Abstract
The present study evaluated the dementia risk after carbon monoxide poisoning (CO poisoning).
Using the National Health Insurance Research Database of Taiwan, a total of 9041 adults newly diagnosed with CO poisoning from 2000 to 2011 were identified as the CO poisoning cohort. Four-fold (N = 36,160) of non-CO poisoning insured people were randomly selected as controls, frequency-matched by age, sex, and hospitalization year. Incidence and hazard ratio (HR) of dementia were measured by the end 2011.
The dementia incidence was 1.6-fold higher in the CO exposed cohort than in the non-exposed cohort (15.2 vs 9.76 per 10,000 person-years; n = 62 vs 174) with an adjusted HR of 1.50 (95% CI = 1.11–2.04). The sex- and age-specific hazards were higher in male patients (adjusted HR = 1.74, 95% CI = 1.20–2.54), and those aged <=49 years (adjusted HR = 2.62, 95% CI = 1.38–4.99). CO exposed patients with 7-day or longer hospital stay had an adjusted HR of 2.18 (95% CI = 1.42, 3.36). The CO poisoning patients on hyperbaric oxygen (HBO2) therapy had an adjusted HR of 1.80 (95% CI = 0.96–3.37).
This study suggests that CO poisoning may have association with the risk of developing dementia, which is significant for severe cases. The effectiveness of HBO2 therapy remains unclear in preventing dementia. Patients with CO poisoning are more prevalent with depression.
INTRODUCTION
The Global Burden of Disease Study has ranked dementia the 5th common chronic disease.1 The Institute for Health Metrics and Evaluation (IHME) has estimated that there was a 112.8% increase in the disability-adjusted life-year cost for dementia in 2010 compared that in 1990. It was anticipated that the cost would increase further for more than 80% by 2030.1 The Alzheimer Disease International (ADI) Report has also estimated that approximately 44.4 million people were affected by dementia in 2010, and this number would increase to 75.6 million by 2030 and 135.5 million by 2050. In 2010, the Global total social costs for dementia were estimated to be US $ 604 billion, corresponding to 1.0% of the global GDP.2–4 Dementia is more important than Alzheimer disease alone in causing a huge economic burden.5–7 Dementia is the brain disease characterized by impaired memory and loss of thinking and reasoning, which severely affect the patient's daily functioning.8
The risk of developing dementia has been associated with several conditions, including alcohol abuse (alcohol-related dementia), chronic traumatic encephalopathy, drug side effects, depression, and other central nervous system disorders.9–15 Establishing a link between associated risk factors and dementia may provide appropriate means of treatment and counseling, thereby reducing the risk of dementia and costs of treatment.
Carbon monoxide (CO) poisoning is a common and important cause of death among populations, including populations in the United States, and England and Wales.16 Approximately 15,000 unintentional nonfire related episodes of CO poisoning have been reported annually in England and Wales, leading to 50,000 emergency department visits and 500 deaths.17 The memory difficulty and confusion are initial neurological symptoms of acute CO poisoning in addition to headache, weakness, dizziness, nausea, and vomiting.15 Furthermore, unconsciousness, respiratory arrest, and even death could develop for the CO poisoning victims.17 Previous studies have reported that up to 30% of patients result in the sequelae of neurologic complications after the poisoning.18,19 Intellectual function impairments, short-term memory loss, dementia, amnesia, psychosis, irritability, dysfunctional gait, speech disorders, Parkinson disease-like syndrome, cortical blindness, and depression are among the poisoning sequelae.20,21 Victims of CO poisoning may suffer from brain hypoxia, inflammation, and subsequent injuries.22 Patients with ischemia-reperfusion injury exposing to high oxygen may aggravate the oxidative damage after recovery from CO poisoning.23,24 Encephalopathy and other neuropsychiatric disorders are other sequelae in victims. It remains unclear whether such abnormalities increase the risk of dementia.25–28 In an earlier Japanese study, Mimura et al28 found considerable sequelae in CO poisoning patients after follow-up for 33 years. No large-scale long-term follow-up study has ever evaluated this relationship. The present study was conducted to evaluate whether patients with CO poisoning are at an elevated risk of developing dementia.
METHODS
Data Source
We conducted this study using the National Health Insurance Research Database obtained from the National Health Insurance program of Taiwan, which was initiated in 1995 to provide comprehensive medical care to the general population. This insurance system has covered approximately 99% of the entire 23 million population of Taiwan (http://www.nhi.gov.tw/english/index.aspx). The Taiwan National Health Research Institutes is responsible to manage the claims data and secure the confidentiality of the insured individuals according to the directives of the insurance authority. The diagnostic codes were determined based on the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM). To assure the accuracy of diagnosis, the Bureau of National Health Insurance sifted and validated medical records review that including clinical manifestations, physical examination, and laboratory and image studies. Because patient identifications were scrambled into surrogate numbers to protect patient privacy, no patients consent was required for this study, which has been approved by the Research Ethics Committee at China Medical University and Hospital (CMUH104-REC2-115).
