Supplemental Digital Content is available in the text
Abstract
Evidence regarding the association between body mass index (BMI) and mortality in TB patients is limited and inconsistent. We investigated the effect of BMI on TB-specific and non-TB-specific mortality in TB patients.
All adult Taiwanese with TB in Taipei, Taiwan, during 2011 to 2012 were included in this retrospective cohort study. Multinomial logistic regression was used to evaluate associations of BMI with cause of death in TB patients.
Of the 1608 eligible patients, 83.6% (1345) were successfully treated, 3.3% (53) died of TB-specific causes, and 13.1% (210) died of non-TB-specific causes. Mean age was 64.6 years, and 67.5% of patients were male. After controlling for potential confounders, underweight was significantly associated with higher risks of all-cause mortality (adjusted odds ratio [AOR], 1.66; 95% confidence interval [CI], 1.21–2.30), TB-specific mortality (AOR, 2.14; 95% CI, 1.18–3.89), and non-TB-specific mortality (AOR, 1.58; 95% CI, 1.11–2.25) during TB treatment, while overweight was not. When gender differences on the association of BMI with mortality were considered, underweight only significantly increased risks of TB-specific (AOR, 2.37; 95% CI, 1.19–4.72) and non-TB-specific mortality (AOR, 1.58; 95% CI, 1.05–2.37) during treatment in male patients, but not female subjects.
The present findings indicate that underweight was associated with higher risks of TB-specific and non-TB-specific mortality during TB treatment, particularly in male patients.
INTRODUCTION
Tuberculosis (TB) remains a common and deadly disease globally.1 Over 8 million people develop TB, and almost 1 million people die of it, each year.2
Nutrition disequilibrium (eg, underweight) can impair the immune system (eg, T cell suppression)3 and thus might affect TB incidence. Prior studies have demonstrated that the incidence of TB infection decreased with increasing body mass index (BMI).4–6 Nutrition disequilibrium may also affect treatment outcome in TB patients due to the decline of immunity. Although many studies have evaluated risk factors for mortality in TB patients,7 evidence regarding the association between BMI and mortality in this population is limited and inconsistent.8–11 Three studies found that lower BMI was significantly associated with a higher risk of mortality among TB patients,9–11 but another found no such association.8 Moreover, 1 study found that overweight was associated with lower mortality among TB patients.9
The effect of BMI on TB mortality might differ between male and female patients. Prior study showed that the body composition (eg, fat and body cell mass) for men and women with TB disease varied at the same BMI.12 Also, men have lower fat body mass and less energy reserve than women at the time of TB diagnosis,12 which may increase the mortality in male TB patients. However, there has been little study to evaluate the gender difference on the association between BMI and mortality during TB treatment.
The World Health Organization (WHO) defines a death as a patient who dies for any reason during treatment.13 However, many TB patients die of malignancy or cerebrovascular disease rather than of TB. A 2011 review found that few studies of TB treatment outcomes distinguished between TB-specific and non-TB-specific mortality.7 Indeed, no published studies to date have examined the relationship between BMI and cause-specific mortality in TB patients.
Development of interventions that improve TB outcomes requires better understanding of factors associated with the cause of death among TB patients. This population-based study thus investigated the impact of body weight on TB-specific and non-TB-specific mortality during treatment in TB patients.
METHODS
Study Population and Data Source
A retrospective cohort study was conducted to analyze TB surveillance data from Taipei, Taiwan. All adult Taiwanese (age ≥18 years) with the diagnosis of TB during 2011to 2012 were included in the analysis. TB was defined according to clinical and/or laboratory findings.14 A clinical diagnosis required manifestations consistent with TB and exclusion of other differential diagnoses by diagnostic and clinical evaluation.14 Laboratory definitions required the positivity of Mycobacterium tuberculosis culture or the positivity of acid-fast bacilli (AFB) smear for patients with clinical symptoms of TB. This study was approved by the Institutional Review Board of Taipei City Hospitals.
Data Collection
When TB patients reported to the Taipei TB Prevention Center, trained case managers used a structured questionnaire to ask patients about their sociodemographic characteristics, clinical findings, and underlying diseases. The sociodemographic factors included age, sex, BMI, marital status, education level, smoking status, alcohol use, and employment status. TB patients in Taipei are required by law to be monitored until treatment success, death, or loss to follow-up. To monitor treatment response, case managers followed up all TB cases by phone or in person once every 2 weeks.
Outcome Variable
The outcome variable of interest was treatment outcome, which was categorized as successful treatment or mortality. Cause of death was used to classify mortality as TB-specific or non-TB-specific. TB-specific death was defined as any underlying cause of death due to TB in the Taiwan Death Certification Registry (International Classification of Diseases, 9th Revision [ICD-9]: A010–A018; ICD-10: A15–A19). Non-TB-specific death was defined as any underlying cause of death other than TB. The successful-treatment group was used as the reference.
