Abstract
The mosquito Aedes aegypti, the principal vector of dengue virus, has recently been infected experimentally with Wolbachia: intracellular bacteria that possess potential as dengue biological control agents. Wolbachia depend on their hosts for nutrients they are unable to synthesize themselves. Consequently, competition between Wolbachia and their host for resources could reduce host fitness under the competitive conditions commonly experienced by larvae of Ae. aegypti in the field, hampering the invasion of Wolbachia into natural mosquito populations. We assess the survival and development of Ae. aegypti larvae under starvation conditions when infected with each of three experimentally-generated Wolbachia strains: wMel, wMelPop and wAlbB, and compare their fitness to wild-type uninfected larvae. We find that all three Wolbachia infections reduce the survival of larvae relative to those that are uninfected, and the severity of the effect is concordant with previously characterized fitness costs to other life stages. We also investigate the ability of larvae to recover from extended food deprivation and find no effect of Wolbachia on this trait. Aedes aegypti larvae of all infection types were able to resume their development after one month of no food, pupate rapidly, emerge at a large size, and exhibit complete cytoplasmic incompatibility and maternal transmission. A lowered ability of Wolbachia-infected larvae to survive under starvation conditions will increase the threshold infection frequency required for Wolbachia to establish in highly competitive natural Ae. aegypti populations and will also reduce the speed of invasion. This study also provides insights into survival strategies of larvae when developing in stressful environments.
Author Summary
Dengue is currently the most important arboviral disease in the world. With no effective treatment or commercial vaccine available, strategies to control dengue focus on its mosquito vectors, primarily Aedes aegypti. A recent effort to reduce the burden of dengue aims to replace native Ae. aegypti with those refractory to the virus. This is achieved by infecting mosquitoes with Wolbachia, bacteria which can invade insect populations by exploiting host reproduction. Some strains of Wolbachia have harmful effects on the mosquito host which can inhibit its ability to spread. While these costs have been characterized comprehensively in the laboratory, we must also consider any impacts when mosquitoes experience stresses that commonly occur in nature. For instance, Ae. aegypti larvae often develop in highly-occupied habitats where food is scarce. We investigated the effects of Wolbachia on mosquito larvae when they develop under extremely nutrient-limited conditions and found costs to survival for all strains. This will translate to a reduced ability of Wolbachia-infected mosquitoes to replace native populations in competitive habitats.
Introduction
Dengue fever is an increasing threat to global health. An estimated 50 to 390 million new cases of dengue occur annually, with 2.5 billion people living in areas at risk of infection [1,2]. At present, dengue lacks an effective treatment or vaccine that protects against all serotypes of the virus. Thus, strategies to reduce infection incidence must rely on the control of its mosquito vector, principally Aedes aegypti [3,4]. While permanent eradication is unlikely to be achieved, many emerging genetic and biological approaches aim to reduce mosquito vectorial capacity [5,6].
A promising new approach to dengue control utilizes the obligate intracellular bacterium, Wolbachia. Wolbachia are maternally inherited [7] and usually manipulate the reproduction of their hosts to enhance their own transmission [8]. The most common manipulation induced by Wolbachia is cytoplasmic incompatibility; a mechanism where embryonic lethality occurs when an infected male mates with a female that is not infected with Wolbachia, providing infected females with a relative reproductive advantage [9,10]. Many Wolbachia infections also provide protection to their host against pathogens, including RNA viruses [11–13]. These traits have enabled Wolbachia to be implemented in strategies to both suppress [14,15] and replace [16–18] insect populations.
While Ae. aegypti does not harbour a natural Wolbachia infection [19,20], three infections have been stably introduced into the vector: the wMelPop and wMel strains originating from Drosophila melanogaster [21,22] and wAlbB from the mosquito Aedes albopictus [23]. All three infections are transmitted vertically at high rates and exhibit complete cytoplasmic incompatibility [21–23], and these effects have remained stable after many years in the novel host [24–26]. Crucially, they also suppress the replication and transmission of dengue virus in Ae. aegypti [22,27,28], giving them potential to reduce dengue incidence in transformed populations. Establishment of Wolbachia in a field population is facilitated largely by maternal transmission and cytoplasmic incompatibility [16,29,30]. However, because Wolbachia-infected mosquitoes must survive and reproduce in competition with the native inhabitants, lower relative fitness of infected mosquitoes can hamper the invasibility of Wolbachia [31–33].
The experimental Wolbachia infections established in Ae. aegypti vary considerably in their effects on mosquito life-history traits. The wMel infection is relatively benign and has invaded both caged [22] and field [18] populations. wMel remains at a high frequency in mosquitoes collected from the release sites, three years after releases of wMel ceased in two suburbs of Cairns, Australia[24]. Conversely, the wMelPop infection tends to overreplicate in host cells, leading to rapid tissue degeneration and early death [34–36]. It exacts a high fitness cost on Ae. aegypti; wMelPop shortens adult lifespan [21,37], while fecundity [38], blood feeding success [39,40] and quiescent egg viability [37,38,41] deteriorate rapidly with age. wMelPop also modifies behaviour and metabolism [42], reduces the response of larvae to light stimulation [43], delays larval development, and decreases viability and adult size when reared under crowded conditions [44]. The wAlbB infection has intermediate fitness costs, likely due to its moderate density in host tissues that lies between that of wMel and wMelPop [26].
While each of these infections can invade caged populations of Ae. aegypti [22,23,26], the mosquitoes were not exposed to many of the selective pressures that exist in the field [6]. Suitable habitats for immature development in the field are limited; as a consequence, larvae are often subjected to competition for space and nutrition [45–48]. Though Wolbachia infection has no clear effect on Ae. aegypti larval development in the absence of stress [22,26,37,38], some costs emerge when larvae are crowded [44]. Many fitness costs of Wolbachia in Ae. aegypti also tend to become clearer with age in both adults and eggs [26,37,39]. As larval development times can reach several weeks, or even months in the field [49] and often experience periods of food limitation [47,48], deleterious effects of Wolbachia on larvae undetected in laboratory studies could emerge when development times are prolonged, impacting Wolbachia’s invasive potential. This could explain a lack of invasion success by wMelPop in natural populations despite multiple attempts to establish the infection in the field [50].
Aedes aegypti larvae are adapted to nutrient poor-habitats as food limitation is a major regulator of their population size [47,51]. Larvae decrease their rate of development in response to food scarcity, delaying metamorphosis until reaching a critical threshold of nutritional reserves [52–55], and larvae can resist starvation for several weeks at a time [51,56–58]. This is achieved largely by expending their accumulated reserves [59–61], though larvae also scavenge on dead conspecifics [62,63] and may even prey on younger larvae [64] to increase their chance of survival. Wolbachia depend on their hosts for a wide range of resources they cannot synthesize themselves [65–68]. Since Wolbachia increase the activity and metabolic rate of Ae. aegypti in adults, at least for the wMelPop infection [42], we hypothesize that Wolbachia may also increase the rate at which energy reserves are depleted in larvae without food. Aedes aegypti breeding containers typically have low productivity and high food intermittency because leaf litter, animal detritus and the microorganisms that break them down are the primary source of nutrition [62,69,70]. Thus, the ability to survive periods of limited food is a critical aspect of larval fitness [47,51]. In the field, competition between Wolbachia and Ae. aegypti for resources could substantially reduce the survival of larvae, limiting the potential for Wolbachia to invade and persist in natural populations.
In this study we investigate the effects of wMel, wAlbB and wMelPop infection on the ability of Ae. aegypti larvae to survive and develop under extreme nutrient stress. We compare the survival and development of Wolbachia-infected and uninfected larvae under starvation conditions when held in groups, when infected and uninfected larvae are together in the same container, or when isolated, and test their ability to recover when an influx of resources is provided. We also examine the ability of Wolbachia to express their reproductive effects when Ae. aegypti larvae are held under starvation conditions for extended periods. We then consider the likely impact of any fitness costs imposed by Wolbachia on the potential for these infections to invade highly competitive populations.
Methods
Colony maintenance and mosquito strains
Aedes aegypti mosquitoes were sourced from Cairns, Queensland and maintained under laboratory conditions for at least two generations before use in experiments. Wolbachia-infected lines were generated by crossing male uninfected Cairns mosquitoes to laboratory-reared female mosquitoes infected with wMel [22], wAlbB [23] or wMelPop [21] to maintain a similar genetic background (>98%) between colonies. Mosquitoes were kept in the laboratory at 26°C ± 1°C and 80–90% relative humidity with a 12:12 light: dark photoperiod, and maintained according to methods described by Axford et al. [26]. Within one week of emerging, female adults were allowed to feed to repletion on the forearm of a single human volunteer. Blood feeding of female mosquitoes on human volunteers for this research has been approved by the University of Melbourne Human Ethics Committee (approval 0723847). All adult subjects provided informed written consent (no children were involved).
Rearing regime
Larvae were reared under a common regime before initiating the food-deprivation period for all experiments. At the beginning of each experiment, wMel-infected, wMelPop-infected, wAlbB-infected and uninfected eggs were hatched synchronously in separate trays containing 3 L of RO (reverse osmosis) water, 2–3 grains of yeast and one crushed tablet of TetraMin tropical fish food (Tetra, Melle, Germany). Within three hours of hatching, cohorts of 200 1st instar larvae were transferred to plastic trays filled with 700 mL of RO water and fed TetraMin ad libitum for 72 hours. This rearing environment was chosen as development times do not differ significantly between Wolbachia-infected and uninfected larvae with abundant nutrition at this density. After the feeding period, larvae were pipetted into fresh trays of RO water. To remove any remaining food particles, larvae were rinsed by passing them through two additional trays of water before being added to experimental containers. All experiments used 72 hour old 3rd instar larvae of approximately the same size, and were conducted at 26°C ± 1°C and 80–90% relative humidity with a 12:12 light: dark photoperiod.