Study Population
We identified 9041 new patients diagnosed with CO poisoning (ICD-9 code 986) from the inpatient claims data in 2000 to 2011. The date of the first hospitalization for CO poisoning was designated as the index date. Patients with a history of dementia (ICD-9-CM 290, 294.1, and 331.0–331.2) before the index date or those with incomplete age or sex information were excluded from the study. From inpatient individuals without the history of CO poisoning and/or dementia, we randomly selected 36,160 persons as the non-CO poisoning cohort frequency-matched against CO poisoning patients by age (every 5 years), sex, and the year of hospitalization.
Outcome and Comorbidities
Individuals in both study cohorts were followed up until the date of diagnosis of dementia, loss to follow-up, death, withdrawal from the insurance program, or the end of 2011, whichever occurred earlier. We identified the baseline information on comorbidity and health care utilizations as covariates including diabetes (ICD-9-CM code 250), hypertension (ICD-9-CM code 401–405), head injury (ICD-9-CM code 310.2, 800, 801, 803, 804, 850, 851, 853, and 854), depression (ICD-9-CM code 296.2, 296.3, 296.82, 300.4, and 311), cerebrovascular diseases (ICD-9-CM code 430–438), chronic obstructive pulmonary disease (COPD) (ICD-9-CM codes 490–492, 494, and 496), cognitive impairment (ICD-9-CM codes 31.83, 438.0, 310.8, and 294.9), urinary tract infection (ICD-9-CM code 599.0), and pneumonia ((ICD-9-CM codes 480–487).29
Statistical Analysis
The Chi-square test was used to examine the differences in the categorical demographic variables and comorbidities between CO poisoning and non-CO poisoning cohorts, whereas the Student's t-test was used to examine mean ages between the 2 cohorts. Comorbidities included diabetes, hypertension, head injury, depression, stroke, COPD, cognitive impairment, urinary tract infection, and pneumonia. The overall sex, age, and comorbidity specific incidence rates (per 10,000 person-years) were calculated for each cohort. The Kaplan–Meier method plotted cumulative incidences of dementia for both CO poisoning and non-CO poisoning cohorts and the proportions were examined using the log-rank test. Univariate and multivariate Cox proportional hazards regression analyses were performed to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) of dementia for patients of the CO poisoning, compared with the non-CO poisoning subjects. The multivariable Cox model, in addition to age and gender, included comorbidities of head injury, depression, COPD, and cognitive impairment, which were significantly different in distributions at the baseline between the 2 cohorts. We further evaluated whether sex, age, and comorbidity interacted with CO poisoning for the dementia risk. The effect of hyperbaric oxygen (HBO2) therapy (Procedure Code 93.95) on the risk of dementia was evaluated for CO poisoning patients who had received the treatment within 3 days after the hospital admission. We further evaluated the dementia risk by the severity of CO poisoning using 7 days of hospital stay as a cut-off to distinct severity. The SAS software (Version 9.3 for Windows; SAS Institute Inc., Cary, NC) was used to perform all the data analyses. Kaplan–Meier curves were plotted using the R software (Version 2.14.1; R Development Core Team, Vienna, Austria). P values < 0.05 were considered statistically significant.
RESULTS
The CO poisoning and non-CO poisoning cohorts were similar in distributions of sex and age (Table 1). Near 80% of study subjects in both cohort aged less than 50 years old, with a mean age of 39.8 years (standard deviation = 13.8). The CO poisoning cohort exhibited higher comorbidities of depression and COPD, but lower head injury and cognitive impairment than did the non-CO poisoning cohort (all P-values < 0.05). After a 12-year follow-up period, the CO poisoning cohort revealed a higher cumulative incidence of dementia than did the non-CO poisoning cohort (P = 0.008, Figure 1). The mean follow-up years for the CO poisoning and non-CO poisoning cohorts were 4.53 and 4.93 years, respectively.
TABLE 1.
Characteristics of Patients With Carbon Monoxide Poisoning and Frequency Matched Patients Without Carbon Monoxide Poisoning
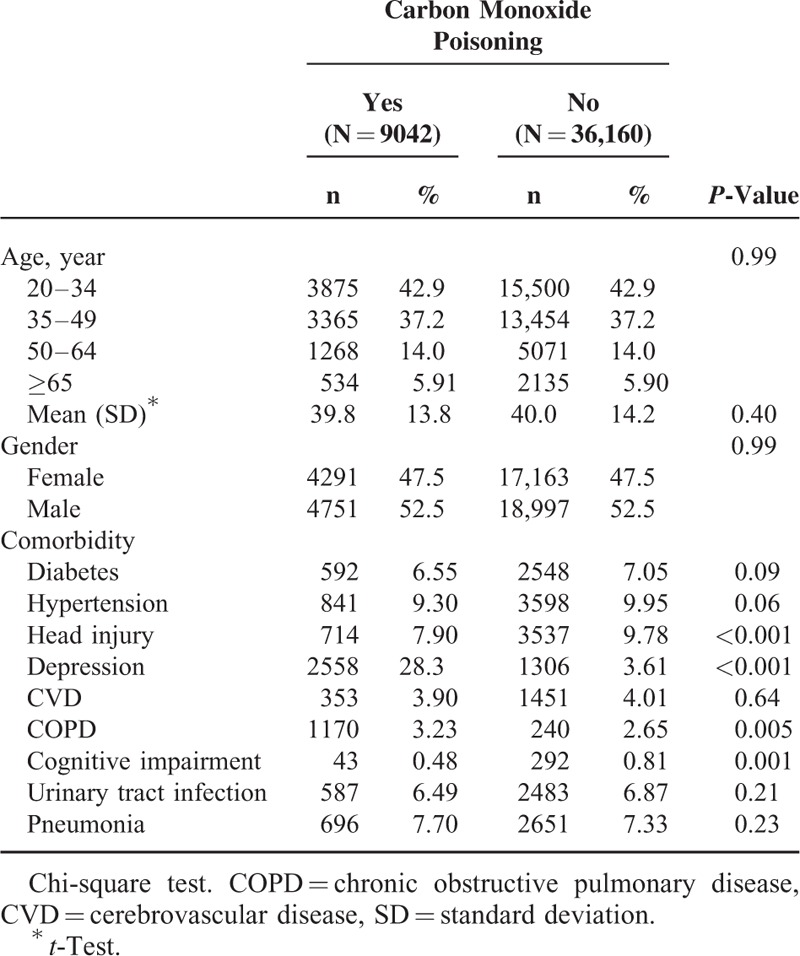
FIGURE 1.
