Abstract
Medications degrade over time, and degradation is hastened by extreme storage conditions. Current procedures ensure that medications aboard the International Space Station (ISS) are restocked before their expiration dates, but resupply may not be possible on future long-duration exploration missions. For this reason, medications stored on the ISS were returned to Earth for analysis. This was an opportunistic, observational pilot-scale investigation to test the hypothesis that ISS-aging does not cause unusual degradation. Nine medications were analyzed for active pharmaceutical ingredient (API) content and degradant amounts; results were compared to 2012 United States Pharmacopeia (USP) requirements. The medications were two sleep aids, two antihistamines/decongestants, three pain relievers, an antidiarrheal, and an alertness medication. Because the samples were obtained opportunistically from unused medical supplies, each medication was available at only 1 time point and no control samples (samples aged for a similar period on Earth) were available. One medication met USP requirements 5 months after its expiration date. Four of the nine (44% of those tested) medications tested met USP requirements 8 months post expiration. Another three medications (33%) met USP guidelines 2–3 months before expiration. One compound, a dietary supplement used as a sleep aid, failed to meet USP requirements at 11 months post expiration. No unusual degradation products were identified. Limited, evidence-based extension of medication shelf-lives may be possible and would be useful in preparation for lengthy exploration missions. Only analysis of flight-aged samples compared to appropriately matched ground controls will permit determination of the spaceflight environment on medication stability.
Electronic supplementary material
The online version of this article (doi:10.1208/s12248-015-9834-5) contains supplementary material, which is available to authorized users.
KEY WORDS: microgravity, pharmaceutical, space, spaceflight, stability
INTRODUCTION
It is known that medications degrade over time, particularly with exposure to light, oxygen, or humidity; poor storage conditions will hasten their degradation. The temperature and humidity conditions of the International Space Station (ISS) have been shown to be within the ideal ranges for medication storage (1), but the effects of other environmental factors unique to spaceflight (e.g., microgravity or chronic elevated exposure to radiation) have not yet been evaluated with respect to either degradation rates or formation of unusual degradation products. Current operational procedures ensure that medications aboard the ISS are restocked before their expiration date, but resupply may not be possible on long-duration exploration missions. For this reason, medications that have been exposed to long-duration storage on the ISS are worthy of study before lengthy explorations begin.
Results from an earlier multi-year medication stability study on the ISS showed that most spaceflight-aged medications fail to meet United States Pharmacopeia (USP) guidelines for active pharmaceutical ingredient (API) content around the time of their expiration dates (1). There were six exceptions that failed earlier than their expiration dates: the antimicrobials amoxicillin/clavulanate, levofloxacin, and trimethoprim/sulfamethoxazole; the diuretic furosemide; and the synthetic hormone levothyroxine. Du and colleagues reported analysis of only a single sample of each drug, so it is not possible to assign statistical significance to their results, but their results indicate that at least some drugs in the flight medical kit merit further attention. Also, Du et al. measured only the content of the active ingredient from each sample; no screening for appearance (or toxicity) of breakdown products was performed (1). The study described in this current report was conducted to expand upon their earlier results. We took advantage of unused medical supplies as a source of additional ISS-aged samples that could be used to test the hypothesis that medication degradation on the ISS does not differ from what is typically seen on Earth.
