Canada) with a 30-MHz probe designed for examination of small rodents as previously described (27;28). The examination was performed on mice under general anesthesia with inhalation of 1–2% isoflurane as previously described (28). Left and right parasternal long axis views were used to obtain B-mode two-dimensional cinematic images at 50–70 Hz, from which measurements were made of LV and RV chamber area and cross-sectional area of the LV walls. B-mode images were used to position cursors for high speed (1 KHz) M-mode imaging and pulse wave (PW) doppler measurements. M-mode measurements of ventricular chamber diameter and wall thicknesses were made in a line perpendicular to the long axis of the chamber passing through the tip of the left posterior papillary muscle. RV chamber dilatation (RV end-diastolic dimension, RVEDD), RV hypertrophy (RV free wall thickness at end-diastole, RVFWd and RVFW thickness at end-systole, RVFWs) and RV systolic function (RVFW thickening) were measured.
Doppler echocardiography of the pulmonary outflow was also utilized to estimate pulmonary artery (PA) pressure in mice non-invasively (27;28). Doppler recordings of the main PA were obtained in the right parasternal long axis position. The PA blood flow velocity was measured at the main PA root of the mice. The PA acceleration time (PAAT) was determined from the start to the peak of the flow signal.
3.2. In vitro characterization of pulmonary smooth muscle cells
Pulmonary arteries from wild type and TLR4−/− mice were isolated by microdissection; and PASMC were obtained by explantation and confirmed by immunohistochemical staining of α-SMA as we previously described (29;30). Experiments were performed with SMC maintained in culture for 3 to 5 passages. In all experiments, PASMC were seeded at 80% confluence and starved in serum-free media (DMEM/F12) for 6 hours; subsequentially cells were placed into an incubator with 2% oxygen or normal air for 24 and 48 hours.
3.2.1. ROS measurement
The production of reactive oxygen species (ROS) in PASMC from control and TLR4−/− mice were determined by assessing 2′,7′-dichlorofluorescin diacetate (DCF-DA), an intercellular indicator for oxidative stress using flow cytometry analysis as we previously described (31).
3.2.2. Real-time PCR
The expressions of NOX enzymes in mouse PASMC with or without exposure to hypoxia were determined by Real-time PCR with specific primers: Nox1, F-5′-TGGCTAAATCCCATCCAGTC-3′ and R-5′-CCCAAGCTCTCCTCTGTTTG-3′; Nox4, F-5′-ACTTTTCATTGGGCGTCCTC-3′ and R-5′-AGAACTGGGTCCACAGCAGA-3′; NOXO1, F-5′-TTCCTGATGCTCCATTGCTG-3′ and R-5′-GGTTGGGATAAGGGCTCCTC-3′; NOXA1, F-5′-AGCTGCAGAGGTTCCAGGAG-3′and R-5′-GATGTCTTGAGCCCCCTCTG-3′. The expression of TLR4 was determined using primers: F-5′-GCTTTCACCTCTGCCTT CAC-3′; and R-5′-CGAGGCTTTTCCATCCAATA-3′. The values of each determination were normalized to those of GAPDH, assessed using primers for GAPDH: 5′-TCATCCCTGCATC CACTGGT-3′ and 5′-CTGGGATGACCTTGCC CAC-3′.
3.2.3. Proliferation and apoptosis
The effect of TLR4 signals on apoptosis and proliferation of PASMC under basal and hypoxia conditions were determined by exposure of WT and TLR4−/− PASMC to hypoxia or normal air. The NADPH oxidase inhibitor, diphenyleneiodonium (DPI, Sigma), was used to determine the effects of NADPH oxidase activity on hypoxia-stimulated PASMC proliferation. WT PASMC were incubated with or without DPI (0.1, 0.5 or 2.5μM) and exposed to normal air or hypoxia conditions. Cell proliferation was determined after 24 hours by BrdU incorporation (Calbiochem), and apoptosis was determining by Western blot analysis of the cleaved form of caspase-3 using specific anti-caspase-3 antibody (Enzo lifescience).
3.2.4. Western Blot analysis
Western blot analysis was performed as we previously described. The specific antibodies for Nox1 and Nox4 were purchased from Santa Cruz Biotechnology Inc. The expression of GAPDH (anti-GAPDH antibody, Research Diagnostics Inc.) was used for loading control.
3.3. Statistical analysis
All the data are expressed as means ± SD. Differences in data between two groups were compared with Student’s paired 2-tailed t test. For multiple groups comparison, by one-way analysis of variance followed by a Student-Newman-Keuls test was performed. A p value less than 0.05 was considered statistically significant.
4. RESULTS
4.1. TLR4 deficient mice develop pulmonary hypertension, which is not enhanced by hypoxia
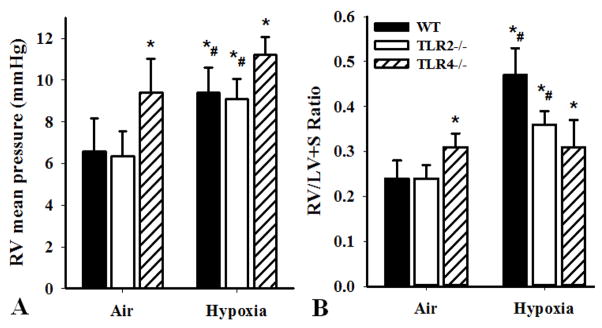
To determine the role of TLR4 in the development of pulmonary hypertension, we compared mice with three genotypes, including TLR2 knock out (TLR2−/−), TLR4 knock out (TLR4−/−) and wild type C57BL6 (WT), in a hypoxia-induced pulmonary hypertension model as previously described (25;26). The development of pulmonary hypertension (PAH) and right ventricle (RV) hypertrophy were determined by the mean RV pressure (Figure 1A) and the ratio of RV/ (LV+S) (Figure 1B) calculated by the weights of RV to left ventricle plus septum (LV+S) in mice exposed to hypoxia or for 6 weeks. We found that hypoxia (12% oxygen) induced pulmonary hypertension in WT and TLR2−/− mice, as indicated by increased RV pressure and RV hypertrophy (Figure 1A&B), compared with air (20% oxygen). In contrast, TLR4−/− mice exposed to air exhibited signs of PAH, including increased RV pressure and RV/ (LV+S) ratio compared with those of WT and TLR2−/− mice exposed to normal air (Figure 1A&B). Furthermore, hypoxia did not enhance PAH in TLR4−/− mice (Fig 1A&B, 3rd bars). These results suggested that TLR4, but not TLR2, signaling is important in the development of PAH.