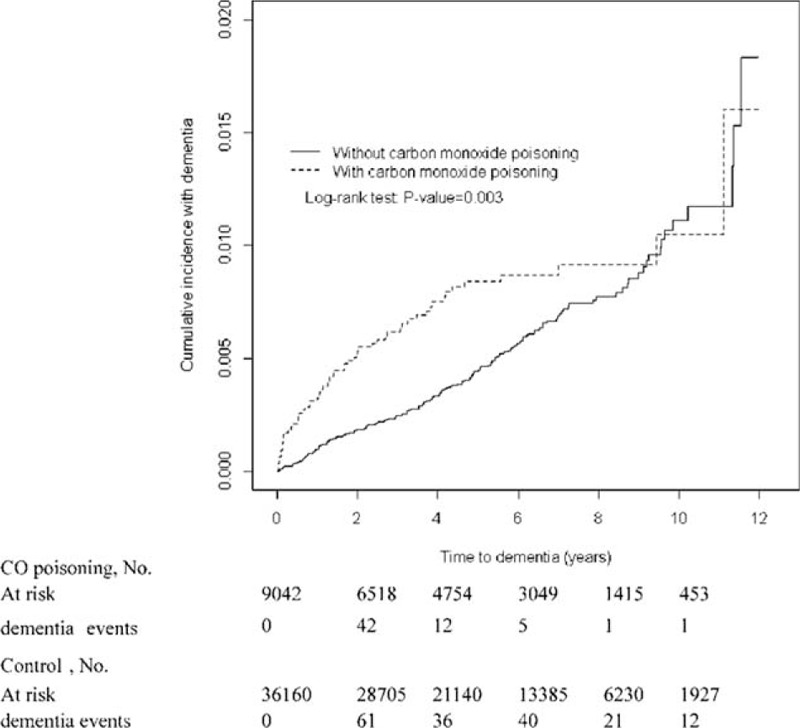
Cumulative incidence of dementia in patients with and without carbon monoxide poisoning.
The overall incidence rate of dementia was 1.56-fold higher in the CO poisoning cohort than in the non-CO poisoning cohort (15.2–9.76 per 10,000 person-years; n = 62 vs 174), with an adjusted HR of 1.50 (95% CI = 1.11–2.04) (Table 2). In both cohorts, men exhibited a higher incidence of dementia than did women; moreover, the incidence increased with age and was higher for patients with comorbidity than for those without comorbidity. The sex-specific hazards showed a higher in male patients (adjusted HR = 1.74, 95% CI = 1.20–2.54), and the age-specific hazards showed a higher for those aged <49 years (adjusted HR = 2.62, 95% CI = 1.38–4.99). Among study population without comorbidities, patients with CO poisoning had a higher risk of dementia compared to the non-CO poisoning cohort.
TABLE 2.
