Abstract
To characterize the clinical, virological, and immunological status at presentation as well as the outcome of patients diagnosed with HIV above the age of 50.
A retrospective study of 418 patients newly diagnosed with HIV in 1 Israeli center, between the years 2004 and 2013.
Patients with new HIV diagnosis ≥50 years of age defined as “older" and <50 defined as “younger." Patients were evaluated every 1 to 3 months (mean follow-up 53 ± 33 months). Patients with <2 CD4/viral-load measurements or with <1 year of follow-up were excluded. Time of HIV infection was estimated by HIV sequence ambiguity assay. Ambiguity index ≤0.43 indicated recent (≤1 year) HIV infection.
Eighty nine (21%) patients were diagnosed with HIV at an older age. Those older patients presented with significant lower CD4 cell counts and higher viral-load compared with the younger patients. At the end of the study, the older patients had higher mortality rate (21% vs 3.5%; P < 0.001) and lower CD4 cell counts (381 ± 228 vs 483 ± 261cells/μL; P < 0.001) compared with the younger patients. This difference was also observed between older and younger patients with similar CD4 cell counts and viral load at the time of HIV diagnosis and among patients with a recent (≤1 year) HIV infection.
One-fifth of HIV patients are diagnosed at older age (≥50 years). Those older patients have less favorable outcome compared with the younger patients. This point to the need of educational and screening programs within older populations and for a closer follow-up of older HIV patients.
INTRODUCTION
Over the years, the proportion of HIV patients older than 50 years of age is constantly increasing.1–4 It is estimated that by the end of 2015, more than half of HIV patients in the United States will be older than 50 years of age.5,6 Currently, about 15% of HIV infected adults in Sub-Saharan Africa are older than 50 but the prevalence of older HIV patients in that area is expecting to increase in the coming decades.7 The main reason for the increasing age of patients living with HIV is the improvement of specific antiretroviral treatment (highly active antiretroviral treatment (HAART)) and its expanding accessibility.6 Better treatment of HIV complications and comorbidities such as diabetes mellitus (DM) and hypertension also contributes to the improving survival of HIV-infected patients.8 Thus, HIV patients grow older and live longer with a life expectancy approaching that of the general population.9
Currently, the majority of the older (≥50 years of age) HIV patients were infected and diagnosed with HIV in youth or in middle age.10 In the United States, 10% to 15% of all patients newly diagnosed with HIV diagnosed patients are above the age of 50.6 A similar prevalence (11%) was reported in Spain.11 It is still unknown whether HIV diagnosed at older age represents delayed diagnosis or a real new (recent) HIV infection. Several studies had demonstrated differences between older and younger patients with new HIV diagnosis regarding their demographic, risk behavior, clinical and immunological characteristics.12–15 Some studies suggested worse prognosis with a rapid disease progression, despite usage of HAART, in patients diagnosed with HIV at older age as compared to patients who were diagnosed with HIV at a younger age,12,13 however, other studies failed to show such a difference.14,15
The aim of the present study is to characterize the demographic, risk behavior, clinical, virological, and immunological status at the time of HIV diagnosis as well as the prognosis and outcome of patients with new HIV diagnosis at older age (≥50) compared with young patients (HIV diagnosis <50) between the years 2004 and 2013 in a major HIV/AIDS center in Israel.
METHODS
Patients
All patients with a new HIV diagnosis during the years 2004 to 2013 in our HIV-AIDS center were recruited to the study. HIV was diagnosed by ELISA and confirmed by Western Blot analysis.16 Patients with a prior positive HIV ELISA test, patients <18 years at the time of diagnosis, patients with <1 year of follow-up or with less than 2 CD4 cell counts or 2 viral load (VL) measurements, and patients with acute HIV infection were excluded from the study. Patients who died during the first year of follow-up but had at least 2 CD4 and 2 VL assays were included. Patients were stratified according to their age at the time of HIV diagnosis. The “older” patients—newly diagnosed HIV patients who were ≥50 years of age at the time of diagnosis and the “younger” group—patients diagnosed with HIV below the age of 50. For each enrolled patient we obtained epidemiological (age, sex, mode of HIV acquisition), immunological (CD4 cell counts), virological (VL), and clinical (AIDS defining illnesses and comorbidities) parameters at the time of HIV diagnosis (study entry). AIDS defining illnesses were defined according to CDC criteria.17 Comorbidities included 1 or more of the following: hypertension, DM, ischemic heart disease, and cerbrovascular disease.
All patients were followed every 1 to 3 months with a periodical clinical and laboratory (CD4 cell counts and VL) evaluations. The follow-up period of time was determined from the time of HIV diagnosis to the last documented visit or death. HAART was initiated according to the AIDSinfo guidelines.18 Adherence was defined according to clinical judgment (physician's follow-up interviews) and the rate of consecutive drug acquisition from the pharmacy by the patients. The study was approved by the Kaplan Hospital Ethics committee.