MATERIALS AND METHODS
FDA-approved medications were obtained by the Johnson Space Center (JSC) Pharmacy from commercial pharmacy distributors and were repackaged by JSC pharmacists to conserve mass and volume according to typical mission practices. All medication doses were part of the ISS crew medical kit and were transported to the ISS via Russia’s Progress vehicle. After 550 days of storage, the medications were removed from the supply chain and returned to Earth on a Dragon (SpaceX) capsule. The samples were shipped by boat to California, then flown to Houston, TX. The elapsed time from capsule splashdown to sample arrival at Johnson Space Center was ∼58 h. During travel, the samples were stored in double cold bags with +4°C cold packs. Returned medications were transferred to temperature- and humidity-controlled environmental chambers (Darwin, St. Louis, MO; 20–25°C, 35–65% RH) until they were analyzed (3–5 months later). No specific environmental monitoring was in place during the time that these samples were onboard, another caveat of this unplanned study. Environmental monitoring in place during previous inflight medication storage showed that temperature (20–26°C) and humidity (30–50% RH) were within the ranges desired for medication storage. It should be noted that temperature and humidity are well controlled on the ISS for crew health reasons. A previous medication study on the ISS showed that radiation exposure at the longest time point of about 2.5 years was 110 mGy (1); which permits approximation of a 70 mGy exposure during the 550-day storage time of the medications in this study.
Nine medications were available for study (Supplemental Table I). The medications included several of those most heavily used by US crewmembers: two sleep aids, an antihistamine, a decongestant, three pain relievers, an antidiarrheal, and an alertness medication. Each medication was available at a single time point; typical stability study protocol (analysis of the same medication at multiple time points) was not possible. Because the samples examined in this study were obtained opportunistically from medical supplies, no control samples (that is, samples aged for a similar period on the ground) were available. All medications were solid dosage forms.
Medications were analyzed using the methods described in the USP to measure the amount of intact active ingredient, identify degradation products when possible, and measure their amounts. Each medication was analyzed according to USP methods for content of active ingredient as well as USP-recognized impurities (2). It should be noted that these methods may fail to distinguish many impurities; we undertook no additional analyses aimed at measurements of impurities. These analyses were conducted in the spring and summer of 2013; USP guidelines active for that time period were used for both methodology and interpretation of results. For every medication in this study, the USP guidelines stipulated that the content of API be between 90 and 110% of the amount specified on the label; note that stricter API guidelines have now been adopted for several medications including acetaminophen and ibuprofen. Additional impurities have been identified and limitations added for ibuprofen and zolpidem. The methodological details of analysis are unique to each medication, but briefly, samples of solid dosage forms were solubilized and separations were performed with high-pressure liquid chromatography followed by ultraviolet detection mass spectroscopy. Some analyses (zolpidem, modafinil, and pseudoephedrine) were performed at Johnson Space Center on a Waters Alliance 2795 HT HPLC with PAD detector and MassLynx 4.1 software (Waters, Milford, MA); all other analyses were performed on an Agilent 1100s with UV DAD detector and Chemstation software (Agilent, Foster City, CA) by an independent analytical laboratory (Velesco Pharmaceutical Servicers, Plymouth, MI).
To meet USP guidelines, multiple tablets (20 each, except for loratadine, whose standard is 10 tablets) were crushed together to make each sample. Analyses were performed separately on five different samples each composed of multiple tablets. Due to the ad hoc nature of this study, insufficient unit doses were available to meet these guidelines for loperamide and pseudoephedrine. In these cases, analyses were performed in four samples composed of 10 tablets each (loperamide) and four samples composed of 20 tablets each (pseudoephedrine).
All medications were repackaged for flight by the JSC Pharmacy and were given expiration dates of 2/2012 in the new packaging; they were all analyzed 12 to 16 months after this repackaging expiration date. However, in all but one case, the original manufacturers’ expiration date was recorded and we include this date as a reference point.
RESULTS
To assess potency and purity, nine aged medications were analyzed with respect to active pharmaceutical ingredient (API) and degradant amounts (Table I). Results were compared to the USP requirements for API and the content of degradants and impurities (2). For medications having USP-specified criteria for impurities or related substances, known impurities and degradation products were investigated and assessed against USP criteria.
Table I.
