Abstract
To evaluate the evidence of effects and safety of magnesium sulfate on neuroprotection for preterm infants who had exposure in uteri.
We searched electronic databases and bibliographies of relevant papers to identify studies comparing magnesium sulfate (MgSO4) with placebo or other treatments in patients at high risk of preterm labor and reporting effects and safety of MgSO4 for antenatal infants. Then, we did this meta-analysis based on PRISMA guideline. The primary outcomes included fatal death, cerebral palsy (CP), intraventricular hemorrhage, and periventricular leukomalacia. Secondary outcomes included various neonatal and maternal outcomes.
Ten studies including 6 randomized controlled trials and 5 cohort studies, and involving 18,655 preterm infants were analyzed. For the rate of moderate to severe CP, MgSO4 showed the ability to reduce the risk and achieved statistically significant difference (odd ratio [OR] 0.61, 95% confidence interval [CI] 0.42–0.89, P = 0.01). The comparison of mortality rate between the MgSO4 group and the placebo group only presented small difference clinically, but reached no statistical significance (OR 0.92, 95% CI 0.77–1.11, P = 0.39). Summarily, the analysis of adverse effects on babies showed no margin (P > 0.05). Yet for mothers, MgSO4 exhibited obvious side-effects, such as respiratory depression, nausea and so forth, but there exited great heterogeneity.
MgSO4 administered to women at high risk of preterm labor could reduce the risk of moderate to severe CP, without obvious adverse effects on babies. Although there exit many unfavorable effects on mothers, yet they may be lessened through reduction of the dose of MgSO4 and could be tolerable for mothers. So MgSO4 is both beneficial and safety to be used as a neuroprotective agent for premature infants before a valid alternative was discovered.
INTRODUCTION
Preterm infants, defined as infants who are born at more than 24 gestational weeks (wk) but <37 wk, are at high risk of dying in early life. If they fortunately survive, they are usually at great risk of neurological impairments, such as cerebral palsy (CP), gross motor dysfunction, deafness, blindness, developmental delay, and intellectual impairment.1 Manuck et al2 reported approximately 1 in 4 preterm children (<34 wk) had neurodevelopmental impairment at age 2 years. Among these, CP is the leading cause of neurologic impairment. CP is a nonprogressive neurological disorder affecting motor function, including a number of different morbid conditions that can arise at any time during brain development and may involve a disorder of motor function that is permanent but may change over time. Ninety-two percent of affected children can survive to 20 years old, contributing substantially to the burden of illness into adulthood.3
In recent decades, with the advances in medical and health conditions, such as the widespread use of surfactant and antenatal steroids, and improvements in ventilation management, the survival rate of preterm infants is sharply rising. Concomitantly, the number of infants with subsequent neurological impairments and disabilities is increasing, resulting in that more children require intensive postnatal medical care and costly developmental services.4,5 So the therapy which can have a substantial effect on reducing the risk of neurological impairments is eagerly needed.
Luckily, magnesium sulfate (MgSO4) shunts light upon such a head-scratching problem. In 1992, Kuban et al6 found MgSO4 to be associated with a reduction in risk of intraventricular hemorrhage (IVH). A few years later, a case–control study demonstrated MgSO4 had an effect in decreasing the risk of subsequent development of CP among preterm infants.7 However, 2 observational studies reported that prenatal use of MgSO4 had no effects in reducing risk of IVH or CP.8,9 Subsequently, researches regarding the association between MgSO4 and CP mushroomed. To date, there have been 2 randomized control trials (RCTs) supporting that antenatal exposure to MgSO4 could significantly decrease the risk of CP for preterm infants.10,11 However, a meta-analysis suggested there were no significant effects of antenatal MgSO4 therapy on combined rates of mortality with CP, and there were higher rates of minor maternal side effects in the MgSO4 group.12 Regardless of MgSO4's uncertain neuroprotective effects, some study hold that it was its adverse effects on the mother, such as palpitations, hypotension, oliguria or renal failure, absent or reduced tendon reflexes, which may place gravida in life-threatening conditions, that restricted its use in clinics. As you witnessed, the issue concerning the effects and safety of MgSO4 used in clinics still remained controversial. Considering this, we sought to probe the correlation between MgSO4 and neuroprotective effects, as well as fetal and maternal adverse outcomes based on a large population.
MATERIALS AND METHODS
Search Strategy
We searched PubMed, China National Knowledge Infrastructure (CNKI), the Cochrane Library, and bibliographies of relevant papers published up to August 2015, without language limitations, using the keywords and combinations of the following search terms “magnesium sulfate” and “preterm labor/labor/delivery” or “antenatal” and “neuroprotection” or “cerebral palsy.”
Study Selection Criteria
All published articles about women at risk of preterm labor given MgSO4 administered intravenously, intramuscularly or orally comparing with those using either placebo or tocolytic were included. And the reported outcomes should include primary outcomes or secondary outcomes.
Study Exclusion Criteria
Studies without a control group were excluded. Abstracts, reviews, protocols, letters, and comments were excluded because of the absence of details concerning study methods and results. Studies were surely ineligible if there was no information provided on any of the outcomes of focus, if data were not reported regarding the intention to deal with.
Data Extraction, Synthesis, and Analysis
If the abstract described a study that did not meet the eligible criteria, the study was not reviewed any further. Eligible articles were reviewed in details. The review of articles was undertaken independently by 2 reviewers (XZ and YX) who decided on which article was eligible. Any disagreements were resolved by discussing with a third reviewer (QT).
Two reviewers extracted data independently. The primary outcomes were death of preterm infants (neonatal, fetal, or later death during follow-up period), CP (moderate to severe, or mild), IVH (grade III–IV or any), periventricular leukomalacia (PVL), white matter injury (WMI). Secondary outcomes were infant outcomes (Apgar score <7 at 5 min, tracheal intubation, mechanical ventilation, neonatal convulsions/seizures, necrotizing enterocolitis (NEC), in special care baby unit (ISCU), need for supplemental oxygen at 36 wk, neurologic disability and developmental delay) and maternal complications (hypotension, absent or reduced tendon reflexes, muscle weakness, blurred vision, flushing, nausea or vomiting, sweating).
