Abstract
The gaseous phytohormone ethylene participates in the regulation of root growth and development in Arabidopsis. It is known that root growth inhibition by ethylene involves auxin, which is partially mediated by the action of the WEAK ETHYLENE INSENSITIVE2/ANTHRANILATE SYNTHASE α1 (WEI2/ASA1), encoding a rate-limiting enzyme in tryptophan (Trp) biosynthesis, from which auxin is derived. However, the molecular mechanism by which ethylene decreases root growth via ASA1 is not understood. Here we report that the ethylene-responsive AP2 transcription factor, ETHYLENE RESPONSE FACTOR1 (ERF1), plays an important role in primary root elongation of Arabidopsis. Using loss- and gain-of-function transgenic lines as well as biochemical analysis, we demonstrate that ERF1 can directly up-regulate ASA1 by binding to its promoter, leading to auxin accumulation and ethylene-induced inhibition of root growth. This discloses one mechanism linking ethylene signaling and auxin biosynthesis in Arabidopsis roots.
Author Summary
Ethylene is a gaseous phytohormone that plays critical roles in plant development and defense. It is well known that ethylene inhibits primary root elongation through effects on auxin. However, it is not clear how ethylene signal is translated into auxin. In this report, the highly ethylene-responsive transcription factor ETHYLENE RESPONSE FACTOR1 (ERF1) is demonstrated to positively regulate ASA1, encoding ANTHRANILATE SYNTHASE α1, a rate-limiting enzyme in Trp biosynthesis where auxin is derived, by directly binding to its promoter and activating ASA1. Consequently, auxin biosynthesis is promoted, leading to increased auxin accumulation and thus inhibition of primary root elongation. This study unravels a molecular mechanism that bridges ethylene signaling and auxin biosynthesis in primary root elongation.
Introduction
Phytohormones are central regulators of plant root growth and development. Each root development process is determined by a network of interacting signals to give the final architecture of the root[1]. Ethylene and auxin have been shown to regulate some of the same developmental processes, including primary root elongation[2–4]. Although the crosstalk between ethylene and auxin in regulating primary root elongation is well characterized[3,5–9], there is still a significant lack of understanding of the molecular mechanism that links ethylene signaling and auxin biosynthesis.
Ethylene, a gaseous plant hormone, acts as a key regulatory signal during the plant life cycle[10,11]. The biosynthesis of ethylene begins from methionine, which is converted to S-adenosyl-methionine (SAM) by SAM synthetase. Then a family of 1-aminocyclopropane-1-carboxylic acid (ACC) synthases (ACS) converts SAM to ACC. This reaction is a rate-limiting step and a key regulatory point in ethylene biosynthesis[12]. Finally, ACC is converted to ethylene by ACC oxidase (ACO)[13,14]. In the Arabidopsis genome, there are 12 members in ACS gene family, which display overlapping temporal and spatial expression patterns and are responsive to a variety of biotic and abiotic stresses and hormones, such as auxin[15,16]. Apparently all major components of ethylene signal transduction have been identified by the successful isolation of a series of ethylene response mutants and a precise ethylene signaling pathway has been established[17–21]. Once ethylene is synthesized, it is perceived by any of five membrane bound protein receptors ETHYLENE RESPONSE1 (ETR1), ETR2, ETHYLENERESPONSE SENSOR1 (ERS1), ERS2, and ETHYLENEINSENSITIVE4 (EIN4), which possess sequence similarity to bacterial two-component His kinases[22–24]. The binding of ethylene to its receptor results in inhibition of a Raf-like Ser/Thr protein kinase CONSTITUTIVE TRIPLE RESPONSE1(CTR1)[25]. Inhibited CTR1 loses its ability to phosphorylate and repress a positive component of the ethylene signal pathway, the membrane protein ETHYLENE INSENSITIVE2 (EIN2)[26]. The active form of EIN2 stabilises the transcription factors of the EIN3 family located in the nucleus. The EIN3 proteins subsequently bind to the promoters of the ERF genes and activate their transcription[27,28]. Thus a transcriptional cascade commencing with the sensing of ethylene is triggered to produce the ethylene response.
ERFs, which contain an AP2 DNA-binding domain, form a plant-specific superfamily of 122 transcriptional factors in Arabidopsis[29]. ERFs influence a variety of functions involved in plant development and also play important roles in response to biotic and abiotic stresses[30–34], through specifically binding to sequences containing GCCGCC motifs (GCC-box) in the regulatory region of downstream genes[35]. It was reported that GCC-box is not well conserved in ethylene responsive genes, suggesting that other types of transcription factors may also be activated by EIN3 and involve in transcriptional cascade caused by ethylene[7]. ERF1 (AT3G23240) is a downstream component of the ethylene signaling pathway and is directly regulated by EIN3 at the transcriptional level[27]. It is well known that ERF1 is a key integrator of the jasmonic acid (JA) and ethylene signaling pathways involved in the regulation of defence response genes such as b-CHI and PDF1.2[36]. Ethylene signaling is also involved in plant responses to both salt and water stress, as ethylene insensitive mutants were more salt sensitive[37–39]. ERF1 also plays a positive role in abiotic stress responses such as salt, drought, and heat stress[40]. In addition to responding to biotic and abiotic stress, ERF1 further mediates ethylene responses in developmental processes, such as the inhibition of primary root growth and hypocotyl elongation in the dark. This has been confirmed by the production of transgenic plants with constitutively activated ERF1, which displayed phenotypes similar to that are observed in ctr1 mutant, EIN3-overexpressing plants, and wild-type plants treated with ethylene[25,27,41]. Recently, ERF109 was shown to mediate crosstalk between JA signaling and auxin biosynthesis[42].
Root growth relies on two essential developmental processes: cell division in the root meristem and elongation of cells produced by the root meristem[43]. Root cell elongation can be affected by diverse endogenous and exogenous factors such as ethylene[3], auxin[44], and calcium[45]. Ethylene, and its precursor ACC, reduces root elongation in a concentration-dependent manner by inhibition of the cell elongation process[4,6]. The crosstalk between ethylene and auxin has been well investigated[3,6]. The most interesting discovery for auxin/ethylene crosstalk in recent years is that Arabidopsis pyridoxal-phosphate -dependent aminotransferase, VAS1, uses the ethylene biosynthetic intermediate methionine as an amino donor and the auxin biosynthetic intermediate indole-3-pyruvic acid as an amino acceptor to produce L-tryptophan and 2-oxo-4-methylthiobutyric acid[46]. Many mutants that affect auxin synthesis, distribution, or signaling also result in abnormal responses to ethylene[8,47–50], such as, mutants of AUX1 and EIR1/AGR/PIN2 involved in auxin transport, AXR2/IAA7 and AXR3/IAA17 in the auxin signal pathway, or the auxin receptor TIR1, which all exhibit ethylene-insensitive root growth[5,20,49,51–53]. Auxin biosynthetic genes encoding enzymes such as WEI2/ ANTHRANILATE SYNTHASE α1 (ASA1), WEI7/ASB1, TAA1, and TAR1, which are regulated by ethylene, also exhibit ethylene-insensitive root growth[8,9]. YUC genes also play an important role in root responses to ethylene[54]. These studies suggest that the inhibition of primary root growth caused by ethylene requires auxin biosynthesis, transport, or signaling. WEI2 encodes the α-subunit of the enzyme anthranilate synthase in Trp-dependent auxin biosynthesis. Its expression in roots can be induced by ethylene and wei2 mutations cause ethylene-insensitive root growth phenotypes[8]. In Arabidopsis roots, ethylene promotes auxin biosynthesis in a ASA1-dependent manner[8], this is an important molecular mechanism by which ethylene exerts its effect on promoting auxin biosynthesis. However, the molecular mechanism for regulation of ASA1 by ethylene is not well understood.
Here, we report that ERF1, a downstream AP2 transcription factor in the ethylene signaling pathway, positively regulates auxin biosynthesis during inhibition of ethylene-mediated primary root growth. Transgenic plants with constitutive expression or knockdown of ERF1 displayed similar root development phenotype to mutants of ethylene signaling. ERF1 affected auxin accumulation through directly binding to the ASA1 promoter and positively regulating ASA1 expression. Our results indicate that ERF1 plays a pivotal role in the inhibition of ethylene-induced primary root growth in Arabidopsis and acts as the crosstalk node between ethylene and auxin in primary root elongation.
Results
The expression of ERF1 is responsive to ethylene and dependent on ethylene signaling
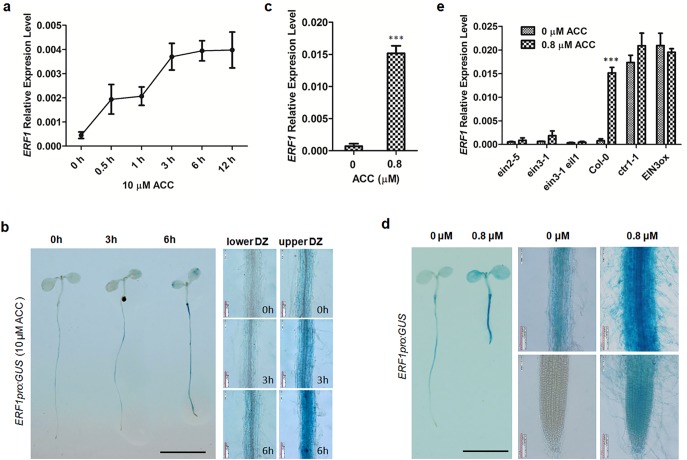
To study the role of ERF1 in ethylene response, we examined the ethylene-induced expression of ERF1 in 5-day-old wildtype seedlings (Col-0) grown on Murashige and Skoog (MS) medium with 10 μM ACC for 0–12 h. The ERF1 transcript level in whole seedlings was measured by quantitative real-time PCR (qRT-PCR). Consistent with previous studies[36,40], the ERF1 transcript level was rapidly induced by ACC treatment. ERF1 increased in a biphasic manner showing a 4-fold increase after 0.5 h, and a second plateau increasing by approximately 8-fold at 3 h. Thereafter, the ERF1 transcript levels remained high (Fig 1A).
