Abstract
luxS mutants of Borrelia burgdorferi strain 297 naturally colonized their arthropod (Ixodes scapularis) vector, were maintained in ticks throughout the molting process (larvae to nymphs), were tick transmitted to uninfected mice, and elicited histopathology in mice indistinguishable from that induced by wild-type B. burgdorferi.
Borrelia burgdorferi, the causative agent of Lyme disease, persists in nature via a complex enzootic life cycle that involves both warm-blooded mammalian hosts and Ixodes sp. arthropod (tick) vectors (24). Because B. burgdorferi must adapt to these two very diverse niches (11, 18, 22, 23), it may utilize any number of differential expression systems to regulate its virulence factors for each parasitic environment. One regulatory system operative in certain other bacterial pathogens is the LuxS/autoinducer-2 (AI-2) quorum-sensing system (9, 15). In selected bacteria, the product of the luxS gene is an enzyme involved in the generation of an AI (a furanosyl borate diester) (7) which, in turn, induces gene expression when the AI reaches a sufficiently high concentration. The increased local concentration of AI-2 is proportional to the increased density of the bacteria synthesizing AI-2, thereby allowing the AI to serve as a surrogate signal of population density (9, 15).
In B. burgdorferi, a homolog of luxS (BB0377) was identified from genome sequence information for strain B31 (10). Studies have since demonstrated that expression of borrelial luxS is enhanced during tick feeding or during the growth of spirochetes under in vitro conditions that mimic tick feeding (e.g., elevated temperature) (16, 18). These observations, combined with the finding that the expression of certain membrane lipoproteins of B. burgdorferi (e.g., OspC, Mlp's, P35, and P7.5) is influenced by spirochete cell density (14, 21, 30), have led to the provocative notion that B. burgdorferi may utilize a LuxS/AI-2 quorum-sensing system to regulate differentially factors that sustain the organism in one or more phases of its complex life cycle in ticks and mammals (25, 26). Along these lines, it has been reported that the B. burgdorferi luxS ortholog can functionally complement a luxS deficiency in Escherichia coli (13, 25). However, thus far, AI-2 activity has not been detectable within B. burgdorferi culture supernatants or concentrated cell lysates (13, 25). Furthermore, infection of mice via needle inoculation revealed that a luxS mutant of B. burgdorferi strain 297 was fully infectious for susceptible animals at levels comparable to those of wild-type B. burgdorferi (13). Although the latter results are inconsistent with the notion that luxS plays an essential role in the infectious phenotype of B. burgdorferi, it remained possible that luxS might contribute at some other strategic phase in the natural transmission cycle of the spirochete, particularly one involving B. burgdorferi's arthropod vector. For example, upon tick engorgement, resident spirochetes in the tick midgut undergo a replicative burst to higher cell density (19, 20), a condition ostensibly more conducive to quorum sensing. This population change may be critical for signaling alterations in borrelial gene expression, thereby prompting the spirochetes to migrate to the salivary glands of the tick (where they are subsequently transmitted into the mammalian host). The present study was thus undertaken to assess the potential role(s) of borrelial luxS in the ability of B. burgdorferi to (i) colonize ticks naturally, (ii) be tick transmitted to naïve mice, and (iii) cause disease in the murine model of Lyme borreliosis.
Colonization of Ixodes scapularis with B. burgdorferi strains.