The diagnose of CP, neurologic disability, and developmental delay almost made by expert pediatricians at more than 18 months of corrected age. Whereby, 4 RCTs13–15 were did at 18 months of corrected age, 2 RCTs16,17 were did at 24 months of corrected age, the 2 cohort studies18,19 were did at school ages. Baseline data were depicted explicitly if possible.
Statistical analyses were conducted using the program “Review Manager 5.2.” We calculated a summary odds ratio (OR) and 95% confidence interval (CI) for dichotomous variables, using Mantel–Haenszel and fixed/random-effects mode.20 Statistical heterogeneity between trials was tested using the I2 statistic. If substantial heterogeneity was found (I2 > 25%), we used a random-effects Model. The OR was calculated as the ratio of the number of events using magnesium sulfate over that using placebo. If the 95% CI did not encompass 1.0 for OR or if the P value was <0.05, then the results were considered to be statistically significant. Homogeneity of tests among pooled results were performed using simple chi-squared test. Methodologic quality assessment of the trials was conducted based on the modified scoring system.21 Points were awarded on the basis of the quality of randomization, blinding, and follow-up. In addition, we also assessed concealment of allocation. The methodologic quality of included trials was assessed. The funnel plot was used to examine publication bias.22
Ethical Approval
The ethical approval was not necessary because our study was a meta-analysis that belongs to secondary researches.
RESULTS
This research generated 387 pieces of paper totally. However, 338 articles were excluded undoubtedly after screening the abstracts. Among the remaining 49 articles, 39 articles were excluded because of reviews, letters, comments, and unavailable data. The included 10 articles including 6 RCTs and 5 cohort studies (3 follow-up studies and 2 retrospective studies) were reviewed carefully. Because the article written by Mittendorf et al13 had 2 arms (tocolytic and neuroprotective), we considered it as 2 separate studies. Finally, 11 studies including 18,655 preterm infants were analyzed (Figure 1).
FIGURE 1.
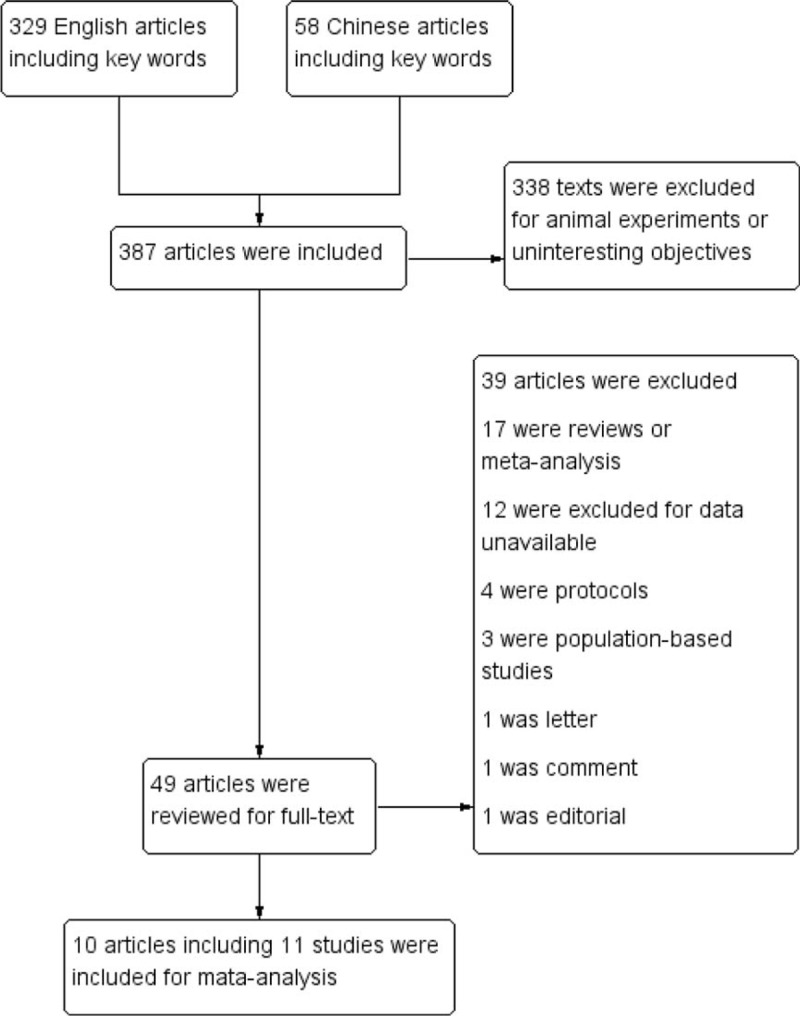
Search algorithm.