Figure 1.
Hypoxia-induced pulmonary hypertension in mice. WT, TLR2−/− and TLR4−/− mice (10 weeks old) were exposed to hypoxia (12% oxygen) or air (20% oxygen) for 6 weeks. A). RV mean pressure was measured in situ by a closed-chest technique. B). The RV/ (LV+S) ratio was determined. Results shown are means±SD (n=8 mice in each group, #p<0.05). One-way analysis of variance and Student-Newman-Keuls test were used to identify differences among groups (*p<0.05 compared with WT AIR conditions, the first solid bar in Fig 1A or Fig 1B).
4.2. TLR4 deficiency increases pulmonary arterial muscularization
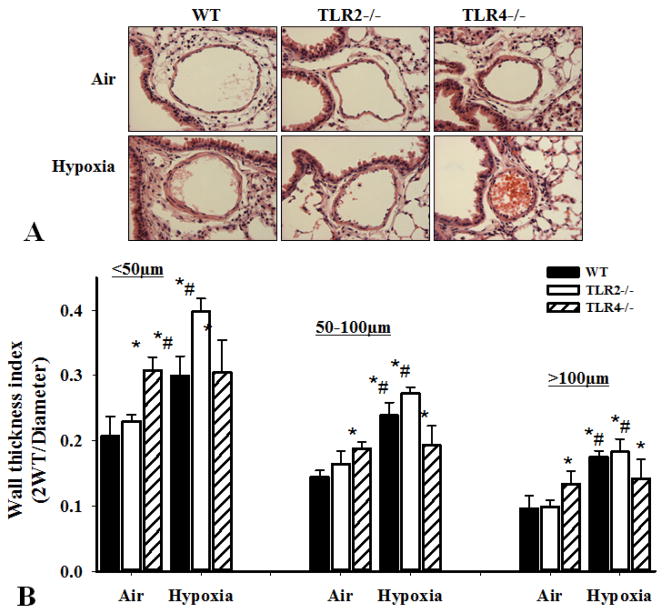
To assess the effect of TLR4 deficiency on hypoxia-induced vascular remodeling, we determined pulmonary arterial muscularization from WT, TLR2−/− and TLR4−/− mice exposed to air or hypoxia for 6 weeks. Histological analysis of the hematoxylin and eosin (H&E) staining of lung sections demonstrated that TLR4−/− mice had increased pulmonary arterial wall thickness in normoxia compared to WT and TLR2−/− mice. Hypoxia increased pulmonary artery wall thickness index in WT and TLR2−/−mice (Figure 2A&B, #p<0.05, hypoxia vs. normal air) but did not further enhance thickening in TLR4−/− mice (Figure 2A&B, #p<0.05 hypoxia vs. normal air).
Figure 2.
Effect of TLR deficiency on vascular muscularization. WT, TLR2−/− and TLR4−/− mice at 10 weeks old were exposed to hypoxia or normal air for 6 weeks. After measurements of RV pressure, lungs were isolated, fixed, and sectioned and immunohistochemical analyses were done. A). Representative pictures of hematoxylin and eosin staining of the lung sections (showing the pulmonary arteries) from the WT, TLR2−/− and TLR4−/− mice exposed to air (upper) and hypoxia (lower). B). Wall thickness index of pulmonary arteries. 10 high-power lung fields were examined in each mouse. Wall thickness was analyzed in three groups of arteries based on their diameters: less than 50μm, 50–100μm and greater than 100μm. Results shown are means±SD (n=8 mice for each group, #p<0.05 hypoxia vs. normal air for each genotype; *p<0.05 compared with WT air in each group).
4.3. Effect of TLR4 deficiency on hemodynamics and RV function
Echocardiography was performed on WT and TLR4−/− mice to characterize the role of TLR4 deficiency on the RV function (Figure 3). Heart rate was 483±14 bpm for WT mice and 454±22 bpm for TLR4−/− mice. We found that the RVFW thickness in TLR4−/− mice at both end-diastole and end-systole was significantly increased compared to WT mice (Figure 3C). RVEDD decreased slightly in the TLR4−/− mice as RVFWd increased, thereby demonstrating a concentric RV chamber hypertrophy with normal wall thickening, indicative of preserved RV systolic function. Thus, these studies demonstrated that TLR4 deficiency in mice was associated with RV hypertrophy with well preserved systolic function at this stage.
Figure 3.
Echocardiography evaluation of right ventricle and pulmonary arteries of wild type and TLR4−/− mice. A). Representative WT mouse B-mode long axis of RV and LV. B). M-mode tracing of the RV captured in a line perpendicular to the long axis of the chamber passing through the tip of the left posterior papillary muscle (left). RVEDD and RVFW thickness at end-diastole (left lines) and systole (right lines). C). In vivo Echo M-mode RV measurements for parasternal long axis of the WT and TLR4−/− mice. D). Representative Doppler echocardiography tracings of pulmonary artery blood flow velocity in the main pulmonary artery. E). Illustration of the measurement of pulmonary arterial acceleration time (PAAT). The PAAT was determined from the start to the peak of the flow signal of three consecutive beats. Two areas were measured for each mouse. F). PAAT in WT (n=4 mice) and TLR4−/− mice (n=3, *p<0.05).