Incidence and Hazard Ratio of Dementia in Patients With and Without Carbon Monoxide Poisoning
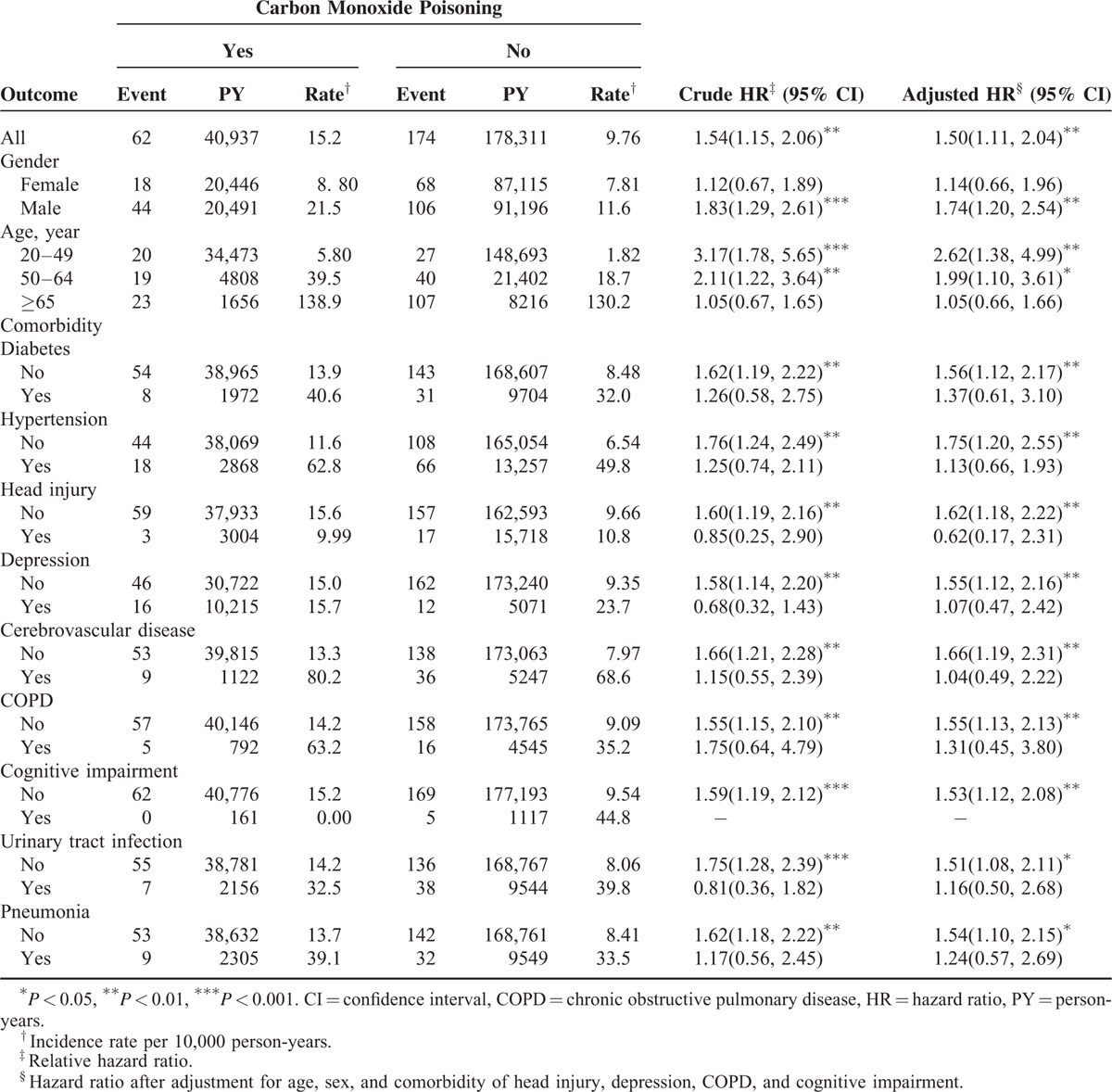
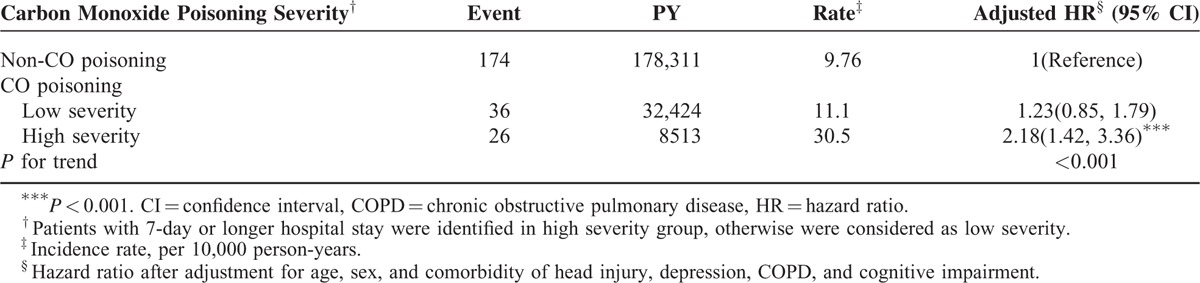
Table 3 summarizes the HRs of dementia associated with the interaction between CO poisoning and sex, age, comorbidity, and HBO2 therapy. Compared with women in the control cohort, men with CO poisoning patients exhibited an adjusted HR of 2.33 developing dementia (95% CI = 1.57–3.46). The HR of dementia increased with age, higher for those with CO poisoning than those without poisoning, but not for the elderly. The CO poisoning patients with a comorbidity had an adjusted HR of 1.75 (95% CI = 1.08–2.83) for dementia, compared with persons without poisoning and comorbidity. Compared with the non-CO poisoning cohort, the CO poisoning patients without HBO2 therapy had an adjusted HR of 1.45 (95% CI = 1.05–2.01) to develop dementia. CO poisoning patients receiving HBO2 therapy had an adjusted HR of 1.80, but not significant. Table 4 shows the risk of dementia increased with the severity of the poisoning. The severe cases had an adjusted HR of 2.18 (95% CI = 1.42–3.36).