Fig 1. ERF1 expression is responsive to ethylene.
(a) Ethylene-induced ERF1 expression in wildtype. Seeds of Col-0 were germinated on MS medium for 5 d then treated with 10 μM ACC for 0, 0.5, 1, 3, 6, and 12 h. The transcriptional level of ERF1 was detected by quantitative RT-PCR (qRT-PCR). Values are mean ± SD of three replicates. ACC, 1-aminocyclopropane-1-carboxylic acid (precursor of ethylene biosynthesis). (b) Ethylene-induced expression of ERF1pro:GUS. Five-day-old seedlings of transgenic lines of were treated with 10 μM ACC for 0, 3, and 6 h before GUS staining. Upper DZ and lower DZ represent different primary root regions. Scale bar, 0.5 cm. (c) Ethylene-induced expression of ERF1 in wildtype. Seeds of Col-0 were germinated on MS medium with 0 or 0.8 μM ACC for 5 d, and relative ERF1 transcription levels measured by qRT-PCR. Values are mean ± SD of three replicates (***P<0.001). Asterisks indicate Student’s t-test significant differences. (d) Ethylene-activated expression in ERF1pro:GUS lines. Transgenic plants were grown MS medium with either 0 or 0.8 μM ACC for 5 d before GUS staining assay. Scale bar, 0.5 cm. (e) The relative ERF1 expression level was determined in ethylene signaling related mutants ein2-5, ein3-1, ein3-1eil1and compared to wildtype (Col-0) seedlings. Seedlings carrying constructs for either constitutive (ctr1-1) or inducible EIN3-FLAG (iE/qm) (EIN3ox) expression were also examined. Seedlings were geminated on MS medium with either 0 or 0.8 μM ACC for 5 d. Seeds of EIN3ox were grown on medium containing 1 μM β-estradiol and 0 or 0.8 μM ACC. Roots of seedlings were used for qRT-PCR analysis. Values are mean ± SD of three replicas (***P<0.001). Asterisks indicate Student’s t-test significant differences.
The spatial expression pattern of ERF1, was determined by assaying ß-glucuronidase (GUS) activity of transgenic plants carrying an ERF1pro:GUS construct, in which a GUS reporter gene was under the control of the ERF1 promoter (ERF1pro, a 3.0kb promoter fragment). ERF1 was mainly expressed in the maturation zone of the primary root of seedlings (Fig 1B). To examine the response of ERF1 to ethylene, we treated 5-day-old ERF1pro:GUS seedlings with 10 μM ACC for 0–6 h and found that the expression of ERF1 was induced in the maturation zone and cotyledons as the ACC treatment time increased. Stronger induction was observed in the upper maturation zone (Fig 1B).
To determine the long-term response of ERF1 to ethylene, we grew wildtype seedlings on MS medium with either 0 or 0.8 μM ACC for 5 d and examined the relative transcript levels. The abundance of ERF1 increased about 21 times by ACC treatment compared with the 0 μM ACC control (Fig 1C). Transgenic seeds carrying ERF1pro:GUS were directly germinated on MS supplemented with or without 0.8 μM ACC for 5 d before GUS analysis. Strikingly in ACC-supplemented seedlings, GUS staining increased along the entire root and with weaker staining was also visible in the primary root tip compared to the 0 μM ACC control (Fig 1D).
GUS staining of ERF1pro:GUS transgenic lines showed that ERF1 expression was mainly present in roots and old leaves (S1A–S1E Fig). ERF1 nuclear-specific subcellular localization was demonstrated in transgenic plants carrying a CaMV 35S-driven ERF1 construct fused to GFP (S1F Fig). These results suggest that ERF1 may play a role in root development in response to ethylene signaling.
To confirm ethylene-dependent induction of ERF1 expression, we analysed the ERF1 transcript level in the ethylene signaling mutants ein2-5, ein3-1, ein3-1eil1 and compared them to wildtype seedlings. ERF1 transcription was also measured in lines of constitutive or β-estradiol inducible expression, ctr1-1 and EIN3-FLAG (iE/qm) (EIN3ox), respectively. Expression of ERF1 could not be induced by ACC in the ein2-5, ein3-1 and ein3-1eil1 mutants, while it was constitutively expressed in the ctr1-1 and EIN3ox seedlings (Fig 1E). These results suggested that ethylene-induced expression of ERF1 is dependent on the ethylene signaling pathway.
Genetic analysis of ERF1 in primary root elongation
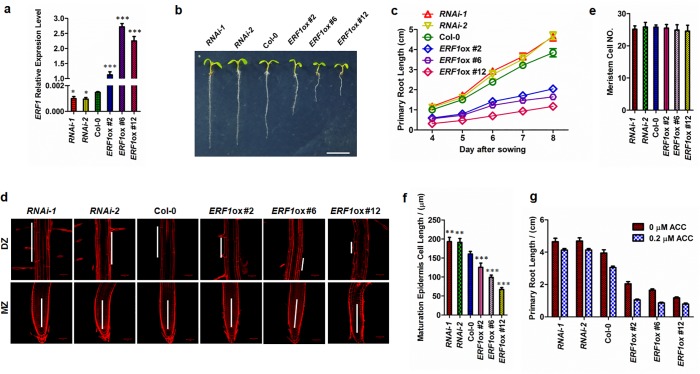
To investigate the function of ERF1 in Arabidopsis root growth, we generated transgenic knockdown and overexpression lines. The phenotype of these plants was confirmed with the analysis of ERF1 expression (S2 Fig). From three overexpression lines and two RNAi knockdown lines (Fig 2A), we found that the primary roots of the lines overexpressing ERF1 (ERF1ox) were stunted. In contrast, the RNAi lines showed longer primary roots compared to the wildtype controls (Fig 2B and 2C). Similar results of root elongation were observed in etiolated seedlings (S3 Fig). Root elongation in ERF1ox lines was similar to mutants with an activated ethylene signal pathway, while root elongation in ERF1 RNAi lines was similar to mutants with defects in ethylene signal pathway (S4A Fig).
Fig 2. Role of ERF1 in ethylene-mediated inhibition of primary root elongation.
(a-c) Primary root phenotypes of ERF1 knockdown (RNAi-1, RNAi-2) and overexpression (ERF1ox #2, ERF1ox #6, ERF1ox #12) transgenic lines. The expression level of ERF1 in 5 day-old-seedlings was tested by qRT-PCR (a). Values are mean ± SD of three replicas (*P<0.05, ***P<0.001). Asterisks indicate Student’s t-test significant differences. The representative seedlings were photographed (b). Scale bar, 1 cm. The primary root length of these related transgenic lines and Col-0 was measured from 4 to 8 d (c). Data shown are average and SD (Values are mean±SD, n = 20). (d-f) Epidermal cell length within the DZ was affected in ERF1 knockdown (RNAi-1, RNAi-2) and overexpression (ERF1ox #2, ERF1ox #6, ERF1ox #12) transgenic lines. The primary root MZ and DZ of the 5-day-old seedlings of indicated lines were photographed. Representative images are shown (d). Root meristem cell number (e) and epidermal cell length of the maturation zone (f) were measured. The root meristem cell number was counted from the QC to the first elongation cell in the cortex file. The mean ± SD (n = 20) is shown, **P<0.01, ***P<0.001). Asterisks indicate Student’s t-test significant differences. (g) Primary root length of ERF1 knockdown (RNAi-1, RNAi-2) and overexpression lines grown on medium with or without ACC treatment. Five-day-old seedlings grown on MS medium were transferred to MS medium containing 0 or 0.2 μM ACC and growth vertically for 3 d. The mean ± SD is shown (n = 50).
Ethylene signaling mutants ein2-5, ein3-1, ein3-1eil1, ctr1-1, and EIN3ox also display abnormal primary root development with or without ACC treatment[25,26,55–58]. The roots of ein2-5, ein3-1 and ein3-1eil1 seedlings, in which the corresponding genes positively regulate the ethylene signal pathway, were longer and more insensitive to ACC compared to wildtype (S4A Fig). However, the roots of ctr1-1 and EIN3ox, in which ethylene signaling was significantly active, were shorter than wildtype roots.
To explore the primary root elongation of the ERF1-related transgenic lines, we investigated the primary root in three developmental zones, the differentiation zone (DZ, also known as maturation zone), the elongation zone (EZ), and the meristem zone (MZ). Root meristem size was measured as the cell number from the quiescent centre (QC) to the first elongated cell in the cortex[59]. No significant change in meristem cell number was observed among the transgenic lines compared to the wildtype (Fig 2D and 2E). However, cell length had dramatically decreased in the DZ of ERF1ox lines, whereas it was greater in the DZ of the RNAi lines compared to wildtype (Fig 2D and 2F). These results suggest that ERF1 contributes to root length via altering cell elongation but not cell division (Fig 2D–2F), which is a similar effect to that produced by exogenously applied auxin[60]. This agrees with the root inhibition mechanism resulting from ACC treatment and the ethylene signal pathway mutants, ein2-5 and ctr1-1[3,6,61].
Since ERF1 is downstream of the ethylene signal pathway, we proposed that the inhibition of root elongation by ethylene might be ERF1-dependent. To analyze this, wildtype, ERF1ox, and RNAi lines were grown on MS plates for 5 d, then transferred to plates with or without ACC for 3 d, and primary root lengths were measured. We found that the primary root lengths of the RNAi lines were greater than controls, but those of ERF1ox lines were shorter than controls (Fig 2G). Furthermore, the difference between ERF1 expression levels in ERF1 RNAi lines and wildtype was augmented by ACC treatment (S4B Fig). Taken together, these results suggest that ethylene inhibits primary root elongation mainly through ERF1. ERF1, as a positive regulator of ethylene signaling, appears to play a negative role in regulating root cell elongation, leading to dramatically shortened primary roots under conditions of hyperactive ethylene signaling.