In addition, Doppler echocardiography of the pulmonary outflow was utilized to estimate pulmonary artery (PA) pressure in mice non-invasively (27;28). Doppler recordings of the main PA in the right parasternal long axis position were obtained. As indicated in Figure 3D, the PA blood flow velocity was measured at the main PA of the WT and TLR4−/− mice (Figure 3A), and the PA acceleration time (PAAT) was determined from the start to the peak of the flow signal as indicated (Figure 3E). PAAT of the TLR4−/− mice exhibited a 23% decrease compared with WT mice (Figure. 3F). The shorter PAAT in TLR4−/− is consistent with increased RV and pulmonary pressure, which has been used for estimating PAH in mouse and rat models (27;32). Taken together, the short PAAT and RV hypertrophy in the TLR4−/− mice compared to WT mice are consistent with pulmonary hypertension.
4.4. Increased ROS production in PASMC from TLR4−/− mice
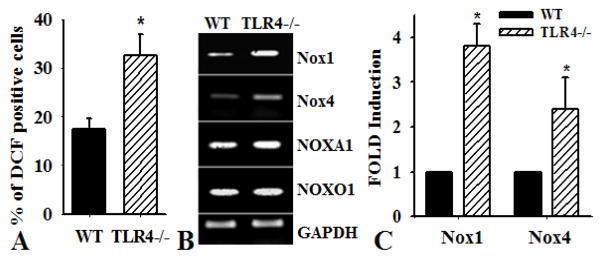
PASMC from TLR4−/− mice exhibited increased intracellular ROS production compared to PASMC from WT mice (Figure 4A, n=4, *p<0.001). To identify the NADPH oxidase that was responsible for the increased ROS, we characterized the expression of Nox subunits that are most abundant in smooth muscle cells, Nox1 and Nox4. Increased expression of Nox1 and Nox4 was demonstrated in PASMC from TLR4−/− mice compared with that in WT and TLR2−/− mice, as determined by RT-PCR (Figure 4B). The expression of Nox1 activation subunit NOXA1, an important component of NADPH oxidase in SMC (33), increased; whereas NOXO1 was not affected. Real-time PCR analysis confirmed increased the expression of Nox1 and 4 in TLR4−/− PASMC compared with that in WT PASMC (Figure 4C).
Figure 4.
TLR4 deficiency increases the expression of Nox1 and Nox4 in PASMC. PASMC were isolated from pulmonary arteries of mice deficient in TLR4 (TLR4−/−) or wild type (WT) mice. A). Oxidative stress in PASMC from WT and TLR4−/− mice cultured in growth media for 48 hours was determined by assessing DCF-DA by flow cytometry. Results show the percentage of cells that positively stained for DCF (n=4, *p<0.001). B). RT-PCR analysis was performed to determine the expression of NOX enzymes in WT and TLR4−/− PASMC. The primers for mouse NADPH oxidase genes are described in materials and Methods. The expression of GAPDH was used as a control. C). Real-time PCR analysis of the expression of Nox1 and Nox4 in WT and TLR4−/− PASMC (n=3, *p<0.001).
4.5. Hypoxia inhibits TLR4 expression and induces NADPH expression in PASMC
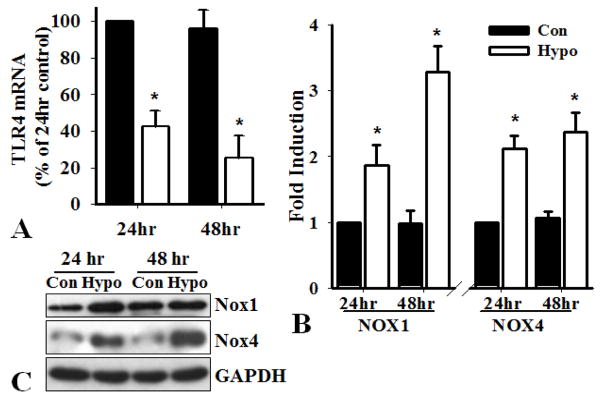
To determine whether hypoxia affects the expression of TLR4, PASMC from WT mice were incubated in a hypoxia chamber (2% oxygen) for 24 and 48 hours. Real-time PCR analysis showed a 58% and 75% decrease of TLR4 mRNA after PASMC were exposed to hypoxia for 24 or 48 hours, respectively (Figure 5A). These results are consistent with the previous observation that hypoxia diminishes the expression of TLR4 through ROS in endothelial cells (22). By contrast, hypoxia induced the expression of Nox1 and Nox4, as determined by real-time PCR as well as Western blot analysis (Figure 5B&C). Therefore, decreased expression of TLR4, genetically or hypoxia-induced, and increased expression of Nox1 and 4 in PASMC are likely to contribute to the development of PAH.
Figure 5.
Hypoxia decreases the expression of TLR4 and induces Nox1 and Nox4. PASMC from WT mice were seeded at 80% confluence and exposed to control (20% oxygen, Con) or hypoxia (2% oxygen, Hypo) condition for 24 and 48 hours. Real-time PCR analysis of the expression of A). TLR4 using primers for mouse TLR4: F-5′-GCTTTCACCTCTGCCTTCAC; R-5′-CGAGGCTTTTCCATCCAATA; B). Nox1 and Nox4 using specific primers shown in Fig 4. Results shown are the expressions of TLR4, Nox1 and Nox4 mRNA (normalized by GAPDH mRNA) as percentage of that in cells exposed to condition for 24 hours, defined as 100% (n=3, *p<0.05). C). Western blot analysis of the expression of Nox1 and Nox4 proteins. The expression of GAPDH was used as a loading control.