Cause of death among TB patients in this study was determined by the Taiwan Death Certification Registry.15 Taiwanese law requires that a death certificate with an ICD-9 or ICD-10 code be registered within 30 days after a person dies. Because trained medical registrars review all death certificates at the central office of the National Death Certification Registry, cause-of-death coding in Taiwan is considered highly accurate.16
Main Explanatory Variable
The main explanatory variable was BMI (in kg/m2), which was recorded when case managers interviewed TB patients at the time of TB notification to the Taipei TB Prevention Department. BMI was categorized according to the WHO International Classification of Adult Body Weight as underweight (<18.5), normal (18.5–24.9), and overweight (≥25).17
Control Variables
Covariates identified in previous studies as risk factors for mortality in TB patients were assessed in the analyses.18,19 The control variables included sociodemographic factors (eg, education level, employment status, and smoking status), clinical findings (AFB smear status, TB culture, cavities on chest radiograph, pleural effusion, and extrapulmonary TB), and comorbidities (including malignancies, diabetes mellitus, and end-stage renal disease), which were recorded at the time of TB notification to the Taipei TB Prevention Department. Education level was categorized as uneducated, elementary school, high school, and university or higher. Smoking status was categorized as never smoker, former smoker, and current smoker.
Statistical Analysis
First, the characteristics of TB patients were described according to the BMI category. Chi-square test was conducted to examine the association between 2 categorical variables and two-sample t-test was used to compare the means of continuous variables for 2 groups.
In bivariate analysis, Chi-square test was used to assess associations of selected factors with mortality. Multivariate analyses were conducted using death versus survival as the outcome with BMI as the main explanatory variable. Factors found to be associated with mortality on bivariate analysis at an alpha level of <0.1 were considered for inclusion in the multiple logistic regression analysis. Backward stepwise multiple logistic regression analysis was conducted to estimate odds ratios (ORs) and 95% confidence intervals (CIs) after controlling for potential confounders. To identify factors associated with cause-specific mortality, the backward stepwise multinomial regression analysis was used to identify factors associated with TB-specific and non-TB-specific mortality in TB patients, respectively. Variables with P < 0.05 were included in the final model.
To examine the gender difference on the association of BMI and mortality, this study analyze the data after stratifying study subjects by gender. The sensitivity analyses were conducted by a Cox proportional hazard model to examine the robustness of the main findings. All data management and analyses were performed using the SAS 9.4 software package (SAS Institute, Cary, NC).
RESULTS
TB Patient Characteristics
A total of 1869 TB cases were reported to the Taipei TB Prevention Department in 2011 to 2012. Of these, 46 died before the start of TB treatment, 20 were lost to follow-up during treatment, 176 had incomplete data, and 19 were still receiving treatment at the time of this study (Fig. 1). The remaining 1608 were included in subsequent analyses. Overall, the mean age was 64.6 years (range 18–112 years), 11 (0.7%) of the subjects were coinfected with human immunodeficiency virus (HIV), and 1086 (67.5%) of the subjects were male (Table 1). Using WHO BMI definitions, 25.5%, 63.3%, and 11.2% of the TB patients were classified as underweight, normal-weight, and overweight, respectively. During the study follow-up period, 145 (14.2%) deaths occurred in normal-weight patients, 99 (24.4%) deaths occurred in underweight patients, and 19 (10.3%) deaths occurred in overweight patients (Table 1). Table 1 shows the distribution of mortality by cause of death among the study subjects.
FIGURE 1.
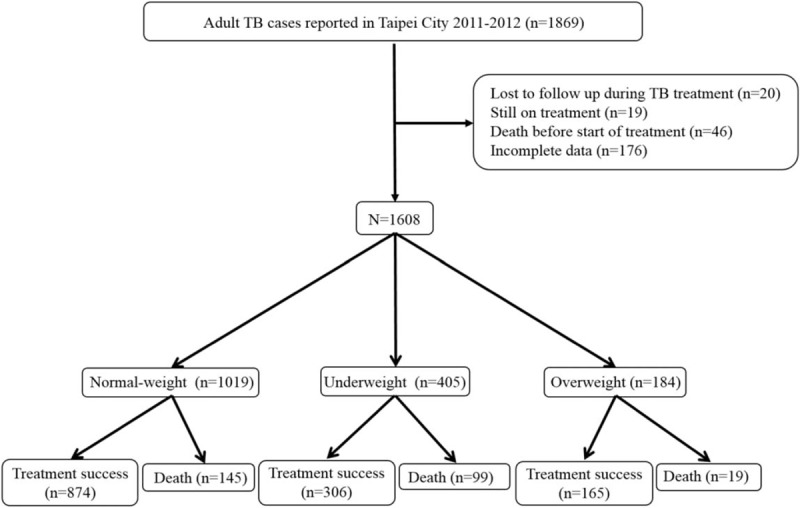
Study population. TB = tuberculosis.