ERF1 enhances auxin accumulation in roots
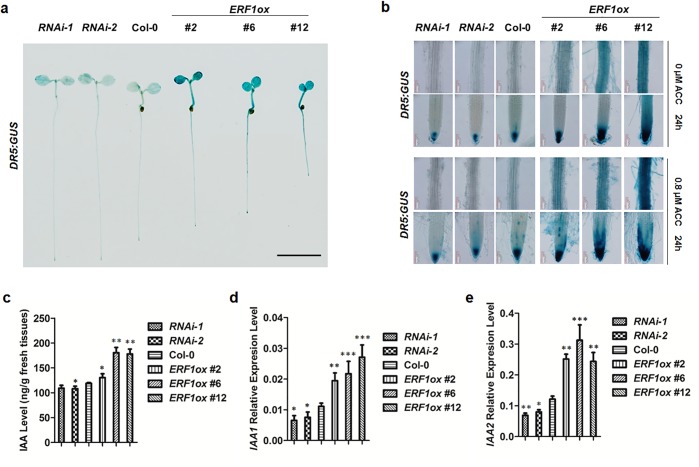
The reduced primary root elongation phenotype in ERF1 overexpression lines was similar to wildtype plants grown on the medium containing auxin[6], consistent with the proposal that ethylene enhances auxin biosynthesis to inhibits root elongation [3,6]. To study whether ERF1 enhances auxin accumulation in Arabidopsis roots, Firstly, we introduced a DR5:GUS reporter, an auxin reporter responding to endogenous auxin[62], into ERF1 knockdown and overexpression lines (Fig 3A and S5A Fig). Expression of DR5:GUS in primary roots, including the MZ and DZ was significantly increased in the ERF1 overexpression background. GUS expression occurred even in the absence of added ACC, in the root as well as in the cotyledons and hypocotyl (Fig 3A and 3B).The distribution of GUS staining in the primary root tip extended to other tissues compared to wildtype, i.e., also occurring in the epidermal cells of the MZ (Fig 3B, #12). In addition, DR5:GUS expression in DZ occurred not only in the xylem as for wildtype, but also in epidermis, cortex and endodermal tissues (Fig 3B, #2, #6, and #12). In contrast, the GUS activity decreased in the primary root tip and stele in ERF1 knockdown background compared with wildtype (Fig 3B, RNAi-1 and -2 compared with Col-0, respectively). The difference of DR5:GUS expression in these lines were more evident when treated with ACC for 24 h (Fig 3B). Moreover, the IAA content was higher in the ERF1 overexpression lines and lower in the RNAi lines compared with wildtype (Fig 3C). In accordance with the increased auxin, the expression of IAA1 and IAA2, which are auxin-responsive marker genes, was activated in overexpression lines of ERF1 and down regulated in knockdown lines (Fig 3D and 3E). Our results indicate that, ERF1 restrains primary root elongation by increasing auxin.
Fig 3. ERF1 enhances auxin accumulation in roots.
(a) DR5:GUS expression in 5-day-old seedlings of Col-0,ERF1 knockdown (RNAi-1, RNAi-2) and overexpression lines (ERF1ox #2, ERF1ox #6, ERF1ox #12). Three independent experiments were done, and each replica containing 15 plants for each line. Representative seedlings were photographed. Scale bar, 0.5 cm. (b) DR5:GUS expression in maturation region and root tip of primary root. Five-day-old seedlings of Col-0, ERF1 knock-down (RNAi-1, RNAi-2) and over-expression (ERF1ox #2, ERF1ox #6, ERF1ox #12) lines were treated with 0 or 0.8 μM ACC for 24 h before GUS activity was assayed. More than 20 plants were observed for each line. Representative photos were displayed. (c) Free IAA content. Seeds of ERF1 overexpression and RNAi lines were grown on MS plates for 5 d before root IAA content was measured. The mean ± SD of three replicas is shown (*P<0.05, **P<0.01). Asterisks indicate Student’s t-test significant differences. (d-e) The transcript level of IAA1 and IAA2. Seeds of ERF1 overexpression and RNAi lines were grown on MS plates for 5 d before RNA isolation from the roots. Transcript abundance of IAA1 and IAA2 was measured using qRT-PCR. The mean ± SD of three replicates is shown (*P<0.05, **P<0.01, ***P<0.001). Asterisks indicate Student’s t-test significant differences.
ERF1 directly binds to ASA1 promoter region in vitro and in vivo
Ethylene positively regulates the transcription of ASA1 to increase auxin and inhibit root elongation[8]. This finding prompted us to examine whether ERF1 functions as a direct regulator of ASA1. Previous studies showed that ERF1 binds to a specific cis-element (a GCC-box related sequence) upstream of its target genes, to regulate downstream gene expression[27,40].We analysed the promoter sequence of ASA1 and found one GCC-box at 27 bp upstream of the translational start codon. We sought to test whether this GCC-box could provide a handle for ERF1 to regulate ASA1 directly.
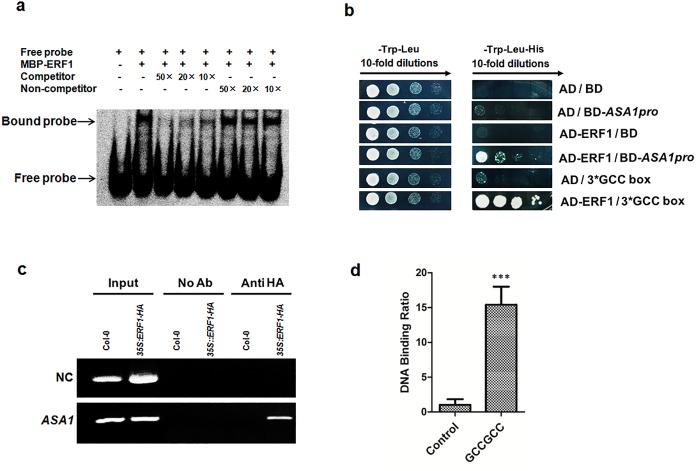
To determine if there was a direct physical interaction of the ERF1 protein with the ASA1 promoter sequence, we conducted an electrophoretic mobility shift assay (EMSA) with the full-length ERF1 protein fused to a maltose binding protein (MBP-ERF1) that was expressed in E. coli and purified through affinity chromatography. As shown in Fig 4A, the ERF1-MBP fusion protein was able to specifically bind digoxigenin-labelled DNA probes that contained the GCC-box motif of the ASA1 promoter. Moreover, the binding specificity was confirmed by competition with native DNA probes, or probes carrying a mutated GCC-box. Unlabelled native DNA probe was used as a competitor and unlabelled promoter fragment containing the mutant form of the GCC-box motif as a non-competitor. The EMSA results showed that ERF1 specifically binds to the promoter sequence containing a GCC-box of ASA1 promoter in vitro, but not to the DNA probe containing the mutant GCC-box sequence (Fig 4A). In addition, we carried out a yeast-one-hybrid assay that showed ERF1 was able to bind to the GCC-box sequence in the ASA1 promoter in yeast cells (Fig 4B).
Fig 4. ERF1 directly binds to ASA1 promoter region in vitro and in vivo.
(a) EMSA assay for binding to GCC-box sequence in the promoter of ASA1 by ERF1 protein in vitro. Dig-labelled probes were incubated with ERF1-MBP protein. As indicated, unlabelled probes were used as competitors, unlabelled probes with mutated GCC-box sequence were used as non-competitors, and the ERF1-MBP protein bound probers were separated from free probes by an acrylamide gel. (b) Yeast-one-hybrid assay. pGADT7/ERF1 (AD-ERF1) and pHIS2/ASA1pro (BD-ASA1pro) constructs were co-transformed into yeast strain Y187. AD-empty and BD-empty, AD-empty and BD-ASA1pro, AD-ERF1 and BD-empty, AD-empty and BD-3*GCC-box were used as negative controls while AD-ERF1 and BD-3*GCC-box were used as a positive control. (c) Chromatin immunoprecipitation-PCR for ASA1 promoter. Roots of 5-day-old 35S:HA:ERF1 and Col-0 seedlings were used. Anti-HA antibodies were used for the enrichment of the DNA fragments containing GCC-box in the promoter of ASA1. The results were determined by real-time PCR. Tub8 was used as a negative control (NC). (d) Quantitative real-time PCR was performed using the same ChIP products and PCR primers flanking GCC-boxes in ASA1 promoter as in c. The region of ASA1 that do not contain GCC-box was used as negative control. Values are mean ± SD of three replicas (***P<0.001). Asterisks indicate Student’s t-test significant differences.
To confirm whether this specific binding occurs in planta, we generated transgenic Arabidopsis plants expressing the 35S promoter-driven HA tagged ERF1 construct (S6 Fig). Transgenic lines showing expected phenotypes were used for chromatin immunoprecipitation (ChIP) with anti-HA antibodies (Roche, USA). As shown in Fig 4C, the chromatin immunoprecipitated with anti-HA antibodies was significantly enriched for the ASA1 promoter containing GCC-box fragments in the ChIP-PCR assay. This was further confirmed by quantitative real-time PCR performed using the same ChIP products and PCR primers flanking GCC-boxes in ASA1 promoter (Fig 4D). These results suggest a specific binding of ERF1 to the promoter of ASA1 in vivo.