Infectious, low-passage B. burgdorferi strain 297 (17) was the representative wild-type strain for this study. luxS mutants of strain 297, BbAH308 and BbAH309, are infectious clones isolated from ear punch biopsy-positive cultures from needle-inoculated mice (13). Previous characterization of these luxS mutants revealed that they express neither mRNA nor LuxS protein (13). B. burgdorferi strain 297 was cultured in BSK-H medium (Sigma) without selective agents, and luxS mutants were grown in BSK-H containing erythromycin (0.06 μg/ml). To obtain B. burgdorferi-colonized ticks, two independent experiments were carried out: one with strains 297 and BbAH308 and a second with strains 297 and BbAH309. Three- to five-week-old female C3H/HeJ mice (five per group) were first needle inoculated intradermally with 104 bacteria of either wild-type B. burgdorferi strain 297 or the luxS mutant (BbAH308 or BbAH309) as previously described (12). After 2 weeks, mice were confirmed to be infected by immunoblotting of their sera against borrelial whole-cell lysates and by culturing of ear punch biopsy specimens in BSK-H medium (12). Approximately 400 pathogen-free I. scapularis larvae, reared and generously provided by Thomas Mather (University of Rhode Island), were then placed on two infected mice per isolate and were allowed to feed to repletion. Engorged larvae were then collected over a 5- to 7-day period, transferred into 16-ml glass vials, and stored at 22°C over a saturated solution of potassium sulfate (to maintain a relative humidity of 97%) and within an incubator with a photoperiod of 16 h of light and 8 h of darkness. Engorged ticks were then allowed to molt to the nymphal stage.
PCR amplification of the B. burgdorferi ospA gene from chromosomal DNA extracted from these flat nymphs (20) revealed that at least 80% of the ticks were infected with either wild-type B. burgdorferi or the luxS mutants (data not shown). However, the number of spirochetes in flat ticks is relatively low (19, 20), thereby making determinations of infection efficiencies less than optimal. To obtain more precise PCR assessments of efficiencies of tick colonization by wild-type B. burgdorferi or the luxS mutants, we took advantage of the fact that spirochete populations markedly expand during tick feeding (19, 20). To accomplish this expansion, naïve 3-week-old female C3H/HeJ mice were each infested with five I. scapularis nymphs infected with either wild-type B. burgdorferi or one of the luxS mutants. Mice were housed individually in cages with wire bottoms above water and monitored until ticks fed to repletion and detached from the mice (ca. 72 h). Fed ticks were collected and stored individually at −70°C.
To isolate chromosomal DNA from fed nymphs, individual ticks were first surface disinfected using consecutive washes of sterile water, 0.5% sodium hypochlorite, 3% hydrogen peroxide, 70% ethanol, and sterile water. Ticks were then transferred into 1.5-ml tubes of a Kontes disposable homogenizer, frozen in liquid nitrogen, and pulverized using a fitted pestle. DNA was purified using the Invitrogen Easy-DNA kit according to the manufacturer's instructions, and mussel glycogen was added to a concentration of 20 μg/ml at the precipitation step. Barrier tips were used during DNA extraction and preparation of PCR mixtures to protect against contamination of samples. The primer set used for detecting borrelial DNA in the extracted tick sample, OspA-2 (5′-GTTTTGTAATTTCAACTGCTGACC) and OspA-4 (5′-CTGCAGCTTGGAATTCAGGCACTT), generates a 156-bp amplicon specific for the ospA gene. As a control for contamination, an unfed naïve I. scapularis nymph was prepared with each set of B. burgdorferi-infected ticks. PCRs were carried out with Takara Ex-Taq, 1 μM (each) primers, and 0.5 mM MgCl2. The optimized reaction conditions required an initial 94°C denaturation for 3 min followed by 45 cycles of the following steps: 94°C for 15 s, 65°C for 15 s, and 72°C for 15 s.
PCR results showed that 100% of nymphs were infected with either wild-type B. burgdorferi strain 297 or the luxS mutant BbAH308 (Table 1). BbAH309 yielded similar tick colonization efficiencies (Table 1). These findings support the conclusion that luxS is not required for either the initial colonization of I. scapularis larvae by B. burgdorferi or the spirochete's persistence within the arthropod during subsequent maturation to the nymphal form.
TABLE 1.
Efficiencies of tick colonization by and mouse infectivity of wild-type B. burgdorferi strain 297 and derivative luxS mutants (BbAH308 and BbAH309)
| Expt | Strain | Tick colonizationa | Mouse infectivityb |
|---|---|---|---|
| 1 | 297 | 23/23 | 8/8 |
| BbAH308 | 23/23 | 5/6 | |
| 2 | 297 | 22/22 | 11/11 |
| BbAH309 | 24/25 | 10/10 |
Number of fed nymphs testing positive by PCR for B. burgdorferi/total number of nymphs tested.