Immunological and Virological Evaluation
HIV-1 subtypes were determined by the REGA HIV-I Subtyping tool (www.hivdb.stanford.edu/hiv). CD4 cell counts were determined by fluorescence-activated cell sorting (FACS) using fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies (IQ Products, Groningen, The Netherlands).19 HIV VL was determined by the COBAS Ampliprep/COBAS AMPLICOR HIV-1 MONITOR Test, version 1.5 (CAP/CA; Roche Molecular Systems, Branchburg, NJ).20 Patients with VL above the level of detection (400 copies/mL during the years 2004–2009 and 20 copies/mL thereafter) were retested within 1 month. Two consecutive VL tests above the level of detection were defined as virological failure, whereas VL test below that level was defined as LDL (lower than detection limit).
Estimating HIV Infection Time by Analyzing Genetic Ambiguity
Duration of HIV infection was estimated by analyzing the sequence ambiguity in a population-based sequencing (TRUGENE, Simens). The number of ambiguous nucleotides found in each patient's sample was determined and an ambiguity index was calculated as described previously.21 An ambiguity cutoff of 0.43 was used to distinguish recent (≤1 year; ambiguity ≤0.43) from chronic infection (>1 year; ambiguity >0.43).22
Statistical Analysis
Data are presented as mean ± standard deviation (SD). Student t test, Fisher exact test, and χ2 were used for statistical analysis. P ≤ 0.05 was considered statistically significant.
RESULTS
Four hundred eighteen HIV patients (men 243 (58%); women 175 (42%)) were included in our retrospective study. The mean age of the patients at the time of HIV diagnosis was 40.4 ± 13.5 (range 18–88) years. The mean age of the women (39 ± 12.6 years), at the time of HIV diagnosis, was significantly lower compared with the age of the men (41.3 ± 14.2 years; P = 0.01). The mean follow-up period (53.6 ± 33.6 (range 13–126) months) was similar for the men and women.
Eighty nine (21%) patients were first diagnosed with HIV at “older age” (≥50 years). Fifty-two of them (59%) were men and 37 (41%) were women. The number and the percentage of the older patients with new HIV diagnosis varied (nonsignificantly) along the study period (15%–34%). It peaked at 2008 (34%) and then decreased gradually (16% at 2013).
Table 1 summarizes the demographic, clinical, immunological, and virological characteristics at the time of HIV diagnosis, of our older and younger patients. As can be seen in the table, the proportion of men and women was similar between the younger and the older patients. On the other hand, the mode of HIV acquisition was significantly different. The prevalence of men who have sex with man (MSM) and intravenous drug users (IVDU) was significantly higher among the younger compared with the older patients. In contrast, 70% of our older patients were heterosexual immigrants from Ethiopia (an HIV endemic country) as compared with only 50% in the younger patients. The HIV subtype was C in the Ethiopian immigrants, B in the MSM (few infected MSM had subtype A), and A in the IVDU patients, regardless of their age.
TABLE 1.
Demographic, Clinical, Immunological, and Virological Characteristics of “Older" Vs “Younger" Patients With a New HIV Diagnosis
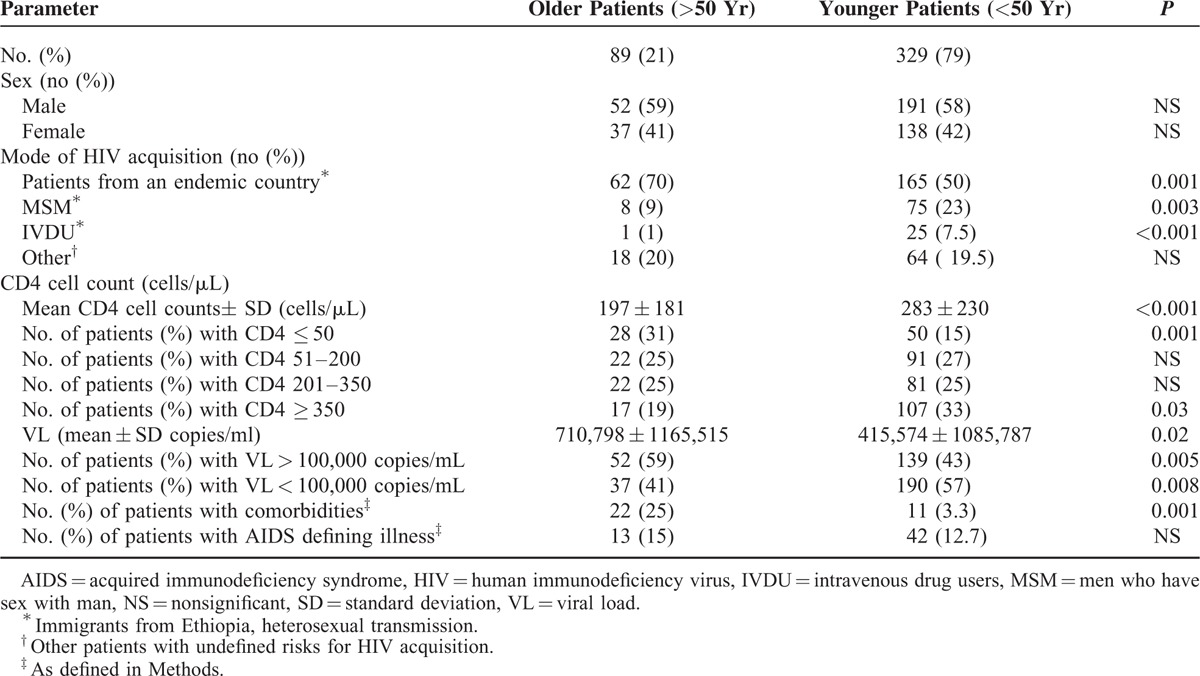
The immune system at the time of HIV diagnosis was significantly impaired in the older compared with the younger group of patients (Table 1). Moreover, 31% of the older patients had CD4 cell counts of less than 50 cells/μL at HIV diagnosis (compared with only 15% in the younger patients; P = 0.001). HIV VL at the time of diagnosis was significantly higher in the older patients. Significantly more older than younger patients had VL above 100,000 copies/mL at the time of diagnosis (Table 1).