Survival of isolated larvae under starvation conditions
We tested the ability of Wolbachia-infected and uninfected larvae to survive starvation conditions in the absence of conspecific larvae, removing any effects of resource competition and also the ability to scavenge on dead larvae. Two independent experiments were conducted; in each, 96 larvae per infection type (see rearing regime) were added individually to wells of Costar 12-well cell culture plates (Corning, Corning, NY) filled with ~4 mL of RO water only. Plates were enclosed in stockings and held in a tray covered with a mesh lid to minimize external sources of food input, and RO water was topped up daily to counter evaporation. For both experiments, wells were monitored for mortality daily until all larvae had died. A larva was considered dead when no movement was observed after fifteen seconds of physical stimulation.
In the first experiment, plates were unmanipulated with the exception of maintaining a consistent volume of water in each well. In the second experiment, water was replaced completely twice per week to reduce the accumulation of microorganisms as a potential source of nutrition (e.g., bacteria, algae, protozoa, fungi) and waste in the water [73]. For this experiment, larvae were removed from wells and rinsed by pipetting through multiple trays of RO water, then returned to wells filled with a fresh change of water.
Survival and development of larvae held in groups under starvation conditions
Two independent experiments tested the ability of Wolbachia-infected and uninfected larvae to survive starvation conditions when held in the presence of conspecific larvae. Larvae (see rearing regime) were added to circular plastic containers (9.5–11.5 cm radius, 7 cm height) with mesh lids and filled with 200 mL of RO water only (no TetraMin was provided). Mortality was scored every second day by temporarily pipetting larvae into a separate container of RO water. Numbers of dead and live larvae were counted before all larvae (including dead larvae) were returned to the original container. Water was refreshed every four days by transferring all larvae to a new container of RO water. In the first experiment, larvae were added to containers in groups of 50. Each container was replicated eight times for the uninfected, wMel, wAlbB and wMelPop strains. The experiment was terminated when all larvae had died or had reached adulthood.
During field releases, preferential mortality of Wolbachia-infected larvae in nutrient-deprived containers could release the remaining larvae from food stress, providing an advantage to uninfected larvae [71,72]. A second experiment was therefore conducted to determine whether there were differences in survival when Wolbachia-infected and uninfected larvae were held together in mixed proportions within the same container. Cohorts of larvae were added to plastic containers filled with 200 mL of RO water in the following proportions (Wolbachia-infected to uninfected): 12:36, 24:24 and 36:12. Additional cohorts of 48 Wolbachia-infected and 48 uninfected larvae were set up as controls. Treatments (mixed proportions) were replicated eight times each, while the controls (pure cohorts) were replicated four times, and the experiment was repeated for the wMel, wAlbB and wMelPop infections. Containers were monitored as per the previous experiment, with the exception that the five longest surviving larvae in each container were removed and screened for their Wolbachia infection status (see DNA extraction and Wolbachia detection). The proportion of individuals infected with Wolbachia in the longest surviving larvae was then compared with the initial proportion of larvae infected with Wolbachia in each container (see statistical analysis).
In both experiments, a few percent of larvae were able to reach the pupal and adult stages due to the availability of dead conspecific larvae as a food resource. All adults emerging throughout the two group experiments were stored in ethanol for wing length measurement and later tested for their Wolbachia infection status (see wing length measurements and DNA extraction and Wolbachia detection). Their development time and sex were also recorded.
Recovery from food deprivation
An experiment was carried out to test the ability of Wolbachia-infected and uninfected larvae to recover from starvation conditions after providing an influx of resources. Larvae (see rearing regime) were added to RO water in groups of 50 (see survival and development of larvae held in groups under starvation conditions). Containers were then divided into two treatments; larvae were re-fed TetraMin ad libitum after either 15 or 25 days of surviving starvation conditions. These two time points were chosen based on when substantial starvation-induced mortality had occurred; approximately 25% and 10% of larvae were remaining on Days 15 and 25 respectively (S1 Fig). For each infection type and treatment, the following observations were recorded: the number of surviving larvae upon the resumption of feeding, rates of pupation and survival to the pupal stage after re-feeding, rates of adult emergence and survival to adulthood, and the body size (see wing length measurements) and sex ratio of emerging adults. Containers were replicated between six and eight times for each infection type and treatment.
Cytoplasmic incompatibility when larvae are food-deprived then re-fed
We ran a series of experiments to determine if the reproductive effects caused by Wolbachia remain robust when larvae are held under starvation conditions for an extended period. To test the level of cytoplasmic incompatibility induced by Wolbachia-infected males, larvae (see rearing regime) were added to containers of RO water and their development was suspended for ~30 days by maintaining them in the absence of TetraMin. After this period larvae were again fed TetraMin ad libitum until pupation. Pupae were sexed (males are smaller than females), and male pupae pipetted into small cups of RO water and allowed to emerge in 1.5 L plastic containers with mesh sides and a stocking lid. Female pupae emerging from this treatment were set aside for an additional experiment on reproductive effects (see fecundity and maternal transmission). After confirming the sex of all males as adults, newly-emerged uninfected females that were reared under standard laboratory conditions (see colony maintenance and mosquito strains) were added to each cage and allowed to mate freely with Wolbachia-infected males. Seven Wolbachia-infected males and seven uninfected females were held in each experimental cage, and crosses were replicated eight times for the wMel, wAlbB and wMelPop infections. Cages of adults were provided access to 10% sucrose solution and water throughout the experiment. Crosses between standard laboratory-reared Wolbachia-infected males and uninfected females were set up as controls, as these crosses are known to produce no viable offspring [21–23]. Females were then blood fed and eggs were collected according to Axford et al. [26] for three gonotrophic cycles.
Maternal transmission and fecundity when larvae are food-deprived then re-fed
This experiment assessed the rate at which Wolbachia-infected females transmit the infection to their offspring when their development time is greatly extended. Food-deprived and re-fed larvae from the wMel, wAlbB and wMelPop lines (see cytoplasmic incompatibility) were sorted by sex, and 100 females per infection type were added to 12 L plastic cages and provided with 10% sucrose solution and a source of water. 100 uninfected males reared under standard laboratory conditions were then aspirated into each cage and allowed to mate freely. Females were then blood fed and isolated for oviposition according to Axford et al. [26], and their progeny reared to adulthood and stored in absolute ethanol.
Ten progeny each from 30 isolated females per infection type were tested for the presence of Wolbachia using PCR to determine maternal transmission efficiency (see DNA extraction and Wolbachia detection). A set of control crosses was also completed for each infection type where both Wolbachia-infected females and uninfected males were reared under standard laboratory conditions. Ten progeny from 15 Wolbachia-infected females were tested for each of the wMel, wAlbB and wMelPop infections. These crosses have expected maternal transmission rates of close to 100% [21–23]. All female parents from the treatments and controls were scored for their fecundity, with a sample also measured for wing length (see wing length measurements). Data from uninfected females reared under standard laboratory conditions from a concurrent experiment were included as a point of comparison.
Wing length measurements
Linear measurements of wings were taken to give an indication of body size [74,75]. The right wing was removed from each adult and fixed on a slide under a 10 mm circular coverslip (Menzel-Gläser, Braunschweig, Germany) using Hoyer’s solution (dH2O: gum arabic: chloral hydrate: glycerin in the ratio 5: 3: 20: 2) [76]. Wings were observed under a dissecting microscope fitted with a camera and measured using NIS-Elements BR (Nikon Instruments, Japan). Wing length was determined by calculating the distance from the alular notch to the intersection of the radius 3 vein and outer margin, excluding the wing fringe scales [77]. Measurements in pixels were converted to millimetres by calibration with a graticule before the start of each set of measurements. Each measurement was repeated independently so that length represented the average of two measurements. Damaged or folded wings were excluded from the analysis.
DNA extraction and Wolbachia detection
To test for the presence of Wolbachia in adult and immature mosquitoes, we carried out DNA extraction and Wolbachia detection according to methods described previously [24,26,78]. DNA from whole adults or larvae was extracted using 150 μL of 5% Chelex 100 resin (Bio-Rad Laboratories, Hercules, CA). The PCR assay was conducted using a LightCycler 480 system (Roche Applied Science, Indianapolis, IN); mosquitoes were considered positive for Wolbachia when the mRpS6 (Aedes universal) and aRpS6 (Ae. aegypti-specific) primer sets were successfully amplified in addition to the appropriate Wolbachia-specific primer set (wMel, wAlbB or wMelPop). Wolbachia-free mosquitoes tested positive for mRpS6 and aRpS6 and negative for all Wolbachia-specific primer sets.
Statistical analysis
All data were analysed using SPSS statistics version 21.0 for Windows (SPSS Inc, Chicago, IL). Survival data were investigated using Kaplan-Meier analysis; log-rank tests compared rates of mortality between lines and treatments. Wolbachia infection frequency was calculated as the proportion of individuals that tested positive for Wolbachia. For containers where both Wolbachia-infected and uninfected larvae were present, deviations from expected infection frequencies in larvae and adults were analysed using Chi-squared tests. Maternal transmission rates of Wolbachia were expressed as the proportion of infected offspring produced by infected mothers, for which 95% binomial confidence intervals were calculated. All other data were tested for normality using Shapiro-Wilk tests. Data that were not normally distributed were arcsine square-root transformed (proportional data) or square-root transformed and tested again. Normally distributed data were then analysed with one-way ANOVA and Tukey’s honest significant difference tests, while data that failed Shapiro-Wilk tests were analysed with non-parametric Kruskal-Wallis and Mann-Whitney U tests. Associations between wing length and development time were assessed with Pearson’s correlation if data were normally distributed or Spearman’s rank-order correlation where data could not be transformed for normality.