TABLE 1.
Characteristics of Tuberculosis Patients and Deaths, by BMI Category
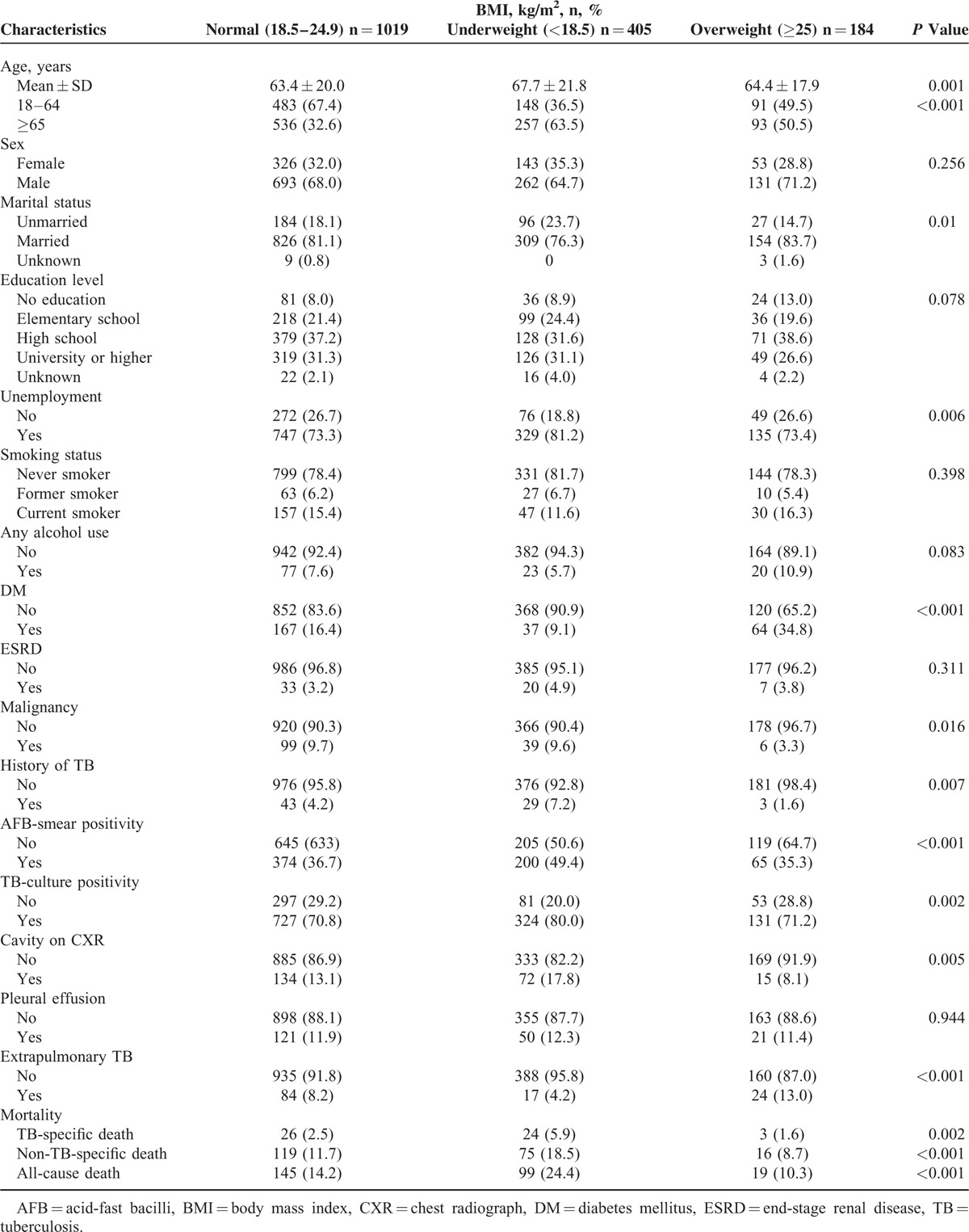
Univariates Analyses of Risk Factors for All-Cause Mortality
Table 2 shows univariate analyses of factors for all-cause mortality in TB patients. Risk factors associated with all-cause mortality included underweight, aged ≥65 years, male gender, married status, unemployment, ESRD, malignancy, AFB smear positivity, TB culture positivity, and pleural effusion on CXR. Also, factors associated with a lower risk of all-cause mortality included high school, university or higher education, current smoking, any alcohol use, cavities on CXR, and extrapulmonary TB.