Comorbidities were more commonly observed in our older patients (25%) as compared with only 3.3% in the younger group of patients. AIDS defining illnesses were observed in 55 of our patients at the time of HIV diagnosis, with a similar prevalence in both age groups. The main AIDS defining illnesses were TB (mycobacterium tuberculosis; 34%), and pneumocystis jirovecii pneumonia (PCP, 23%). Lymphoma, brain toxoplasmosis, esophageal candidiasis, Kaposi sarcoma, and cryptococal meningitis occurred less frequently.
HAART was initiated according to the AIDS info guidelines18 regardless of the age of the patients. The HARRT regiments were based on 2 reverse transcriptase inhibitors (mainly Combivir, Truvada, or Kivexa) and either a nonnucleoside reverse transcriptase inhibitor (Efaviranze) or a boosted protease inhibitor (mainly Kaletra, boosted Atazanavir, or boosted Darunavir) or an integrase inhibitor (Isentress). There were no differences in the recommended HAART regimes between the older and the younger patients. In the years of the study, we did not recommend HAART to patients with CD4 > 350 cells/μL. The prevalence of such patients was significantly higher among the younger group of patients (33% vs 19% in younger and older patients, respectively; P = 0.03). Adherence to HAART medications during the follow-up period was similar in the 2 groups of patients (about 85%–90%).
The mean follow-up period (53 ± 33 months) was similar in both age groups (Table 2). During that period, 31 of our patients had died. The mortality rate was significantly higher in the older compared with the younger group of patients (Table 2). The mortality rate among older men (28%) was significantly higher than that of the older women (11%; P = 0.04). No such sex difference was observed in the younger group of patients (4.1% and 3.5% mortality for younger men and women; respectively; P = NS). As was observed at the time of HIV diagnosis (Table 1), at the end of the study comorbidities were more often observed in the older group of patients (Table 2).
TABLE 2.
Immunological, Virological, and Clinical Characteristics of “Older” Vs “Younger” HIV Patients at the End of Follow-Up Period
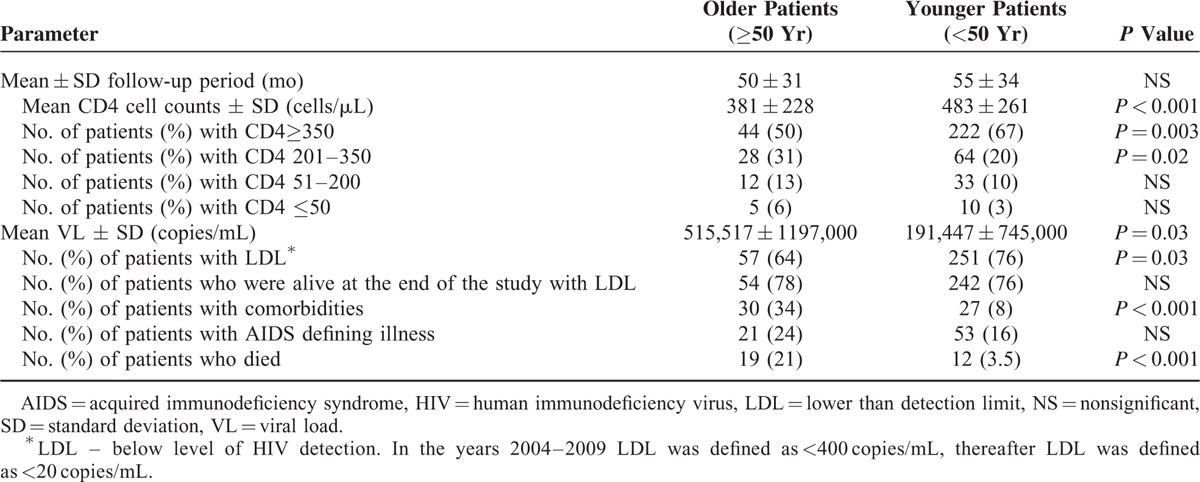
At the end of the follow-up period, the CD4 cell counts were significantly lower in the older, compared with the younger, group of patients. Furthermore, half of the older patients had CD4 cell counts below 350 cells/μL as compared with 33% of the younger patients (Table 2). As can be seen in Figure 1 the mean change in CD4 cell counts (ΔCD4) was lower in the older (183 cells/μL) compared with the younger patients (200 cells/μL) though without statistical significance. However, in the subgroups of patients with initial CD4 cell counts of less than 50 cells/μL, and in patients with initial CD4 cell counts between 201 and 350 cells/μL, ΔCD4 was significantly lower (P = 0.05 and P = 0.02; respectively) in the older compared with the younger group of patients (Figure 1).