TABLE 3.
Cox Proportional Hazards Regression Analysis for Hazard Ratio of Dementia-Associated Carbon Monoxide Poisoning With Interaction of Gender, Age, and Comorbidity
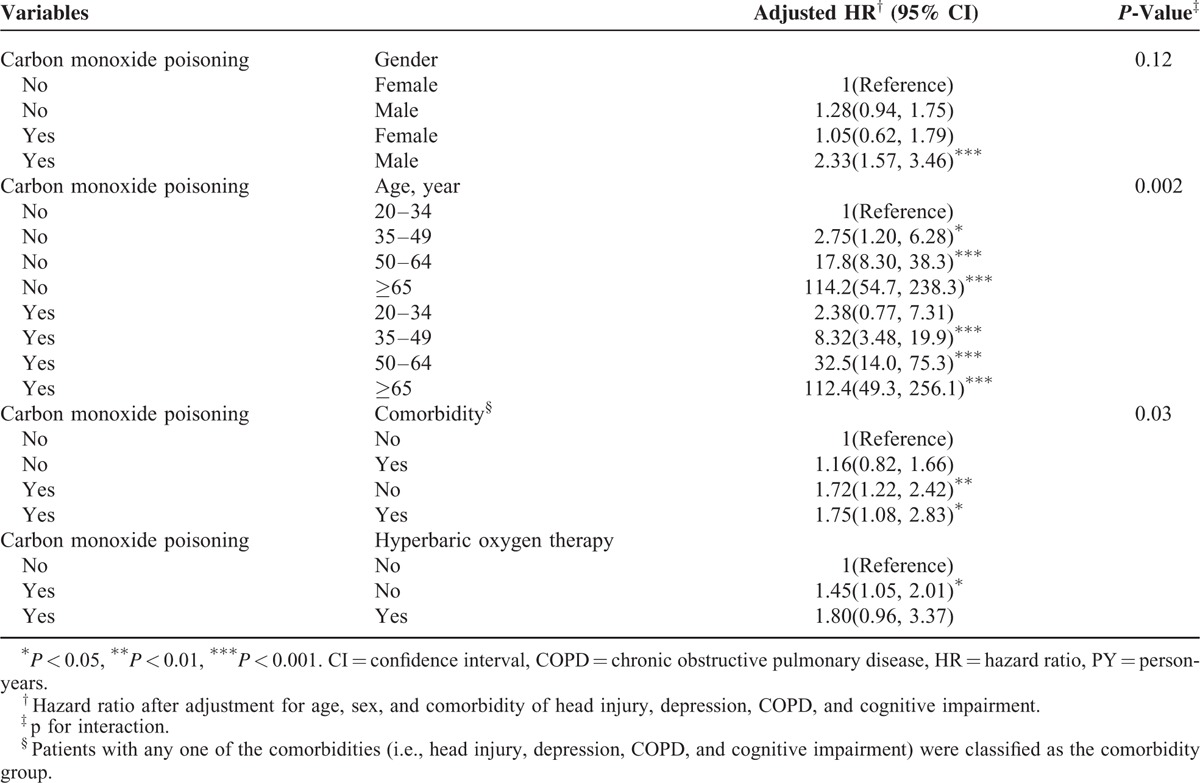
TABLE 4.
Incidence and Hazard Ratio for Dementia Stratified by the Severity of Carbon Monoxide Poisoning
DISCUSSION
An earlier study has reported that the mortality from intentional CO poisoning was 1.5-fold higher than that from accidental poisoning30 and the suicide rate was higher in young men.31,32 Particularly, charcoal-burning suicides increased markedly in Taiwan in the first decade of the 21st century.33,34 Our study shows that the majority patients of CO poisoning are young, more men than women, and prevalent with depression at baseline (Table 1). Whether the younger and men are at the increased likelihood of voluntarily poisoning requires further research. Prevention strategies are needed to avoid the exposure to CO.35,36
A previous large, multicenter, long-term population-based study on the dementia risk showed that self-perceived poor health increased the risk of dementia for near 4 folds.9 The risk can be typically aggravated by alcohol, drugs, smoking, infection, brain trauma, stroke, and chronic diseases.9–15 A recent prospective cohort study revealed that patients receiving general anesthesia and surgery developed dementia in a short period with an HR of 1.99.14 Another study shows that patients with trauma brain injury are also at an increased risk of dementia with an adjusted HR of 3.26 (95% CI = 2.69–3.94).15 In the present study, the adjusted HR of dementia was 1.75 (95% CI = 1.08, 2.83) for CO poisoning patients with comorbidities, compared with the non-CO poisoning cohort without comorbidity (Table 3). We found the dementia risk was 2.4-fold greater for men than for women (21.5 vs 8.80 per 100,000 person-years) in the CO exposed cohort (Table 2). The age-specific analysis shows a 2.6-fold greater hazard of developing dementia for young poisoned patients than controls. There are more men and much larger number of young people suffer from CO poisoning, representing a proportion loss of healthy life.