TABLE 2.
Univariates Analyses of Risk Factors for All-cause Mortality in TB Patients, Taipei, Taiwan (2011–2012)
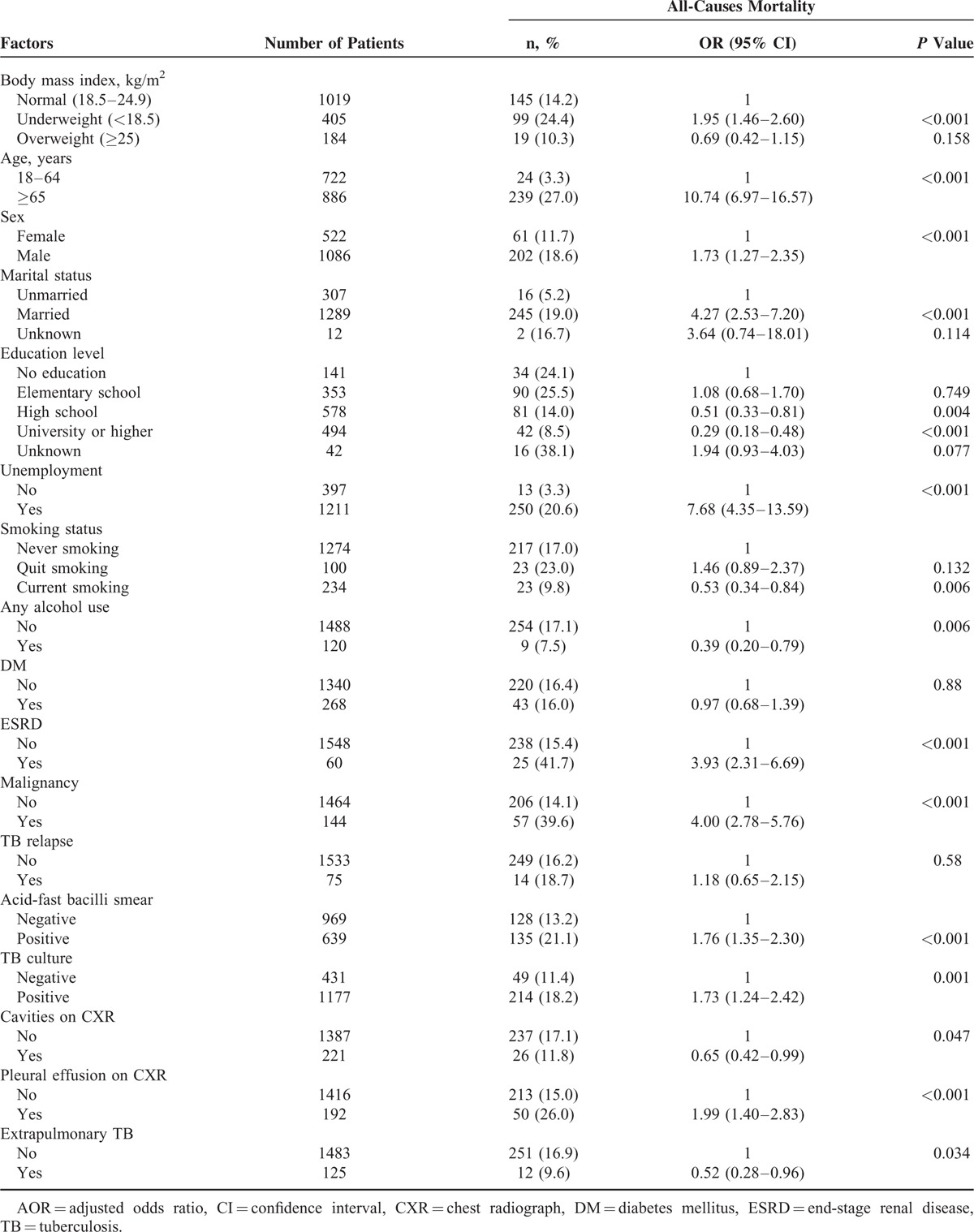
Multivariate Analyses for the Factors Associated With All-Cause Mortality in TB Patients
Backward stepwise logistic regression analysis showed that – after controlling for subject age, sex, clinical findings, and comorbidities – the risk of all-cause mortality was significantly higher for underweight patients (adjusted odds ratio [AOR], 1.66; 95% CI, 1.21–2.30; P = 0.002) than for normal-weight patients (Table 3). Overweight was not significantly associated with all-cause mortality in TB patients. Other risk factors associated with all-cause mortality included aged ≥65 years (AOR, 5.82; 95% CI, 3.62–9.36), male gender (AOR, 1.52; 95% CI, 1.07–2.15), unemployment (AOR, 2.36; 95% CI, 1.26–4.43), ESRD (AOR, 3.02; 95% CI, 1.66–5.49), malignancy (AOR, 3.54; 95% CI, 2.34–5.35), AFB smear positivity (AOR, 1.82; 95% CI, 1.34–2.46), and pleural effusion on CXR (AOR, 1.84; 95% CI, 1.23–2.75). Also, factors associated with a lower risk of all-cause mortality included university or higher education (AOR, 0.56; 95% CI, 0.32–0.97).