Results of Medication API Assays
| Medication | Method | Results per sample | Mean ± Std | % Label |
|---|---|---|---|---|
| Aspirin (325 mg) | USP monograph: Aspirin tablets | A 314.1 | 313.7 ± 0.5 | 96.5 |
| B 313.0 | ||||
| C 314.0 | ||||
| D 313.5 | ||||
| E 314.1 | ||||
| Acetaminophen (325 mg) | USP monograph: Acetaminophen tablets | A 314.7 | 315.4 ± 3.2 | 97.0 |
| B 321.1 | ||||
| C 313.5 | ||||
| D 313.4 | ||||
| E 314.4 | ||||
| Ibuprofen (400 mg) | USP monograph: Ibuprofen tablets | A 403.0 | 399.6 ± 7.1 | 99.9 |
| B 403.0 | ||||
| C 387.0 | ||||
| D 404.0 | ||||
| E 399.6 | ||||
| Loratadine (10 mg) | USP monograph: Loratadine tablets | A 9.6 | 10.0 ± 0.3 | 99.9 |
| B 10.1 | ||||
| C 10.1 | ||||
| D 10.1 | ||||
| Loperamide (2.0 mg) | USP monograph: Loperamide hydrochloride tablets | A 1.9 | 2.0 ± 0.6 | 96.3 |
| B 1.9 | ||||
| C 2.0 | ||||
| D 2.0 | ||||
| Pseudoephedrine hydrochloride (120 mg) | USP monograph: Pseudoephedrine hydrochloride extended-release tablets | A 114.8 | 118.9 ± 3.3 | 99.0 |
| B 119.4 | ||||
| C 122.7 | ||||
| D 118.5 | ||||
| Melatonin (3 mg) | Dietary supplement: Melatonin tablets | A 2.6 | 2.7 ± 0.1 | 89.2 |
| B 2.7 | ||||
| C 2.7 | ||||
| D 2.7 | ||||
| E 2.7 | ||||
| Zolpidem (10 mg) | USP monograph: Zolpidem tablets | A 10.16 | 10.07 ± 0.09 | 100.6 |
| B 10.16 | ||||
| C 9.96 | ||||
| D 10.07 | ||||
| E 9.99 | ||||
| Modafinil (200 mg) | USP monograph: Modafinil tablets | A 197.8 | 201.2 ± 2.4 | 100.6 |
| B 203.2 | ||||
| C 202.2 | ||||
| D 201.4 |
USP criteria for all of these medications in 2012 was 90.0–110.0% of the amount stated on the product label. Results are given per individual sample and as mean ± standard deviation. Percentage of label claim (mean API amount/label claim) is given for each medication for reference
Over-the-Counter Pain Relievers
Aspirin samples 9 months beyond the original manufacturer expiration date were found to contain active ingredient well within USP guidelines (96.5% of the label claim of 325 mg per tablet; Table I). Free salicylic acid was found at less than 0.05% in each sample; the USP limit for this impurity is no more than (NMT) 0.3% (Fig. 1a).
Fig. 1.