Number of mice yielding ear punch biopsy cultures positive for B. burgdorferi/total number of mice tested.
Infectivity of the luxS mutants for mice challenged via tick bite.
The presence of a luxS gene was not required for B. burgdorferi colonization of I. scapularis larvae or maintenance of spirochetes in nymphs. To examine whether the luxS mutant was deficient for subsequent transmission of B. burgdorferi into a mammalian host, naïve 3-week-old female C3H/HeJ mice were each infested with five I. scapularis nymphs infected with B. burgdorferi (the fewest number of ticks that has been demonstrated to result in consistent 100% infection by strain 297) (data not shown). Mice were housed individually in cages with wire bottoms above water. Three weeks after tick infestation, ear punch biopsy specimens from mice were cultured in BSK-H medium with 1× Borrelia antibiotic mixture (Sigma). One to two weeks later, dark-field microscopy was used to examine cultures for the presence of motile spirochetes. In the first set of experiments, motile spirochetes were observed in 100% of the cultures from mice infected with B. burgdorferi strain 297 (Table 1). Cultures from five (83%) of the six mice infected with BbAH308 via tick infestation were also positive for growth of B. burgdorferi (Table 1). Histopathological analysis of the single culture-negative BbAH308-infected mouse revealed pathology identical to that of mice infected with B. burgdorferi strain 297 (data not shown), suggesting that this mouse indeed was infected (raising the infection efficiency for BbAH308 to 100%). A second set of mouse infestation experiments, comparing B. burgdorferi strain 297 with BbAH309, showed 100% infection in both groups (Table 1). An aliquot of each ear punch biopsy culture from the above-described experiments was used to inoculate a larger volume of BSK-H medium without selection by erythromycin. Once these cultures reached an adequate cell density (ca. 107 spirochetes/ml), chromosomal DNA was extracted and the genotypes of the recovered spirochetes were confirmed using a set of primers (priAH166, 5′-GTGACCGGTCAATGGGAAAAATTAGATTTTGTATTTGTAAAAAAAATAC, and priAH167, 5′-GAAAAGGTACCTTACTAAGGATATTTTAAATTTTCTTCTTTAATATTG) that flank the region of luxS where the erythromycin marker was inserted. In all cases, the cultures derived from mice infected with B. burgdorferi strain 297 via tick infestation yielded the wild-type 546-bp amplicon, whereas PCR amplification of DNA extracted from the cultures of the mice infected with the luxS mutants (with insertionally disrupted luxS genes) resulted in the larger 1.5-kb luxS::ermC PCR product (data not shown). The combined data suggest that the luxS mutants were fully capable of being transmitted from ticks to mice and, furthermore, were competent for establishing disseminated infection in the murine model of Lyme borreliosis.
Histopathology in mice infected with the luxS mutants via tick bite.
Previous studies have established that borrelial infectivity and pathogenicity are dissociable, inasmuch as isolates of B. burgdorferi strain N40 can retain the ability to infect C3H/HeJ mice but are unable to induce arthritis or carditis (1, 27). To examine whether a deficiency in luxS perhaps allowed infectivity at the expense of pathogenicity, histological examinations were performed on heart and joint tissues from mice infected with either wild-type B. burgdorferi strain 297 or the luxS mutants. Three weeks post-tick infestation, the heart and right hind limb were harvested from each mouse, fixed in 10% neutralized formalin, and embedded in paraffin. A blind histological assessment was then performed with tissue sections stained with hematoxylin and eosin. Arthritis of the knee joint was evaluated for thickening of the tendon sheath, severity of inflammation, leukocyte infiltration, synovial proliferation, and involvement of adjacent bone or muscle tissue (3-5). Cardiac sections were examined for the severity of inflammation, leukocyte infiltration, and fibroblastic proliferation, as well as involvement of the aortic epithelium, the epicardium, and the endocardium (2, 4, 5).