TABLE 3.
Adjusted Odds Ratios for Factors Associated with All-Cause Mortality in TB Patients, Taipei, Taiwan (2011–2012).∗
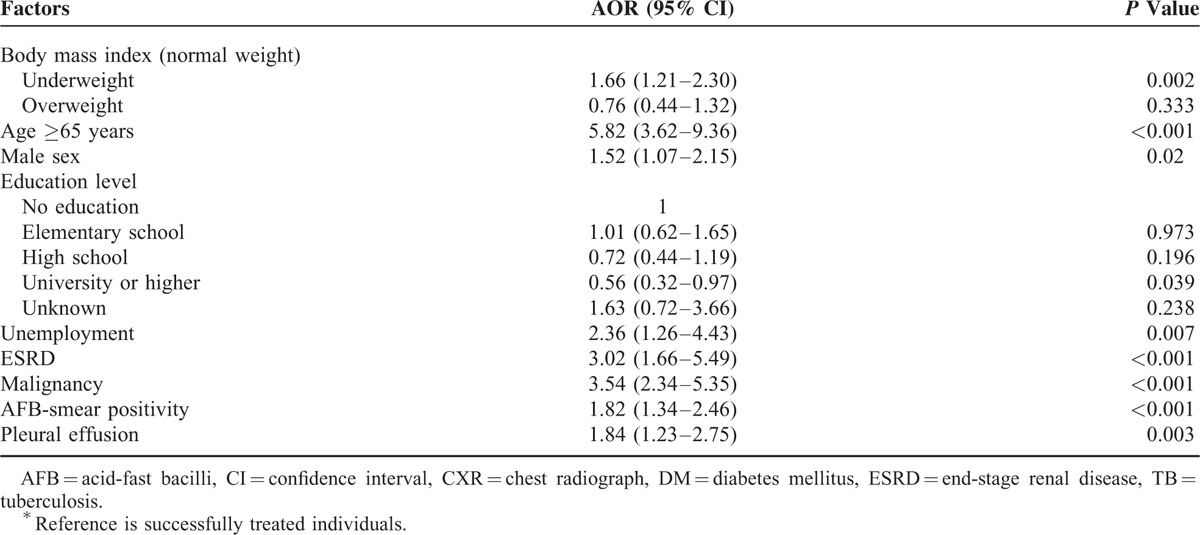
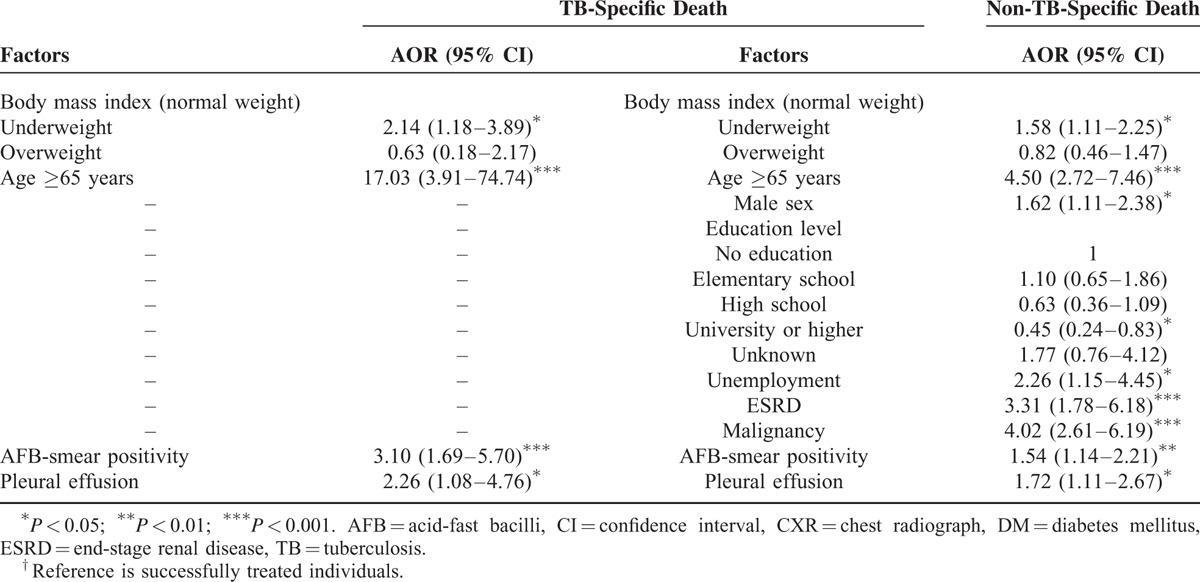
Association Between BMI and Cause-Specific Mortality
Backward stepwise multinomial regression showed that, after controlling for other variables, underweight was significantly associated with higher risks of TB-specific mortality (AOR, 2.14; 95% CI, 1.18–3.89; P = 0.012) and non-TB-specific mortality (AOR, 1.58; 95% CI, 1.11–2.25; P = 0.011) during treatment, while overweight was not (Table 4). Other factors associated with a higher risk of TB-specific and non-TB-specific mortality included aged ≥65 years, AFB smear positivity, and pleural effusion on CXR. Also, male gender, unemployment, ESRD, and malignancy were associated with a higher risk of non-TB-specific mortality, but university or higher education was associated with a lower risk of non-TB-specific mortality.