4.6. Hypoxia-induced PASMC proliferation is decreased by an NADPH inhibitor
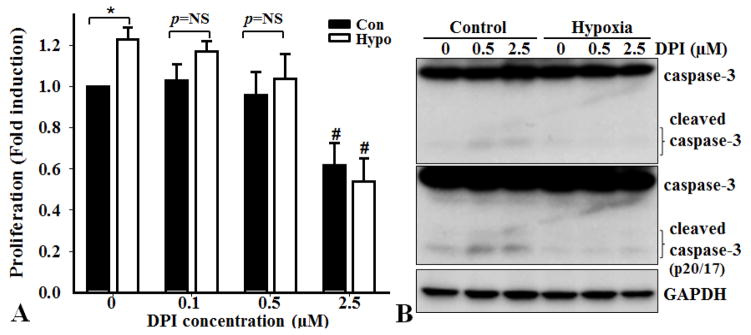
As ROS-induced abnormal PASMC proliferation contributes to vascular remodeling, we determined the effect of hypoxia on PASMC proliferation and the effect of an NADPH oxidase inhibitor, DPI. Hypoxia induced the proliferation of PASMC, which was inhibited by DPI in a concentration-dependent manner, as demonstrated by BrdU incorporation assays (Fig 6). Furthermore, the effect of DPI on PASMC proliferation was not due to induction of apoptosis, because DPI did not induce cleavage/activation of caspase-3, an indicator for apoptosis. These results confirmed that increased activation of NADPH oxidase mediated hypoxia-induced PASMC proliferation.
Figure 6.
Inhibition of NADPH oxidase activity decreases hypoxia-induced proliferation of PASMC. PASMC from WT mice were seeded at 80% confluence and exposed to control (Con) or hypoxia (Hypo) condition with or without NADPH oxidase inhibitor DPI for 24 hours. A). Proliferation was determined by BrdU incorporation (n=3, *p<0.05; p=NS, no significant difference; #p<0.05 compared with their related control conditions without DPI). B). Western blot analysis of caspase-3. The blot in the middle is a longer exposure of the upper one, to show the cleaved form of caspase-3. The expression of GAPDH was used as a loading control. Representative blots of three independent experiments are shown.
5. DISCUSSION
In animal models of hypoxia-induced PAH, vascular remodeling and right ventricle hypertrophy are prominent characteristics (9;25). In the present study, we demonstrated that mice deficient in TLR4 spontaneously developed PAH, featuring increased RV pressure and hypertrophy of pulmonary arteries compared with wild type mice or mice with TLR2 deficiency. In contrast to hypoxia-induced PAH in the wild type and TLR2−/− mice, spontaneously developed PAH in TLR4−/− mice was not increased by hypoxia, supporting a role of TLR4 in mediating hypoxia-induced PAH. Consistent with previous observations in endothelial cells that hypoxia decreases the expression of TLR4 via increased ROS (22), we found hypoxia decreased TLR4 expression in PASMC, supporting that hypoxia may contribute to PAH via down-regulation of TLR4.
Hypoxia-induced ROS production regulates vascular remodeling in PAH. Therefore, increased ROS production in PASMC from TLR4−/− mice may contribute to PAH in the TLR4−/− mice. As the major source of ROS in the vasculature, the NADPH oxidase family of enzymes is distributed throughout all three layers of the vascular wall (4;5). NADPH oxidase-dependent ROS generation has been described in pulmonary endothelial cells and smooth muscle cells (3;34–36). Nox4 and Nox1 are predominant NADPH oxidases in smooth muscle cells. Nox-derived ROS is thought to be a hypertensive signaling element in the vasculature (5;37–39). Hypoxia-induced activation of Nox1 and Nox4 leads to ROS production that plays an important role in pulmonary hypertension (4;7;9;10;36;40;41). Previous studies have demonstrated increased expression of NOX4 in patients with PAH; as well as in lungs and pulmonary artery of mice after chronic exposure to hypoxia (36). With the use of PASMC, we found that hypoxia induced the expression of Nox1 and Nox4. Furthermore, increased expression of Nox1 and Nox4 were determined in TLR4−/− PASMC. Accordingly, increased ROS and NADPH oxidases may contribute to hypoxia-induced PAH, as well as PAH in the TLR4−/− mice. However, the molecular mechanisms linking TLR4 and Nox1 and Nox4 activation are not known.
TLR4 was first characterized through studies of LPS hyporesponsive C3H/HeJ mice (42), which possess a point mutation that renders the receptor unable to transduce a signal (43). In addition to LPS, increasing evidence has demonstrated that host-derived endogenous ligands of TLRs such as heat shock protein 60, oxidized low-density lipoproteins and fibronectin are also able to activate immune responses through TLR4 and induce an inflammatory reaction that leads to atherosclerotic and injured arteries (20;44;45). In general, TLR4 deficiency protects mice from endotoxin, ischemia-reperfusion, and ozone-induced injury (24;46;47). As TLR4 has been well-studied as a microbial pattern receptor for human body’s first line of defense against infectious agents, one would anticipate that TLR4 deficiency will result in hyposensitivity of host cells to bacteria or virus infection, a common risk for heart and lung disease. Therefore, we analyzed the serum concentrations of 22 cytokines and chemokines, including interleukins 1–17, interferon-γ, MCP-1 and TNFα, using Bio-Plex Protein Array System and a Mouse Cytokine 22-plex Panel (Bio-Rad). However, we found that TLR4 deficiency did not affect serum levels of the tested cytokines and chemokines (data not shown), suggesting additional signals independent of the role of TLR4 as a receptor for LPS might be involved in promoting PAH.