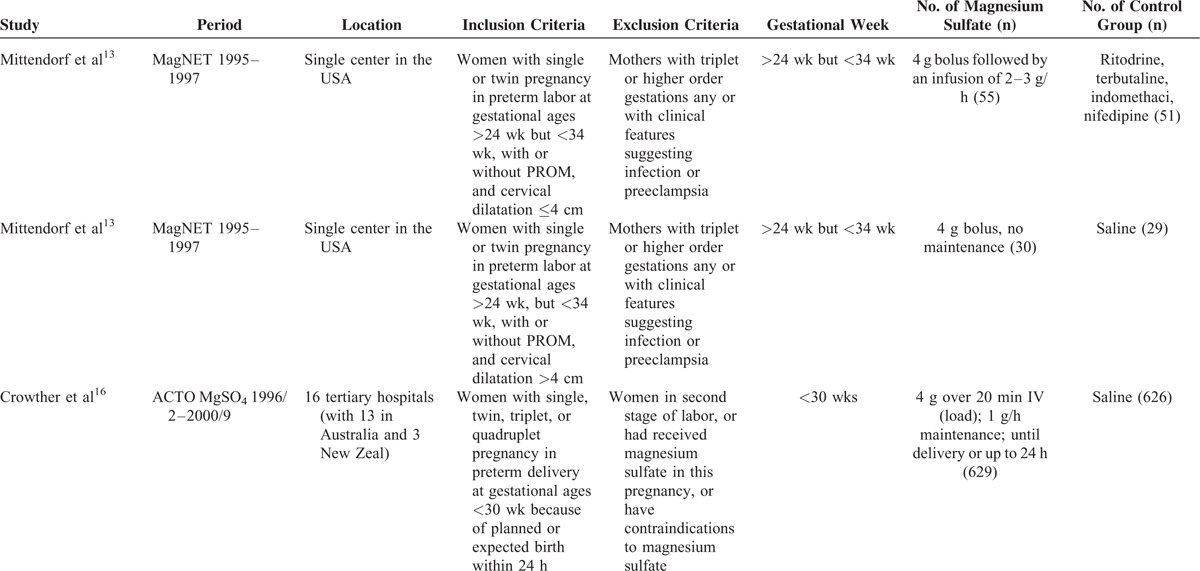
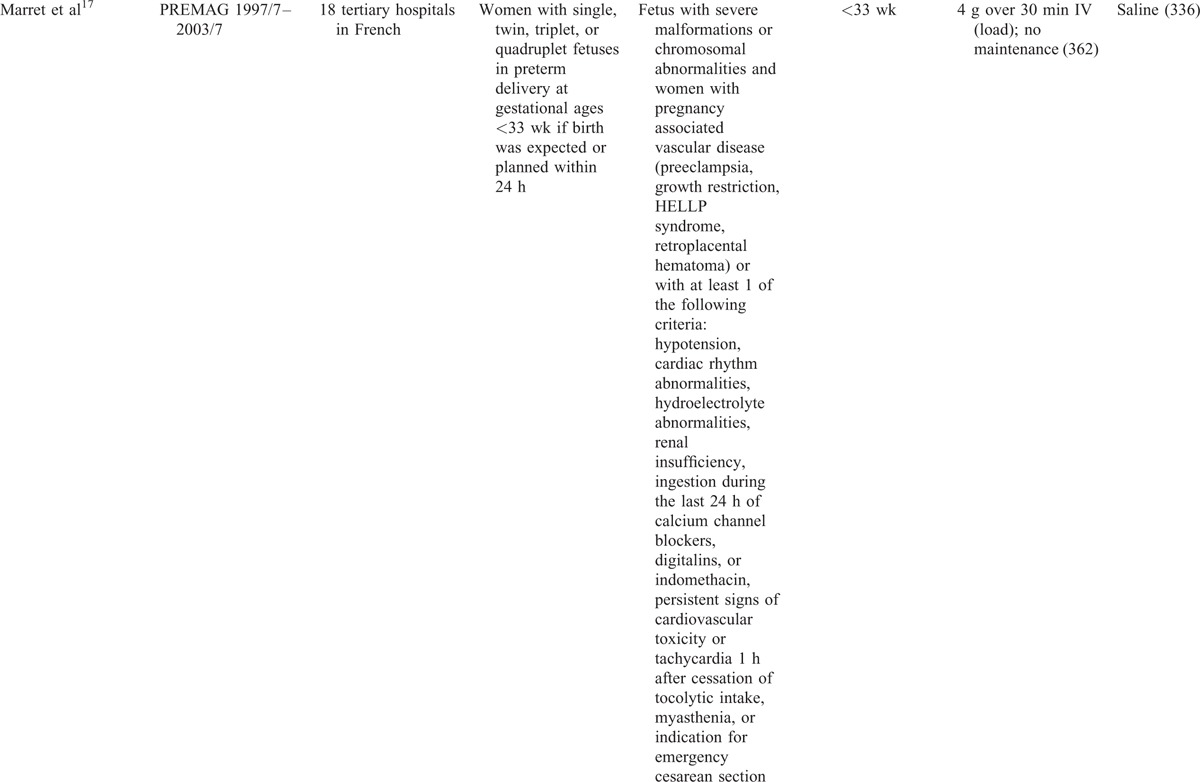
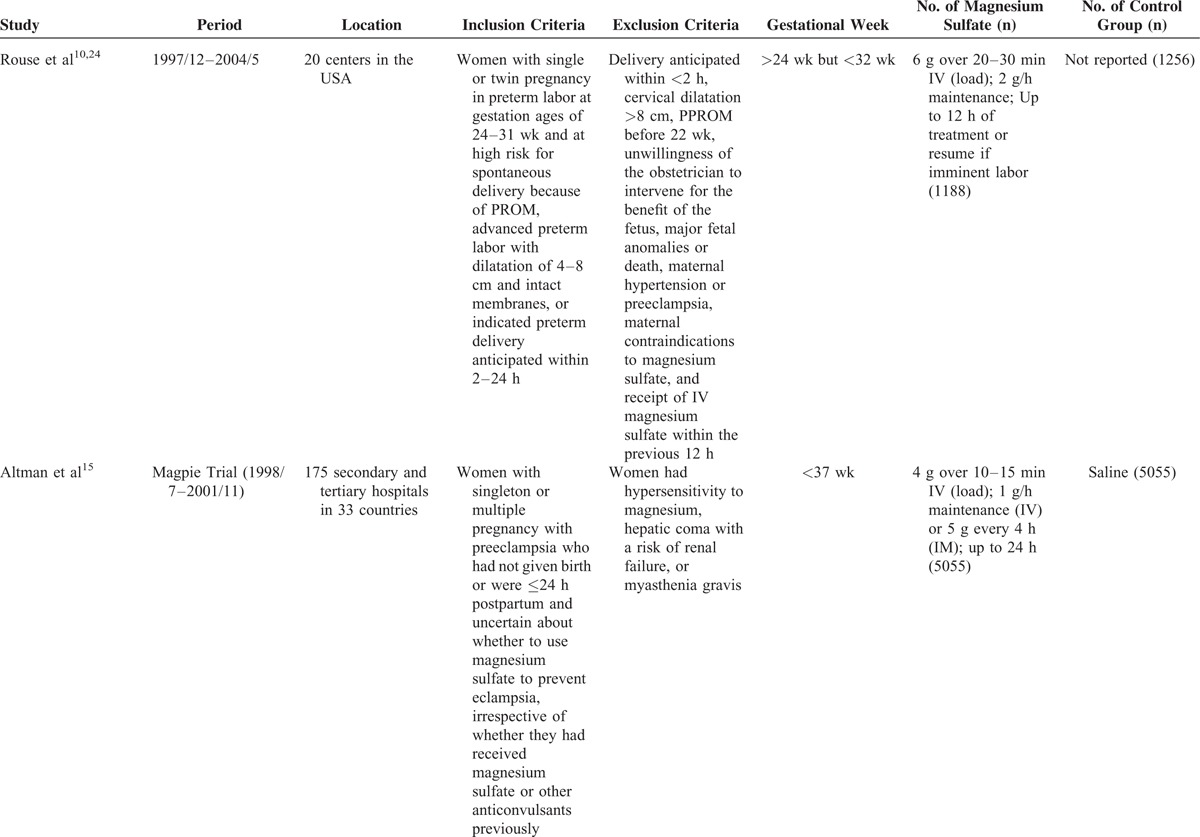
The characteristics of the included studies were exhibited in Table 1 . The largest number of objectives was 10,110, more than 100 times of the smallest number. The earliest RCT started in 1995, the latest RCT ended in 2004, the duration of 6 RCTs ranged from 3 to 7 years. The 6 RCTs were conducted before 2004, while the 5 cohort studies were did in the lasted few years except 1.23 Apart from 2 RCTs13 and 2 retrospective studies,18,19 the rest were did in multicenters. The gestational ages at randomization were almost <34 wk except 1 was <37 wk.15 The dose of MgSO4 was 4 grams (g) bolus load only, or followed by an infusion of 1 to 3 g per hour (1–3 g/h) in 5 RCTs. While in one RCT and one retrospective study,24,25 the dose was 6 g bolus load, followed by an infusion of 2 g/h. Another retrospective study23 used 5 g bolus load, followed by an infusion of one g/h. Four RCTs applied saline in the control group, one RCT,13 and one retrospective study23 exploited tocolytic, the remaining RCT24 and one retrospective study25 did not report.