FIGURE 1.
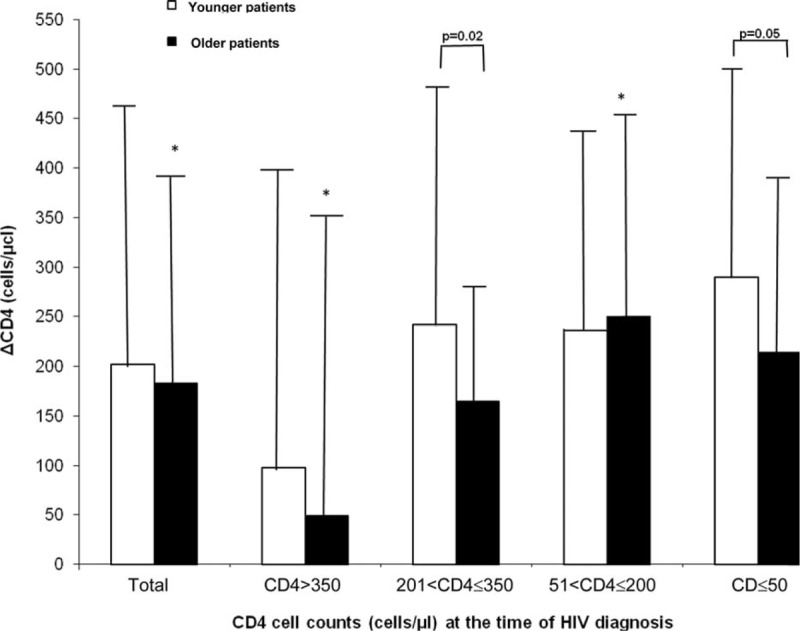
CD4 cell counts increments (ΔCD4) of older and younger patients during the study period. Patients were stratified into 4 subgroups according to their initial CD4 cell counts at the time of HIV diagnosis. ΔCD4 was determined as the mean ± SD differences between CD4 cell counts at the end of the study (last visit) and the initial CD4 (at HIV diagnosis) cell counts for each patient. ∗P not significant.
The older patients revealed, at the end of the study, significantly higher VL levels as compared with the younger group. In addition, significantly, fewer older patients achieved a good virological response (LDL). However, among the patients who were still alive at the end of the study, LDL rates were similar in both age groups (Table 2).
To rule out the possibility that the unfavorable outcome of the older patients resulted, mainly, from their initially worse virological and immunological status (Table 1) rather than from their age, we further stratified our older and younger patients into 4 subgroups according to their initial CD4 cell counts at the time of HIV diagnosis. Group I—patients with initial CD4 ≤ 50 cells/μL; Group II—with initial CD4 51 to 200 cells/μl; Group III with initial CD4 201 to 350 cells/μL, and group IV with initial CD4 cell counts above 350 cells/μL. Table 3 summarizes the immunological, virological, and clinical outcomes in those “older” and “younger” subgroups of patients. As can be seen in the table, in each subgroup the outcome of the older patients was less favorable than that of the younger patients. Thus, at the end of the study, the older group of patients demonstrated higher VL, lower CD4 counts, higher mortality rates, and higher rates of AIDS defining illnesses as compared with the younger subgroup of patients who were presented with a similar CD4 cell counts at the time of HIV diagnosis. Similarly, the older patients with VL > 100,000 copies/mL at diagnosis demonstrated worse prognosis compared with the younger patients who had a similar high initial VL levels. Thus, end of study comparison between older and younger patients who were presented with a similar initial high VL (>100,000 copies/mL) demonstrated significantly lower CD4 cell counts (360 ± 259 vs 437 ± 288 cells/μL for older and younger patients respectively; P = 0.04), higher VL (343,834 ± 1009,060 vs 100,537 ± 579,618 copies/mL for older and younger patients respectively; P = 0.04) and a higher mortality rate (32% vs 6% for older and younger patients respectively; P < 0.0001) in the older group of patients.
TABLE 3.