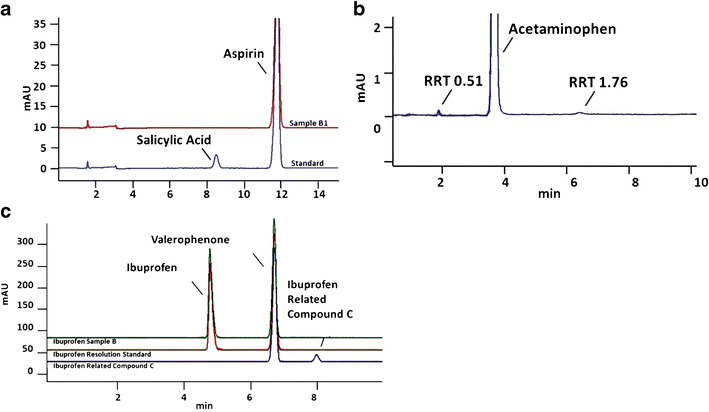
Representative chromatograms from analysis of pain-relief medications. a A commercial standard that includes a common contaminant, salicylic acid, is shown in the lower trace for comparison, with the free salicylic acid peak labeled at ∼RT = 8.5 min. Results from analysis of sample B1 are shown superimposed. Note the absence of a salicylic acid peak in the aged medication sample. Aspirin samples 9 months beyond their original manufacturer expiration date were found to contain active ingredient well within USP guidelines (96.5% of the label claim of 325 mg per tablet). Free salicylic acid was found at less than 0.05% in each sample; the USP limit for this impurity is no more than 0.3%. b Acetaminophen samples that were analyzed 5 months after their original manufacturer expiration date were found to contain active ingredient at 96.5% of the label claim of 325 mg per tablet, which is within the USP at the time these samples were analyzed, but outside the new USP requirements of 98–102%. Although two additional peaks were observed, 0.17 and 0.25% (by peak area), they were not identified. If one of them was paraaminophenol, this sample would also have exceeded the limit on this impurity (NMT 0.005%). c Ibuprofen is known to have several potential ingredients in addition to the active ingredient, so multiple standards were run for comparison. Ibuprofen samples analyzed 3 months prior to their original expiration date also met USP criteria with active ingredient well within USP guidelines, 99.9% of the label claim of 400 mg per tablet. USP has identified and determined limits for an impurity in ibuprofen, called ibuprofen related compound C. Ibuprofen related compound C was found at less than 0.001% in each sample; the USP limit for this impurity is 0.25%
Acetaminophen samples that were analyzed 5 months after their original manufacturer expiration date were found to contain active ingredient at 97.0% of the label claim of 325 mg per tablet, well within 2012 USP guidelines (Table I). Two additional peaks were observed (Fig. 1b), 0.17 and 0.25% (by peak area). The USP monograph for acetaminophen has been updated; these samples do not meet current requirements for API content (98–102% of label).
Ibuprofen samples analyzed 3 months before their original expiration date also met USP criteria, with the active ingredient at 99.9 % of the label claim of 400 mg per tablet (Table I). USP has identified and determined limits for an impurity in ibuprofen called ibuprofen-related compound C. Ibuprofen-related compound C was found at less than 0.001% in each sample; the USP limit for this impurity is 0.25% (Fig. 1c). The USP monograph for ibuprofen has been updated; these samples meet current requirements for API content (97–103% of label).
Other Commonly Used Over-the-Counter Medications
Antihistamine loratadine samples were found to contain active ingredient well within USP guidelines at 8 months beyond the manufacturer expiration date, 99.9% of the label claim of 10 mg per tablet (Table I). Three additional peaks, one of which had a retention-time match for loratadine-related compound B, were each observed at ≤0.03% with a total of ≤0.07% (Fig. 2). Identification or other analysis of these additional peaks was not possible due to the small amount of material. USP guidelines limit individual impurities to ≤0.1% and total impurities to 0.1% (Table II). Total impurities in these samples were within, but close to, the USP limits.
Fig. 2.
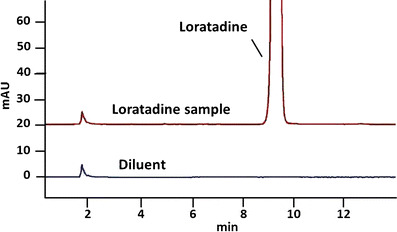
Representative chromatograms from analysis of antihistamine loratadine. Antihistaminergic loratadine samples were found to contain active ingredient well within USP guidelines at 8 months beyond their manufacturer’s expiration date, 99.9% of the label claim of 10 mg per tablet. The USP-identified impurity 4-(8-chloro-11-fluoro-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridine-11-yl)-1-piperidinecarboxylate ethyl (RRT 0.79) was not detected
Table II.