TABLE 4.
Multinomial Regression Analysis of the Association of BMI With TB-Specific and Non-TB-Specific Death in TB Patients†
Association Between BMI and Mortality in Male and Female Patients
Figure 2 shows the results of analysis of the association between BMI and mortality after stratifying patients by sex. Underweight was significantly associated with higher risks of all-cause (AOR, 1.70; 95% CI, 1.17–2.46), TB-specific (AOR, 2.37; 95% CI, 1.19–4.72), and non-TB-specific death (AOR, 1.58; 95% CI, 1.05–2.37) during treatment in male patients, but not female subjects.
FIGURE 2.
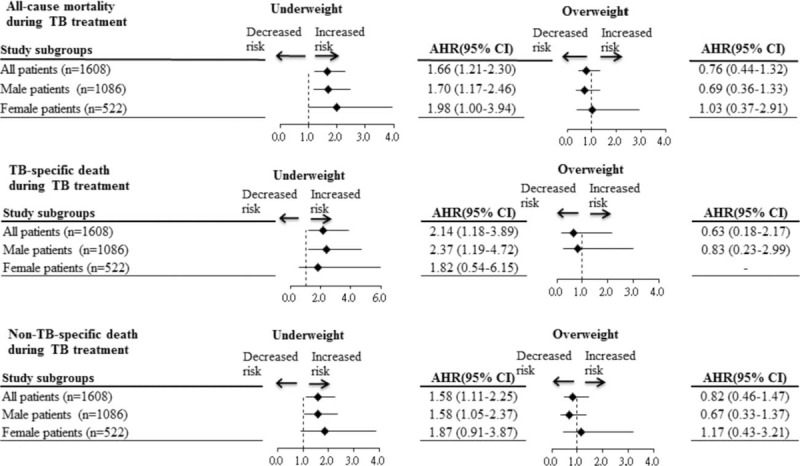
Subgroup analysis of the association between BMI and mortality after stratifying patients by gender. Values greater than 1.0 indicate increased risk. AHR = adjusted hazard ratio, BMI = body mass index, TB = tuberculosis.
Sensitivity Analysis of the Association Between BMI and Mortality
Sensitivity analysis of the association between BMI and mortality was conducted by the Cox proportional hazard model. After controlling for potential confounders, underweight was significantly associated with a higher risk of all-cause mortality during treatment (AHR, 1.54; 95% CI, 1.18–2.01) (Table S1). Also, multinomial Cox regression analysis showed that underweight was significantly associated with higher risks of TB-specific mortality (AHR, 1.82; 95% CI, 1.01–3.27) and non-TB-specific mortality (AHR, 1.48; 95% CI, 1.08–2.02) during treatment, while overweight was not (Table S2).
DISCUSSION
In this cohort study of 1608 Taiwanese adults with TB, overall mortality was 16.4% in 2011 to 2012. After controlling for potential confounders, underweight was significantly associated with higher risks of all-cause, TB-specific, non-TB-specific mortality during treatment, while overweight was not. When gender differences on the association of BMI with mortality were considered, underweight only significantly increased risks of TB-specific and non-TB-specific mortality during treatment in male patients, but not female subjects.