Immunological, Virological, and Clinical Characteristics at the End of the Study of “Older” Vs “Younger” Patients as Related to Their Initial CD4 Cell Counts at the Time of HIV Diagnosis
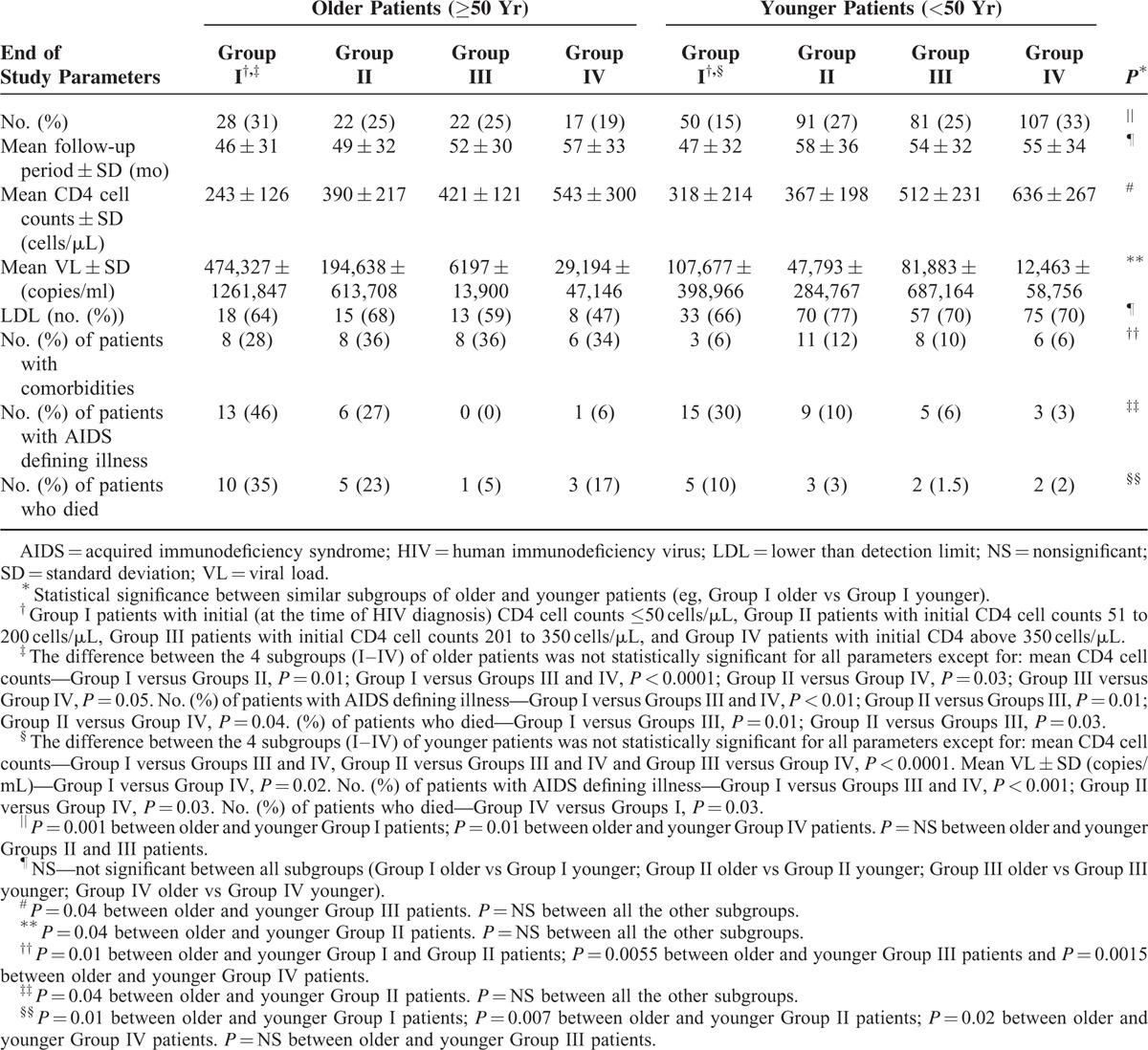
Delayed HIV diagnosis can affect the immunological, virological, and clinical status of the patients at the time of diagnosis as well as their outcome. Therefore, it was important to determine whether our older patients were indeed recently (≤1 year) infected with HIV or were already infected for a long period of time prior to their diagnosis. To this end, we applied the recency algorithm, which estimates the time of HIV infection according to viral ambiguity.23 To validate the algorithm in older patients, we first analyzed HIV sequences obtained from 20 older patients (not included in our study) more than 1 year after their HIV diagnosis. In all cases, the assay confirmed the chronic (>1 year) nature of their disease. Viral sequences, obtained at the time of HIV diagnosis, were available for 34 older and 65 younger patients. As shown in Table 4, about 40% of our patients, regardless of their age, presented with a recent (≤1 year) HIV infection at the time of their diagnosis. In the other patients the sequencing ambiguity-based analysis suggested a more chronic (>1 year) disease (delayed HIV diagnosis).
TABLE 4.