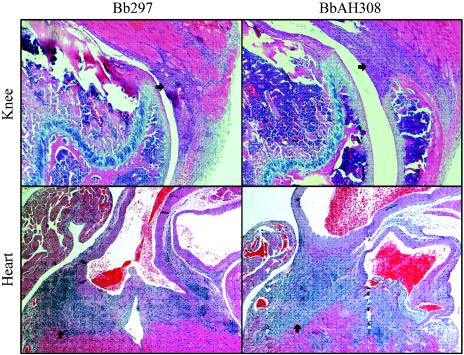
Cardiac and knee tissues from mice infected with B. burgdorferi strain 297 (n = 8) or either of the luxS mutants (n = 8; four mice from each group) were virtually indistinguishable (Fig. 1). In all specimens from mice infected with either wild-type B. burgdorferi or the luxS mutants, there was significant inflammation within the myocardium at the base of the heart surrounding the aorta, pulmonary artery, and coronary arteries. The inflammatory response consisted of macrophages admixed with lymphocytes, plasma cells, and occasional polymorphonuclear cells. In more severely affected animals in all groups, inflammation extended into the mediae of the arteries, resulting in proliferation and inflammation of the overlying intimae. Articular lesions consisted primarily of those of tendonitis. The tendon sheaths were infiltrated with macrophages and fibroblasts admixed with lymphocytes, plasma cells, and small numbers of degenerating neutrophils. There was mild proliferation of the synovial linings of the knee joints associated with infiltration of small numbers of neutrophils and lymphocytes.
FIG. 1.
Histopathology of cardiac and knee tissues from mice infected with either wild-type B. burgdorferi strain 297 (Bb297) or the luxS mutant BbAH308. Results were identical for the luxS mutant BbAH309. Arrows denote areas of pronounced immune cell infiltration.
Summary and implications.
Increased recognition of the importance of LuxS/AI-2 quorum-sensing systems for bacterial gene regulation (7, 9, 15) has prompted the hypothesis that the luxS gene product of B. burgdorferi also may be involved in regulating borrelial gene expression in a LuxS/AI-2-dependent manner (25, 26). However, the relevance of observations implicating LuxS/AI-2 as a regulatory system in B. burgdorferi (25, 26) remains unclear. Furthermore, Winzer et al. (29) have cautioned strongly about the pitfalls of proposing AI-2-dependent quorum sensing as a regulatory system for any bacterium largely, if not solely, because of the mere presence of a luxS gene and/or the production of an AI-2-like molecule(s). To garner more direct evidence for the potential involvement of LuxS in the infectivity of B. burgdorferi for mammalian hosts, it was previously reported that a LuxS-deficient mutant of B. burgdorferi readily infects mice via intradermal (needle) inoculation (13); this finding suggests that LuxS function is not required for the mammalian phase of infection or, at the very least, adaptation of B. burgdorferi to the mammalian host. Herein we now show that the same luxS mutants are fully competent for (i) colonization of their natural arthropod (I. scapularis) vector, (ii) maintenance in I. scapularis throughout the molting process (from larval to nymphal forms), (iii) transfer from ticks to uninfected mice, and (iv) after dissemination in mice, elicitation of histopathology indistinguishable from that induced by wild-type B. burgdorferi. Of note, this is not the first study to show that a bacterial luxS mutant can retain pathogenicity; luxS mutants of both Porphyromonas gingivalis and Shigella flexneri are not attenuated in their respective models (6, 8).
All studies by us to date have failed to garner support for the contention that B. burgdorferi requires a luxS gene product for any key regulatory aspect(s) of either its complex life cycle in ticks and mammals or its ability to produce disease in a susceptible mammalian host. Although the precise functional role of LuxS in B. burgdorferi remains unknown, until direct evidence indicates otherwise, it should be considered more plausible that the major, if not sole, function for LuxS is the conversion of S-ribosylhomocysteine to homocysteine and 4,5-dihydroxy-2,3-pentanedione. The latter product likely represents a dispensable by-product of methionine metabolism that ultimately is excreted to reduce toxicity and maintain a proper metabolic state (28, 29).
Acknowledgments
We thank Anette Hubner for generation of the luxS mutants, Dena Nolen for technical assistance, and Thomas Mather for supplying I. scapularis larvae.