Our study showed robust associations between BMI and mortality by conducting the sensitivity analysis. The Cox proportional hazard model showed the same findings as those in the multiple logistic regression analysis. Underweight was significantly associated with higher risks of all-cause, TB-specific, and non-TB-specific mortality during treatment in the Cox proportional hazard model, but overweight was not.
The association between BMI and mortality in TB patients has not been extensively studied, and existing evidence is inconsistent.5–8 Zachariah et al11 found that TB patients with a BMI <17.0 had a higher mortality risk than those with a BMI ≥18.5. A 1-unit increase in BMI was significantly associated with lower mortality risk in Indian TB patients.10 In patients coinfected with HIV and TB, underweight significantly increased the risk of mortality, but obesity and overweight decreased the risk.9 Also, in TB/HIV coinfected patients under antiretroviral treatment, underweight patients had slow recoveries of immunity, which may increase the risk of mortality in this population.20 Another study of TB patients in the miliary found that those with a lower BMI (<18.5) did not have a higher risk of death within 90 days after starting treatment, as compared to those with a higher BMI (≥18.5).8 The present study found that overweight was not significantly associated with mortality risk; however, underweight was significantly associated with higher risks of TB-specific and non-TB-specific mortality during treatment. These findings suggest that, to reduce TB-specific and non-TB specific mortality, comprehensive care should be offered to underweight patients during TB treatment.
Only underweight was significantly associated with higher risks of TB-specific and non-TB-specific mortality during TB treatment. This higher mortality among underweight TB patients may be due to decreased immunity and the greater severity of TB infection in this population. Underweight can suppress lymphocyte stimulation and reduce Th1 cytokine secretions (the Th1 cytokines interleukin-2, interferon-γ, and tumor necrosis factor-α),21 which could cause the higher burden of TB infection and increase the severity of TB disease in underweight patients. In animal studies, malnourished animals had immune system impairment (reduction of reactive nitrogen intermediates) in response to Mycobacterium infection.22 In addition, malnourished animals had a higher bacterial burden of TB and died of infection earlier.22
We found that the risk of all-cause mortality was lower among overweight TB patients than among those of normal weight (10.3% vs 14.2%, respectively), although the difference was not statistically significant. This association has not been extensively studied; however, 1 study found that overweight/obesity was associated with decreased mortality among patients coinfected with HIV and TB.9 TB patients with small or moderate increases in BMI may have higher daily protein and energy intakes, which could improve immune function and reduce mortality. However, more studies are needed in order to evaluate the effects of overweight/obesity on all-cause and cause-specific mortality and timing of death in TB patients.
Some limitations should be considered when interpreting the findings of this citywide population-based study. First, 25.7% of TB cases were identified by clinical diagnosis rather than by AFB smear or culture, which might have resulted in overdiagnosis of TB. However, overdiagnosis of TB is not very likely in this study because the Taipei TB Control Department convenes monthly expert committee meetings to discuss ambiguous TB diagnoses.23 Second, this study only measured the BMI at the baseline. Since BMI may be changed during TB treatment,24 future studies will be needed to evaluate the time-varying effect of BMI on mortality in TB patients. Finally, because this study is a retrospective cohort study, some important information on TB patients (eg, intravenous drug use) was not available. Nevertheless, the study is strengthened by its inclusion of all eligible TB patients in the analysis, which means that sample size was not based on considerations of statistical power.
CONCLUSION
This study found that the mortality was high in TB patients in Taipei, Taiwan, during 2011 to 2012. After controlling for other covariates, underweight significantly increased risks of all-cause, TB-specific, and non-TB-specific mortality during TB treatment. Overweight, however, was not significantly associated with all-cause or cause-specific death during treatment. When gender differences on the association of BMI with mortality were considered, underweight only significantly increased risks of TB-specific and non-TB-specific mortality during treatment in male patients, but not female subjects. These findings suggest that underweight was associated with a higher risk of TB-specific and non-TB-specific mortality during TB treatment. Comprehensive care should be offered to underweight TB patients to reduce mortality, particularly in male patients.
Supplementary Material
Acknowledgements
The authors thank Ministry of Science and Technology, Taiwan (MOST103-2314-B-010-008) for the support. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors are grateful to the members of the Research Office for Health Data, Department of Education and Research, Taipei City Hospital, Taiwan for their valuable contributions in data management and statistical analysis.
Footnotes
Abbreviations: AFB = acid-fast bacilli, AOR = adjusted odds ratio, BMI = body mass index, CI = confidence interval, HIV = human immunodeficiency virus, TB = tuberculosis, WHO = World Health Organization.