Results of Medication Related Compounds Assays
| Medication | Method | Criteria | Results per sample | |||||
|---|---|---|---|---|---|---|---|---|
| Loratadine | USP monograph: Loratadine tablets | Individual NMT 0.1% | RRT | A | B | C | D | |
| Total NMT 0.1% | 0.40 | ND | ND | 0.01 | ND | |||
| 1.35 | 0.03 | 0.03 | 0.03 | 0.03 | ||||
| 1.96 | 0.02 | 0.03 | 0.03 | 0.03 | ||||
| Tot | 0.05 | 0.06 | 0.07 | 0.06 | ||||
| Melatonin | Dietary supplement: Melatonin tablets | Individual NMT 0.1% | RRT | A | B | C | D | E |
| Total NMT 1.0% | 1.13 | 0.08 | ND | 0.08 | 0.08 | 0.08 | ||
| 1.97 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | |||
| 2.04 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | |||
| 2.11 | 0.12 | 0.11 | 0.12 | 0.12 | 0.11 | |||
| 2.15 | 0.08 | 0.08 | 0.08 | 0.08 | 0.10 | |||
| 2.17 | 0.06 | 0.06 | 0.06 | 0.06 | 0.06 | |||
| 2.22 | 0.06 | 0.06 | 0.05 | 0.05 | 0.05 | |||
| 2.24 | 0.14 | 0.14 | 0.14 | 0.14 | 0.14 | |||
| 2.61 | 0.10 | 0.11 | 0.11 | 0.10 | 0.09 | |||
| Tot | 0.96 | 0.77 | 0.88 | 0.85 | 0.84 | |||
Assays were USP-related Compounds (% w/w) for each medication. USP criteria are no more than (NMT) 0.1% by weight for individual compounds in either medication. In the case of melatonin, an amount of related compounds up to a total of 1.0% by weight is acceptable, but for loratadine the total limit is the same as the individual limit, 0.1%. Relative retention time (RRT) is used to define each peak (listed vertically under “Results per sample”), with each different sample identified by A–E. Results are given in % w/w
ND not detectable
Samples of loperamide, an opioid compound with little central nervous system activity used to treat diarrhea, were analyzed 2 months before the manufacturer expiration date. They contained active ingredient at 96.3% of the label claim of 2 mg per tablet (Table I). Additional, unidentified peaks were observed bracketing the main peak at ≤0.1 and ≤0.8%, respectively (Fig. 3), the second of which (at 0.8%), exceeds the new limits of 0.1%. The USP-identified impurity 4-(8-chloro-11-fluoro-6,11-dihydro-5H-benzo[5,6]cyclohepta[1,2-b]pyridine-11-yl)-1-piperidinecarboxylate ethyl (RRT 0.79) was not detected.
Fig. 3.
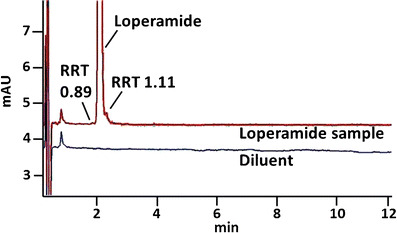
Representative chromatograms from analysis of loperamide. Samples of loperamide, an opioid compound with little central nervous system activity used to treat diarrhea, were analyzed 2 months before the manufacturer expiration date. They contained active ingredient at 96.3% of the label claim of 2 mg per tablet; the lowest amount in a sample was 93.2%. Two additional peaks were observed bracketing the main peak at ≤0.1 and ≤0.8%, respectively
Pseudoephedrine samples were analyzed 9 months after the manufacturer expiration date and found to contain active ingredient at 99.0% of the label claim of 120 mg per tablet (Table I).
The melatonin samples in this study were 11 months beyond the manufacturer expiration date, the oldest samples in this study. They were found to contain API at 89.2% of the label claim of 10 mg per tablet and thus failed to meet USP standards for API content. Ten additional peaks were observed at ≤0.16% with a total of ≤0.96% (Table II and Fig. 4). USP guidelines limit individual impurities to ≤0.1% and total impurities to 1.0% (Table II). Although total impurities in these samples were within (but very close to) limits, the individual impurity limits were exceeded in multiple samples for five of the 10 impurities detected. Samples of this sleep supplement thus failed to meet USP criteria for both impurities and API.