This work was supported by grants AI-45538 and AI-29735 from the Lyme disease program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and by a grant (Advanced Research Program) from the Texas Higher Education Coordinating Board.
Editor: D. L. Burns
REFERENCES
- 1.Anguita, J., S. Samanta, B. Revilla, K. Suk, S. Das, S. W. Barthold, and E. Fikrig. 2000. Borrelia burgdorferi gene expression in vivo and spirochete pathogenicity. Infect. Immun. 68:1222-1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong, A. L., S. W. Barthold, D. H. Persing, and D. S. Beck. 1992. Carditis in Lyme disease susceptible and resistant strains of laboratory mice infected with Borrelia burgdorferi. Am. J. Trop. Med. Hyg. 47:249-258. [DOI] [PubMed] [Google Scholar]
- 3.Barthold, S. W., D. S. Beck, G. M. Hansen, A. A. Terwilliger, and K. D. Moody. 1990. Lyme borreliosis in selected strains and ages of laboratory mice. J. Infect. Dis. 162:133-138. [DOI] [PubMed] [Google Scholar]
- 4.Barthold, S. W., M. S. de Souza, J. L. Janotka, A. L. Smith, and D. H. Persing. 1993. Chronic Lyme borreliosis in the laboratory mouse. Am. J. Pathol. 143:959-971. [PMC free article] [PubMed] [Google Scholar]
- 5.Barthold, S. W., D. H. Persing, A. L. Armstrong, and R. A. Peeples. 1991. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am. J. Pathol. 139:263-273. [PMC free article] [PubMed] [Google Scholar]
- 6.Burgess, N. A., D. F. Kirke, P. Williams, K. Winzer, K. R. Hardie, N. L. Meyers, J. Aduse-Opoku, M. A. Curtis, and M. Camara. 2002. LuxS-dependent quorum sensing in Porphyromonas gingivalis modulates protease and haemagglutinin activities but is not essential for virulence. Microbiology 148:763-772. [DOI] [PubMed] [Google Scholar]
- 7.Chen, X., S. Schauder, N. Potier, A. Van Dorsselaer, I. Pelczer, B. L. Bassler, and F. M. Hughson. 2002. Structural identification of a bacterial quorum-sensing signal containing boron. Nature 415:545-549. [DOI] [PubMed] [Google Scholar]
- 8.Day, W. A., and A. T. Maurelli. 2001. Shigella flexneri LuxS quorum-sensing system modulates virB expression but is not essential for virulence. Infect. Immun. 69:15-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Kievit, T. R., and B. H. Iglewski. 2000. Bacterial quorum sensing in pathogenic relationships. Infect. Immun. 68:4839-4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J.-F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. Weidman, T. Utterback, L. Watthey, L. McDonald, P. Artiach, C. Bowman, S. Garland, C. Fujii, M. D. Cotton, K. Horst, K. Roberts, B. Hatch, H. O. Smith, and J. C. Venter. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580-586. [DOI] [PubMed] [Google Scholar]
- 11.Gilmore, R. D., M. L. Mbow, and B. Stevenson. 2001. Analysis of Borrelia burgdorferi gene expression during life cycle phases of the tick vector Ixodes scapularis. Microbes Infect. 3:799-808. [DOI] [PubMed] [Google Scholar]
- 12.Hagman, K. E., P. Lahdenne, T. G. Popova, S. F. Porcella, D. R. Akins, J. D. Radolf, and M. V. Norgard. 1998. Decorin-binding protein of Borrelia burgdorferi is encoded within a two-gene operon and is protective in the murine model of Lyme borreliosis. Infect. Immun. 66:2674-2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hubner, A., A. T. Revel, D. M. Nolen, K. E. Hagman, and M. V. Norgard. 2003. Expression of a luxS gene is not required for Borrelia burgdorferi infection of mice via needle inoculation. Infect. Immun. 71:2892-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Indest, K. J., R. Ramamoorthy, M. Sole, R. D. Gilmore, B. J. B. Johnson, and M. T. Philipp. 1997. Cell-density-dependent expression of Borrelia burgdorferi lipoproteins in vitro. Infect. Immun. 65:1165-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller, M. B., and B. L. Bassler. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165-199. [DOI] [PubMed] [Google Scholar]