Upregulation and activation of Nox1 or Nox4 NADPH oxidases have been linked to activation of TLR4 signaling, in which TLR4 functions as a receptor for ligands such as LPS (48–52). Upregulation of Nox1 by Helicobacter pylori-derived LPS in guinea pig gastric pit cells is mediated through TLR4-dependent pathways (51;52). On the other hand, Nox4 has been shown to be a major downstream effector of TLR4 signaling in human aortic endothelial cells and SMC (48–50). Nox4 knockdown by siRNA inhibits TLR4 signaling-induced ROS production and NFκB signaling (48–50). Therefore these studies with LPS, a potent TLR4 activator, suggest a role of the Nox1 and Nox4 NADPH oxidase in mediating TLR4-activated signaling.
Our finding that TLR4−/− mice developed PAH is consistent with previous observations that TLR4 is essential for maintaining normal homeostasis of gut, lung and vasculature independent of its well established role as the LPS receptor that mediates the inflammatory signals (23;53;54). TLR4 deficiency has been shown to confer susceptibility to lethal oxidant lung injury (53). Lack of TLR4 or MyD88 causes emphysema in mice (23). Furthermore, TLR4 and MyD88 are necessary for intestinal epithelial homeostasis and protection from injury (54). We found that spontaneously developed PAH in the TLR4−/− mice was not enhanced by hypoxia, which is consistent with the observations by Young et al that TLR4 deficiency conferred resistance to hypoxia-induced pulmonary hypertension (55). Of note, different strains of mice were used in that study; and the basal level of right ventricular pressure and RV weight seem higher in the TLR4−/− mice (55). Recent studies from Bauer et al demonstrated that platelet but not myeloid-derived cells contributed to the effects of TLR4 in hypoxia-induced PAH (56). They also found that high mobility group box 1 medicated the function of TLR4 in PAH (57). However, the underlying mechanisms by which TLR4 maintains basal vascular homeostasis have not been fully elucidated. TLR4 deficiency in lungs confers susceptibility to lethal oxidant lung injury (53), which is associated with increased expression of anti-apoptotic singles, such as Bcl-2 and phosphorylated-AKT protein (53). TLR4 deficiency was associated with decreased inflammatory response induced by hypoxia (55). Increased oxidative stress, as determined by ROS production, as well as Nox expression, has been found in lung endothelial cells from TLR4 deficient mice (23). Similar to this later observation, we found increased ROS production in the PASMC from TLR4−/− mice, which contributed to increased proliferation of these cells.
NOX-derived ROS have also been shown to have a pro-survival effect (11–13). In addition, ROS serves as second messengers to induce cell proliferation in a variety of cells (11;58–60). Increased production of ROS is associated with greater proliferation in tumor cells (61). In SMC, increased Nox4-derived ROS promotes proliferation; whereas inhibition of Nox4 reduces ROS production and SMC proliferation (50;60). The increased ROS production in TLR4−/− PASMC was associated with increased expression of Nox1 and Nox4, the two predominant NADPH oxidase isoforms in the SMC. The proliferative role of Nox1 and Nox4 in SMC is supported by many studies. NOX1 has been referred as a “mitogenic oxidase 1”, which is related to its mitogenic effect on SMC proliferation (62). Nox1 induces the generation of hydrogen peroxide that promotes cell growth and transformation (40). Overexpression of NOX1 enhances vascular hypertrophy (38). In Nox1-deficient mice, angiotensin II-induced SMC proliferation was inhibited (56). Several recent studies have demonstrated an important role of Nox4 in regulating SMC proliferation (36;50;60;63;64). In resting SMC, NADPH oxidase activity largely depends on NOX4 expression (63). The expression of NOX4 is upregulated in patients with PAH (36). Hypoxia upregulates NOX4 in homogenized lung tissue, pulmonary arterial vessels and medial PASMC (36), supporting an important role of NOX4 in pulmonary vascular remodeling in chronic hypoxia-induced PAH. Treatment of PASMC with Nox4 siRNA reduced ROS production and cell proliferation induced by transforming growth factor-beta1 (60). Consistently, increased Nox4 promotes proliferation and inhibition of Nox4 reduces proliferation of PASMC (50;60). Using a NADPH oxidase inhibitor, we confirmed that hypoxia-induced PASMC proliferation was mediated by NADPH oxidase. Therefore, upregulation of Nox1 and Nox4 expression in PASMC from TLR4−/− mice and in PASMC exposed to hypoxia may mediate increased PASMC proliferation and thus vascular remodeling in PAH.
Taken together, the present studies demonstrating that TLR4-deficient mice spontaneously develop PAH that is not enhanced by hypoxia, and that hypoxia reduces TLR4 expression, suggest that TLR4 plays an important role in maintaining normal pulmonary vasculature and hypoxia induces PAH via TLR4. Inhibition of TLR4 in PASMC, by genetic ablation or hypoxia was associated with increased expression of the Nox1 and Nox4 NADPH oxidase, which contributed to increased PASMC proliferation and vascular remodeling. These results demonstrate a novel function of TLR4 in regulating the development of PAH and identify a novel regulatory axis that is responsible for TLR4 deficiency-induced vascular hypertrophy and remodeling. Our studies provide novel insights into the crosstalk between TLR4 and oxidative stress signaling in regulating cell proliferation, which will likely be useful for our understanding of other heart and lung diseases that are characterized by increased vascular hypertrophy and remodeling.
Acknowledgments
This work was supported by VA Merit Review Award BX000369 (YC). We also thank grant support from National Institutes of Health HL092215 and DK100847 (YC), HL092906 (NA), and VA BX001591 (project 2, YC).