Results
Survival of isolated larvae under starvation conditions
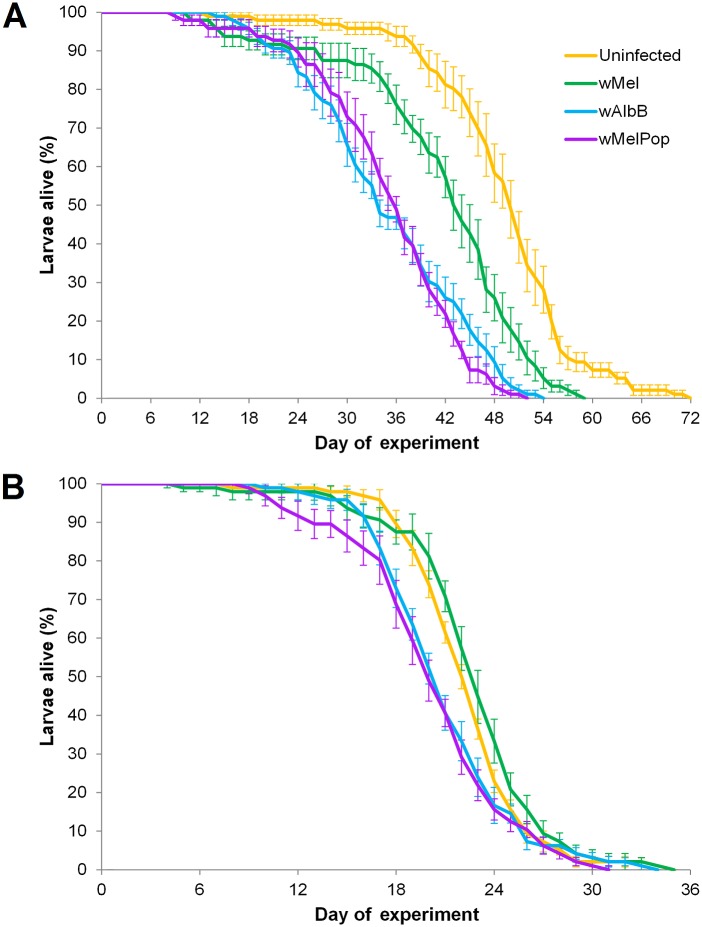
Kaplan-Meier (KM) analysis revealed a significant effect of Wolbachia infection type (KM: χ2 = 123.273, df = 3, P < 0.0001) and water-replacement regime (KM: χ2 = 678.532, df = 1, P < 0.0001) on the survival of larvae when isolated under starvation conditions. Whether water was refreshed in each well or left unmanipulated had a dramatic effect on survival, with the former (mean ± SE = 20.682 ± 0.221 days) reducing the mean survival time of larvae by half compared with unmanipulated experimental wells (40.286 ± 0.573 days, S2 Fig). An increased survival in the latter experiment is likely due to the build-up of microorganisms which are an important resource for mosquito larvae [70,73,79].
When water was not replaced, all three Wolbachia infections reduced survival; the wMel, wAlbB and wMelPop infections decreased mean survival 15.8, 28.8 and 28.7% compared with uninfected larvae (Fig 1A). All pairwise comparisons between the infection types were highly significant (KM: all χ2 > 24.087, df = 1, all P < 0.0001), with the exception that wMelPop and wAlbB did not differ significantly in their survival patterns under starvation conditions (χ2 = 0.717, df = 1, P = 0.397).
Fig 1. Survival of Ae. aegypti larvae when isolated under starvation conditions.
(A) Survival of larvae when there was no manipulation of the wells. (B) Survival of larvae when the water in each well was replaced every four days. Error bars are standard errors. Note that (A) and (B) differ in their x-axis values.
Although there was a significant effect of Wolbachia infection type in both experiments, survival differences between Wolbachia-infected and uninfected larvae were reduced markedly when water was replaced every four days (KM: χ2 = 17.939, df = 3, P = 0.0005) compared with wells that were unmanipulated (χ2 = 150.024, df = 3, P < 0.0001, Fig 1). When water was replaced, all pairwise comparisons between infection types were significant (KM: all χ2 > 4.262, df = 1, all P ≤ 0.039) except for between uninfected and wMel (KM: χ2 = 1.707, df = 1, P = 0.191), and wAlbB and wMelPop (KM: χ2 = 0.630, df = 1, P = 0.427) (Fig 1B). No pupae or adults emerged in either experiment where larvae were isolated.
Survival and development of larvae held in groups under starvation conditions
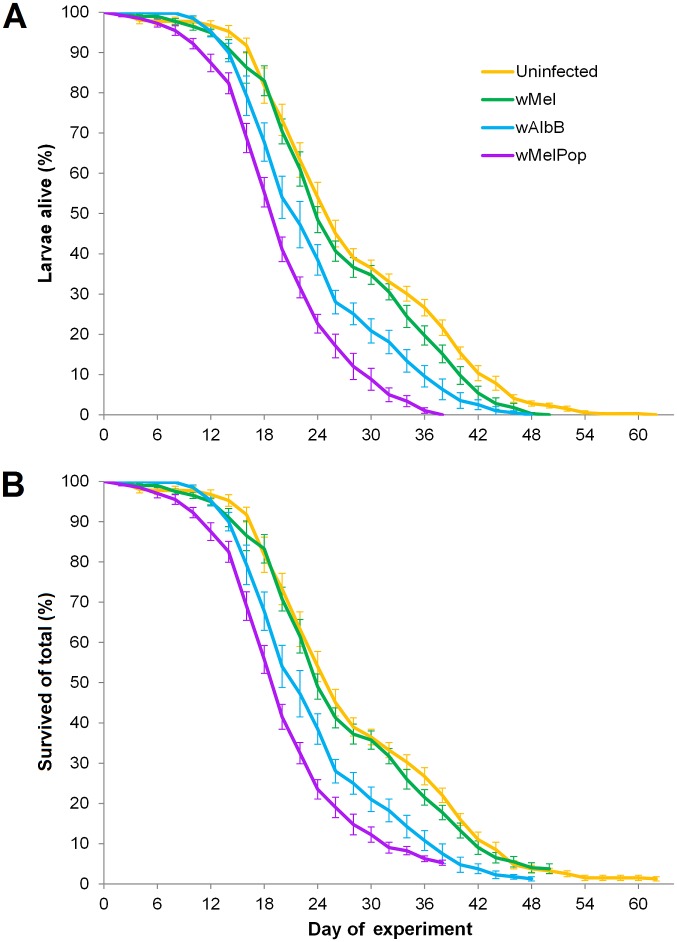
Wolbachia infection type also had a substantial effect on survival when larvae were held under starvation conditions in groups of 50 (KM: χ2 = 225.821, df = 3, P < 0.0001). Uninfected larvae had the greatest mean time of survival (mean ± SE = 28.289 ± 0.532 days), with the wMel, wAlbB and wMelPop infections reducing survival times by 5.7, 15.7 and 29.5% respectively (Fig 2). All pairwise comparisons between lines were significant (KM: all χ2 > 7.411, df = 1, all P ≤ 0.006). Note that emerging adults were excluded from Kaplan-Meier analyses rather than censored because the rate and number of adults emerging differed between infection types.
Fig 2. Survival of Ae. aegypti larvae under starvation conditions in groups of 50 per container.
(A) Shows only larval mortality for each line and excludes those larvae that emerged as adults, while (B) is adjusted so that emerging adults are included in the survivors. Error bars are standard errors.
Larvae from both Wolbachia-infected and uninfected lines readily consumed dead conspecifics throughout the experiment. We inferred scavenging based on observations that the number of dead larvae in each container fluctuated with mortality rather than increasing proportionally (S3 Fig). Distributions of necrophagy closely matched larval mortality, with the mean time for larval consumption occurring less than one day after the mean time of death for both Wolbachia-infected and uninfected lines (S4 Fig). Necrophagy likely contributed to increased survival time; larvae lived for longer in groups compared with larvae kept in isolation under otherwise similar conditions. While survival began to decline earlier in the group experiment, rates of mortality became considerably slower when the majority of larvae had died (S2 Fig).
Less than five percent of larvae reached pupation or adulthood during this experiment (Table 1). Wolbachia infection type had a significant effect on the total number of larvae that survived to both the pupal (one-way ANOVA: F3, 28 = 3.417, P = 0.031) and adult (F3, 28 = 5.647, P = 0.004) stages, and also affected the development times of those pupae (Kruskal-Wallis: χ2 = 31.499, df = 3, P < 0.0001) and adults (χ2 = 14.200, df = 3, P = 0.003). Despite uninfected larvae having greater survival times under starvation conditions (Fig 2), they developed more slowly and pupated less often than Wolbachia-infected larvae, with the wMelPop infection displaying the greatest proportion of larvae reaching adulthood and the most rapid development on average (Table 1, S5 Fig). This observation is likely due to an earlier availability and greater abundance of conspecific carcasses as a source of nutrition in containers with wMelPop-infected larvae.
Table 1. Pupation and adult emergence from Ae. aegypti larvae held under starvation conditions in groups of 50.
| Survival (%) ± SE | Development time (days) ± SE | |||
|---|---|---|---|---|
| Infection type | Pupae* | Adults* | Pupae† | Adults† |
| Uninfected | 2.25 ± 0.35 a | 1.25 ± 0.26 a | 43.33 ± 2.58 a (n = 9) | 43.20 ± 3.93 a (n = 5) |
| wMel | 4.75 ± 0.80 ab | 3.75 ± 0.61 ab | 29.47 ± 3.19 b (n = 19) | 29.47 ± 3.72 ab (n = 15) |
| wAlbB | 3.00 ± 0.50 ab | 1.25 ± 0.26 a | 33.17 ± 0.87 b (n = 12) | 33.20 ± 1.02 b (n = 5) |
| wMelPop | 7.50 ± 0.53 b | 5.25 ± 0.32 b | 25.47 ± 1.45 c (n = 30) | 26.86 ± 1.98 c (n = 21) |
* Within a column, values with the same letter in bold are not significantly different from each other (P > 0.05, by Tukey’s honest significant difference test)
† Within a column, values with the same letter in bold are not significantly different from each other (P > 0.05, by Mann-Whitney U tests on data pooled across replicates)
A second experiment was conducted where Wolbachia-infected and uninfected larvae were held together in the same container under starvation conditions. Control containers, where 48 larvae from each infection type were held separately, had a shorter starved survival period than in the previous experiment despite nearly identical methods, though the relative performance of each infection type was similar (S2 Fig). In each treatment container, the five longest-lived larvae were screened for their infection status to test for differential survival between infected and uninfected larvae when held together at different frequencies. The wAlbB and wMelPop infections were significantly underrepresented in the surviving larvae for all treatments, while for wMel there were no significant deviations from any starting ratio (Table 2).