ERF1 positively regulates the expression of ASA1 in the roots
To investigate the consequence of ERF1 binding to the GCC-box of the ASA1 promoter, we measured the ASA1 expression level in ERF1 transgenic lines by qRT-PCR. As predicted, the transcript level of ASA1 in ERF1 overexpression lines was significantly increased compared to wildtype without additional treatment. A small but statistically significant reduction of ASA1 transcripts in ERF1 knockdown lines was observed (Fig 5A). The difference in ASA1 expression levels between knockdown lines of ERF1 and wildtype was further increased by ACC treatment (S4C Fig). In addition, the increased expression level of ASA1 in 35Spro:ERF1 plants agreed with the microarray results in a previous report that ASA1 was upregulated in 35S:ERF1 transgenic plants[36]. These results suggest that ASA1 is indeed a downstream target of ERF1.
Fig 5. ERF1 up-regulates the expression of ASA1 in roots.
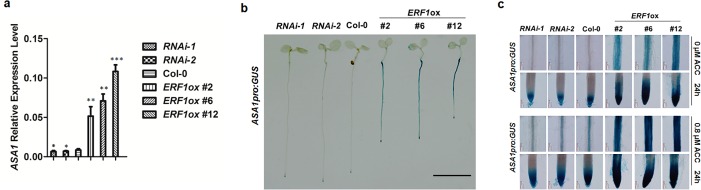
(a) Transcript levels of ASA1 in 5-day-old Col-0, ERF1 knockdown (RNAi-1, RNAi-2), and overexpression (ERF1ox #2, ERF1ox #6, ERF1ox #12) seedlings. Values are mean ± SD of three replicas (*P<0.05, **P<0.01, ***P<0.001). Asterisks indicate Student’s t-test significant differences. (b) ASA1pro:GUS expression in 5-day-old Col-0 and ERF1 knockdown (RNAi-1, RNAi-2) and over-expression (ERF1ox #2, ERF1ox #6, ERF1ox #12) seedlings. Three independent experiments were done, and each replica containing 15 plants for each genotype. Representative seedlings were photographed. Scale bar, 0.5 cm. (c) ASA1pro:GUS expression in primary root maturation region and root tip of 5-day-old Col-0, ERF1 knockdown (RNAi-1, RNAi-2), and overexpression (ERF1ox #2, ERF1ox #6, ERF1ox #12) seedlings without or with ACC treatment. More than 20 plants were observed for each genotype. Representative photos were displayed.
To confirm further whether the expression level of ASA1 was affected by ERF1, we introduced the ASA1pro:GUS reporter into ERF1 knockdown and overexpression background and examined the primary root phenotype (Fig 5B and S5B Fig). Consistent with the ASA1 expression level in ERF1 transgenic lines (Fig 5A), we found that the intensity of GUS staining in ASA1pro:GUS significantly increased in an ERF1 overexpression background and slightly reduced in the ERF1 knockdown lines compared to wildtype (Fig 5C). Moreover, this difference was further exaggerated in response to ACC treatment (Fig 5C). Furthermore, the GUS staining patterns produced by ASA1pro:GUS in the ERF1 knockdown lines was weaker than that of wildtype with ACC treatment. Taken together, these results suggest that ERF1 positively regulates ASA1 expression in response to ethylene.
The inhibition of primary root growth by ethylene is ASA1-dependent
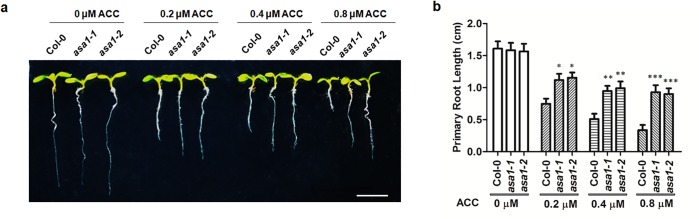
Given that ethylene-inhibited root elongation involved auxin biosynthesis which is enhanced by ERF1 through regulating ASA1 expression, we predicted that loss of ASA1 would decrease the sensitivity to ethylene. To test this, we grew wildtype and asa1 mutants (asa1-1 and asa1-2) on MS medium with or without ACC. The asa1 mutants grown on media containing different concentrations of ACC displayed longer primary roots than wildtype seedlings (Fig 6A and 6B). Our results support the notion that inhibition of root elongation by ethylene is ASA1-dependent. This observation is consistent with a previous finding that asa1 mutants are insensitive to ethylene under dark conditions[8]. Furthermore, as shown in S7 Fig, ERF1-RNAi lines, asa1, and ein2-5 showed reduced response to ACC with respect to the effect of the ethylene on primary root growth compared to Col-0. For the reduced sensitivity of root to ACC, asa1 and ein2 were more evident than ERF1-RNAi lines, which might be due to the limitation of ERF1-RNAi materials, and/or some other factors participating in this process.
Fig 6. Root elongation of asa1mutants in response to ACC treatment.
(a) Root elongation of Col-0 and asa1 without and with ACC treatment. CS16398 (asa1-1) and CS16397 (asa1-2) are two different loss-of-ASA1 mutants. Seeds of Col-0 and asa1 were grown on MS plates without or with ACC for 5 d. Three independent experiments were displayed with similar results. Representative seedlings were photographed. Scale bar, 0.5 cm. (b) The primary root length of 5-day-old Col-0 and asa1 mutants grown on medium without or with ACC. Values are mean ± SD of three replicas (*P<0.05, **P<0.01, ***P<0.001). Each replica contains 30 plants for each line. Asterisks indicate Student’s t-test significant differences.
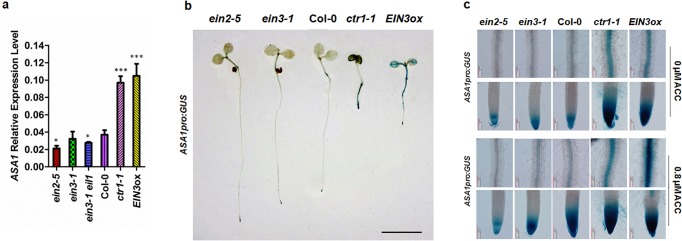
The primary root elongation of mutants (ein2-5, ein3-1eil1 and ein3-1) was less sensitive to ACC compared to wildtype. The mutants with enhanced ethylene signals (ctr1-1, EIN3ox) showed dramatically shortened primary roots under normal conditions, mimicking wildtype seedlings treated with ACC[25,26] (S4A Fig). Since ASA1 functions downstream of CTR1[8], we measured the ASA1 transcript levels in these mutants in normal conditions and found that the expression level of ASA1 in ein2-5, ein3-1eil1 and ein3-1 was lower than that of wildtype and conversely higher in ctr1-1 and EIN3ox lines (Fig 7A). To confirm this further, we introduced ASA1pro:GUS reporter into ein2-5, ein3-1, ctr1-1, and EIN3ox backgrounds and found that the GUS staining pattern closely correlated with the qRT-PCR results (Fig 7B and 7C). Strong GUS activity in the root and cotyledons was observed in ctr1-1 and EIN3ox backgrounds (Fig 7B). These results indicated that ASA1 is downstream of these ethylene signal pathway components.
Fig 7. The expression of ASA1 was regulated by ethylene signal pathway.
(a) The expression of ASA1 in ethylene signal pathway mutants. Ethylene signal pathway-related mutants ein2-5, ein3-1, ein3-1eil1, ctr1-1, and Col-0 were grown on MS medium for 5 d while the seeds of EIN3-FLAG (iE/qm) (EIN3ox) were grown on MS medium supplementing with 1 μM β-estradiol for 5 d. Total RNA was extracted from roots. The expression level of ASA1 was checked by qRT-PCR. Values are mean ± SD of three replicas (*P<0.05, **P<0.01, ***P<0.001. Asterisks indicate Student’s t-test significant differences). (b) ASA1pro:GUS expression in 5-day-old seedlings. Three independent experiments were performed with each replica containing 15 plants for each line. Representative seedlings were photographed. Scale bar, 0.5 cm. (c) ASA1pro:GUS expression in primary root maturation region and root tip of 5-day-old seedlings without or with ACC treatment. More than 20 plants were observed for each genotype. Representative photos were displayed.
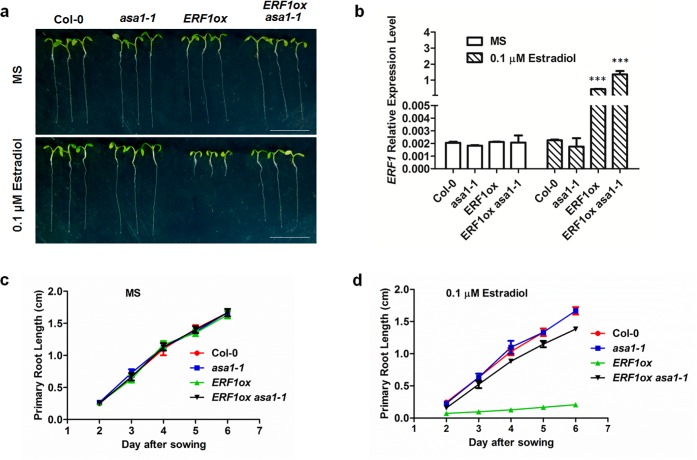
To confirm this genetically, we introduced an estradiol-inducible ERF1 overexpression in asa1 mutant background (ERF1ox asa1-1). When ERF1 is overexpressed, the primary root of ERF1ox asa1-1 is significantly longer than that of ERF1ox and a little shorter than Col-0 (Fig 8, S8 Fig). The result demonstrates that ASA1 is downstream of ERF1, and suggests that ethylene-inducible ERF1 controls root elongation through regulating ASA1 expression. There may be other targets of ERF1 which also participate this process since some difference in primary root length was observed between ERF1ox asa1-1 and asa1-1 (Fig 8D).
Fig 8. ASA1 acts downstream of ERF1.