TABLE 1.
Characteristics of Included Studies
As to the quality assessment (Table 2), all RCTs described randomized assignment, allocation concealment, methods of blinding, and follow-up status, gaining a total score of 4 to 8 points (2 RCTs were 4 points, 2 RCTs were 7 points, 2 RCTs were 8 points). Among these, 3 RCTs achieved satisfactory follow-up rate, in addition to 2 RCTs untold.
TABLE 1 (Continued).
Characteristics of Included Studies
Seven studies evaluated the prevalence of CP between children who exposed to MgSO4 in uteri and those did not. For the rate of CP, MgSO4 seemingly showed the ability to reduce the risk of CP, but there was no statistically significant difference (OR 0.96, 95% CI 0.78–1.17, P = 0.66; Figure 2). As to the individual analysis of mild CP and moderate to severe CP, the former did not generate statistically significant difference (OR 0.76, 95% CI 0.53–1.11, P = 0.16), while the latter demonstrated obvious statistical difference (OR 0.61, 95% CI 0.42–0.89, P = 0.01). Simultaneously, infant mortality was analyzed in details and no statistical significance was found (OR 0.92, 95% CI 0.77–1.11, P = 0.39; Figure 3). The rates of whole mortality, death <28 days or >28 days, death after discharge all showed a reduction in preterm infants who had exposure to MgSO4, but significant difference was not found (P > 0.05). Moreover, there was no effect on the rates of death before discharge and still birth. In terms of IVH, IVH (III–IV), PVL, and WMI, there was no evidence showing whether MgSO4 would exert an effect on increasing or decreasing the risk (Table 3).
FIGURE 2.
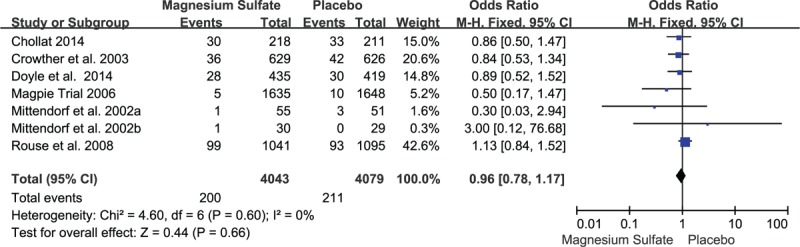
Meta-analysis of data about cerebral palsy from 7 studies using a fixed-effect model. CI = confidence interval, OR = odds ratio.
FIGURE 3.
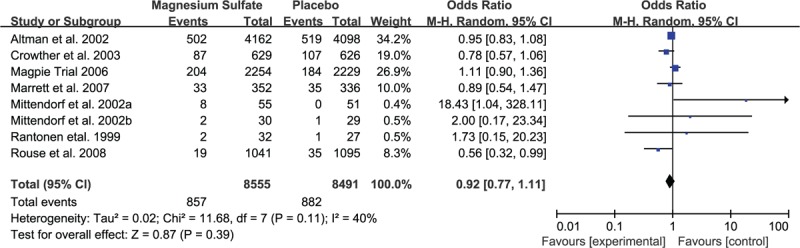
Meta-analysis of data about death from 8 studies using a random-effect model. CI = confidence interval, OR = odds ratio.
TABLE 1 (Continued).
Characteristics of Included Studies
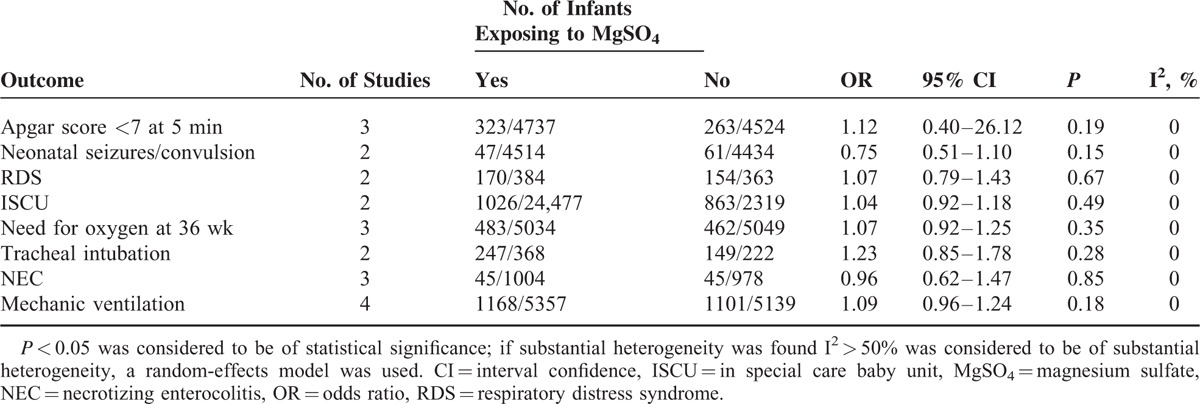
Researchers also performed a comparison of negative effects on neonates between the MgSO4 group and placebo group based on limited available data. The risk of Apgar score <7 at 5 min, need for oxygen at 36 wk, NEC and mechanic ventilation apparently went up for neonates in MgSO4 group, but no statistically significant difference was witnessed. Furthermore, neonatal seizures/convulsion, respiratory distress syndrome (RDS), ISCU, and tracheal intubation were dependently assessed by 2 studies, without achieving any statistical significance (Table 4).