Table 2. Wolbachia infection frequencies in surviving Ae. aegypti larvae when held at different initial proportions under starvation conditions.
| Observed proportion Wolbachia-infected: uninfected | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Initial proportion Wolbachia-infected: uninfected | wMel: uninfected | wAlbB: uninfected | wMelPop: uninfected | |||||||
| Treatment* | Expected | Observed† | χ2‡ | P§ | Observed† | χ2‡ | P§ | Observed† | χ2‡ | P§ |
| 36:12 | 30:10 | 30:10 | 0 | 1 | 23:17 | 6.53 | 0.011 | 12:28 | 43.20 | < 0.0001 |
| 24:24 | 20:20 | 16:24 | 1.60 | 0.206 | 6:34 | 19.60 | < 0.0001 | 6:34 | 19.60 | < 0.0001 |
| 12:36 | 10:30 | 7:33 | 1.20 | 0.273 | 4:36 | 4.80 | 0.029 | 2:38 | 8.53 | 0.004 |
| Total | 60:60 | 53:67 | 1.63 | 0.201 | 33:87 | 24.30 | < 0.0001 | 20:100 | 53.33 | < 0.0001 |
* Cohorts of larvae were set up with initial ratios of 36:12, 24:24 and 12:36 (Wolbachia-infected: uninfected) and held under starvation conditions until five larvae per container were left alive.
† Observed proportion of Wolbachia-infected: uninfected in the longest five surviving larvae of each container
‡ Chi-squared tests assessed deviations from expected ratios which were based on the initial proportion of Wolbachia-infected larvae in each container. Deviations from an expected 1:1 ratio were also tested when all treatments for each infection type were combined
§ P-values in bold denote significant deviations from expected ratios where all df = 1
Less than two percent of larvae from this experiment emerged as adults. Expected ratios of Wolbachia-infected and uninfected adults emerging were based on the initial proportion of larvae in each container. We found no significant deviations from expected proportions of adults for all treatments (Chi-squared test: all χ2 < 3.267, df = 1, all P > 0.071), except for the wMelPop infection which was significantly underrepresented when larvae were held in the ratio 36:12 (wMelPop: uninfected) (Chi-squared test: χ2 = 24.2, df = 1, P < 0.0001).
All adults that emerged from larvae held in groups were measured for wing length to test for effects on body size. Due to low numbers of adults, data were pooled across both experiments as they did not differ significantly (Student’s t test: P = 0.795). Wing length was not associated with development time for either males (Spearman’s rank-order correlation: ρ = 0.071, P = 0.455, n = 56) or females (ρ = -0.009, P = 0.924, n = 58). As expected, there was a significant effect of sex on wing length (one-way ANOVA: F1,106 = 285.910, P < 0.0001), where males (mean ± SE = 1.659 ± 0.009 mm) were considerably smaller than females (1.973 ± 0.015 mm). However, we found no effect of Wolbachia infection type (one-way ANOVA: F3,106 = 0.360, P = 0.782); wings of mosquitoes with any infection type were approximately the same size (Table 3).
Table 3. Wing lengths of Ae. aegypti adults emerging from groups of larvae held under starvation conditions.
| Wing length (mm) ± SE | ||
|---|---|---|
| Infection type | Males | Females |
| Uninfected | 1.657 ± 0.013 (n = 21) | 1.973 ± 0.024 (n = 24) |
| wMel | 1.649 ± 0.019 (n = 11) | 2.020 ± 0.024 (n = 8) |
| wAlbB | 1.671 ± 0.021 (n = 7) | 1.971 ± 0.042 (n = 10) |
| wMelPop | 1.664 ± 0.020 (n = 17) | 1.950 ± 0.027 (n = 16) |
Data are pooled across experiments where infection types were held both separately and in mixed proportions. No values within a column differed significantly from each other by one-way ANOVA.
Recovery from food deprivation
25.5% and 12.3% of larvae across all infection types survived after 15 and 25 days of exposure to starvation conditions respectively. Wolbachia infection type had a significant effect on the number of larvae surviving after both 15 (one-way ANOVA: F3,56 = 4.152, P = 0.010) and 25 days (F3,26 = 4.114, P = 0.016). The wMelPop infection had the lowest survival at both time points (S1 Fig), consistent with other experiments (Figs 1B and 2).
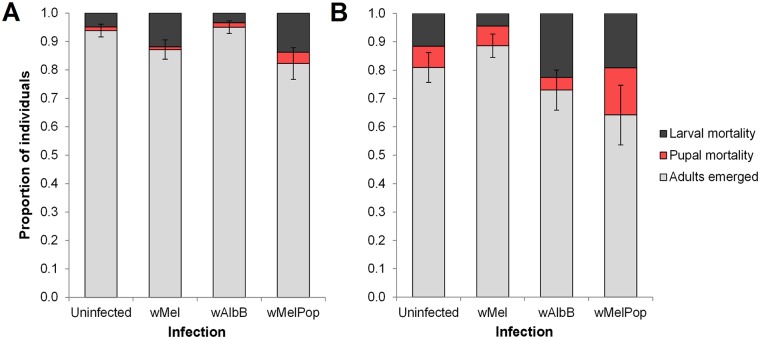
Recovery from food deprivation was assessed by scoring the proportion of surviving larvae that pupated and reached adulthood upon resuming feeding. The majority of surviving larvae were able to recover, though larval and pupal mortality occurred across both treatments for all infection types (Fig 3). We found a significant effect of treatment (day of re-feeding) (one-way ANOVA: F1,52 = 5.576, P = 0.022), but not Wolbachia infection type (F3,52 = 1.461, P = 0.236), on the proportion of surviving larvae that reached adulthood. Surviving larvae that were deprived of food for 25 days were less likely to reach adulthood than larvae deprived for 15 days, with the percentage surviving of larvae that died after re-feeding averaging 10.4% and 22.9% respectively. This is, in part, due to an increase in pupal mortality at the later time point (2.1% for Day 15, 9.0% for Day 25, Student’s t test: P = 0.042, Fig 3). The proportion of surviving larvae that reached adulthood was less for wMelPop than for other infection types, though this difference was not significant (Fig 3). Larvae that reached pupation before re-feeding (33.3% of wMelPop-infected larvae and 3.3% of wMel-infected larvae) were counted as survivors. However, these individuals were excluded from development time and wing length analyses (see below) as they pupated before food was provided again ad libitum, and were similar in size to adults emerging from larvae held in groups under starvation conditions (Table 3).
Fig 3. Proportion of Ae. aegypti larvae developing when fed ad libitum after extended food deprivation.
Larvae were provided with TetraMin ad libitum after (A) 15 and (B) 25 days of food deprivation. Light grey bars denote the proportion of surviving larvae that reached adulthood, while black and red bars correspond to the proportion of larval and pupal mortality respectively. Error bars are standard errors for the proportion of larvae that survived to adulthood. Within treatments, no proportions differed significantly from each other (P > 0.05, by Tukey’s honest significant difference test).
The number of days taken for larvae to reach pupation after re-feeding was significantly affected by infection type (one-way ANOVA: F3, 488 = 5.377, P = 0.001) but not treatment (day of re-feeding) (F1, 488 = 2.128, P = 0.145), though infection types within treatments did not differ significantly from each other (Table 4). Development times of both male and female adults were unaffected by infection type and treatment (one-way ANOVA: all P > 0.053). Female wing length was significantly affected by treatment (one-way ANOVA: F1, 251 = 6.696, P = 0.010) but not infection type (F3, 251 = 1.432, P = 0.234). Females re-fed after 25 days of food deprivation were smaller than those fed after 15 days for all infection types, though no pairwise comparisons were significant (Table 4). Conversely, male wing length was unaffected by both infection type (F3, 194 = 0.844, P = 0.471) and treatment (F1, 194 = 0.032, P = 0.859). We found no correlation between development time and wing length for both males and females for each treatment (Pearson correlation: all P > 0.175).
Table 4. Mean development time and wing length of Ae. aegypti when fed ad libitum after extended food deprivation.
| Development time (days after re-feeding) ± SE | Wing length (mm) ± SE | ||||
|---|---|---|---|---|---|
| Infection type | Pupae | Males | Females | Males | Females |
| Re-fed on Day 15 | |||||
| Uninfected | 4.302 ± 0.042 ab (n = 89) | 6.075 ± 0.063 a (n = 47) | 6.530 ± 0.105 a (n = 33) | 2.192 ± 0.011 a (n = 43) | 2.867 ± 0.022 a (n = 33) |
| wMel | 4.145 ± 0.022 a (n = 109) | 5.877 ± 0.058 a (n = 54) | 6.257 ± 0.074 a (n = 54) | 2.185 ± 0.009 a (n = 54) | 2.838 ± 0.016 a (n = 54) |
| wAlbB | 4.379 ± 0.026 ab (n = 99) | 5.953 ± 0.071 a (n = 38) | 6.612 ± 0.084 a (n = 59) | 2.192 ± 0.013 a (n = 35) | 2.869 ± 0.018 a (n = 58) |
| wMelPop | 4.309 ± 0.055 ab (n = 55) | 6.129 ± 0.142 a (n = 21) | 6.482 ± 0.149 a (n = 32) | 2.161 ± 0.015 a (n = 21) | 2.827 ± 0.022 a (n = 32) |
| Re-fed on Day 25 | |||||
| Uninfected | 4.478 ± 0.089 b (n = 42) | 6.199 ± 0.256 a (n = 11) | 6.883 ± 0.089 a (n = 29) | 2.161 ± 0.020 a (n = 11) | 2.831 ± 0.022 a (n = 29) |
| wMel | 4.264 ± 0.079 ab (n = 55) | 6.140 ± 0.127 a (n = 26) | 6.668 ± 0.086 a (n = 26) | 2.194 ± 0.014 a (n = 25) | 2.788 ± 0.014 a (n = 25) |
| wAlbB | 4.404 ± 0.106 ab (n = 34) | 6.354 ± 0.171 a (n = 8) | 6.632 ± 0.139 a (n = 24) | 2.196 ± 0.029 a (n = 7) | 2.800 ± 0.027 a (n = 24) |
| wMelPop | 4.250 ± 0.204 ab (n = 13) | 5.876 ± 0.281 a (n = 6) | 6.392 ± 0.311 a (n = 4) | 2.169 ± 0.020 a (n = 6) | 2.743 ± 0.084 a (n = 4) |
Larvae were re-fed TetraMin ad libitum after either 15 (top) or 25 (bottom) days of food deprivation. Development time is defined as the number of days taken for larvae to reach pupation or adulthood after re-feeding. Within a column, values with the same letter in bold are not significantly different from each other (P > 0.05, by Tukey’s honest significant difference test).