(a) The primary root phenotypes of Col-0, asa1-1, ERF1ox and ERF1ox asa1-1 seedlings grown on MS medium with either 0 or 0.1 μM estradiol for 5 d. ERF1ox is the transgenic plants expressing ERF1 protein under control of the estradiol-inducible promoter in Col-0 background. Scale bar, 1 cm. (b) qRT–PCR analysis of transcriptions of ERF1. The roots of 5-day-old Col-0, asa1-1, ERF1ox and ERF1ox asa1-1 seedlings grown on MS medium with either 0 or 0.1 μM estradiol were used. Values are mean ± SD of three replicas (***P<0.001. Asterisks indicate Student’s t-test significant differences). (c-d) Primary root length of Col-0, asa1-1, ERF1ox and ERF1ox asa1-1 seedlings grown on MS medium with either 0 or 0.1 μM estradiol were measured at the fifth days. Data shown are average and SD (Values are mean ± SD, n = 20).
Discussion
One of the best studied effects of ethylene on roots is the inhibition of root elongation[5,20]. A number of studies have indicated that ethylene inhibits root development through interaction with auxin. Ethylene has been shown to increase auxin synthesis, auxin transport to the elongation zone, and auxin signaling at the root tip[3,5–9,47,52]. ERF1, a downstream transcription factor in the ethylene signal pathway was reported to reduce primary root growth in the dark when constitutively expressed[27]. Until now, no detailed and explicit mechanism has been provided for its role in primary root elongation. In this study, we demonstrated that ERF1 directly regulates the expression of ASA1, a key enzyme in Trp biosynthesis where auxin is derived and known to play an important role in ethylene-regulated root development[8]. This work elaborates the mechanism by which the transcription factor ERF1 participates in primary root development and directly mediates crosstalk between ethylene and auxin biosynthesis during root elongation.
Through analyses of root response to ethylene with overexpression and knockdown lines of ERF1 (Fig 2A–2C), we found that the length of the primary root was closely correlated to ERF1 expression. These results imply that ERF1 is involved in ethylene-mediated root elongation.
ERFs belong to a large gene family. Only a few ERF mutants show obvious phenotypes, probably due to functional redundancy. However, ERF1 knockdown lines displayed longer root under normal and added ACC conditions (Fig 2B, 2C and 2G), indicating that ERF1 plays an important role in ethylene-inhibited root elongation. Furthermore, ERF1 controls primary root elongation by reducing cell elongation, but not cell division (Fig 2D–2F), which is consistent with ethylene signal pathway mutants[6].
Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root elongation[6]. High auxin levels are known to reduce root growth[3]. Some auxin biosynthesis genes are ethylene responsive, and if mutated, cause some defects in root growth in the presence of ACC[8,9]. To understand how ERF1 mediates ethylene signaling in primary root elongation and particularly auxin biosynthesis, we analysed the promoters of all genes which participate in auxin biosynthesis, and found that two genes including ASA1 and YUCCA2 contained a GCC-box which can be specifically bound by ERF1. A recent study showed that ERF109, another member of the ERF family that is highly responsive to JA signaling, directly regulates both ASA1 and YUC2 and mediates crosstalk between JA and auxin biosynthesis[42]. The primary root elongation of the yucca2 mutant, in the presence of added ACC, did not differ from wildtype. Considering that ASA1 is ethylene responsive, asa1 is ethylene insensitive in root-elongation[8], and there is a GCC-box in the promoter of ASA1 which could be bound by ERF1, we hypothesized that ASA1 might be a direct target of ERF1.
To confirm our hypothesis, we conducted in vitro binding (EMSA), yeast-one-hybrid, and chromatin immunoprecipitation (ChIP) experiments and confirmed that ERF1 could directly bind a conserved GCC-box element in the promoter of ASA1 in vitro and in vivo (Fig 4). We further confirmed our hypothesis by analyzing ASA1pro:GUS in ERF1 knockdown and overexpression background. As we expected, the expression of ASA1 was remarkably increased in ERF1 overexpression background but reduced in the knockdown lines (Fig 5). Upon ACC treatment, the staining of ASA1pro:GUS in ERF1 knockdown background becomes darker but still relatively weaker than that in Col-0 background at the root tip and DZ (Fig 5C), indicating that the ethylene responsiveness was not completely removed. In the ERF1 knockdown lines, the induction of ASA1 by ethylene was reduced but some induction was still retained compared to the wildtype (S4C Fig), which is consistent with the primary root phenotype (Fig 6). This may be due to incomplete suppression of ERF1 by RNAi technique, alternatively, there may be additional components involved in this process. For instance, EIN3 was suggested to directly regulate ASA1 based on the data of EIN3 ChIP-Seq experiments [63].
Meanwhile, analyses of ASA1pro:GUS reporter in ethylene signal pathway mutation background (ein2-5, ein3-1, ctr1-1, EIN3ox) showed that when ethylene signal pathway was enhanced, the expression of ASA1 was also enhanced. Conversely, if ethylene signal pathway was blocked, the expression of ASA1 was reduced and the induction by ACC was also impaired (Fig 7). These results explicated that ASA1 is downstream of these ethylene signal pathway components.
Taken together, our results support a model in which ethylene stimulates auxin biosynthesis in roots through ethylene-responsive transcription factor ERF1 that positively regulates ASA1. As a consequence of activating ASA1 expression, ERF1 increases the accumulation of auxin, which in turn decreases root elongation and alters root architecture.
Methods
Plant growth conditions and material
Surface-sterilised Arabidopsis seeds were treated for 10 min in 10% bleach and cold-treated for 3–4 d at 4°C, and grown on Murashige and Skoog (MS, 1 x salts) medium with 1% sucrose for the indicated days (see Figure legends). Seedlings were then transferred to plates supplemented with or without ACC for the indicated days, and the plates were placed vertically in a growth room. ACC (Sigma-Aldrich) was dissolved in water and prepared as a 2 mM stock solution. Arabidopsis thaliana 7-d-old seedlings were transferred to soil and grown to maturity in a growth room. All plants were grown under long-day conditions (16-h light / 8-h dark) at 22–24°C. Every experiment was repeated at least three times.
Arabidopsis thaliana ecotype Columbia-0 (Col-0) was used. Some plant materials used in this study were previously described: ein2-5[26], ein3-1[41], ein3-1 eil1-1[52], ctr1-1[25], EIN3-FLAG (iE/qm) (EIN3ox) [64], ASA1pro:GUS [8], DR5:GUS[65].
Plants were obtained from transformation of Col-0 plants with the ERF1pro:GUS construct, which contained a 3.0-kb promoter sequence with primers (S1 Table). ERF1 over-expressing plants (ERF1ox #2, #6, and #12) were obtained from transformation of Col-0 with the 35Spro:ERF1 construct, which contains full-length ERF1 (At3g23240). ERF1 knockdown plants were obtained from transformation of Col-0 with the 35Spro:ERF1:RNAi construct. 35S:HA-ERF1 were obtained from transformation of Col-0 plants with a vector comprised of a 35S promoter and HA sequence in front of the full-length ERF1 cDNA. 35Spro:ERF1-GFP transgenic lines were constructed in Col-0 Arabidopsis. ERF1ox transgenic plants used in genetic analysis of crossing ERF1ox with asa1 were obtained from transformation of Col-0 plants with the vector comprised of an estradiol-inducible promoter in front of the full-length ERF1.
DNA constructs and plant transformation
To prepare the ERF1pro:GUS construct, the primer set ERF1pro-F and ERF1pro-R (S1 Table), was used to amplify a 3.0 kb sequence from the ERF1 promoter region. This was cloned into the binary vector pCB308R. To prepare the ERF1 over-expression construct, the primer set, ERF1-F and ERF1-R and full-length ERF1 fragments were amplified and cloned into the binary vector pCB2004[66]. To prepare the ERF1 knock-down construct, the primer set ERF1RNAiF and ERF1RNAiR was used to amplify a 0.18 kb sequence from the ERF1 coding region, and cloned into the binary vector pCB2004B. To prepare the HA:ERF1 over-expression construct, the primer set ERF1HAF and ERF1HAR was used to amplify the HA-ERF1 coding sequence, and cloned into the binary vector pCB2004. To prepare the 35Spro:ERF1-GFP construct, the product amplified by ERF1GFPF and ERF1GFPR was cloned into the binary vector pGWB5[67]. All of these constructs utilised the gateway system technology. To generate the ERF1ox transgenic plants with estradiol-inducible promoter, the PCR product amplified by pER8-ERF1 F and pER8-ERF1 R primers was cloned into the vector pER8 for transformation[68].These constructs were then individually transformed into Agrobacterium tumefaciens Strain (C58C1), and introduced into Arabidopsis plants by the floral dip method[69]. More than 30 transgenic lines were obtained for each construct.
Histochemical GUS staining and confocal microscopy analysis
Histochemical staining for GUS activity in transgenic plants was conducted as previous described[70]. Seedlings were grown on MS medium as above, and stained in fresh GUS staining solution (1 mg/mL X-glucuronide in 0.1 M potassium phosphate, pH 7.2, 0.5 mM ferrocyanide, 0.5 mM ferricyanide, and 0.1% Triton X-100) at 37°C in the dark for the indicated time. After incubation, seedlings were cleared with a series of ethanol solutions (100%, 50% and 30%) and photographed. Images were captured using an OLYMPUS IX81 microscope and HiROX (Japan) MX5040RZ. For in planta GFP analysis, seedlings were stained in 10 mg/mL propidium iodide for 8 min and washed twice in water. Propidium iodide fluorescence and GFP were imaged under a ZEISS710 confocal laser scanning microscope: 488-nm and 543-nm lines of the laser were used for excitation, and emission was detected at 510 nm and 620 nm, respectively. More than 15 seedlings were examined for each transgenic line, and at least three independent experiments were displayed.