CO poisoning may increase the risk of subsequent morbidity and mortality. A recent study found the mortality was 5.24 times higher in patients with CO poisoning than the non-CO poisoning cohort.35 Information on subsequent neurological and cognitive sequelae after the poisoning is limited.20,21,36 CO poisoning causes brain hypoxia which may contribute inflammation and subsequent injury.22 An American study found from 63 suspected cases that CO poisoning could cause considerable inflammation responses, even for patients with brief exposure.37 Studies have hypothesized that reactive oxygen species, free radicals, and neuronal nitric oxide induce oxidative injury38 and lead to neuropathologic effects post-CO poisoning.39 A recent retrospective cohort study showed that victims of CO poisoning exhibited a 9.08-fold increased risk for Parkinsonism.40 Other studies also found patients with CO poisoning are at a higher risk of developing cardiovascular complications.33,41 However, there are conflicting findings about the risk of cardiovascular deaths after CO poisoning.42,43 More evidences are needed to evaluate whether CO poisoning affects small vessel diseases leading to dementia.
HBO2 therapy is the primary treatment for CO poisoning to restore the oxygen level in the blood and to prevent the neuropsychiatric syndrome44–46 and hypoxic encephalopathy.26,46–48 HBO2 does much more in CO poisoning than just treat hypoxia, by preventing the inflammatory cascade and later complications.49 However, the beneficial effects of HBO2 therapy for CO poisoning remain debatable.49 Our study showed an increased HR of dementia on HBO2 therapy in patients with CO poisoning, but not significant. Our further data analysis showed severe CO poisoned patients with longer hospital stay are at higher HR of developing dementia. It is possible that patients receiving HBO2 therapy are sicker and the risk of dementia may be less likely associated with the CO exposure. Studies that have investigated the epidemiological relationship between CO poisoning and dementia are scarce. The CO-poisoning related sequelae needs much more studies.50
LIMITATIONS
This study was subject to limitations. First, this study used retrospective cohort study design, which relies on the data available. Information on lifestyle, body mass index, physical activity, and family health history were not available to measure their potential confounding effects in this study. COPD is a disorder associated with lifestyles. We therefore used COPD in the data analysis as controlling variable to substituting lifestyles. Second, this study used ICD-9-CM coding system to identify disorders. To avoid coding errors, we excluded data with the diagnosis appeared only once. An ad hoc committee established by the insurance authority could monitor claims data to insure the accuracy. Therefore, coding errors have been minimized. Third, patients with CO poisoning may have association with other late neuropsychiatric disorders other than dementia. We did not have enough power to measure other disorders as we were able to identify only 62 cases of dementia. We therefore did not differentiate between Alzheimer disease, vascular dementia, and dementia related to other causes. Forth, laboratory data were unavailable in our database. We, therefore, are unable to evaluate whether amyloid and TAU formation, and alpha-synuclein are associated in the CO-related dementia. Finally, in the present cohort study, the severity of CO poisoning was not coded in the claims record. No detailed information was available in the claims data to clarify whether the events were voluntarily or accidentally, acute or chronic poisoning, and co-ingestions. The level of CO exposure was unknown. Patients who were cared at outpatient clinics could be less severe cases and were not included in this study. HBO2 therapy is available at medical centers and large community hospitals. However, reasons of choosing and not choosing HBO2 therapy might somewhat reflect the severity of CO poisoning. We therefore are unable to make any robust conclusion about the effectiveness of the HBO2 therapy for dementia patients.
In conclusion, this study suggests that CO poisoned patients may associate with an increased risk of dementia. It is important to note there is a large number of CO poisoning events, which occur to mainly young populations. Further data analysis showed the cumulative mortality over 12 years (2000–2011) was approximate 10.2% (922/9041) in the CO poisoning cohort and 32.3% (20/62) in those with dementia developed. People suffering from depression may need more medical attention, particularly for the young population. Further study needs to differentiate between late neuropsychiatric effects of CO poisoning and dementia. The limitations left many unanswered questions. Different dementias are associated with other etiological factors. These would take future investigators a great amount of research efforts to study.
Footnotes
Abbreviations: CI = confidence interval, CO = carbon monoxide, HR = hazard ratio.
C-Y L and Y-W H contributed equally to this work.
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039-006); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
Conception and design: C-YL, Y-WH, C-HK; Administrative support: C-HK; Collection and assembly of data, Data analysis and interpretation, and Final approval of manuscript: All authors.
All authors have no conflicts of interest to disclose.