Cytoplasmic incompatibility, maternal transmission and fecundity when larvae are food-deprived then re-fed
Males deprived of food for 30 days as larvae and then re-fed were tested for their ability to induce cytoplasmic incompatibility when crossed to uninfected females. All food-deprived and re-fed Wolbachia-infected males exhibited complete cytoplasmic incompatibility, with no viable offspring produced across three gonotrophic cycles (Table 5). Control crosses using standard laboratory-reared adults were also completely sterile, with the exception that a low proportion of eggs hatched in the wMelPop control cross due to contamination with uninfected males (Table 5).
Table 5. Percentage of hatching eggs from crosses between Wolbachia-infected males and uninfected female Ae. aegypti.
| Gonotrophic cycle† | |||
|---|---|---|---|
| Cross* | 1 | 2 | 3 |
| Controls‡ | |||
| Uninfected ♀ × wMel ♂ | 0 (248.50 ± 25.67) | 0 (247.38 ± 35.71) | 0 (249.38 ± 41.28) |
| Uninfected ♀ × wAlbB ♂ | 0 (223.75 ± 24.56) | 0 (188.625 ± 37.45) | 0 (272.75 ± 18.25) |
| Uninfected ♀ × wMelPop ♂ | 0.30 (246.88 ± 22.96) | 0.75 (234.63 ±14.30) | 0.23 (221.75 ± 29.13) |
| Treatments§ | |||
| Uninfected ♀ × wMel ♂ | 0 (298.00 ± 53.84) | 0 (258.50 ± 21.18) | 0 (206.88 ± 39.91) |
| Uninfected ♀ × wAlbB ♂ | 0 (272.75 ± 39.88) | 0 (211.00 ± 20.71) | 0 (194.38 ± 37.70) |
| Uninfected ♀ × wMelPop ♂ | 0 (174.88 ± 39.79) | 0 (219.25 ± 34.71) | 0 (184.38 ± 41.13) |
* Eight cages with each containing seven males and seven females were tested per cross. All females were reared under standard laboratory conditions
† Percentage hatch rates across three gonotrophic cycles are given, followed by the mean number of eggs laid per cross in parentheses, with standard errors
‡ Wolbachia-infected males were reared under standard laboratory conditions
§ Wolbachia-infected males were fed ad libitum as larvae for 72 hours, deprived of food for 30 days, then fed ad libitum until pupation
We also tested maternal transmission rates of Wolbachia when infected females were held under starvation conditions for 30 days as larvae and then re-fed. The wMel, wAlbB and wMelPop infections were transmitted with perfect fidelity by both standard laboratory-reared females (All infection types: maternal transmission rate = 1, lower 95% binomial confidence interval = 0.976), and females that were food-deprived then re-fed (All infection types: maternal transmission rate = 1, lower 95% binomial confidence interval = 0.988).
Female parents were also measured for their fecundity and wing length. Both Wolbachia infection type (one-way ANOVA: F3, 227 = 33.011, P < 0.0001) and treatment (F1, 227 = 8.787, P = 0.003) had significant effects on fecundity. The food-deprivation treatment reduced the mean fecundity of wMel, wAlbB and wMelPop-infected females by approximately 5–6 eggs relative to the controls, though no pairwise comparisons were significant (Table 6). All Wolbachia-infected females had considerably reduced fecundity compared with uninfected standard laboratory-reared females, regardless of the rearing treatment (Table 6). Female wing length was also significantly affected by both Wolbachia infection type (one-way ANOVA: F3, 108 = 6.935, P = 0.0003) and treatment (F1, 108 = 8.852, P = 0.004). For all infection types, females held under starvation conditions and then re-fed were smaller than standard laboratory-reared females, though only the wAlbB comparison was significant (Table 6).
Table 6. Average wing length and fecundity of isolated female Ae. aegypti tested for their maternal transmission fidelity.
| Infection type | Feeding regime | Wing length (mm) ± SE* | Fecundity ± SE* |
|---|---|---|---|
| Uninfected | Control† | 2.854 ± 0.018 a (n = 19) | 68.21 ± 2.16 a (n = 39) |
| wMel | Control† | 2.757 ± 0.020 abc (n = 16) | 50.68 ± 3.73 b (n = 22) |
| Treatment‡ | 2.738 ± 0.027 bc (n = 16) | 44.38 ± 2.34 bc (n = 42) | |
| wAlbB | Control† | 2.817 ± 0.019 ab (n = 16) | 50.71 ± 2.46 b (n = 24) |
| Treatment‡ | 2.688 ± 0.022 c (n = 16) | 44.65 ± 1.81 bc (n = 43) | |
| wMelPop | Control† | 2.703 ± 0.021 c (n = 16) | 38.86 ± 1.86 cd (n = 22) |
| Treatment‡ | 2.665 ± 0.042 c (n = 16) | 34.21 ± 1.36 d (n = 42) |
* Within a column, values with the same letter in bold are not significantly different from each other (P > 0.05, by Tukey’s honest significant difference test)
† Wolbachia-infected females were reared under standard laboratory conditions
‡ Wolbachia-infected females were fed ad libitum as larvae for 72 hours, deprived of food for 30 days, then fed ad libitum until pupation
Discussion
We have demonstrated that Wolbachia infection reduces the tolerance of Ae. aegypti larvae to starvation conditions. Because Ae. aegypti larvae survive nutrient-poor conditions primarily by expending their own accumulated energy reserves [59,60], we suspect that Wolbachia reduce survival by increasing the rate at which these reserves are depleted. Wolbachia do not appear to affect the rate at which larvae accumulate reserves because development times are unaffected by infection when larvae are well-fed [26,37,38]. However, when food is limited, Wolbachia may increase the drain on host reserves due to various nutritional requirements [65–68]. Indeed, Wolbachia increase the metabolism of Ae. aegypti adults, at least for the wMelPop infection [42], though this remains to be tested in larvae.
All three infections negatively affected the survival patterns of nutrient-deprived larvae but differed in their severity; wMelPop was highly costly to survival across all experiments, wMel either had a slightly deleterious or no significant effect relative to uninfected larvae, and wAlbB had an intermediate effect. These relative costs are consistent with their effects on mosquito adults and eggs; wMelPop drastically reduces adult lifespan and quiescent egg viability [21,37,38], wMel has relatively minor costs or no detectable effect [22,24], and wAlbB has an intermediate cost to these traits [26]. Here, we demonstrate that infections with higher virulence in these life stages also have greater costs to the survival of larvae under starvation conditions. The differences between Wolbachia infections in terms of their deleterious effects are likely to be attributed to their density in mosquito tissues [80]. High bacterial densities and broad tissue tropisms in host cells are often implicated in increasing fitness costs imposed by Wolbachia infection, both in Ae. aegypti [22,26] and other insects [81–84].
We found that as the survival period of larvae increased, the deleterious effects of Wolbachia became clearer. In adults and eggs of Ae. aegypti, the fitness costs of Wolbachia are also enhanced with age; wMelPop has relatively little cost to the reproductive success of young females, but fecundity [38] and rates of successful probing [39,40] decline severely with subsequent gonotrophic cycles. Additionally, the wAlbB and wMelPop infections impose increased costs on the viability of quiescent eggs over time [26,37]. If these age effects also occur in larvae as suggested by our results, virulent Wolbachia infections could have difficulty invading populations where resources are scarce and thus development times are lengthened.
Adults emerging from starvation conditions were small in size, even in comparison with those produced through extreme crowding or nutrient limitation (e.g. [75,85,86]). Adult sizes were at the lowest end of natural variation found in Australian field populations of Ae. aegypti, from where these mosquitoes were sourced [87,88]. Adult body size reflects the feeding history of larvae after reaching a critical weight [54]; therefore adults emerging from starvation conditions likely obtained only the minimum nutritional reserves required for pupation. In contrast, larvae that were deprived of food for extended durations and then fed ad libitum emerged nearly as large as mosquitoes fed ad libitum throughout development, suggesting that they were able to attain a close approximation of their maximum weight despite the long interruption to feeding [52,53,55,58].
We found that Ae. aegypti larvae, regardless of Wolbachia infection type, recover well from long periods of nutrient deprivation. While the ability of larvae to resume their development has been reported previously [54,56,59], we show that larvae exhibit low mortality, pupate rapidly and emerge at a large size when fed again after being deprived of food for as long as three weeks. In addition, infected males deprived of food as larvae for one month exhibited complete cytoplasmic incompatibility and females transmitted Wolbachia to their offspring with perfect fidelity despite a greatly extended development time. Maternal transmission rates of Wolbachia also remain high when eggs are held in a quiescent state for several weeks [41]. In insects, the maternal transmission efficiency of Wolbachia [35,89–91] and the strength of cytoplasmic incompatibility [36,92–95] are known to be affected by bacterial density. Because environmental factors such as temperature [96–98] and nutrition [68,90,99,100] modulate Wolbachia density, extreme stress in the field could lead to changes in host effects derived from Wolbachia. However, the wMel infection of Ae. aegypti established in Australian field populations has so far remained stable in terms of its reproductive effects, fitness costs and dengue blockage [24,101].