Demographic, Immunological, Virological, and Clinical Outcome (End of Study) of “Older” Vs “Younger” HIV Patients as Related to the Time of Their HIV Infection∗
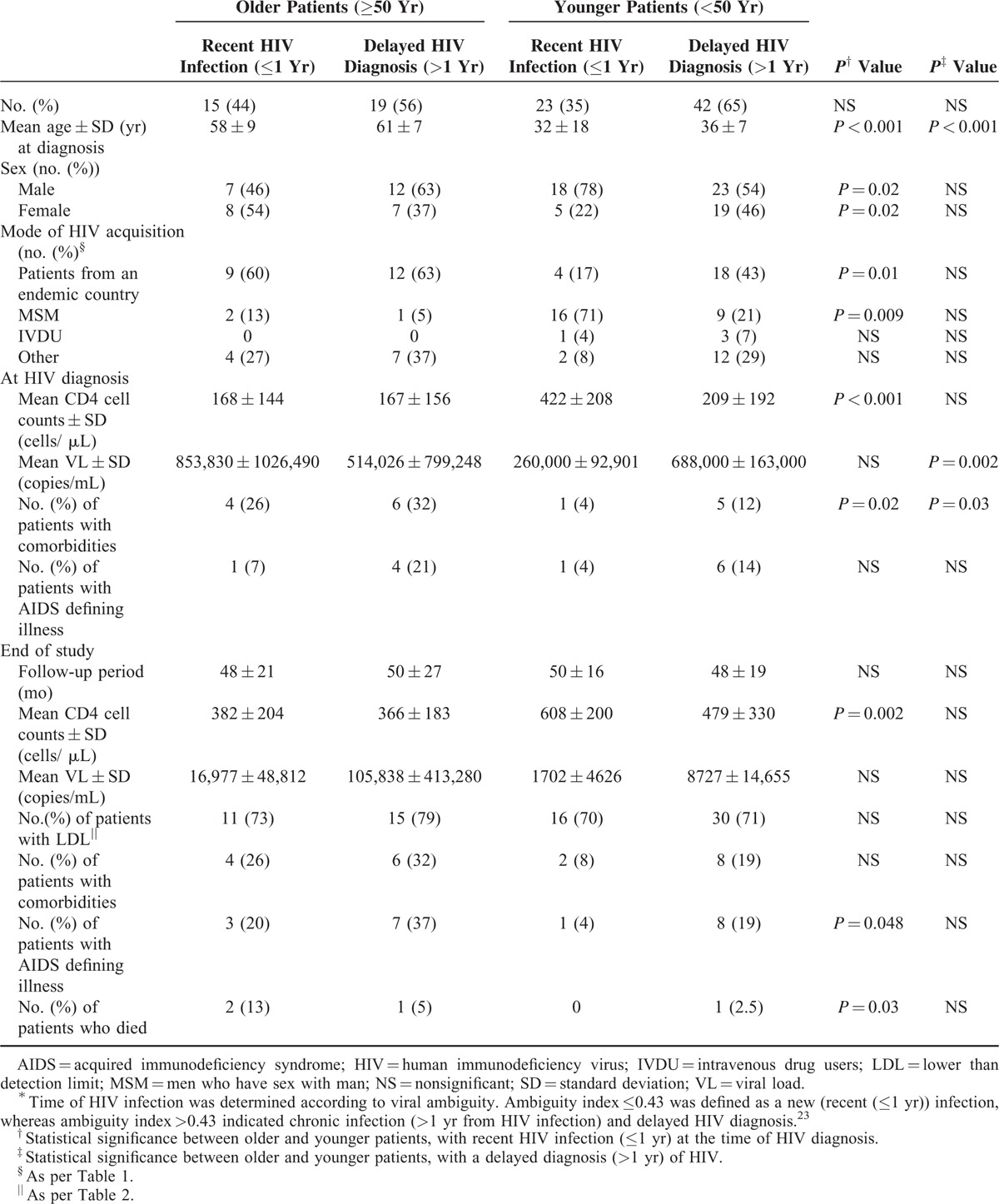
In both age groups, patients with delayed diagnosis were diagnosed at an older age compared with those with a recent HIV infection. In the younger group of patients, significantly more MSM (P = 0.009) and fewer immigrants from Ethiopia (P = 0.01) were estimated to have a recent HIV infection at the time of diagnosis. Younger patients with delayed diagnosis demonstrated significantly higher VL (P = 0.002) and lower CD4 cell counts (P < 0.001) compared with the younger patients with a recent HIV infection. No such differences were observed within the group of older patients (Table 4). The older patients with a recent HIV infection presented with a significantly lower CD4 cell count, and more comorbidities compared with the younger patients with a recent HIV infection (Table 4). Moreover, the outcome in the latter 2 subgroups of patients deferred significantly. Thus, at the end of the study, the older patients with a recent HIV infection at diagnosis demonstrated significantly lower CD4 cell counts and higher rates of AIDS defining illness and mortality compared with the younger patients with a recent HIV infection at diagnosis. Similar outcome differences trends, though without statistical significance, were observed between older and younger patients who were presented with a delayed HIV diagnosis (Table 4). Interestingly, although VL levels were higher among older patients with recent HIV infection (compared with the younger patients) at the time of diagnosis and at the end of the study, it was not statistically significant. Moreover, the rate of patients with LDL (at the end of the study) was similar in the older and younger groups of patients, regardless of the recency (recent or delayed) of HIV infection at the time of diagnosis (Table 4).
DISCUSSION
The main findings of the present study are that one-fifth (21%) of the patients newly diagnosed with HIV are above the age of 50. Those older patients presented with higher VL and lower CD4 cell counts compared with the younger patients. Moreover, the older patients had less favorable outcome with high mortality rate and more impairment of their immune system, compared with young patients.
Since in Israel all HIV-positive ELISA sera are validated in 1 central laboratory, we were able to exclude patients with a prior positive HIV test. Thus, our 418 patients were indeed patients with a new HIV diagnosis. As was also shown by others,12,14 MSM and IVDU were more common in the younger group, whereas heterosexual (including immigrants from endemic countries) HIV acquisition was more prevalent among older HIV patients (Table 1). A different sexual behavior between young and old MSM and heterosexuals is probably the cause for that difference.