Fig. 4.
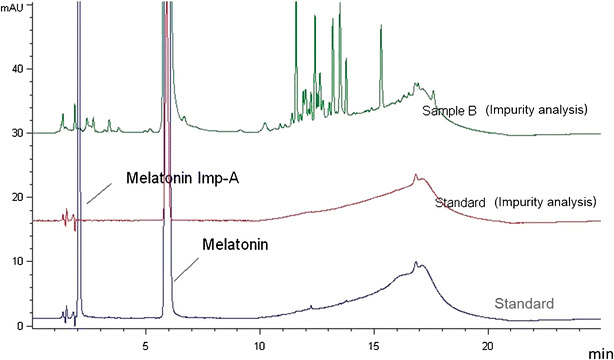
Representative chromatograms from analysis of melatonin. Ten additional peaks (including melatonin impurity-A) were observed in the melatonin samples at ≤0.16% with a total of ≤0.96%. USP guidelines limit individual impurities to ≤0.1% and total impurities to 1.0%. Although total impurities in these samples were within (but very close to) limits, the individual impurity limits were exceeded in multiple samples for five of the 10 impurities detected. Samples of this sleep supplement failed to meet USP criteria for both impurities and API
Prescription Medications for Sleep and Alertness
Zolpidem samples that were 9 months beyond their expiration date were found to contain active ingredient at 100.6% of the label claim of 10 mg per tablet, well within USP guidelines. Seven impurities were detected, totaling 0.29% by peak area and 0.24 by peak height. This is less than the USP limit of 0.5%. The most abundant was zolpidem-related compound B (0.12% by peak area and 0.10% by peak height). Three of the impurities were unspecified degradation products, the most abundant at 0.02% by peak area and by peak height (Table II).
Samples of modafinil, an alertness medication, were analyzed 2 months before the original manufacturer expiration date and found to contain active ingredient at 100.6% of the label claim of 200 mg per tablet, well within USP guidelines (Table I). Five impurities were detected, totaling 0.17%, about 10% of the USP standard of 1.5%. The most abundant was modafinil sulfate (0.14%), for which the USP specifies a limit of 0.5%. Three of the impurities were unspecified products that totaled 0.01%.
DISCUSSION
Analysis of each medication at a single time point provides limited information on the API and impurities contents of a specific lot of a medication from a particular manufacturer stored in specified conditions; it is not possible to extrapolate the results to other lots, other manufacturers or other storage conditions. The Shelf Life Extension Program (SLEP), a >20 years cooperative study of the Department of Defense and the Food and Drug Administration, has clearly shown medication stability is dependent on manufacturer and formulation and can even vary from one manufacturing lot to another (3). Although it is tempting to conclude from the current results, each based on a single time point, that aspirin, pseudoephedrine, and zolpidem remain safe and effective for more than 6 months after expiration, other studies have made it clear that limited results like these cannot be extrapolated to other time points, brands, or even lots of the same medication (3, 4).
These limitations notwithstanding, five of the nine medications tested (56%) met 2012 USP requirements for API and degradants and impurities at least 5 months past their expiration dates (Fig. 5). Four of the nine (44% of those tested) medications tested met 2012 USP requirements up to 8 months post expiration. Another three medications (33% of those tested) met USP guidelines 2 to 3 months before expiration. Some common degradation products with specific USP guidelines were noted in aspirin, ibuprofen, loratadine, and zolpidem. Other degradation products were noted in other samples, at amounts too small for further analysis with USP methods and, with the exception of melatonin, within 2012 USP guidelines for total impurities for those medications. It is important to note that standards for medication are updated regularly as new information becomes available and analytical techniques improve. Under current guidelines, both the acetaminophen and loratadine samples, would fail. Taken together, these preliminary findings support our hypothesis and suggest that aging of the samples on the ISS did not result in either unusually rapid degradation or in degradation via unusual pathways. One medication, a compound classified by the FDA as a dietary supplement and sometimes used as a sleep aid, failed to meet USP requirements at 11 months post expiration. This compound is not regulated as strictly as prescription medications are during manufacture; it is unknown if this medication would have met the requirements before flight. Notably, it was also the furthest beyond its expiration date.