References
- 1.Pietra GG, Capron F, Stewart S, Leone O, Humbert M, Robbins IM, Reid LM, Tuder RM. Pathologic assessment of vasculopathies in pulmonary hypertension. J Am Coll Cardiol. 2004;43:25S–32S. doi: 10.1016/j.jacc.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 2.McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation. 2006;114:1417–1431. doi: 10.1161/CIRCULATIONAHA.104.503540. [DOI] [PubMed] [Google Scholar]
- 3.Hanze J, Weissmann N, Grimminger F, Seeger W, Rose F. Cellular and molecular mechanisms of hypoxia-inducible factor driven vascular remodeling. Thromb Haemost. 2007;97:774–787. [PubMed] [Google Scholar]
- 4.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 5.Griendling KK, Sorescu D, Ushio-Fukai M. NAD (P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Yang F, Yang XP, Jankowski M, Pagano PJ. NAD (P)H oxidase mediates angiotensin II-induced vascular macrophage infiltration and medial hypertrophy. Arterioscler Thromb Vasc Biol. 2003;23:776–782. doi: 10.1161/01.ATV.0000066684.37829.16. [DOI] [PubMed] [Google Scholar]
- 7.Katsuyama M, Fan C, Yabe-Nishimura C. NADPH oxidase is involved in prostaglandin F2alpha-induced hypertrophy of vascular smooth muscle cells: induction of NOX1 by PGF2alpha. J Biol Chem. 2002;277:13438–13442. doi: 10.1074/jbc.M111634200. [DOI] [PubMed] [Google Scholar]
- 8.Djordjevic T, BelAiba RS, Bonello S, Pfeilschifter J, Hess J, Gorlach A. Human urotensin II is a novel activator of NADPH oxidase in human pulmonary artery smooth muscle cells. Arterioscler Thromb Vasc Biol. 2005;25:519–525. doi: 10.1161/01.ATV.0000154279.98244.eb. [DOI] [PubMed] [Google Scholar]
- 9.Liu JQ, Zelko IN, Erbynn EM, Sham JS, Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox) Am J Physiol Lung Cell Mol Physiol. 2006;290:L2–10. doi: 10.1152/ajplung.00135.2005. [DOI] [PubMed] [Google Scholar]
- 10.Grobe AC, Wells SM, Benavidez E, Oishi P, Azakie A, Fineman JR, Black SM. Increased oxidative stress in lambs with increased pulmonary blood flow and pulmonary hypertension: role of NADPH oxidase and endothelial NO synthase. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1069–L1077. doi: 10.1152/ajplung.00408.2005. [DOI] [PubMed] [Google Scholar]
- 11.Guichard C, Pedruzzi E, Fay M, Ben Mkaddem S, Coant N, Daniel F, Ogier-Denis E. The Nox/Duox family of ROS-generating NADPH oxidases. Med Sci (Paris) 2006;22:953–959. doi: 10.1051/medsci/20062211953. [DOI] [PubMed] [Google Scholar]
- 12.Kim YS, Morgan MJ, Choksi S, Liu ZG. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol Cell. 2007;26:675–687. doi: 10.1016/j.molcel.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 13.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 14.Deshpande SS, Angkeow P, Huang J, Ozaki M, Irani K. Rac1 inhibits TNF-alpha-induced endothelial cell apoptosis: dual regulation by reactive oxygen species. FASEB J. 2000;14:1705–1714. doi: 10.1096/fj.99-0910com. [DOI] [PubMed] [Google Scholar]
- 15.Mochizuki T, Furuta S, Mitsushita J, Shang WH, Ito M, Yokoo Y, Yamaura M, Ishizone S, Nakayama J, Konagai A, Hirose K, Kiyosawa K, Kamata T. Inhibition of NADPH oxidase 4 activates apoptosis via the AKT/apoptosis signal-regulating kinase 1 pathway in pancreatic cancer PANC-1 cells. Oncogene. 2006;25:3699–3707. doi: 10.1038/sj.onc.1209406. [DOI] [PubMed] [Google Scholar]
- 16.Clement MV, Stamenkovic I. Superoxide anion is a natural inhibitor of FAS-mediated cell death. EMBO J. 1996;15:216–225. [PMC free article] [PubMed] [Google Scholar]
- 17.Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J Immunol. 2004;172:2522–2529. doi: 10.4049/jimmunol.172.4.2522. [DOI] [PubMed] [Google Scholar]
- 18.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 19.Liew FY, Xu D, Brint EK, O’Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 20.Xu XH, Shah PK, Faure E, Equils O, Thomas L, Fishbein MC, Luthringer D, Xu XP, Rajavashisth TB, Yano J, Kaul S, Arditi M. Toll-like receptor-4 is expressed by macrophages in murine and human lipid-rich atherosclerotic plaques and upregulated by oxidized LDL. Circulation. 2001;104:3103–3108. doi: 10.1161/hc5001.100631. [DOI] [PubMed] [Google Scholar]
- 21.Vink A, Schoneveld AH, van der Meer JJ, Van Middelaar BJ, Sluijter JP, Smeets MB, Quax PH, Lim SK, Borst C, Pasterkamp G, de Kleijn DP. In vivo evidence for a role of toll-like receptor 4 in the development of intimal lesions. Circulation. 2002;106:1985–1990. doi: 10.1161/01.cir.0000032146.75113.ee. [DOI] [PubMed] [Google Scholar]
- 22.Ishida I, Kubo H, Suzuki S, Suzuki T, Akashi S, Inoue K, Maeda S, Kikuchi H, Sasaki H, Kondo T. Hypoxia diminishes toll-like receptor 4 expression through reactive oxygen species generated by mitochondria in endothelial cells. J Immunol. 2002;169:2069–2075. doi: 10.4049/jimmunol.169.4.2069. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Shan P, Jiang G, Cohn L, Lee PJ. Toll-like receptor 4 deficiency causes pulmonary emphysema. J Clin Invest. 2006;116:3050–3059. doi: 10.1172/JCI28139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 25.Chen YF, Feng JA, Li P, Xing D, Zhang Y, Serra R, Ambalavanan N, Majid-Hassan E, Oparil S. Dominant negative mutation of the TGF-beta receptor blocks hypoxia-induced pulmonary vascular remodeling. J Appl Physiol. 2006;100:564–571. doi: 10.1152/japplphysiol.00595.2005. [DOI] [PubMed] [Google Scholar]