We acknowledge some limitations of our laboratory study that should be addressed in future experiments. We were somewhat limited in our ability to discern any effects of Wolbachia on larval development time and survival to adulthood when held under starvation conditions, due to low pupation rates. Future experiments testing these traits specifically should use larger cohorts with greater replication. Furthermore, we demonstrated the fitness costs of Wolbachia under rather arbitrary and specific scenarios. Nutrient input in the field is dynamic [102], but in this study larvae were fed for a single time period before either being deprived of food completely or re-fed at a later point. Breeding containers in the field are often populated by multiple cohorts [45,103,104], and Suh and Dobson [43] recently reported differential survival of Wolbachia-infected and uninfected 1st instar Ae. aegypti larvae in the presence of later instars. Because predatory behaviour is more likely to occur under nutrient-poor conditions [64], future experiments on survival under starvation conditions should also test interactions between larvae of mixed age classes. Our experiments also were conducted over multiple generations, and while all infection types were outcrossed to an uninfected colony, the number of generations spent in the laboratory varied between experiments. Laboratory adaptation can have substantial effects on fitness [6,105], which could explain why larvae in some experiments had reduced survival under similar conditions (see S2 Fig)
Nevertheless, our study demonstrates consistent deleterious effects of Wolbachia on the survival of Ae. aegypti larvae under starvation conditions. To predict the impact on the invasion dynamics of Wolbachia in highly resource-limited habitats, we estimate changes to the unstable equilibrium frequency, denoted , when this cost to larval viability is considered. For Wolbachia to reach fixation in a population its frequency must reach or exceed ; larger values thus decrease the likelihood and speed of invasion, and will additionally reduce the potential for spatial spread once established in a population [31,106,107].
Based on the mean survival time of larvae under starvation conditions (averaged across all experiments where larvae were held in groups), we estimate the relative fitness of the wMel, wAlbB and wMelPop infections to be 92.3, 81.3 and 68.5% that of uninfected respectively. We detected no significant costs for other traits, thus only the cost to survival patterns under starvation conditions is considered. Following equation 17b of Turelli [108], this produces a of 0.08, 0.19 and 0.32 for wMel, wAlbB and wMelPop respectively in the absence of any other fitness costs, assuming complete cytoplasmic incompatibility and no maternal transmission leakage as indicated by our results. Previous laboratory studies have estimated the fitness costs of the wMel, wAlbB and wMelPop infections to be approximately ~24% [22], ~15% [23,26] and ~43% [37,108] respectively. Using these estimates, increases to 0.30, 0.31 and 0.61 for wMel, wAlbB and wMelPop respectively when both the costs to larval viability under starvation conditions and deleterious effects on other life stages are considered.
In a more extreme scenario, where larvae are deprived of food for 25 days before being provided access to food ad libitum, the invasive potential of Wolbachia decreases further. Assuming Wolbachia-infected larvae are equally as capable of recovering from food deprivation as suggested by our results, the relative fitness of the wMel, wAlbB and wMelPop infections decrease to 90.6, 73.9 and 42.5% that of uninfected respectively. This corresponds to increases of to 0.31, 0.37 and 0.75 when taking into account other fitness costs. The deleterious effects demonstrated here could in part explain why wMelPop was able to establish in semi-field cages [22,41] but has had great difficulty invading wild mosquito populations, both in Australia and Vietnam [50]. In semi-field cages, any costs of Wolbachia infection to larval viability under nutrient stress were likely to be masked by the fact that larvae were relatively well-fed. On the other hand, survival of larvae under starvation conditions was likely to be a critical fitness component in the field releases. The deleterious effects of Wolbachia demonstrated here will, therefore, have an impact on the potential for these infections to invade natural mosquito populations where competition for resources is the major limiting factor of population size, particularly for wMelPop.
Supporting Information
(DOCX)
Points A and B denote when larvae were re-fed TetraMin for the experiment. Survival curves are based on 12–16 replicates for each line until Day 15 and 6–8 replicates after Day 15. Error bars are standard errors.
(TIF)
Larvae of Ae. aegypti were held under starvation conditions in isolation when water was replaced every four days (solid red line) or when water was left unmanipulated (dashed red line). Experiments where larvae were held in groups (grey lines) were conducted under similar conditions (water was replaced), but the mixed cohort (dashed grey line) and recovery (dotted grey line) experiments were conducted at a later time on different generations. Data are averaged across all four infection types. Error bars are standard errors.
(TIF)
Rates of larval mortality are shown by solid lines while the numbers of dead larvae observed are shown by dotted lines. The dotted line being below the solid line suggests that mortality is occurring at a slower rate than the consumption of larvae.
(TIF)
Rates of larval mortality are shown by solid lines while the numbers of dead larvae inferred to be consumed are shown by dashed lines. The delay between distributions of larval mortality and consumption provide an estimate of the rate of necrophagy in group containers. Mean delays between mortality and consumption are as follows: Uninfected, 0.60 days; wMel, 0.32 days; wAlbB, 0.44 days; wMelPop, 0.81 days.
(TIF)
Number of (A) pupae and (B) adults emerging in total from eight containers of 50 larvae for each infection type.
(TIF)
Acknowledgments
The authors thank Jason Axford for experimental design and technical advice.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The research was supported by the National Health and Medical Research Council (1037003, www.nhmrc.gov.au) and the Australian Research Council (FL100100066, www.arc.gov.au). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.WHO (2009) Dengue: guidelines for diagnosis, treatment prevention and control. Geneva: World Health Organization. [PubMed] [Google Scholar]
- 2.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, et al. (2013) The global distribution and burden of dengue. Nature 496: 504–507. 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capeding MR, Tran NH, Hadinegoro SRS, Ismail HIHJM, Chotpitayasunondh T, et al. (2014) Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. The Lancet 384: 1358–1365. [DOI] [PubMed] [Google Scholar]
- 4.Brown D, James A (2014) Dengue vector control: new approaches In: Gubler DJ, Ooi EE, Vasudevan S, Farrar J, editors. Dengue and Dengue Hemorrhagic Fever. 2 ed: CAB International; pp. 519–536. [Google Scholar]
- 5.Franz AWE, Balaraman V, Fraser MJ (2015) Disruption of dengue virus transmission by mosquitoes. Curr Opin Insect Sci 8: 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leftwich PT, Bolton M, Chapman T (2015) Evolutionary biology and genetic techniques for insect control. Evol Appl: 10.1111/eva.12280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffmann AA, Turelli M, Simmons GM (1986) Unidirectional incompatibility between populations of Drosophila simulans. Evolution 40: 692–701. [DOI] [PubMed] [Google Scholar]
- 8.Werren JH, Baldo L, Clark ME (2008) Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 6: 741–751. 10.1038/nrmicro1969 [DOI] [PubMed] [Google Scholar]
- 9.Yen JH, Barr AR (1973) The etiological agent of cytoplasmic incompatibility in Culex pipiens. J Invertebr Pathol 22: 242–250. [DOI] [PubMed] [Google Scholar]
- 10.Tram U, Sullivan W (2002) Role of delayed nuclear envelope breakdown and mitosis in Wolbachia-induced cytoplasmic incompatibility. Science 296: 1124–1126. [DOI] [PubMed] [Google Scholar]
- 11.Panteleev DY, Goryacheva II, Andrianov BV, Reznik NL, Lazebny OE, et al. (2007) The endosymbiotic bacterium Wolbachia enhances the nonspecific resistance to insect pathogens and alters behavior of Drosophila melanogaster. Russ J Genet 43: 1066–1069. [PubMed] [Google Scholar]
- 12.Teixeira L, Ferreira A, Ashburner M (2008) The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol 6: e1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hedges LM, Brownlie JC, O'Neill SL, Johnson KN (2008) Wolbachia and virus protection in insects. Science 322: 702–702. 10.1126/science.1162418 [DOI] [PubMed] [Google Scholar]
- 14.Laven H (1967) Eradication of Culex pipiens fatigans through cytoplasmic incompatibility. Nature 216: 383–384. [DOI] [PubMed] [Google Scholar]
- 15.O'Connor L, Plichart C, Sang AC, Brelsfoard CL, Bossin HC, et al. (2012) Open release of male mosquitoes infected with a Wolbachia biopesticide: field performance and infection containment. PLoS Negl Trop Dis 6: e1797 10.1371/journal.pntd.0001797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curtis CF (1976) Population replacement in Culex fatigans by means of cytoplasmic incompatibility: 2. Field cage experiments with overlapping generations*. Bull World Health Organ 53: 107–119. [PMC free article] [PubMed] [Google Scholar]
- 17.Dobson SL, Marsland EJ, Rattanadechakul W (2002) Mutualistic Wolbachia infection in Aedes albopictus: accelerating cytoplasmic drive. Genetics 160: 1087–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, et al. (2011) Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476: 454–457. 10.1038/nature10356 [DOI] [PubMed] [Google Scholar]
- 19.Kittayapong P, Baisley KJ, Baimai V, O’Neill SL (2000) Distribution and diversity of Wolbachia infections in Southeast Asian mosquitoes (Diptera: Culicidae). J Med Ent 37: 340–345. [DOI] [PubMed] [Google Scholar]
- 20.Popovici J, Moreira LA, Poinsignon A, Iturbe-Ormaetxe I, McNaughton D, et al. (2010) Assessing key safety concerns of a Wolbachia-based strategy to control dengue transmission by Aedes mosquitoes. Mem Inst Oswaldo Cruz 105: 957–964. [DOI] [PubMed] [Google Scholar]