Fig. 5.
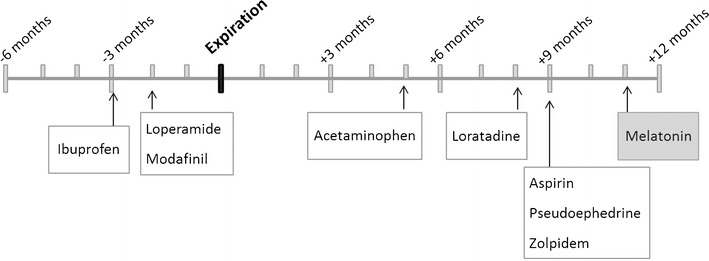
Timeline of medication ages relative to their expiration dates. All medications were aged on the ISS for 550 Earth days. Assays were performed after sample return to Earth at the time points indicated above, relative to their original manufacturer expiration date (shown in bold). Most (white boxes) passed 2012 USP requirements for amount of active pharmaceutical ingredient (API) and for degradants/impurities. Melatonin (shaded box) failed both API and degradants/impurities assays. Note that the acetaminophen, loratadine, and loperamide samples would fail according to updated USP guidelines
The results reported here agree with those of other studies of medication potency. Several, but not all, samples of various pharmaceuticals in their original unopened containers discovered in a pharmacy more than 28 years after their expiration dates were found to contain adequate amounts of their active ingredient (5). It is worth noting that the samples in our study had been repackaged into bags to reduce the overall volume of the ISS medication kit, whereas the samples from the Cantrell and SLEP studies were stored in their unopened original manufacturer’s packaging. Packaging is a critical factor in long-term stability of medications; aspirin lost 7% of its active ingredient after 1 week in a dosette box in accelerated aging conditions (40°C, 75% relative humidity (6)). Even an enteric coating on a tablet may improve product stability (7).
Medications are known to exhibit highly variable stability depending on their formulation. Aspirin has been shown to have compromised stability in combination with alumina or silica, while formulations containing starch, stearate, cellulose, or dextrose were more stable (8). The aspirin samples analyzed here and found to meet USP requirements 9 months after expiration included starch, but no alumina or silica. An elegant study comparing globally available brands of ibuprofen linked faster degradation with the inactive ingredients in the formulation. The brand of ibuprofen examined here contained hypromellose, polyethylene glycol, and polysorbate, three excipients associated with faster degradation of ibuprofen (4). However, our finding that the ISS-aged ibuprofen samples passed both API and impurities testing 3 months before expiration is consistent with results reported by Du and colleagues (1); their results indicated that ibuprofen samples passed API analysis at every time point examined, even after about 2.5 years of ISS aging. Unfortunately, it is not possible to perform additional comparisons of our current results with those of Du and colleagues (1); ibuprofen was the only one of the medications available for this analysis that was also examined in their study. Our finding that acetaminophen met all USP requirements at 5 months post expiration is in good agreement with results from the SLEP study (3), but unfortunately, it was the only medication in common between these studies.