- 26.Ambalavanan N, Bulger A, Murphy-Ullrich J, Oparil S, Chen YF. Endothelin-A receptor blockade prevents and partially reverses neonatal hypoxic pulmonary vascular remodeling. Pediatr Res. 2005;57:631–636. doi: 10.1203/01.PDR.0000159512.55862.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsen KO, Sjaastad I, Svindland A, Krobert KA, Skjonsberg OH, Christensen G. Alveolar hypoxia induces left ventricular diastolic dysfunction and reduces phosphorylation of phospholamban in mice. Am J Physiol Heart Circ Physiol. 2006;291:H507–H516. doi: 10.1152/ajpheart.00862.2005. [DOI] [PubMed] [Google Scholar]
- 28.Finsen AV, Christensen G, Sjaastad I. Echocardiographic parameters discriminating myocardial infarction with pulmonary congestion from myocardial infarction without congestion in the mouse. J Appl Physiol. 2005;98:680–689. doi: 10.1152/japplphysiol.00924.2004. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Kelm RJ, Jr, Budd RC, Sobel BE, Schneider DJ. Inhibition of apoptosis and caspase-3 in vascular smooth muscle cells by plasminogen activator inhibitor type-1. J Cell Biochem. 2004;92:178–188. doi: 10.1002/jcb.20058. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y, Budd RC, Kelm RJ, Jr, Sobel BE, Schneider DJ. Augmentation of proliferation of vascular smooth muscle cells by plasminogen activator inhibitor type 1. Arterioscler Thromb Vasc Biol. 2006;26:1777–1783. doi: 10.1161/01.ATV.0000227514.50065.2a. [DOI] [PubMed] [Google Scholar]
- 31.Wiles ME, Wagner TL, Weglicki WB. Effect of acute magnesium deficiency (MgD) on aortic endothelial cell (EC) oxidant production. Life Sci. 1997;60:221–236. doi: 10.1016/s0024-3205(96)00619-4. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka N, Dalton N, Mao L, Rockman HA, Peterson KL, Gottshall KR, Hunter JJ, Chien KR, Ross J., Jr Transthoracic echocardiography in models of cardiac disease in the mouse. Circulation. 1996;94:1109–1117. doi: 10.1161/01.cir.94.5.1109. [DOI] [PubMed] [Google Scholar]
- 33.Ambasta RK, Schreiber JG, Janiszewski M, Busse R, Brandes RP. Noxa1 is a central component of the smooth muscle NADPH oxidase in mice. Free Radic Biol Med. 2006;41:193–201. doi: 10.1016/j.freeradbiomed.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 34.Brar SS, Kennedy TP, Sturrock AB, Huecksteadt TP, Quinn MT, Murphy TM, Chitano P, Hoidal JR. NADPH oxidase promotes NF-kappaB activation and proliferation in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2002;282:L782–L795. doi: 10.1152/ajplung.00206.2001. [DOI] [PubMed] [Google Scholar]
- 35.Parinandi NL, Kleinberg MA, Usatyuk PV, Cummings RJ, Pennathur A, Cardounel AJ, Zweier JL, Garcia JG, Natarajan V. Hyperoxia-induced NAD (P)H oxidase activation and regulation by MAP kinases in human lung endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;284:L26–L38. doi: 10.1152/ajplung.00123.2002. [DOI] [PubMed] [Google Scholar]
- 36.Mittal M, Roth M, Konig P, Hofmann S, Dony E, Goyal P, Selbitz AC, Schermuly RT, Ghofrani HA, Kwapiszewska G, Kummer W, Klepetko W, Hoda MA, Fink L, Hanze J, Seeger W, Grimminger F, Schmidt HH, Weissmann N. Hypoxia-dependent regulation of nonphagocytic NADPH oxidase subunit NOX4 in the pulmonary vasculature. Circ Res. 2007;101:258–267. doi: 10.1161/CIRCRESAHA.107.148015. [DOI] [PubMed] [Google Scholar]
- 37.Zalba G, San Jose G, Moreno MU, Fortuno MA, Fortuno A, Beaumont FJ, Diez J. Oxidative stress in arterial hypertension: role of NAD (P)H oxidase. Hypertension. 2001;38:1395–1399. doi: 10.1161/hy1201.099611. [DOI] [PubMed] [Google Scholar]
- 38.Dikalova A, Clempus R, Lassegue B, Cheng G, McCoy J, Dikalov S, San Martin A, Lyle A, Weber DS, Weiss D, Taylor WR, Schmidt HH, Owens GK, Lambeth JD, Griendling KK. Nox1 overexpression potentiates angiotensin II-induced hypertension and vascular smooth muscle hypertrophy in transgenic mice. Circulation. 2005;112:2668–2676. doi: 10.1161/CIRCULATIONAHA.105.538934. [DOI] [PubMed] [Google Scholar]
- 39.Matsuno K, Yamada H, Iwata K, Jin D, Katsuyama M, Matsuki M, Takai S, Yamanishi K, Miyazaki M, Matsubara H, Yabe-Nishimura C. Nox1 is involved in angiotensin II-mediated hypertension: a study in Nox1-deficient mice. Circulation. 2005;112:2677–2685. doi: 10.1161/CIRCULATIONAHA.105.573709. [DOI] [PubMed] [Google Scholar]
- 40.Arnold RS, Shi J, Murad E, Whalen AM, Sun CQ, Polavarapu R, Parthasarathy S, Petros JA, Lambeth JD. Hydrogen peroxide mediates the cell growth and transformation caused by the mitogenic oxidase Nox1. Proc Natl Acad Sci U S A. 2001;98:5550–5555. doi: 10.1073/pnas.101505898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Serrander L, Cartier L, Bedard K, Banfi B, Lardy B, Plastre O, Sienkiewicz A, Forro L, Schlegel W, Krause KH. NOX4 activity is determined by mRNA levels and reveals a unique pattern of ROS generation. Biochem J. 2007;406:105–114. doi: 10.1042/BJ20061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 43.Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem. 1999;274:10689–10692. doi: 10.1074/jbc.274.16.10689. [DOI] [PubMed] [Google Scholar]