Real-time qRT-PCR analysis
Total RNA was extracted with TRIzol reagent (Invitrogen) from seedlings or root samples. cDNA was prepared by TransScript RT kit (Invitrogen) for RNA reverse transcription, and was used for real-time quantitative RT-PCR. All quantitative RT-PCR assays were performed with a SYBR Premix Ex Taq II kit on StepOne real-time PCR system (Applied Biosystems) according to the manufacturer’s instructions. Expression levels of target genes were normalized to Arabidopsis UBQ5. All qRT-PCR experiments were performed at least three biological replicates. The primers used in this study have been listed in S1 Table.
IAA content measurement
The free total IAA content was measured by ELISA as described[71].
Yeast-one-hybrid assay
The procedures of the yeast one-hybrid assay were displayed as described previously with minor modifications[72]. A DNA fragment encoding ERF1 was amplified with the primers ERF1Y1H F and ERF1Y1H R, and cloned into pAD-GAL4-2.1 (AD vector) to produce the pAD/ERF1 plasmid. Two complementary core 30-bp DNA single strands including GCC-box in the promoter of ASA1, named ASA1Y1H F and ASA1Y1H R, were annealed and cloned into the reporter plasmid via SacI and MluI restriction sites, pHIS2 (BD vector), which contained the nutritional reporter gene, HIS3.Two complementary DNA strands with three copies of the DNA sequence GCC-box, named GCCY1H F and GCCY1H R were annealed and cloned into plasmid pHIS2 through SacI and MluI restriction sites as a positive control. The pAD/ERF1 construct containing the ERF1 cDNA sequence and the pHIS2 reporter construct containing the GCC-box cis-element were co-transformed into Y187 yeast cells. For the negative control, the pAD/ERF1 plasmid and the pHIS2 empty plasmid were co-transformed into Y187 yeast cells. Yeast was cultured in SD/–Trp–Leu medium and then transferred to SD/–Trp–Leu–His medium containing10 mM 3-aminotriazole (Sigma) with dilutions as indicated. The plates were incubated at 30°Cfor 4 d and the results were observed. Growth of yeast cells on the SD/–Trp–Leu–His medium in the presence of 10 mM 3-aminotriazole indicated that the transcription factor could bind this cis-element, and activated relevant gene expression.
ChIP-PCR assay
One gram each of six-day-old 35S:HA-ERF1seedlings and Col-0 plants were harvested for ChIP experiments. The procedures were conducted essentially as previously described[73]. The enrichment of DNA fragments in the ASA1 promoter was amplified by PCR using the following primer pairs: ChIP-ASA1pro F, ChIP-ASA1pro R, and β-tubulin8 was used as negative control. The resultant PCR-products were resolved by electrophoresis on 2% agarose gels. The results displayed above represent at least three independent repeats.
To analyse the binding quantitatively, a qRT-PCR assay was performed on the basis of the procedure described previously[74]. The relative quantity value is presented as the DNA binding ratio. The same primers for the above PCR analysis were used for qRT-PCR.
Electrophoretic mobility shift assay
The fragment of the full-length ERF1 coding sequence was amplified by PCR with primers ERF1EMSA F and ERF1EMSA R, and cloned into the pMAL-C2 vector via EcoRI and XbaI restriction sites, to construct a plasmid for the expression of recombinant MYC2 protein in Escherichia coli.
Five individual synthetic 30-bp single-stranded DNA molecules containing the GCC-box were used, namely ASA1EMSA F-DIG, ASA1EMSA R, ASA1EMSA F, ASA1EMSA-Mu F and ASA1EMSA-Mu R. These DNA fragments were annealed with their complementary oligonucleotides, ASA1EMSA F-DIG with ASA1EMSA R for EMSA labelled probe, ASA1EMSA R with ASA1EMSA F for EMSA competitive probe, ASA1EMSA-Mu F with ASA1EMSA-Mu R for EMSA non-competitive probe. EMSA was performed according to a DIG Gel Shift kit, 2nd Generation (Roche).
Supporting Information
(a-c) Seeds of ERF1pro:GUS were germinated on MS medium and stained for GUS expression. ERF1 expression pattern in 4- (a), 6- (b), and 15-day-old (c) plants. Scale bar, 0.5 cm. (d) Twenty-five-day-old ERF1pro:GUS transgenic seedlings grown in soil. (e) Stem, siliques and flowers of 35-day-old ERF1pro:GUS transgenic lines grown in soil. (f) The cellular localization of ERF1 protein revealed by the 35Spro:ERF1-GFP transgenic plants. The roots of 5-day-old 35Spro:ERF1-GFP transgenic plants were photographed using a ZEISS710 confocal laser scanning microscope. Scale bar = 50 μm.
(DOC)
(a) Seeds of the transgenic lines and wildtype were germinated vertically on MS medium for 5 days, and the representative seedlings were photographed. Scale bar, 1 cm. (b) The primary root length of the transgenic lines and wildtype was measured from 4 to 8 days. Data shown are average and SD (n = 20, *P<0.05, **P<0.01, ***P<0.001. Asterisks indicate Student’s t-test significant differences). (c) The expression level of ERF1 in these materials was tested by qRT-PCR. Values are mean ± SD of three replicates (*P<0.05, ***P<0.001. Asterisks indicate Student’s t-test significant differences).
(DOC)
(a) Images of representative 5-d-old etiolated seedlings grown in the MS medium are displayed. Genotypes are as indicated. Scale bar, 1 cm.(b) The primary root length of 5-d-old etiolated seedlings was measured. Data shown are average and SD (*P<0.05, ***P<0.001. Asterisks indicate Student’s t-test significant differences).
(DOC)
(a) The primary root elongation phenotype in the mutants as indicated. Ethylene signalling pathway-related mutants ein2-5, ein3-1, ein3-1eil1, ctr1-1, and wildtype (Col-0) were geminated on MS medium with 0 or 0.8 μM ACC for 5 d. The seeds of EIN3ox (EIN3-FLAG (iE/qm)) were grown on medium containing 1 μM β-estradiol and 0 or 0.8 μM ACC for 5 d, respectively. Scale bar, 1cm. (b-c) ERF1 and ASA1 expression levels in ERF1 knockdown lines (RNAi-1, RNAi-2) and wildtype seedlings with or without 0.2 μM ACC treatment from 5 to 8 d. Total RNA was extracted from roots. The expression level of ERF1 and ASA1 in these materials was detected by qRT-PCR. Values are mean ± SD of three replicates.
(DOC)
The expression level of ERF1 in 5-day-old Col-0 wildtype, ERF1 knockdown (RNAi-1, RNAi-2) and overexpression (ERF1ox #2, ERF1ox #6, ERF1ox #12) seedlings in DR5:GUS (a) and ASA1pro:GUS (b) backgrounds was tested by qRT-PCR. Values are mean ± SD of three replicas (*P<0.05, ***P<0.001. Asterisks indicate Student’s t-test significant differences).
(DOC)
(a) The root phenotype of 5-day-old Col-0 wildtype and 35S:HA-ERF1. Scale bar, 0.5 cm. (b) The ERF1 expression level in 5-day-old wildtype and 35S:HA-ERF1 seedlings. Values are mean ± SD of three replicas (***P<0.001. Asterisks indicate Student’s t-test significant differences).
(DOC)
(a) Images of representative 5-d-old seedlings grown in the presence of 0, 0.2, 0.5, 1, and 10 μM ACC are displayed. Genotypes are as indicated. Scale bar, 1 cm. (b) Relative root length of 5-d-old seedlings (Col-0, RNAi-1, asa1, ein2-5) grown in the presence of 0, 0.2, 0.5, 1, and 10 μM ACC. The response of each genotype to ACC was expressed as the percentage of the root length at a particular concentration of ACC with respect to the average length of root in the absence of ACC. Data shown are average and SD (*P<0.05, **P<0.01, ***P<0.001. Asterisks indicate Student’s t-test significant differences).
(DOC)
(a) The primary root phenotypes of Col-0, asa1-1, ERF1ox and ERF1ox asa1-1 seedlings grown on MS medium with either 0 or 1 μM ACC for 5 d. Scale bar, 1 cm. (b-c) Primary root length of Col-0, asa1-1, ERF1ox and ERF1ox asa1-1 seedlings grown on MS medium with either 0 or 1 μM ACC were measured at the fifth days. Data shown are average and SD (Values are mean ± SD, n = 20).