- 21.McMeniman CJ, Lane RV, Cass BN, Fong AW, Sidhu M, et al. (2009) Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science 323: 141–144. 10.1126/science.1165326 [DOI] [PubMed] [Google Scholar]
- 22.Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, et al. (2011) The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 476: 450–453. 10.1038/nature10355 [DOI] [PubMed] [Google Scholar]
- 23.Xi Z, Khoo CC, Dobson SL (2005) Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science 310: 326–328. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann AA, Iturbe-Ormaetxe I, Callahan AG, Phillips BL, Billington K, et al. (2014) Stability of the wMel Wolbachia infection following invasion into Aedes aegypti populations. PLoS Negl Trop Dis 8: e3115 10.1371/journal.pntd.0003115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeap HL, Axford JK, Popovici J, Endersby NM, Iturbe-Ormaetxe I, et al. (2014) Assessing quality of life-shortening Wolbachia-infected Aedes aegypti mosquitoes in the field based on capture rates and morphometric assessments. Parasit Vectors 7: 58 10.1186/1756-3305-7-58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Axford JK, Ross PA, Yeap HL, Callahan AG, Hoffmann AA (2015) Fitness of wAlbB Wolbachia infection in Aedes aegypti: parameter estimates in an outcrossed background and potential for population invasion. Am J Trop Med Hyg: In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, et al. (2009) A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 139: 1268–1278. 10.1016/j.cell.2009.11.042 [DOI] [PubMed] [Google Scholar]
- 28.Bian G, Xu Y, Lu P, Xie Y, Xi Z (2010) The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog 6: e1000833 10.1371/journal.ppat.1000833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turelli M, Hoffmann AA (1991) Rapid spread of an inherited incompatibility factor in California Drosophila. Nature 353: 440–442. [DOI] [PubMed] [Google Scholar]
- 30.Kriesner P, Hoffmann AA, Lee SF, Turelli M, Weeks AR (2013) Rapid sequential spread of two Wolbachia variants in Drosophila simulans. PLoS Pathog 9: e1003607 10.1371/journal.ppat.1003607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caspari E, Watson G (1959) On the evolutionary importance of cytoplasmic sterility in mosquitoes. Evolution 13: 568–570. [Google Scholar]
- 32.Brownstein JS, Hett E, O’Neill SL (2003) The potential of virulent Wolbachia to modulate disease transmission by insects. J Invertebr Pathol 84: 24–29. [DOI] [PubMed] [Google Scholar]
- 33.Crain PR, Mains JW, Suh E, Huang Y, Crowley PH, et al. (2011) Wolbachia infections that reduce immature insect survival: predicted impacts on population replacement. BMC Evol Biol 11: 290 10.1186/1471-2148-11-290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Min K-T, Benzer S (1997) Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc Natl Acad Sci U S A 94: 10792–10796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGraw EA, Merritt DJ, Droller JN, O'Neill SL (2002) Wolbachia density and virulence attenuation after transfer into a novel host. Proc Natl Acad Sci U S A 99: 2918–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McMeniman CJ, Lane AM, Fong AW, Voronin DA, Iturbe-Ormaetxe I, et al. (2008) Host adaptation of a Wolbachia strain after long-term serial passage in mosquito cell lines. Appl Environ Microbiol 74: 6963–6969. 10.1128/AEM.01038-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeap HL, Mee P, Walker T, Weeks AR, O'Neill SL, et al. (2011) Dynamics of the "popcorn" Wolbachia infection in outbred Aedes aegypti informs prospects for mosquito vector control. Genetics 187: 583–595. 10.1534/genetics.110.122390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McMeniman CJ, O'Neill SL (2010) A virulent Wolbachia infection decreases the viability of the dengue vector Aedes aegypti during periods of embryonic quiescence. PLoS Negl Trop Dis 4: e748 10.1371/journal.pntd.0000748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turley AP, Moreira LA, O'Neill SL, McGraw EA (2009) Wolbachia infection reduces blood-feeding success in the dengue fever mosquito, Aedes aegypti. PLoS Neglected Tropical Diseases 3: e516 10.1371/journal.pntd.0000516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moreira LA, Saig E, Turley AP, Ribeiro JM, O'Neill SL, et al. (2009) Human probing behavior of Aedes aegypti when infected with a life-shortening strain of Wolbachia. PLoS Negl Trop Dis 3: e568 10.1371/journal.pntd.0000568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ritchie SA, Townsend M, Paton CJ, Callahan AG, Hoffmann AA (2015) Application of wMelPop Wolbachia strain to crash local populations of Aedes aegypti. PLoS Negl Trop Dis 9: e0003930 10.1371/journal.pntd.0003930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evans O, Caragata EP, McMeniman CJ, Woolfit M, Green DC, et al. (2009) Increased locomotor activity and metabolism of Aedes aegypti infected with a life-shortening strain of Wolbachia pipientis. J Exp Biol 212: 1436–1441. 10.1242/jeb.028951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suh E, Dobson SL (2013) Reduced competitiveness of Wolbachia infected Aedes aegypti larvae in intra- and inter-specific immature interactions. J Invertebr Pathol 114: 173–177. 10.1016/j.jip.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ross PA, Endersby NM, Yeap HL, Hoffmann AA (2014) Larval competition extends developmental time and decreases adult size of wMelPop Wolbachia-infected Aedes aegypti. Am J Trop Med Hyg 91: 198–205. 10.4269/ajtmh.13-0576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Southwood T, Murdie G, Yasuno M, Tonn RJ, Reader P (1972) Studies on the life budget of Aedes aegypti in Wat Samphaya, Bangkok, Thailand. Bull World Health Organ 46: 211–226. [PMC free article] [PubMed] [Google Scholar]
- 46.Subra R, Mouchet J (1984) The regulation of preimaginal populations of Aedes aegypti (L.)(Diptera: Culicidae) on the Kenya coast. II. Food as a main regulatory factor. Ann Trop Med Parasitol 78: 63–70. [DOI] [PubMed] [Google Scholar]
- 47.Arrivillaga J, Barrera R (2004) Food as a limiting factor for Aedes aegypti in water-storage containers. J Vector Ecol 29: 11–20. [PubMed] [Google Scholar]
- 48.Barrera R, Amador M, Clark GG (2006) Ecological factors influencing Aedes aegypti (Diptera: Culicidae) productivity in artificial containers in Salinas, Puerto Rico. J Med Ent 43: 484–492. [DOI] [PubMed] [Google Scholar]
- 49.Couret J, Benedict MQ (2014) A meta-analysis of the factors influencing development rate variation in Aedes aegypti (Diptera: Culicidae). BMC Ecol 14: 3 10.1186/1472-6785-14-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nguyen TH, Le Nguyen H, Nguyen TY, Vu SN, Tran ND, et al. (2015) Field evaluation of the establishment potential of wMelPop Wolbachia in Australia and Vietnam for dengue control. Parasit Vectors: In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barrera R, Medialdea V (1996) Development time and resistance to starvation of mosquito larvae. J Nat Hist 30: 447–458. [Google Scholar]
- 52.Chambers G, Klowden M (1990) Correlation of nutritional reserves with a critical weight for pupation in larval Aedes aegypti mosquitoes. J Am Mosq Control Assoc 6: 394–399. [PubMed] [Google Scholar]
- 53.Lan Q, Grier CA (2004) Critical period for pupal commitment in the yellow fever mosquito, Aedes aegypti. J Insect Physiol 50: 667–676. [DOI] [PubMed] [Google Scholar]
- 54.Telang A, Frame L, Brown MR (2007) Larval feeding duration affects ecdysteroid levels and nutritional reserves regulating pupal commitment in the yellow fever mosquito Aedes aegypti (Diptera: Culicidae). J Exp Biol 210: 854–864. [DOI] [PubMed] [Google Scholar]
- 55.Nishiura JT, Burgos C, Aya S, Goryacheva Y, Lo W (2007) Modulation of larval nutrition affects midgut neutral lipid storage and temporal pattern of transcription factor expression during mosquito metamorphosis. J Insect Physiol 53: 47–58. [DOI] [PubMed] [Google Scholar]
- 56.Rasnitsyn S, Yasyukevich V (1989) On the ability of mosquito larvae (Diptera, Culicidae) to endure starvation. Entomol Rev 68: 143–151. [Google Scholar]
- 57.Barrera R (1996) Competition and resistance to starvation in larvae of container-inhabiting Aedes mosquitoes. Ecol Entomol 21: 117–127. [Google Scholar]
- 58.Padmanabha H, Lord CC, Lounibos LP (2011) Temperature induces trade-offs between development and starvation resistance in Aedes aegypti (L.) larvae. Med Vet Entomol 25: 445–453. 10.1111/j.1365-2915.2011.00950.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wigglesworth V (1942) The storage of protein, fat, glycogen and uric acid in the fat body and other tissues of mosquito larvae. J Exp Biol 19: 56–77. [Google Scholar]
- 60.Gilpin ME, McClelland G (1979) Systems analysis of the yellow fever mosquito Aedes aegypti. Fortschr Zool 25: 355 [PubMed] [Google Scholar]
- 61.Perez MH, Noriega FG (2012) Aedes aegypti pharate 1st instar quiescence affects larval fitness and metal tolerance. J Insect Physiol 58: 824–829. 10.1016/j.jinsphys.2012.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Daugherty MP, Alto BW, Juliano SA (2000) Invertebrate carcasses as a resource for competing Aedes albopictus and Aedes aegypti (Diptera: Culicidae). J Med Ent 37: 364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bara J, Clark T, Remold S (2014) Utilization of larval and pupal detritus by Aedes aegypti and Aedes albopictus. J Vector Ecol 39: 44–47. 10.1111/j.1948-7134.2014.12068.x [DOI] [PubMed] [Google Scholar]
- 64.Edgerly J, Willey M, Livdahl T (1999) Intraguild predation among larval treehole mosquitoes, Aedes albopictus, Ae. aegypti, and Ae. triseriatus (Diptera: Culicidae), in laboratory microcosms. J Med Ent 36: 394–399. [DOI] [PubMed] [Google Scholar]
- 65.Wu M, Sun LV, Vamathevan J, Riegler M, Deboy R, et al. (2004) Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: a streamlined genome overrun by mobile genetic elements. PLoS Biol 2: E69–E69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Caragata EP, Rances E, Hedges LM, Gofton AW, Johnson KN, et al. (2013) Dietary cholesterol modulates pathogen blocking by Wolbachia. PLoS Pathog 9: e1003459 10.1371/journal.ppat.1003459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caragata EP, Rances E, O'Neill SL, McGraw EA (2014) Competition for amino acids between Wolbachia and the mosquito host, Aedes aegypti. Microb Ecol 67: 205–218. 10.1007/s00248-013-0339-4 [DOI] [PubMed] [Google Scholar]