- 16.Narasimhan, S., F. Santiago, R. A. Koski, B. Brei, J. F. Anderson, D. Fish, and E. Fikrig. 2002. Examination of the Borrelia burgdorferi transcriptome in Ixodes scapularis during feeding. J. Bacteriol. 184:3122-3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norton Hughes, C. A., C. B. Kodner, and R. C. Johnson. 1992. DNA analysis of Borrelia burgdorferi NCH-1, the first northcentral U.S. human Lyme disease isolate. J. Clin. Microbiol. 30:698-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ojaimi, C., C. Brooks, S. Casjens, P. Rosa, A. Elias, A. Barbour, A. Jasinskas, J. Benach, L. Katona, J. Radolf, M. Caimano, J. Skare, K. Swingle, D. Akins, and I. Schwartz. 2003. Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays. Infect. Immun. 71:1689-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piesman, J., J. R. Oliver, and R. J. Sinsky. 1990. Growth kinetics of the Lyme disease spirochete (Borrelia burgdorferi) in vector ticks (Ixodes dammini). Am. J. Trop. Med. Hyg. 42:352-357. [DOI] [PubMed] [Google Scholar]
- 20.Piesman, J., B. S. Schneider, and N. S. Zeidner. 2001. Use of quantitative PCR to measure density of Borrelia burgdorferi in the midgut and salivary glands of feeding tick vectors. J. Clin. Microbiol. 39:4145-4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramamoorthy, R., and M. T. Philipp. 1998. Differential expression of Borrelia burgdorferi proteins during growth in vitro. Infect. Immun. 66:5119-5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Revel, A. T., A. M. Talaat, and M. V. Norgard. 2002. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc. Natl. Acad. Sci. USA 99:1562-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwan, T. G. 2003. Temporal regulation of outer surface proteins of the Lyme-disease spirochaete Borrelia burgdorferi. Biochem. Soc. Trans. 31:108-112. [DOI] [PubMed] [Google Scholar]
- 24.Steere, A. C. 1993. Current understanding of Lyme disease. Hosp. Pract. 28:37-44. [DOI] [PubMed] [Google Scholar]
- 25.Stevenson, B., and K. Babb. 2002. LuxS-mediated quorum sensing in Borrelia burgdorferi, the Lyme disease spirochete. Infect. Immun. 70:4099-4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevenson, B., K. von Lackum, R. L. Wattier, J. D. McAlister, J. C. Miller, and K. Babb. 2003. Quorum sensing by the Lyme disease spirochete. Microbes Infect. 5:991-997. [DOI] [PubMed] [Google Scholar]
- 27.Thomas, V., J. Anguita, S. Samanta, P. A. Rosa, P. Stewart, S. W. Barthold, and E. Fikrig. 2001. Dissociation of infectivity and pathogenicity in Borrelia burgdorferi. Infect. Immun. 69:3507-3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Winzer, K., K. R. Hardie, N. Burgess, N. Doherty, D. Kirke, M. T. G. Holden, R. Linforth, K. A. Cornell, A. J. Taylor, P. J. Hill, and P. Williams. 2002. LuxS: its role in central metabolism and the in vitro synthesis of 4-hydroxy-5-methyl-3(2H)-furanone. Microbiology 148:909-922. [DOI] [PubMed] [Google Scholar]
- 29.Winzer, K., K. R. Hardie, and P. Williams. 2002. Bacterial cell-to-cell communication: sorry, can't talk now—gone to lunch. Curr. Opin. Microbiol. 5:216-222. [DOI] [PubMed] [Google Scholar]
- 30.Yang, X., M. S. Goldberg, T. G. Popova, G. B. Schoeler, S. K. Wikel, K. E. Hagman, and M. V. Norgard. 2000. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol. Microbiol. 37:1470-1479. [DOI] [PubMed] [Google Scholar]