(DOC)
(DOC)
Acknowledgments
The authors thank Dr. Hongwei Guo for kindly providing EIN3-FLAG (iE/qm) (EIN3ox) and ein3-1eil1, Dr. Jingsong Zhang for ctr1-1 and ein2-5, Dr. Chuanyou Li for ASA1pro:GUS, Dr. Jirong Huang for pGWB5 vector, and the Arabidopsis Biological Resource Center for the seeds of CS16397 (wei2-1), CS16398 (wei2-2), and CS8052 (ein3-1).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by grants from NNSFC (grant no.91417306), MOST (2012CB114304), and Chinese Academy of Science (grant no. KSCX3–YW–N–007). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Gazzarrini S, McCourt P (2003) Cross-talk in plant hormone signalling: what Arabidopsis mutants are telling us. Ann Bot 91: 605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Swarup R, Parry G, Graham N, Allen T, Bennett M (2002) Auxin cross-talk: integration of signalling pathways to control plant development. Plant Mol Biol 49: 411–426. [DOI] [PubMed] [Google Scholar]
- 3.Swarup R, Perry P, Hagenbeek D, Van Der Straeten D, Beemster GT, et al. (2007) Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell 19: 2186–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le J, Vandenbussche F, Van Der Straeten D, Verbelen JP (2001) In the early response of Arabidopsis roots to ethylene, cell elongation is up- and down-regulated and uncoupled from differentiation. Plant Physiol 125: 519–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muday GK, Rahman A, Binder BM (2012) Auxin and ethylene: collaborators or competitors? Trends Plant Sci 17: 181–195. 10.1016/j.tplants.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 6.Ruzicka K, Ljung K, Vanneste S, Podhorska R, Beeckman T, et al. (2007) Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell 19: 2197–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stepanova AN, Yun J, Likhacheva AV, Alonso JM (2007) Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell 19: 2169–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stepanova AN, Hoyt JM, Hamilton AA, Alonso JM (2005) A Link between ethylene and auxin uncovered by the characterization of two root-specific ethylene-insensitive mutants in Arabidopsis. Plant Cell 17: 2230–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stepanova AN, Robertson-Hoyt J, Yun J, Benavente LM, Xie DY, et al. (2008) TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133: 177–191. 10.1016/j.cell.2008.01.047 [DOI] [PubMed] [Google Scholar]
- 10.Lei MG, Zhu CM, Liu YD, Karthikeyan AS, Bressan RA, et al. (2011) Ethylene signalling is involved in regulation of phosphate starvation-induced gene expression and production of acid phosphatases and anthocyanin in Arabidopsis. New Phytologist 189: 1084–1095. 10.1111/j.1469-8137.2010.03555.x [DOI] [PubMed] [Google Scholar]
- 11.Bleecker AB, Kende H (2000) Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol 16: 1–18. [DOI] [PubMed] [Google Scholar]
- 12.Frankowski K, Kesy J, Kopcewicz J (2007) [Regulation of ethylene biosynthesis in plants]. Postepy Biochem 53: 66–73. [PubMed] [Google Scholar]
- 13.Spanu P, Reinhardt D, Boller T (1991) Analysis and cloning of the ethylene-forming enzyme from tomato by functional expression of its mRNA in Xenopus laevis oocytes. EMBO J 10: 2007–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamilton AJ, Bouzayen M, Grierson D (1991) Identification of a tomato gene for the ethylene-forming enzyme by expression in yeast. Proc Natl Acad Sci U S A 88: 7434–7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abel S, Nguyen MD, Chow W, Theologis A (1995) ACS4, a primary indoleacetic acid-responsive gene encoding 1-aminocyclopropane-1-carboxylate synthase in Arabidopsis thaliana. Structural characterization, expression in Escherichia coli, and expression characteristics in response to auxin [corrected]. J Biol Chem 270: 19093–19099. [DOI] [PubMed] [Google Scholar]
- 16.Tsuchisaka A, Theologis A (2004) Unique and overlapping expression patterns among the Arabidopsis 1-amino-cyclopropane-1-carboxylate synthase gene family members. Plant Physiol 136: 2982–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang KL, Li H, Ecker JR (2002) Ethylene biosynthesis and signaling networks. Plant Cell 14 Suppl: S131–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen YF, Etheridge N, Schaller GE (2005) Ethylene signal transduction. Ann Bot 95: 901–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benavente LM, Alonso JM (2006) Molecular mechanisms of ethylene signaling in Arabidopsis. Mol Biosyst 2: 165–173. [DOI] [PubMed] [Google Scholar]
- 20.Stepanova AN, Alonso JM (2009) Ethylene signaling and response: where different regulatory modules meet. Curr Opin Plant Biol 12: 548–555. 10.1016/j.pbi.2009.07.009 [DOI] [PubMed] [Google Scholar]
- 21.Kendrick MD, Chang C (2008) Ethylene signaling: new levels of complexity and regulation. Curr Opin Plant Biol 11: 479–485. 10.1016/j.pbi.2008.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang C, Stadler R (2001) Ethylene hormone receptor action in Arabidopsis. Bioessays 23: 619–627. [DOI] [PubMed] [Google Scholar]
- 23.Chang C, Bleecker AB (2004) Ethylene biology. More than a gas. Plant Physiol 136: 2895–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bleecker AB (1999) Ethylene perception and signaling: an evolutionary perspective. Trends Plant Sci 4: 269–274. [DOI] [PubMed] [Google Scholar]
- 25.Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR (1993) CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell 72: 427–441. [DOI] [PubMed] [Google Scholar]
- 26.Alonso JM, Hirayama T, Roman G, Nourizadeh S, Ecker JR (1999) EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284: 2148–2152. [DOI] [PubMed] [Google Scholar]
- 27.Solano R, Stepanova A, Chao Q, Ecker JR (1998) Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev 12: 3703–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657. [DOI] [PubMed] [Google Scholar]
- 29.Nakano T, Suzuki K, Fujimura T, Shinshi H (2006) Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol 140: 411–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berrocal-Lobo M, Molina A, Solano R (2002) Constitutive expression of ETHYLENE-RESPONSE-FACTOR1 in Arabidopsis confers resistance to several necrotrophic fungi. Plant J 29: 23–32. [DOI] [PubMed] [Google Scholar]
- 31.Zhang JY, Broeckling CD, Blancaflor EB, Sledge MK, Sumner LW, et al. (2005) Overexpression of WXP1, a putative Medicago truncatula AP2 domain-containing transcription factor gene, increases cuticular wax accumulation and enhances drought tolerance in transgenic alfalfa (Medicago sativa). Plant J 42: 689–707. [DOI] [PubMed] [Google Scholar]
- 32.Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, et al. (1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10: 1391–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stockinger EJ, Gilmour SJ, Thomashow MF (1997) Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc Natl Acad Sci U S A 94: 1035–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maruyama Y, Yamoto N, Suzuki Y, Chiba Y, Yamazaki K, et al. (2013) The Arabidopsis transcriptional repressor ERF9 participates in resistance against necrotrophic fungi. Plant Sci 213: 79–87. 10.1016/j.plantsci.2013.08.008 [DOI] [PubMed] [Google Scholar]
- 35.Hao D, Ohme-Takagi M, Sarai A (1998) Unique mode of GCC box recognition by the DNA-binding domain of ethylene-responsive element-binding factor (ERF domain) in plant. J Biol Chem 273: 26857–26861. [DOI] [PubMed] [Google Scholar]
- 36.Lorenzo O, Piqueras R, Sanchez-Serrano JJ, Solano R (2003) ETHYLENE RESPONSE FACTOR1 integrates signals from ethylene and jasmonate pathways in plant defense. Plant Cell 15: 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao WH, Liu J, He XJ, Mu RL, Zhou HL, et al. (2007) Modulation of ethylene responses affects plant salt-stress responses. Plant Physiol 143: 707–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao YR, Chen SY, Zhang JS (2008) Ethylene signaling regulates salt stress response: An overview. Plant Signal Behav 3: 761–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cela J, Chang C, Munne-Bosch S (2011) Accumulation of gamma- rather than alpha-tocopherol alters ethylene signaling gene expression in the vte4 mutant of Arabidopsis thaliana. Plant Cell Physiol 52: 1389–1400. 10.1093/pcp/pcr085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng MC, Liao PM, Kuo WW, Lin TP (2013) The Arabidopsis ETHYLENE RESPONSE FACTOR1 regulates abiotic stress-responsive gene expression by binding to different cis-acting elements in response to different stress signals. Plant Physiol 162: 1566–1582. 10.1104/pp.113.221911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, et al. (1997) Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell 89: 1133–1144. [DOI] [PubMed] [Google Scholar]
- 42.Cai XT, Xu P, Zhao PX, Liu R, Yu LH, et al. (2014) Arabidopsis ERF109 mediates cross-talk between jasmonic acid and auxin biosynthesis during lateral root formation. Nat Commun 5: 5833 10.1038/ncomms6833 [DOI] [PubMed] [Google Scholar]
- 43.Petricka JJ, Winter CM, Benfey PN (2012) Control of Arabidopsis root development. Annu Rev Plant Biol 63: 563–590. 10.1146/annurev-arplant-042811-105501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujita H, Syono K (1996) Genetic analysis of the effects of polar auxin transport inhibitors on root growth in Arabidopsis thaliana. Plant Cell Physiol 37: 1094–1101. [DOI] [PubMed] [Google Scholar]
- 45.Kiegle E, Moore CA, Haseloff J, Tester MA, Knight MR (2000) Cell-type-specific calcium responses to drought, salt and cold in the Arabidopsis root. Plant J 23: 267–278. [DOI] [PubMed] [Google Scholar]