TABLE 1 (Continued).
Characteristics of Included Studies
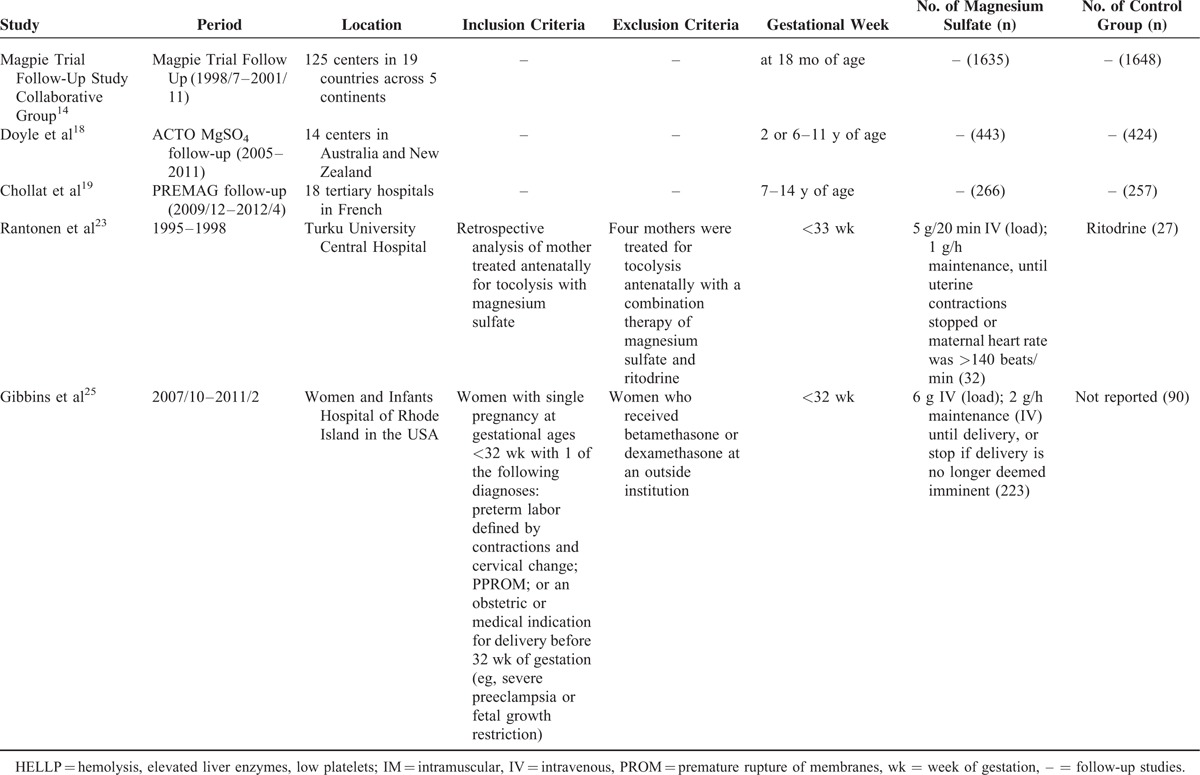
TABLE 4.
Neonatal Outcomes
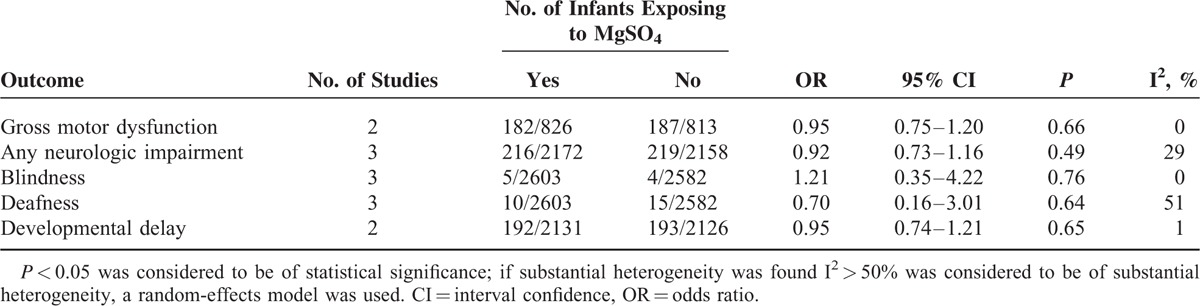
When it came to the results of long-term outcomes for infants, MgSO4 seemingly could increased the risk of gross motor dysfunction, any neurological impairment and developmental delay, despite of no remarkable statistical significance (Table 5).
TABLE 2.
Description of Quality Assessment of Included Studies
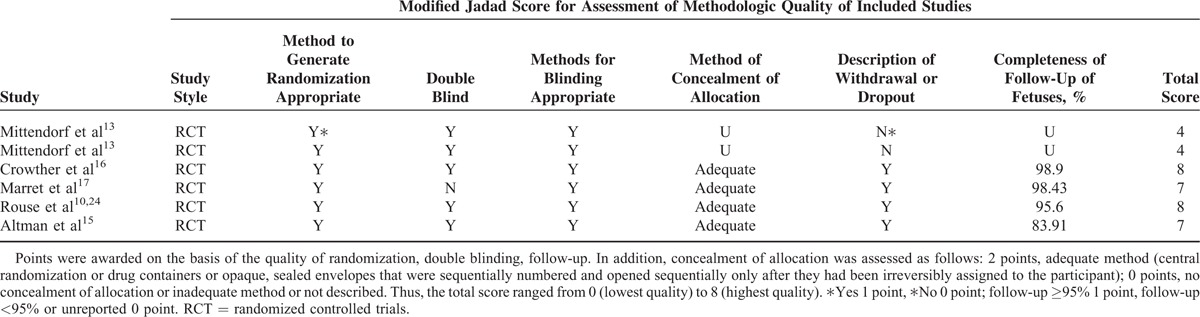
TABLE 5.