- 68.Ponton F, Wilson K, Holmes A, Raubenheimer D, Robinson KL, et al. (2015) Macronutrients mediate the functional relationship between Drosophila and Wolbachia. Proc Biol Sci 282: 20142029 10.1098/rspb.2014.2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kitching RL (2000) Food webs and container habitats: the natural history and ecology of phytotelmata: Cambridge University Press. [Google Scholar]
- 70.Yee DA, Allgood D, Kneitel JM, Kuehn KA (2012) Constitutive differences between natural and artificial container mosquito habitats: vector communities, resources, microorganisms, and habitat parameters. J Med Ent 49: 482–491. [DOI] [PubMed] [Google Scholar]
- 71.Agudelo-Silva F, Spielman A (1984) Paradoxical effects of simulated larviciding on production of adult mosquitoes. Am J Trop Med Hyg 33: 1267–1269. [DOI] [PubMed] [Google Scholar]
- 72.Wilson ML, Agudelo-Silva F, Spielman A (1990) Increased abundance, size, and longevity of food-deprived mosquito populations exposed to a fungal larvicide. Am J Trop Med Hyg 43: 551–556. [DOI] [PubMed] [Google Scholar]
- 73.Yee DA, Kesavaraju B, Juliano SA (2007) Direct and indirect effects of animal detritus on growth, survival, and mass of invasive container mosquito Aedes albopictus (Diptera: Culicidae). J Med Ent 44: 580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nasci RS (1990) Relationship of wing length to adult dry weight in several mosquito species (Diptera: Culicidae). J Med Ent 27: 716–719. [DOI] [PubMed] [Google Scholar]
- 75.Briegel H (1990) Metabolic relationship between female body size, reserves, and fecundity of Aedes aegypti. J Insect Physiol 36: 165–172. [Google Scholar]
- 76.Anderson LE (1954) Hoyer's solution as a rapid permanent mounting medium for bryophytes. Bryologist 7: 242–244. [Google Scholar]
- 77.Huestis DL, Yaro AS, Traore AI, Adamou A, Kassogue Y, et al. (2011) Variation in metabolic rate of Anopheles gambiae and A. arabiensis in a Sahelian village. J Exp Biol 214: 2345–2353. 10.1242/jeb.054668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee SF, White VL, Weeks AR, Hoffmann AA, Endersby NM (2012) High-throughput PCR assays to monitor Wolbachia infection in the dengue mosquito (Aedes aegypti) and Drosophila simulans. Appl Environ Microbiol 78: 4740–4743. 10.1128/AEM.00069-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Merritt R, Dadd R, Walker E (1992) Feeding behavior, natural food, and nutritional relationships of larval mosquitoes. Annu Rev Entomol 37: 349–374. [DOI] [PubMed] [Google Scholar]
- 80.Hoffmann AA, Ross PA, Rašić G (2015) Wolbachia strains for disease control: ecological and evolutionary considerations. Evol Appl 8: 751–768. 10.1111/eva.12286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mouton L, Dedeine F, Henri H, Bouletreau M, Profizi N, et al. (2004) Virulence, multiple infections and regulation of symbiotic population in the Wolbachia-Asobara tabida symbiosis. Genetics 168: 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Duron O, Labbé P, Berticat C, Rousset F, Guillot S, et al. (2006) High Wolbachia density correlates with cost of infection for insecticide resistant Culex pipiens mosquitoes. Evolution 60: 303–314. [PubMed] [Google Scholar]
- 83.Chrostek E, Teixeira L (2015) Mutualism breakdown by amplification of Wolbachia genes. PLoS Biol 13: e1002065 10.1371/journal.pbio.1002065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martinez J, Ok S, Smith S, Snoeck K, Day JP, et al. (2015) Should symbionts be nice or selfish? Antiviral effects of Wolbachia are costly but reproductive parasitism is not. PLoS Pathog 11: e1005021 10.1371/journal.ppat.1005021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Farjana T, Tuno N (2012) Effect of body size on multiple blood feeding and egg retention of Aedes aegypti (L.) and Aedes albopictus (Skuse)(Diptera: Culicidae). Med Entomol Zool 63: 123–131. [Google Scholar]
- 86.Ponlawat A, Harrington LC (2007) Age and body size influence male sperm capacity of the dengue vector Aedes aegypti (Diptera: Culicidae). J Med Ent 44: 422–426. [DOI] [PubMed] [Google Scholar]
- 87.Tun‐Lin W, Burkot T, Kay B (2000) Effects of temperature and larval diet on development rates and survival of the dengue vector Aedes aegypti in north Queensland, Australia. Med Vet Entomol 14: 31–37. [DOI] [PubMed] [Google Scholar]
- 88.Yeap HL, Endersby NM, Johnson PH, Ritchie SA, Hoffmann AA (2013) Body size and wing shape measurements as quality indicators of Aedes aegypti mosquitoes destined for field release. Am J Trop Med Hyg 89: 78–92. 10.4269/ajtmh.12-0719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kittayapong P, Baisley KJ, Sharpe RG, Baimai V, O'Neill SL (2002) Maternal transmission efficiency of Wolbachia superinfections in Aedes albopictus populations in Thailand. Am J Trop Med Hyg 66: 103–107. [DOI] [PubMed] [Google Scholar]
- 90.Dutton TJ, Sinkins SP (2004) Strain‐specific quantification of Wolbachia density in Aedes albopictus and effects of larval rearing conditions. Insect Mol Biol 13: 317–322. [DOI] [PubMed] [Google Scholar]
- 91.Unckless RL, Boelio LM, Herren JK, Jaenike J (2009) Wolbachia as populations within individual insects: causes and consequences of density variation in natural populations. Proc Biol Sci 276: 2805–2811. 10.1098/rspb.2009.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Boyle L, O'Neill SL, Robertson HM, Karr TL (1993) Interspecific and intraspecific horizontal transfer of Wolbachia in Drosophila. Science 260: 1796–1799. [DOI] [PubMed] [Google Scholar]
- 93.Clancy DJ, Hoffmann AA (1998) Environmental effects on cytoplasmic incompatibility and bacterial load in Wolbachia‐infected Drosophila simulans. Entomol Exp Appl 86: 13–24. [Google Scholar]
- 94.Veneti Z, Clark ME, Zabalou S, Karr TL, Savakis C, et al. (2003) Cytoplasmic incompatibility and sperm cyst infection in different Drosophila-Wolbachia associations. Genetics 164: 545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jaenike J (2009) Coupled population dynamics of endosymbionts within and between hosts. Oikos 118: 353–362. [Google Scholar]
- 96.Mouton L, Henri H, Bouletreau M, Vavre F (2006) Effect of temperature on Wolbachia density and impact on cytoplasmic incompatibility. Parasitology 132: 49–56. [DOI] [PubMed] [Google Scholar]
- 97.Wiwatanaratanabutr S, Kittayapong P (2006) Effects of temephos and temperature on Wolbachia load and life history traits of Aedes albopictus. Med Vet Entomol 20: 300–307. [DOI] [PubMed] [Google Scholar]
- 98.Murdock CC, Blanford S, Hughes GL, Rasgon JL, Thomas MB (2014) Temperature alters Plasmodium blocking by Wolbachia. Sci Rep 4: 3932 10.1038/srep03932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Correa CC, Ballard JW (2014) What can symbiont titres tell us about co-evolution of Wolbachia and their host? J Invertebr Pathol 118: 20–27. 10.1016/j.jip.2014.02.009 [DOI] [PubMed] [Google Scholar]
- 100.Serbus LR, White PM, Silva JP, Rabe A, Teixeira L, et al. (2015) The impact of host diet on Wolbachia titer in Drosophila. PLoS Pathog 11: e1004777 10.1371/journal.ppat.1004777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Frentiu FD, Zakir T, Walker T, Popovici J, Pyke AT, et al. (2014) Limited dengue virus replication in field-collected Aedes aegypti mosquitoes infected with Wolbachia. PLoS Negl Trop Dis 8: e2688 10.1371/journal.pntd.0002688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yee DA, Juliano SA (2012) Concurrent effects of resource pulse amount, type, and frequency on community and population properties of consumers in detritus-based systems. Oecologia 169: 511–522. 10.1007/s00442-011-2209-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Walsh R, Facchinelli L, Ramsey J, Bond J, Gould F (2011) Assessing the impact of density dependence in field populations of Aedes aegypti. J Vector Ecol 36: 300–307. 10.1111/j.1948-7134.2011.00170.x [DOI] [PubMed] [Google Scholar]
- 104.Walsh RK, Aguilar CL, Facchinelli L, Valerio L, Ramsey JM, et al. (2013) Regulation of Aedes aegypti population dynamics in field systems: quantifying direct and delayed density dependence. Am J Trop Med Hyg 89: 68–77. 10.4269/ajtmh.12-0378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Koenraadt CJ, Kormaksson M, Harrington LC (2010) Effects of inbreeding and genetic modification on Aedes aegypti larval competition and adult energy reserves. Parasit Vectors 3: 92 10.1186/1756-3305-3-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Turelli M, Hoffmann A (1999) Microbe‐induced cytoplasmic incompatibility as a mechanism for introducing transgenes into arthropod populations. Insect Mol Biol 8: 243–255. [DOI] [PubMed] [Google Scholar]
- 107.Barton NH, Turelli M (2011) Spatial waves of advance with bistable dynamics: cytoplasmic and genetic analogues of allee effects. Am Nat 178: E48–E75. 10.1086/661246 [DOI] [PubMed] [Google Scholar]
- 108.Turelli M (2010) Cytoplasmic incompatibility in populations with overlapping generations. Evolution 64: 232–241. 10.1111/j.1558-5646.2009.00822.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Points A and B denote when larvae were re-fed TetraMin for the experiment. Survival curves are based on 12–16 replicates for each line until Day 15 and 6–8 replicates after Day 15. Error bars are standard errors.
(TIF)
Larvae of Ae. aegypti were held under starvation conditions in isolation when water was replaced every four days (solid red line) or when water was left unmanipulated (dashed red line). Experiments where larvae were held in groups (grey lines) were conducted under similar conditions (water was replaced), but the mixed cohort (dashed grey line) and recovery (dotted grey line) experiments were conducted at a later time on different generations. Data are averaged across all four infection types. Error bars are standard errors.
(TIF)
Rates of larval mortality are shown by solid lines while the numbers of dead larvae observed are shown by dotted lines. The dotted line being below the solid line suggests that mortality is occurring at a slower rate than the consumption of larvae.
(TIF)
Rates of larval mortality are shown by solid lines while the numbers of dead larvae inferred to be consumed are shown by dashed lines. The delay between distributions of larval mortality and consumption provide an estimate of the rate of necrophagy in group containers. Mean delays between mortality and consumption are as follows: Uninfected, 0.60 days; wMel, 0.32 days; wAlbB, 0.44 days; wMelPop, 0.81 days.
(TIF)
Number of (A) pupae and (B) adults emerging in total from eight containers of 50 larvae for each infection type.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.