- 46.Zheng Z, Guo Y, Novak O, Dai X, Zhao Y, et al. (2013) Coordination of auxin and ethylene biosynthesis by the aminotransferase VAS1. Nat Chem Biol 9: 244–246. 10.1038/nchembio.1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pickett FB, Wilson AK, Estelle M (1990) The aux1 Mutation of Arabidopsis Confers Both Auxin and Ethylene Resistance. Plant Physiol 94: 1462–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roman G, Lubarsky B, Kieber JJ, Rothenberg M, Ecker JR (1995) Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: five novel mutant loci integrated into a stress response pathway. Genetics 139: 1393–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luschnig C, Gaxiola RA, Grisafi P, Fink GR (1998) EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev 12: 2175–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rahman A, Amakawa T, Goto N, Tsurumi S (2001) Auxin is a positive regulator for ethylene-mediated response in the growth of Arabidopsis roots. Plant Cell Physiol 42: 301–307. [DOI] [PubMed] [Google Scholar]
- 51.Pickett FB, Wilson AK, Estelle M (1990) The Aux1 Mutation Of Arabidopsis Confers Both Auxin And Ethylene Resistance. Plant Physiology 94: 1462–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alonso JM, Stepanova AN, Solano R, Wisman E, Ferrari S, et al. (2003) Five components of the ethylene-response pathway identified in a screen for weak ethylene-insensitive mutants in Arabidopsis. Proc Natl Acad Sci U S A 100: 2992–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ivanchenko MG, Muday GK, Dubrovsky JG (2008) Ethylene-auxin interactions regulate lateral root initiation and emergence in Arabidopsis thaliana. Plant J 55: 335–347. 10.1111/j.1365-313X.2008.03528.x [DOI] [PubMed] [Google Scholar]
- 54.Won C, Shen X, Mashiguchi K, Zheng Z, Dai X, et al. (2011) Conversion of tryptophan to indole-3-acetic acid by TRYPTOPHAN AMINOTRANSFERASES OF ARABIDOPSIS and YUCCAs in Arabidopsis. Proc Natl Acad Sci U S A 108: 18518–18523. 10.1073/pnas.1108436108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y, Liu C, Li K, Sun F, Hu H, et al. (2007) Arabidopsis EIN2 modulates stress response through abscisic acid response pathway. Plant Mol Biol 64: 633–644. [DOI] [PubMed] [Google Scholar]
- 56.Zhu Z, An F, Feng Y, Li P, Xue L, et al. (2011) Derepression of ethylene-stabilized transcription factors (EIN3/EIL1) mediates jasmonate and ethylene signaling synergy in Arabidopsis. Proc Natl Acad Sci U S A 108: 12539–12544. 10.1073/pnas.1103959108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu Y, Wang J, Zhang Z, Quan R, Zhang H, et al. (2013) Ethylene promotes hypocotyl growth and HY5 degradation by enhancing the movement of COP1 to the nucleus in the light. PLoS Genet 9: e1004025 10.1371/journal.pgen.1004025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.An FY, Zhao QO, Ji YS, Li WY, Jiang ZQ, et al. (2010) Ethylene-Induced Stabilization of ETHYLENE INSENSITIVE3 and EIN3-LIKE1 Is Mediated by Proteasomal Degradation of EIN3 Binding F-Box 1 and 2 That Requires EIN2 in Arabidopsis. Plant Cell 22: 2384–2401. 10.1105/tpc.110.076588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dello Ioio R, Linhares FS, Scacchi E, Casamitjana-Martinez E, Heidstra R, et al. (2007) Cytokinins determine Arabidopsis root-meristem size by controlling cell differentiation. Curr Biol 17: 678–682. [DOI] [PubMed] [Google Scholar]
- 60.Wang Z, Mao JL, Zhao YJ, Li CY, Xiang CB (2014) L-Cysteine inhibits root elongation through auxin/PLETHORA and SCR/SHR pathway in Arabidopsis thaliana. J Integr Plant Biol. [DOI] [PubMed] [Google Scholar]
- 61.De Cnodder T, Vissenberg K, Van Der Straeten D, Verbelen JP (2005) Regulation of cell length in the Arabidopsis thaliana root by the ethylene precursor 1-aminocyclopropane- 1-carboxylic acid: a matter of apoplastic reactions. New Phytol 168: 541–550. [DOI] [PubMed] [Google Scholar]
- 62.Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, et al. (1999) An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99: 463–472. [DOI] [PubMed] [Google Scholar]
- 63.Chang KN, Zhong S, Weirauch MT, Hon G, Pelizzola M, et al. (2013) Temporal transcriptional response to ethylene gas drives growth hormone cross-regulation in Arabidopsis. Elife 2: e00675 10.7554/eLife.00675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.An F, Zhao Q, Ji Y, Li W, Jiang Z, et al. (2010) Ethylene-induced stabilization of ETHYLENE INSENSITIVE3 and EIN3-LIKE1 is mediated by proteasomal degradation of EIN3 binding F-box 1 and 2 that requires EIN2 in Arabidopsis. Plant Cell 22: 2384–2401. 10.1105/tpc.110.076588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ (1997) Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell 9: 1963–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lei ZY, Zhao P, Cao MJ, Cui R, Chen X, et al. (2007) High-throughput binary vectors for plant gene function analysis. Journal Of Integrative Plant Biology 49: 556–567. [Google Scholar]
- 67.Nakagawa T, Kurose T, Hino T, Tanaka K, Kawamukai M, et al. (2007) Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J Biosci Bioeng 104: 34–41. [DOI] [PubMed] [Google Scholar]
- 68.Zuo J, Niu QW, Chua NH (2000) Technical advance: An estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J 24: 265–273. [DOI] [PubMed] [Google Scholar]
- 69.Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743. [DOI] [PubMed] [Google Scholar]
- 70.Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang J, Zhang J, Wang Z, Zhu Q, Wang W (2001) Hormonal changes in the grains of rice subjected to water stress during grain filling. Plant Physiol 127: 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang HW, Zhang B, Hao YJ, Huang J, Tian AG, et al. (2007) The soybean Dof-type transcription factor genes, GmDof4 and GmDof11, enhance lipid content in the seeds of transgenic Arabidopsis plants. Plant J 52: 716–729. [DOI] [PubMed] [Google Scholar]
- 73.Gendrel AV, Lippman Z, Martienssen R, Colot V (2005) Profiling histone modification patterns in plants using genomic tiling microarrays. Nat Methods 2: 213–218. [DOI] [PubMed] [Google Scholar]
- 74.Mukhopadhyay A, Deplancke B, Walhout AJ, Tissenbaum HA (2008) Chromatin immunoprecipitation (ChIP) coupled to detection by quantitative real-time PCR to study transcription factor binding to DNA in Caenorhabditis elegans. Nat Protoc 3: 698–709. 10.1038/nprot.2008.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a-c) Seeds of ERF1pro:GUS were germinated on MS medium and stained for GUS expression. ERF1 expression pattern in 4- (a), 6- (b), and 15-day-old (c) plants. Scale bar, 0.5 cm. (d) Twenty-five-day-old ERF1pro:GUS transgenic seedlings grown in soil. (e) Stem, siliques and flowers of 35-day-old ERF1pro:GUS transgenic lines grown in soil. (f) The cellular localization of ERF1 protein revealed by the 35Spro:ERF1-GFP transgenic plants. The roots of 5-day-old 35Spro:ERF1-GFP transgenic plants were photographed using a ZEISS710 confocal laser scanning microscope. Scale bar = 50 μm.
(DOC)
(a) Seeds of the transgenic lines and wildtype were germinated vertically on MS medium for 5 days, and the representative seedlings were photographed. Scale bar, 1 cm. (b) The primary root length of the transgenic lines and wildtype was measured from 4 to 8 days. Data shown are average and SD (n = 20, *P<0.05, **P<0.01, ***P<0.001. Asterisks indicate Student’s t-test significant differences). (c) The expression level of ERF1 in these materials was tested by qRT-PCR. Values are mean ± SD of three replicates (*P<0.05, ***P<0.001. Asterisks indicate Student’s t-test significant differences).
(DOC)
(a) Images of representative 5-d-old etiolated seedlings grown in the MS medium are displayed. Genotypes are as indicated. Scale bar, 1 cm.(b) The primary root length of 5-d-old etiolated seedlings was measured. Data shown are average and SD (*P<0.05, ***P<0.001. Asterisks indicate Student’s t-test significant differences).
(DOC)
(a) The primary root elongation phenotype in the mutants as indicated. Ethylene signalling pathway-related mutants ein2-5, ein3-1, ein3-1eil1, ctr1-1, and wildtype (Col-0) were geminated on MS medium with 0 or 0.8 μM ACC for 5 d. The seeds of EIN3ox (EIN3-FLAG (iE/qm)) were grown on medium containing 1 μM β-estradiol and 0 or 0.8 μM ACC for 5 d, respectively. Scale bar, 1cm. (b-c) ERF1 and ASA1 expression levels in ERF1 knockdown lines (RNAi-1, RNAi-2) and wildtype seedlings with or without 0.2 μM ACC treatment from 5 to 8 d. Total RNA was extracted from roots. The expression level of ERF1 and ASA1 in these materials was detected by qRT-PCR. Values are mean ± SD of three replicates.
(DOC)
The expression level of ERF1 in 5-day-old Col-0 wildtype, ERF1 knockdown (RNAi-1, RNAi-2) and overexpression (ERF1ox #2, ERF1ox #6, ERF1ox #12) seedlings in DR5:GUS (a) and ASA1pro:GUS (b) backgrounds was tested by qRT-PCR. Values are mean ± SD of three replicas (*P<0.05, ***P<0.001. Asterisks indicate Student’s t-test significant differences).
(DOC)
(a) The root phenotype of 5-day-old Col-0 wildtype and 35S:HA-ERF1. Scale bar, 0.5 cm. (b) The ERF1 expression level in 5-day-old wildtype and 35S:HA-ERF1 seedlings. Values are mean ± SD of three replicas (***P<0.001. Asterisks indicate Student’s t-test significant differences).
(DOC)
(a) Images of representative 5-d-old seedlings grown in the presence of 0, 0.2, 0.5, 1, and 10 μM ACC are displayed. Genotypes are as indicated. Scale bar, 1 cm. (b) Relative root length of 5-d-old seedlings (Col-0, RNAi-1, asa1, ein2-5) grown in the presence of 0, 0.2, 0.5, 1, and 10 μM ACC. The response of each genotype to ACC was expressed as the percentage of the root length at a particular concentration of ACC with respect to the average length of root in the absence of ACC. Data shown are average and SD (*P<0.05, **P<0.01, ***P<0.001. Asterisks indicate Student’s t-test significant differences).
(DOC)
(a) The primary root phenotypes of Col-0, asa1-1, ERF1ox and ERF1ox asa1-1 seedlings grown on MS medium with either 0 or 1 μM ACC for 5 d. Scale bar, 1 cm. (b-c) Primary root length of Col-0, asa1-1, ERF1ox and ERF1ox asa1-1 seedlings grown on MS medium with either 0 or 1 μM ACC were measured at the fifth days. Data shown are average and SD (Values are mean ± SD, n = 20).
(DOC)
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.