Long-Term Outcomes for Preterm Children
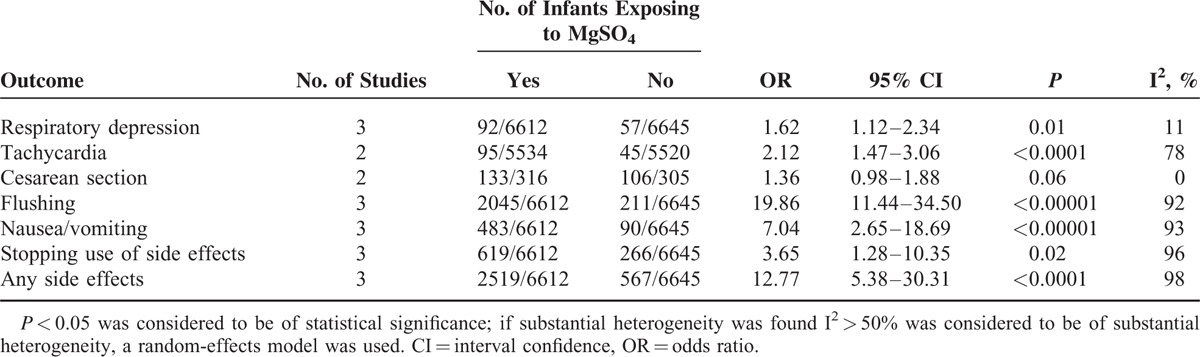
Finally, it is high time to mention the adverse effects of MgSO4 on pregnant women. Compared with women receiving placebo, the OR of respiratory depression for those exposed to MgSO4 was 1.62 (95% CI 1.12–2.34, P = 0.01, I2 = 11%). Besides, MgSO4 appeared to augment the hazard of tachycardia, flushing, and nausea/vomiting, but the heterogeneity among these studies was quite distinct (P < 0.05, I2 > 90%; Table 6).
TABLE 3.
Primary Outcomes
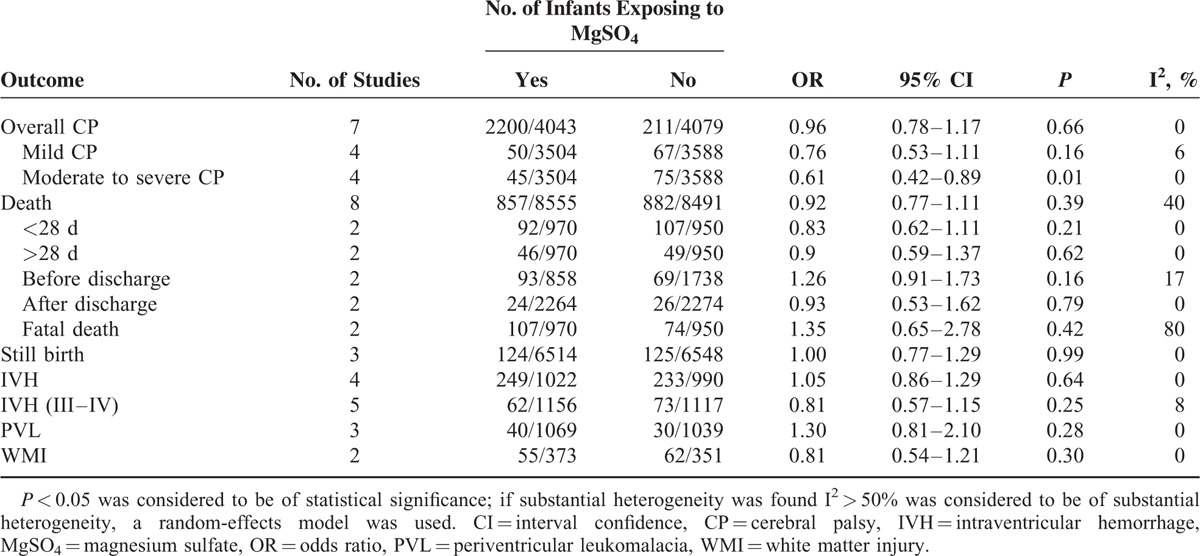
TABLE 6.
Maternal Complications
To evaluate the possibly exiting publication bias for the outcomes, funnel plot was demonstrated to find no evidence of asymmetry, suggesting that publication bias was not present (Figure 4).
FIGURE 4.
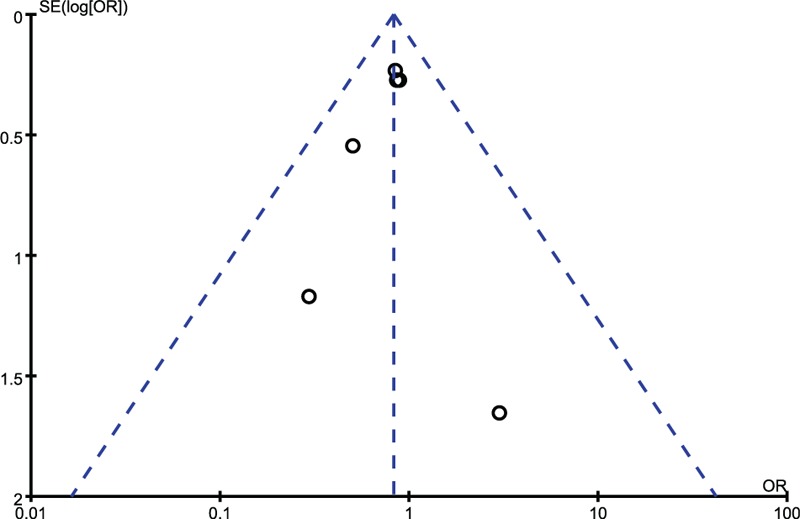
Publication bias is assessed by funnel plot, of which the asymmetry is exhibited by evidence of small studies with higher odds ratio and the paucity of small negative studies in the lower right of the funnel plot.