Although this is not a stability study, the data points presented here are of interest to NASA as the agency prepares for exploration missions beyond low Earth orbit. These missions may last as long as 3 years and there will be no opportunities for resupply. Three years is longer than the shelf life of most medications; this is the motivation for study of medication potency and degradation (1) over long storage periods and (2) during storage in the spaceflight environment. Previous studies have shown that temperature and humidity on the ISS are within terrestrial norms for medication storage (1). Other unusual features of the spaceflight environment are microgravity and increased exposure to radiation. It seems unlikely that storage in a weightless environment would affect medications, but the effects of space radiation on medications are currently unknown. Space radiation is a variable mixture of ionizing and heavy-ion radiation at relatively constant low doses. For example, a sample set of pharmaceuticals aged on the ISS for about 2.5 years received 110 mGy exposure (1). Some medical products, including medications, are treated with acute high doses (typically in the kGy range) of gamma radiation to ensure product sterility (9). It is not known if chronic low-dose, mixed-species radiation is a greater hazard than acute high-dose gamma radiation used for in-package sterilization.
CONCLUSION
Some ISS-aged medications met API and impurities requirements months after their expiration dates. Since the ISS-aged samples used in this study were opportunistically obtained and no age- and lot-matched ground samples were available for analysis, it is not possible to determine effects of the spaceflight environment on these stored medications. Since the current results are based only on measurements at a single time point, they cannot be extrapolated to other storage times. These assessments of safety and efficacy at single time points for a handful of medications provide a useful starting point in data collection, but more rigorous studies will be required to determine if the spaceflight environment causes unusual degradation during storage.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOCX 14 kb)
Acknowledgments
The author wishes to thank Dr. Tina Bayuse and Ms. Kelly Kampe for assistance in obtaining valuable samples from expired ISS stocks, Dr. Zuwei Wang of the JSC Nutritional Biochemistry Laboratory for excellent technical services, and Ms. Vernie Daniels for pharmacy services. The study was funded by the NASA Human Research Program.
Contributions
Dr. Wotring designed the study, obtained the funding, analyzed the data, and wrote the manuscript. Sample analyses were performed under contract by Velesco Pharmaceutical Services and by Dr. Zuwei Wang of the JSC Nutritional Biochemistry laboratory.
References
- 1.Du B, Daniels V, Vaksman Z, Boyd J, Crady C, Putcha L. Evaluation of physical and chemical changes in pharmaceuticals flown on space missions. AAPS J. 2011;13(2):299–308. doi: 10.1208/s12248-011-9270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pharmacopeia US. United States pharmacopeia monographs. 2012.
- 3.Lyon RC, Taylor JS, Porter DA, Prasanna HR, Hussain AS. Stability profiles of drug products extended beyond labeled expiration dates. J Pharm Sci. 2006;95(7):1549–60. doi: 10.1002/jps.20636. [DOI] [PubMed] [Google Scholar]
- 4.Cory WC, Harris C, Martinez S. Accelerated degradation of ibuprofen in tablets. Pharm Dev Technol. 2010;15(6):636–43. doi: 10.3109/10837450903426518. [DOI] [PubMed] [Google Scholar]
- 5.Cantrell L, Suchard JR, Wu A, Gerona RR. Stability of active ingredients in long-expired prescription medications. Arch Intern Med. 2012;172(21):1685–7. doi: 10.1001/archinternmed.2012.4501. [DOI] [PubMed] [Google Scholar]
- 6.Mylrea M, Robertson S, Haywood A, Glass B. Stability of dispersible aspirin tablets repacked into dosette boxes. J Pharm Pract Res. 2012;42:204–7. doi: 10.1002/j.2055-2335.2012.tb00171.x. [DOI] [Google Scholar]
- 7.Mujahid A, Farooq MU, Hameed A, Hussain T, Shah AT, Ahmad S, et al. Quantitative degradation monitoring in core and enteric coated aspirin tablets. Int J Curr Pharm Res. 2013;5(4):68–70. [Google Scholar]
- 8.Ager DJ, Alexander KS, Bhatti AS, Blackburn JS, Dollimore D, Koogan TS, et al. Stability of aspirin in solid mixtures. J Pharm Sci. 1986;75(1):97–101. doi: 10.1002/jps.2600750124. [DOI] [PubMed] [Google Scholar]
- 9.Wotring VE. Space pharmacology. Pelton JN, editor. New York: Springer; 2012.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 14 kb)