The mean age of our new HIV patients increased insignificantly along the years of the study (34 ± 13 years at 2004, 38 ± 12 years at 2013; P = NS). Eighty-nine patients (21%) were diagnosed with HIV at older age (>50 years). Similar rates were also reported by others.12–14 Active sex life with a risky sexual behavior of older men and women is probably the major cause for HIV infection at older age. Therefore, physicians should consider the possibility of HIV diagnosis in older patients. HIV education and prevention programs should be implanted in older populations and not exclusively among young populations.
As was reported by others,13,14 the older patients in our study presented with lower CD4 cell counts and higher VL compared with the younger patients (Tables 1, 3, 4). Previous studies suggested that this is probably due to delayed HIV diagnosis of the older patients.11,14 Using the sequence ambiguity assay,22 we were able to show, for the first time, similar rates (40%) of recent (≤1 year) HIV infection in our older and younger patients (Table 4) excluding the role of late diagnosis as the main cause, for the worse immunological, virological, and clinical status of older patients with a new HIV diagnosis. Furthermore, among the subgroup of patients with a recent HIV infection, the older patients revealed lower CD4 cell counts and higher VL at the time of HIV diagnosis as well as less favorable outcome compared with the younger patients (Table 4). Moreover, the prognosis of older patients was worse (mortality, immunological impairment, AIDS defining illnesses) than that of the younger patients who presented with similar VL and CD4 cell counts at the time of HIV diagnosis (Table 3), further supporting the effect of age, at the time of HIV diagnosis, on the clinical, immunological and virological course of HIV infection.
Despite good HAART adherence (about 85%–90%), the prognosis of the older HIV patients was less favorable compared with young patients. The older patients demonstrated higher mortality rate (Table 2) and less immunological improvement (Figure 1) compared with the younger patients. Our results agree with other studies, which reported high mortality rate in patients diagnosed with HIV above the age of 50,12,15 especially in men.12 Similar to our observations, some studies reported a blunted immunological response of older HIV patients despite good virological response.12,13,23
Several factors, not mutually exclusive, may contribute to the worse prognosis of patients infected with HIV at older age. The level of physician's clinical suspicions of HIV in old age is relatively low. This may lead to delayed diagnosis of HIV in older patients. In our study, this was not a significant factor since the prevalence of patients with delayed HIV diagnosis was similar in our younger and older patients (Table 4). Older patients have more comorbidities compared with young patients (Tables 2–4) which may affect their general clinical condition.24 The therapeutic effects, the pharmacokinetics (including drug–drug interactions), and the tolerability of HAART may differ in different ages although previous studies had demonstrated similar response to HAART in younger and older patients.4,23 Indeed, as was previously reported,13,15,23 we observed good virological suppression (LDL) in our older patients, who had good adherence to HAART, despite a blunted CD4 cells recovery (Tables 2–4). Immunosenescence (ageing of the immune system) may be the main cause for the worse course of older HIV patients. Indeed, older patients, regardless of their HIV status, revealed malfunction of B cells,25 CD4 and CD8 cells26,27 (especially memory B/T cells28,29) and regulatory T cells.30 In agreement, we observed immune impairment at the time of HIV diagnosis (Tables 1, 3) with a blunted T-cell recovery (Figure 1; Tables 2–4) in the older patients compared with the younger HIV patients. Thus, old age (≥50 years) at the time of HIV diagnosis affects the course of the disease by several mechanisms.
The present study has some limitations. First, it is a retrospective study from 1 medical center. Second, the number of the older patients with new HIV diagnosis (89) is relatively small. On the other hand, our strict inclusion and exclusion criteria ascertained that all participants were, indeed, patients with a new HIV diagnosis. To the best of our knowledge, systematic analysis of different subgroups of patients, according to their initial virological and immunological status and to the time of their HIV infection (recent infection vs delayed diagnosis), was not reported previously. Our study enabled us to highlight the significant role of age on the course of HIV infection, overcoming several confounding factors.
To conclude, one-fifth of patients with a new HIV diagnosis are above the age of 50. Those older patients have less favorable outcome compared with patients newly diagnosed with HIV at younger age. This highlights the urgent need for educational and screening programs in older populations, as well as the mandatory need for close follow-up and early HAART initiation in patients diagnosed with HIV at older (≥50 years) age.
Footnotes
Abbreviations: AIDS = acquired immunodeficiency syndrome, CDC = center for disease control and prevention, DM = diabetes mellitus, ELISA = enzyme-linked immunosorbent assay, HAART = highly active antiretroviral treatment, HIV = human immunodeficiency virus, IVDU = intravenous drug users, LDL = lower than detection limit, MSM = men who have sex with man, NS = nonsignificant, PCP = pneumocystis jirovecii pneumonia, SD = standard deviation, US = United States, VL = viral load.