DISCUSSION
The findings indicated that moderate to severe CP occurred significantly less frequently in the MgSO4 group, which was consistent with a previous study.24 And the number needed to treat to prevent one case of disabling CP estimated by one study varied from 30 to 60.26 While MgSO4 did not appear to be helpful in cutting down the mortality rate, which probably because various treatment regimens and durations may yield a difference in the outcomes. But when we excluded the trail using large dose,24 say 6 g/20–30 min IV (load) and followed by 2 g/h (maintenance), the OR of death was 0.82 (95% CI 0.63–1.09, P = 0.17). Similarly, excluding the trail using small dose,23,24 say more than 4 g IV (load), the OR of total mortality was 0.96 (95% CI 0.81–1.14, P = 0.66). This was in line with a study which suggested there was no difference in the rates of overall CP or death regardless of the total duration and doses of MgSO4.27
This study did not show more competence to detect clinically significant differences in many other maternal and neonatal outcomes, because the power was limited for less frequent outcomes such as maternal pulmonary edema, hypotension, postpartum hemorrhage, and infant development, including infant Bayley mental development (BMD) and Bayley psychomotor development (BPD)28 which were routinely used as the quantitative assessment of infant later development. One study24 providing relevant data implied that BMD < 85 was diagnosed in 406 from 876 children in the MgSO4 group (46.34%) and in 427 from 919 children in the placebo group (46.46%), and the result was not statistically compelling (95% CI 0.83–1.20, P = 0.96). Simultaneously, the percentage of BPD < 85 was 34.13% versus 34.27% in the MgSO4 group and placebo group, achieving no statistical significance (95% CI 0.82–1.21, P = 0.99). Additionally, one prevailing study noted that no substantial difference was found on any of the cognitive, academic, attention, executive function, and other neurosensory outcomes.18 Adverse effects may occur in women who become hypermagnesemic during MgSO4 treatment, such as absent or reduced tendon reflexes, headache, itching or tingling, warmth over body, mouth dryness, muscular weakness, sleepiness, and dizziness. So maternal complications were analyzed, resulting in rising risk of any side effects, achieving no difference with inevitable heterogeneity (P < 0.0001, I2 = 98%).
Equally, adverse effects could occur in neonates exposed to MgSO4 antenatally. Generally, we were prone to hypothesize that the risk of RDS, the need for resuscitation, neonatal seizures/convulsion, and Apgar score <7 at 5 min were certain to ascend on account of the elevated MgSO4 concentration in babies’ body. In light of this, the American Academy of Pediatrics and American Heart Association supported Neonatal Resuscitation Program lists MgSO4 among maternally administered medications.29 However, this meta-analysis offered indefinite evidences to associate fetal MgSO4 exposure with potentially possible adverse effects based on limited data (P > 0.05). Furthermore, a recent study aiming at investigating the association between umbilical cord blood MgSO4 concentration and resuscitation of infants showed that MgSO4 for neuroprotection had no effects on additional invasive delivery room resuscitation measures.30 Consequently, the detrimental outcomes of anteneonates cannot be attributed to the effects of MgSO4, for premature newborns were vulnerable to suffer from these hazards instinctively. Similarly, a study published in Lancet conveyed that MgSO4 did not appear to exert substantive harmful effects on mothers and babies in a short term.12 To verify whether there exited long-term effects, a follow-up study reported there were no serious maternal or perinatal complications ascribed to MgSO4.25
Nevertheless, several study limitations must be kept in mind when considering the generalizability of the data. Firstly, from the methodological aspect, the weakness was that the recruited studies included 5 cohort studies that were of low-to-medium quality. Although we searched all the literature worldwide, the 6 RCTs were done 10 years ago. So whether the data collected were suitable for the current requires more newly researches. Besides, there was a wide gap of the number of objects in recruited trails, ranging from 59 to 10,110.13,15 But the analysis including or excluding the smallest or the largest number of study generated the same results. In addition, we failed to appraise the status of neurosensory outcomes in later development, such as cognitive and academic abilities on account of data unavailable. Moreover, the lack of adequate data about the related risks, such as CP or death and other neonate outcomes at different gestational ages, say 24 to 28 wk or 28 to 34 wk, leads to a pity that the analysis was contracted greatly. Further investigations may focus on such issues.
Despite of all the shortages above, the strength of this study is stronger than any single study since the included primary studies are quite homogeneous. This secondary analysis was derived from a large, prospectively collected cohort, providing a relatively large number of women exposed to MgSO4 antenatally. What is more, precedent studies usually used the composition of CP and death as the primary outcomes expecting to obtain supportive result. Nevertheless, we insist on calculating the OR for CP and death separately, because CP and death are divergent endpoints. The accumulative data of CP and death lack scientific theory, because the 2 are rivals. To obtain the best evidence, a meta-analysis based on RCTs perhaps assists a lot. However, the data about the neuroprotective effects of MgSO4 in fetus was collected few years ago and lacked results of long-term follow-up. So in our study, we incorporated 3 follow-up studies and 2 respective studies which provided data regarding outcomes later in life for children surviving the initial neonatal period. The results showed no conspicuous discrepancies. Certainly that lefts a pitiful flaw in view of the methodological aspect. So more large multicenter studies designed to explore the neuroprotective effects and safety of MgSO4 used for preterm infants are in urgent need.
CONCLUSIONS
In conclusion, the effect of MgSO4 in lowering the rate of moderate to severe CP in preterm infants was remarkable without affecting the neonatal and maternal adverse outcomes. Although there were side effects on mothers, yet they could be lessened or removed by reducing the dose. Thus, MgSO4 is beneficial and safe to be used as a special neuroprotective agent for premature infants before discovering a valid alternative.