This work was supported by grants from the Ministry of Science and Technology, Taiwan (MOST103-2314-B-010-008).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Lyons JG, Stewart S. Commentary: tuberculosis, diabetes and smoking: a burden greater than the sum of its parts. Int J Epidemiol 2013; 42:230–232. [DOI] [PubMed] [Google Scholar]
- 2.http://www.who.int/tb/publications/global_report/gtbr12_annex4.pdf [Accessed 2 May 2015]. [Google Scholar]
- 3.Schaible UE, Kaufmann SH. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med 2007; 4:e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tverdal A. Body mass index and incidence of tuberculosis. Eur J Respir Dis 1986; 69:355–362. [PubMed] [Google Scholar]
- 5.Cegielski JP, Arab L, Cornoni-Huntley J. Nutritional risk factors for tuberculosis among adults in the United States, 1971-1992. Am J Epidemiol 2012; 176:409–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lonnroth K, Williams BG, Cegielski P, et al. A consistent log-linear relationship between tuberculosis incidence and body mass index. Int J Epidemiol 2010; 39:149–155. [DOI] [PubMed] [Google Scholar]
- 7.Waitt CJ, Squire SB. A systematic review of risk factors for death in adults during and after tuberculosis treatment. Int J Tuberc Lung Dis 2011; 15:871–885. [DOI] [PubMed] [Google Scholar]
- 8.Kim DK, Kim HJ, Kwon SY, et al. Nutritional deficit as a negative prognostic factor in patients with miliary tuberculosis. Eur Respir J 2008; 32:1031–1036. [DOI] [PubMed] [Google Scholar]
- 9.Hanrahan CF, Golub JE, Mohapi L, et al. Body mass index and risk of tuberculosis and death. AIDS 2010; 24:1501–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhargava A, Chatterjee M, Jain Y, et al. Nutritional status of adult patients with pulmonary tuberculosis in rural central India and its association with mortality. PLoS One 2013; 8:e77979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zachariah R, Spielmann MP, Harries AD, et al. Moderate to severe malnutrition in patients with tuberculosis is a risk factor associated with early death. Trans R Soc Trop Med Hyg 2002; 96:291–294. [DOI] [PubMed] [Google Scholar]
- 12.Mupere E, Zalwango S, Chiunda A, et al. Body composition among HIV-seropositive and HIV-seronegative adult patients with pulmonary tuberculosis in Uganda. Ann Epidemiol 2010; 20:210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balabanova Y, Drobniewski F, Fedorin I, et al. The Directly Observed Therapy Short-Course (DOTS) strategy in Samara Oblast, Russian Federation. Respir Res 2006; 7:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang FD, Chang CH, Su WJ, et al. Screening of hospital workers for pulmonary tuberculosis in a medical center in Taiwan. Infect Control Hosp Epidemiol 2006; 27:510–511. [DOI] [PubMed] [Google Scholar]
- 15.Department of Health: Vital Statistics in Taiwan. Department of Health, Executive Yuan, Taiwan; 1971. [Google Scholar]
- 16.Lu TH, Lee MC, Chou MC. Accuracy of cause-of-death coding in Taiwan: types of miscoding and effects on mortality statistics. Int J Epidemiol 2000; 29:336–343. [DOI] [PubMed] [Google Scholar]
- 17.WHO. Report of a WHO Expert Committee. Geneva: WHO; 1995. Physical status: the use and interpretation of anthropometry. http://whqlibdoc.who.int/trs/WHO_TRS_854.pdf [Accessed 21 April 2015]. [Google Scholar]
- 18.Yen YF, Yen MY, Lin YP, et al. Directly observed therapy reduces tuberculosis-specific mortality: a population-based follow-up study in Taipei, Taiwan. PLoS One 2013; 8:e79644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Low S, Ang LW, Cutter J, et al. Mortality among tuberculosis patients on treatment in Singapore. Int J Tuberc Lung Dis 2009; 13:328–334. [PubMed] [Google Scholar]
- 20.Ezeamama AE, Mupere E, Oloya J, et al. Age, sex, and nutritional status modify the CD4 + T-cell recovery rate in HIV-tuberculosis co-infected patients on combination antiretroviral therapy. Int J Infect Dis 2015; 35:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis 2004; 8:286–298. [PubMed] [Google Scholar]
- 22.Chan J, Tian Y, Tanaka KE, et al. Effects of protein calorie malnutrition on tuberculosis in mice. Proc Natl Acad Sci U S A 1996; 93:14857–14861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taiwan Centers for Disease Control and Prevention (2015) [Promulgated definitions of TB]. Taipei, Taiwan: CDC; http://www.cdc.gov.tw/ [Accessed May 2, 2015]. [Chinese]. [Google Scholar]
- 24.Vasantha M, Gopi PG, Subramani R. Weight gain in patients with tuberculosis treated under directly observed treatment short-course (DOTS). Indian J Tuberc 2009; 56:5–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