IA and KMG contributed equally to this article.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Mack KA, Ory MG. AIDS and older Americans at the end of the Twentieth Century. J Acquir Immune Defic Syndr 2003; 33 Suppl 2:S68–75. [DOI] [PubMed] [Google Scholar]
- 2.Atun RA, Gurol-Urganci I, McKee M. Health systems and increased longevity in people with HIV and AIDS. BMJ 2009; 338:b2165. [DOI] [PubMed] [Google Scholar]
- 3.Lazarus JV, Nielsen KK. HIV and people over 50 years old in Europe. HIV Med 2010; 11:479–481. [DOI] [PubMed] [Google Scholar]
- 4.Kirk JB, Goetz MB. Human immunodeficiency virus in an aging population, a complication of success. J Am Geriatr Soc 2009; 57:2129–2138. [DOI] [PubMed] [Google Scholar]
- 5.Prejean J, Song R, Hernandez A, et al. Estimated HIV incidence in the United States, 2006-2009. PLoS One 2011; 6:e17502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mills EJ, Bärnighausen T, Negin J. HIV and aging-preparing for the challenges ahead. N Engl J Med 2012; 366:1270–1273. [DOI] [PubMed] [Google Scholar]
- 7.Hontelez JA, de Vlas SJ, Baltussen R, et al. The impact of antiretroviral treatment on the age composition of the HIV epidemic in sub-Saharan Africa. AIDS 2012; 26 Suppl 1:S19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greene M, Justice AC, Lampiris HW, et al. Management of human immunodeficiency virus infection in advanced age. JAMA 2013; 309:1397–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 2011; 53:1120–1126. [DOI] [PubMed] [Google Scholar]
- 10.van Sighem AI, Gras LA, Reiss P, et al. ATHENA national observational cohort study. Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. AIDS 2010; 24:1527–1535. [DOI] [PubMed] [Google Scholar]
- 11.Kearney F, Moore AR, Donegan CF, et al. The ageing of HIV: implications for geriatric medicine. Age Ageing 2010; 39:536–541. [DOI] [PubMed] [Google Scholar]
- 12.Navarro G, Nogueras MM, Segura F, et al. HIV-1 infected patients older than 50 years. PISCIS cohort study. J Infect 2008; 57:64–71. [DOI] [PubMed] [Google Scholar]
- 13.Metallidis S, Tsachouridou O, Skoura L, et al. Older HIV-infected patients—an underestimated population in northern Greece: epidemiology, risk of disease progression and death. Int J Infect Dis 2013; 17:e883–e891. [DOI] [PubMed] [Google Scholar]
- 14.Eduardo E, Lamb MR, Kandula S, et al. Characteristics and outcomes among older HIV-positive adults enrolled in HIV programs in four sub-Saharan African countries. PLoS One 2014; 9:e103864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nogueras M, Navarro G, Antón E, et al. Epidemiological and clinical features, response to HAART, and survival in HIV-infected patients diagnosed at the age of 50 or more. BMC Infect Dis 2006; 6:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han N, Wright ST, O’Connor CC, et al. HIV and aging: insights from the Asia Pacific HIV Observational Database (APHOD). HIV Med 2015; 16:152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Branson BM, Handsfield HH, Lampe MA, et al. Centers for Disease Control and Prevention (CDC). Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006; 55:1–17. [PubMed] [Google Scholar]
- 18.http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5710a2.htm. MMWR Dec 5th, 2008/57(RR10); 9. [Google Scholar]
- 19.http://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescenttreatment-guidelines/0.AIDSinfo April 8th 2015. [Google Scholar]
- 20.Ferrara GB, Strelkauskas AJ, Longo A, et al. Markers of human T cell subsets identified by alloantisera. J Immunol 1979; 123:1272–1277. [PubMed] [Google Scholar]
- 21.Bisset LR, Bosbach S, Tomasik Z, et al. Quantification of in vitro retroviral replication using a one-tube real-time RT-PCR system incorporating direct RNA preparation. J Virol Methods 2001; 91:149–155. [DOI] [PubMed] [Google Scholar]
- 22.Kouyos RD, von Wyl V, Hinkley T, et al. Assessing predicted HIV-1 replicative capacity in a clinical setting. PLoS Pathog 2011; 7:e1002321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersson E, Shao W, Bontell I, et al. Evaluation of sequence ambiguities of the HIV-1 pol gene as a method to identify recent HIV-1 infection in transmitted drug resistance surveys. Infect Genet Evol 2013; 18:125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sabin CA, Smith CJ, d’Arminio Monforte A, et al. Response to combination antiretroviral therapy: variation by age. AIDS 2008; 22:1463–1473. [DOI] [PubMed] [Google Scholar]
- 25.Negin J, Martiniuk A, Cumming RG, et al. Prevalence of HIV and chronic comorbidities among older adults. AIDS 2012; 26 Suppl 1:S55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frasca D, Blomberg BB. Effects of aging on B cell function. Curr Opin Immunol 2009; 21:425–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haynes L, Maue AC. Effects of aging on T cell function. Curr Opin Immunol 2009; 21:414–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gibson KL, Wu YC, Barnett Y, et al. B-cell diversity decreases in old age and is correlated with poor health status. Aging Cell 2009; 8:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naylor K, Li G, Vallejo AN, et al. The influence of age on T cell generation and TCR diversity. J Immunol 2005; 174:7446–7452. [DOI] [PubMed] [Google Scholar]
- 30.Tsaknaridis L, Spencer L, Culbertson N, et al. Functional assay for human CD4 + CD25 + Treg cells reveals an age-dependent loss of suppressive activity. J Neurosci Res 2003; 74:296–308. [DOI] [PubMed] [Google Scholar]