REFERENCES
- 1.Prince MJ, Wu F, Guo Y, et al. The burden of disease in older people and implications for health policy and practice. Lancet 2015; 385:549–562. [DOI] [PubMed] [Google Scholar]
- 2.Wimo A, Jonsson L, Winblad B. An estimate of the worldwide prevalence and direct costs of dementia in 2003. Dement Geriatr Cogn Disord 2006; 21:175–181. [DOI] [PubMed] [Google Scholar]
- 3.Wimo A, Winblad B, Jönsson L. An estimate of the total worldwide societal costs of dementia in 2005. Alzheimers Dement 2007; 3:81–91. [DOI] [PubMed] [Google Scholar]
- 4.Wimo A, Winblad B, Jönsson L. The worldwide societal costs of dementia: estimates for 2009. Alzheimers Dement 2010; 6:98–103. [DOI] [PubMed] [Google Scholar]
- 5.Wang G, Cheng Q, Zhang S, et al. Economic impact of dementia in developing countries: an evaluation of Alzheimer-type dementia in Shanghai, China. J Alzheimers Dis 2008; 15:109–115. [DOI] [PubMed] [Google Scholar]
- 6.Allegri RF, Butman J, Arizaga RL, et al. Economic impact of dementia in developing countries: an evaluation of costs of Alzheimer-type dementia in Argentina. Int Psychogeriatr 2007; 19:705–718. [DOI] [PubMed] [Google Scholar]
- 7.Zencir M, Kuzu N, Beşer NG, et al. Cost of Alzheimer's disease in a developing country setting. Int J Geriatr Psychiatry 2005; 20:616–622. [DOI] [PubMed] [Google Scholar]
- 8.APA. Diagnostic and Statistical Manual of Mental Disorders, 5th Edn. (DSM-5). American Psychiatric Association, Arlington, VA, 2013; 2013. [Google Scholar]
- 9.Yip AG, Brayne C, Matthews FE, et al. Risk factors for incident dementia in England and Wales: the medical research council cognitive function and ageing study. A population-based nested case-control study. Age Ageing 2006; 35:154–160. [DOI] [PubMed] [Google Scholar]
- 10.Nordström P, Nordström A, Eriksson M, et al. Risk factors in late adolescence for young-onset dementia in men: a nationwide cohort study. JAMA Intern Med 2013; 173:1612–1618. [DOI] [PubMed] [Google Scholar]
- 11.Ott A, Slooter AJ, Hofman A, et al. Smoking and risk of dementia and Alzheimer's disease in a population-based cohort study: the Rotterdam Study. Lancet 1998; 351:1840–1843. [DOI] [PubMed] [Google Scholar]
- 12.Sampson EL, Blanchard MR, Jones L, et al. Dementia in the acute hospital: prospective cohort study of prevalence and mortality. Br J Psychiatry 2009; 195:61–66. [DOI] [PubMed] [Google Scholar]
- 13.Seitz DP, Shah PS, Herrmann N, et al. Exposure to general anesthesia and risk of Alzheimer's disease: a systematic review and meta-analysis. BMC Geriatr 2011; 11:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen PL, Yang CW, Tseng YK, et al. Risk of dementia after anaesthesia and surgery. Br J Psychiatry 2014; 204:188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee YK, Hou SW, Lee CC, et al. Increased risk of dementia in patients with mild traumatic brain injury: a nationwide cohort study. PLoS One 2013; 8:e62422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thom SR, Keim LW. Carbon monoxide poisoning: a review epidemiology, pathophysiology, clinical findings, and treatment options including hyperbaric oxygen therapy. J Toxicol Clin Toxicol 1989; 27:141–156. [DOI] [PubMed] [Google Scholar]
- 17.Gunnell D, Coope C, Fearn V, et al. Suicide by gases in England and Wales 2001–2011: evidence of the emergence of new methods of suicide. J Affect Disord 2014; 170:190–195. [DOI] [PubMed] [Google Scholar]
- 18.Pepe G, Castelli M, Nazerian P, et al. Delayed neuropsychological sequelae after carbon monoxide poisoning: predictive risk factors in the Emergency Department. A retrospective study. Scand J Trauma Resusc Emerg Med 2011; 19:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ku HL, Yang KC, Lee YC, et al. Predictors of carbon monoxide poisoning-induced delayed neuropsychological sequelae. Gen Hosp Psychiatry 2010; 32:310–314. [DOI] [PubMed] [Google Scholar]
- 20.Jasper BW, Hopkins RO, Duker HV, et al. Affective outcome following carbon monoxide poisoning: a prospective longitudinal study. Cogn Behav Neurol 2005; 18:127–134. [DOI] [PubMed] [Google Scholar]
- 21.Choi IS. Delayed neurologic sequelae in carbon monoxide intoxication. Arch Neurol 1983; 40:433–435. [DOI] [PubMed] [Google Scholar]
- 22.Piantadosi CA, Zhang J, Levin ED, et al. Apoptosis and delayed neuronal damage after carbon monoxide poisoning in the rat. Exp Neurol 1997; 147:103–114. [DOI] [PubMed] [Google Scholar]
- 23.Weaver LK. Carbon monoxide poisoning. Crit Care Clin 1999; 15:297–317.viii. [DOI] [PubMed] [Google Scholar]
- 24.Tomaszewski C. Carbon monoxide poisoning. Early awareness and intervention can save lives. Postgrad Med 1999; 105:39–40.3-8, 50. [DOI] [PubMed] [Google Scholar]
- 25.Choi IS. Delayed neurologic sequelae in carbon monoxide intoxication. Arch Neurol 1983; 40:433–435. [DOI] [PubMed] [Google Scholar]