- 44.Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge. heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 45.Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, Chow JC, Strauss JF., III The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001;276:10229–10233. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- 46.Ke B, Shen XD, Gao F, Busuttil RW, Kupiec-Weglinski JW. Interleukin 13 gene transfer in liver ischemia and reperfusion injury: role of Stat6 and TLR4 pathways in cytoprotection. Hum Gene Ther. 2004;15:691–698. doi: 10.1089/1043034041361244. [DOI] [PubMed] [Google Scholar]
- 47.Kleeberger SR, Reddy SP, Zhang LY, Cho HY, Jedlicka AE. Toll-like receptor 4 mediates ozone-induced murine lung hyperpermeability via inducible nitric oxide synthase. Am J Physiol Lung Cell Mol Physiol. 2001;280:L326–L333. doi: 10.1152/ajplung.2001.280.2.L326. [DOI] [PubMed] [Google Scholar]
- 48.Park HS, Chun JN, Jung HY, Choi C, Bae YS. Role of NADPH oxidase 4 in lipopolysaccharide-induced proinflammatory responses by human aortic endothelial cells. Cardiovasc Res. 2006;72:447–455. doi: 10.1016/j.cardiores.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 49.Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NAD (P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol. 2004;173:3589–3593. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 50.Patel DN, Bailey SR, Gresham JK, Schuchman DB, Shelhamer JH, Goldstein BJ, Foxwell BM, Stemerman MB, Maranchie JK, Valente AJ, Mummidi S, Chandrasekar B. TLR4-NOX4-AP-1 signaling mediates lipopolysaccharide-induced CXCR6 expression in human aortic smooth muscle cells. Biochem Biophys Res Commun. 2006;347:1113–1120. doi: 10.1016/j.bbrc.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 51.Kusumoto K, Kawahara T, Kuwano Y, Teshima-Kondo S, Morita K, Kishi K, Rokutan K. Ecabet sodium inhibits Helicobacter pylori lipopolysaccharide-induced activation of NADPH oxidase 1 or apoptosis of guinea pig gastric mucosal cells. Am J Physiol Gastrointest Liver Physiol. 2005;288:G300–G307. doi: 10.1152/ajpgi.00274.2004. [DOI] [PubMed] [Google Scholar]
- 52.Rokutan K, Kawahara T, Kuwano Y, Tominaga K, Sekiyama A, Teshima-Kondo S. NADPH oxidases in the gastrointestinal tract: a potential role of Nox1 in innate immune response and carcinogenesis. Antioxid Redox Signal. 2006;8:1573–1582. doi: 10.1089/ars.2006.8.1573. [DOI] [PubMed] [Google Scholar]
- 53.Zhang X, Shan P, Qureshi S, Homer R, Medzhitov R, Noble PW, Lee PJ. Cutting edge: TLR4 deficiency confers susceptibility to lethal oxidant lung injury. J Immunol. 2005;175:4834–4838. doi: 10.4049/jimmunol.175.8.4834. [DOI] [PubMed] [Google Scholar]
- 54.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 55.Young KC, Hussein SM, Dadiz R, deMello D, Devia C, Hehre D, Suguihara C. Toll-like receptor 4-deficient mice are resistant to chronic hypoxia-induced pulmonary hypertension. Exp Lung Res. 2010;36:111–119. doi: 10.3109/01902140903171610. [DOI] [PubMed] [Google Scholar]
- 56.Bauer EM, Chanthaphavong RS, Sodhi CP, Hackam DJ, Billiar TR, Bauer PM. Genetic deletion of toll-like receptor 4 on platelets attenuates experimental pulmonary hypertension. Circ Res. 2014;114:1596–1600. doi: 10.1161/CIRCRESAHA.114.303662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bauer EM, Shapiro R, Zheng H, Ahmad F, Ishizawar D, Comhair SA, Erzurum SC, Billiar TR, Bauer PM. High mobility group box 1 contributes to the pathogenesis of experimental pulmonary hypertension via activation of Toll-like receptor 4. Mol Med. 2012;18:1509–1518. doi: 10.2119/molmed.2012.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gavazzi G, Banfi B, Deffert C, Fiette L, Schappi M, Herrmann F, Krause KH. Decreased blood pressure in NOX1-deficient mice. FEBS Lett. 2006;580:497–504. doi: 10.1016/j.febslet.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 59.Petry A, Djordjevic T, Weitnauer M, Kietzmann T, Hess J, Gorlach A. NOX2 and NOX4 mediate proliferative response in endothelial cells. Antioxid Redox Signal. 2006;8:1473–1484. doi: 10.1089/ars.2006.8.1473. [DOI] [PubMed] [Google Scholar]
- 60.Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, Karwande SV, Stringham JC, Bull DA, Gleich M, Kennedy TP, Hoidal JR. Transforming growth factor-beta1 induces Nox4 NAD (P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L661–L673. doi: 10.1152/ajplung.00269.2005. [DOI] [PubMed] [Google Scholar]
- 61.Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 62.Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, Lambeth JD. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 63.Ellmark SH, Dusting GJ, Fui MN, Guzzo-Pernell N, Drummond GR. The contribution of Nox4 to NADPH oxidase activity in mouse vascular smooth muscle. Cardiovasc Res. 2005;65:495–504. doi: 10.1016/j.cardiores.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 64.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]