Footnotes
Abbreviations: BMD = Bayley mental development, BPD = Bayley psychomotor development, CI = 95% confidence interval, CNKI = China National Knowledge Infrastructure, CP = cerebral palsy, g = grams, ISCU = in special care baby unit, IVH = intraventricular hemorrhage, MgSO4 = magnesium sulfate, NEC = necrotizing enterocolitis, OR = odds ratio, PVL = periventricular leukomalacia, RCTs = randomized controlled trials, wk = gestational weeks, WMI = white matter injury.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Wilson-Costello D, Friedman H, Minich N, et al. Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Pediatrics 2005; 115:997–1003. [DOI] [PubMed] [Google Scholar]
- 2.Manuck TA, Sheng X, Yoder BA, et al. Correlation between initial neonatal and early childhood outcomes following preterm birth. Am J Obstet Gynecol 2014; 210:426.e1–426.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hutton JL, Cooke T, Pharoah TOD. Life expectancy in children with cerebral palsy. BMJ 1994; 309:431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vincer MJ, Allen AC, Joseph KS, et al. Increasing prevalence of cerebral palsy among very preterm infants: a population-based study. Pediatrics 2006; 118:e1621–e1626. [DOI] [PubMed] [Google Scholar]
- 5.Himmelmann K, Hagberg G, Beckung E, et al. The changing panorama of cerebral palsy in Sweden. IX. Prevalence and origin in the birth-year period 1995–1998. Acta Paediatr 2005; 94:287–294. [DOI] [PubMed] [Google Scholar]
- 6.Kuban KCK, Leviton A, Pagano M, et al. Maternal toxemia is associated with reduced incidence of germinal matrix hemorrhage in premature babies. J Child Neurol 1992; 7:70–76. [DOI] [PubMed] [Google Scholar]
- 7.Nelson KB, Grether JK. Can magnesium sulfate reduce the risk of cerebral palsy in very low birthweight infants? Pediatrics 1995; 95:263–269. [PubMed] [Google Scholar]
- 8.Canterino JC, Verma UL, Visintainer PF, et al. Maternal magnesium sulfate and the development of neonatal periventricular leucomalacia and intraventricular hemorrhage. Obstet Gynecol 1999; 93:396–402. [DOI] [PubMed] [Google Scholar]
- 9.Weintraub Z, Solovechick M, Reichman B, et al. Effect of maternal tocolysis on the incidence of severe periventricular/intraventricular haemorrhage in very low birthweight infants. Arch Dis Child Fetal Neonatal Ed 2001; 85:F13–F17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rouse DJ, Hirtz DG, Thom E, et al. A randomized controlled trial of magnesium sulfate for the prevention of cerebral palsy. N Engl J Med 2008; 359:895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reeves S, Gibbs R, Clark S. Magnesium for fetal neuroprotection. Am J Obstet Gynecol 2011; 204:e1–e4. [DOI] [PubMed] [Google Scholar]
- 12.Doyle LW, Crowther CA, Middleton P, et al. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst Rev 2009; 1: CD004661. [DOI] [PubMed] [Google Scholar]
- 13.Mittendorf R, Dambrosia J, Pryde PG, et al. Association between the use of antenatal magnesium sulfate in preterm labor and adverse health outcomes in infants. Am J Obstet Gynecol 2002; 186:1111–1118. [DOI] [PubMed] [Google Scholar]
- 14.Magpie Trial Follow-Up Study Collaborative Group. The Magpie Trial: a randomised trial comparing magnesium sulphate with placebo for pre-eclampsia. Outcome for children at 18 months. BJOG 2007; 114:289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altman D, Carroli G, Duley L, et al. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet 2002; 359:1877–1890. [DOI] [PubMed] [Google Scholar]
- 16.Crowther CA, Hiller JE, Doyle LW, et al. Effect of magnesium sulfate given for neuroprotection before preterm birth: a randomized controlled trial. JAMA 2003; 290:2669–7266. [DOI] [PubMed] [Google Scholar]
- 17.Marret S, Marpeau L, Zupan-Simunek V, et al. Magnesium sulphate given before very-preterm birth to protect infant brain: the randomised controlled PREMAG trial. BJOG 2007; 114:310–318. [DOI] [PubMed] [Google Scholar]
- 18.Doyle LW, Anderson PJ, Haslam R, et al. School-age outcomes of very preterm infants after antenatal treatment with magnesium sulfate vs placebo. JAMA 2014; 312:1105–1113. [DOI] [PubMed] [Google Scholar]
- 19.Chollat C, Enser M, Houivet E, et al. School-age outcomes following a randomized controlled of magnesium sulfate for neuroprotecting of preterm infants. J Pediatr 2014; 165:398–400. [DOI] [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188. [DOI] [PubMed] [Google Scholar]
- 21.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17:1–12. [DOI] [PubMed] [Google Scholar]
- 22.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rantonen T, Ekblad U, Grönlund J, et al. Influence of maternal magnesium sulphate and ritodrine treatment on the neonate: a study with six-month follow-up. Acta Paediatr 1999; 88:1142–1146. [DOI] [PubMed] [Google Scholar]
- 24.Rouse DJ, Hirtz DG, Thom E, et al. A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. N Engl J Med 2008; 9:895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibbins KJ, Browning KR, Lopes VV, et al. Evaluation of the clinical use of magnesium sulfate for cerebral palsy prevention. Obstet Gynecol 2013; 121 ((2 Pt 1)):235–240. [DOI] [PubMed] [Google Scholar]
- 26.Rouse DJ. Magnesium sulfate for the prevention of cerebral palsy. Am J Obstet Gynecol 2009; 200:610–612. [DOI] [PubMed] [Google Scholar]
- 27.McPherson JA, Rouse DJ, Grobman WA, et al. Association of duration of neuroprotective magnesium sulfate infusion with neonatal and maternal outcomes. Obstet Gynecol 2014; 124:749–755. [DOI] [PubMed] [Google Scholar]
- 28.Bayley N. Bayley scales of infant development. 2nd ed.New York: Psychological Corporation; 1993. [Google Scholar]
- 29.Kattwinkel J, Boyle D, Bloom RS. Textbook of Neonatal Resuscitation. American Academy of Pediatrics. 5th ed.Elk Grove Village, IL: American Heart Association, American Academy of Pediatrics; 2006. [Google Scholar]
- 30.Johnson LH, Mapp DC, Rouse DJ, et al. Association of cord blood magnesium concentration and neonatal resuscitation. J Pediatr 2012; 160:573–577. [DOI] [PMC free article] [PubMed] [Google Scholar]