- 26.Kudo K, Otsuka K, Yagi J, et al. Predictors for delayed encephalopathy following acute carbon monoxide poisoning. BMC Emerg Med 2014; 14:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katirci Y, Kandis H, Aslan S, et al. Neuropsychiatric disorders and risk factors in carbon monoxide intoxication. Toxicol Ind Health 2011; 27:397–406. [DOI] [PubMed] [Google Scholar]
- 28.Mimura K, Harada M, Sumiyoshi S, et al. Long-term follow-up study on sequelae of carbon monoxide poisoning; serial investigation 33 years after poisoning. Seishin Shinkeigaku Zasshi 1999; 101:592–618. [PubMed] [Google Scholar]
- 29.Magaki S, Yong WH, Khanlou N, et al. Comorbidity in dementia: update of an ongoing autopsy study. J Am Geriatr Soc 2014; 62:1722–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mott JA, Wolfe MI, Alverson CJ, et al. National vehicle emissions policies and practices and declining US carbon monoxide-related mortality. JAMA 2002; 288:988–995. [DOI] [PubMed] [Google Scholar]
- 31.Gunnell D, Middleton N, Whitley E, et al. Why are suicide rates rising in young men but falling in the elderly? – a time-series analysis of trends in England and Wales 1950–1998. Soc Sci Med 2003; 57:595–611. [DOI] [PubMed] [Google Scholar]
- 32.Phillips MR, Li X, Zhang Y. Suicide rates in China, 1995–99. Lancet 2002; 359:835–840. [DOI] [PubMed] [Google Scholar]
- 33.Lee FY, Chen WK, Lin CL, et al. Carbon monoxide poisoning and subsequent cardiovascular disease risk: a nationwide population-based cohort study. Medicine 2015; 94:e624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang SS, Chen YY, Yip PS, et al. Regional changes in charcoal-burning suicide rates in East/Southeast Asia from 1995 to 2011: a time trend analysis. PLoS Med 2014; 11:e1001622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang CC, Chung MH, Weng SF, et al. Long-term prognosis of patients with carbon monoxide poisoning: a nationwide cohort study. PloS One 2014; 9:e105503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weaver LK, Valentine KJ, Hopkins RO. Carbon monoxide poisoning: risk factors for cognitive sequelae and the role of hyperbaric oxygen. Am J Respir Crit Care Med 2007; 176:491–497. [DOI] [PubMed] [Google Scholar]
- 37.Thom SR, Bhopale VM, Milovanova TM, et al. Plasma biomarkers in carbon monoxide poisoning. Clin Toxicol (Phila) 2010; 48:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akyol S, Erdogan S, Idiz N, et al. The role of reactive oxygen species and oxidative stress in carbon monoxide toxicity: an in-depth analysis. Redox Rep 2014; 19:180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thom SR, Bhopale VM, Han ST, et al. Intravascular neutrophil activation due to carbon monoxide poisoning. Am J Respir Crit Care Med 2006; 174:1239–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lai CY, Chou MC, Lin CL, et al. Increased risk of Parkinson disease in patients with carbon monoxide intoxication: a population-based cohort study. Medicine 2015; 94:e869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chung WS, Lin CL, Kao CH. Carbon monoxide poisoning and risk of deep vein thrombosis and pulmonary embolism: a nationwide retrospective cohort study. J Epidemiol Community Health 2015; 69:557–562. [DOI] [PubMed] [Google Scholar]
- 42.Hampson NB, Rudd RA, Hauff NM. Increased long-term mortality among survivors of acute carbon monoxide poisoning. Crit Care Med 2009; 37:1941–1947. [DOI] [PubMed] [Google Scholar]
- 43.Henry CR, Satran D, Lindgren B, et al. Myocardial injury and long-term mortality following moderate to severe carbon monoxide poisoning. JAMA 2006; 295:398–402. [DOI] [PubMed] [Google Scholar]
- 44.Hampson NB, Little CE. Hyperbaric treatment of patients with carbon monoxide poisoning in the United States. Undersea Hyperb Med 2005; 32:21–26. [PubMed] [Google Scholar]
- 45.Weaver LK, Hopkins RO, Chan KJ, et al. Hyperbaric oxygen for acute carbon monoxide poisoning. N Engl J Med 2002; 347:1057–1067. [DOI] [PubMed] [Google Scholar]
- 46.Thom SR, Taber RL, Mendiguren II, et al. Delayed neuropsychologic sequelae after carbon monoxide poisoning: prevention by treatment with hyperbaric oxygen. Ann Emerg Med 1995; 25:474–480. [DOI] [PubMed] [Google Scholar]
- 47.Ziser A, Shupak A, Halpern P, et al. Delayed hyperbaric oxygen treatment for acute carbon monoxide poisoning. Br Med J (Clin Res Ed) 1984; 289:960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu H, Pan X, Wan Y, et al. Factors affecting the prognosis of patients with delayed encephalopathy after acute carbon monoxide poisoning. Am J Emerg Med 2011; 29:261–264. [DOI] [PubMed] [Google Scholar]
- 49.Weaver LK. Hyperbaric oxygen therapy for carbon monoxide poisoning. Undersea Hyperb Med 2014; 41:339–354. [PubMed] [Google Scholar]
- 50.Weaver LK. Clinical practice. Carbon monoxide poisoning. N Engl J Med 2009; 360:1217–1225. [DOI] [PubMed] [Google Scholar]


