Abstract
Infection with Anaplasma phagocytophilum in white-footed mice results in partial protection against reinfection with the same agent. However, humans and domestic animals may be sequentially exposed to different isolates of the agent circulating in the same or adjacent foci. We investigated whether immune response to a tick-borne infection with A. phagocytophilum provides protection against homologous and heterologous challenges. BALB/c mice were infected with one of the two sympatric isolates of A. phagocytophilum via tick bite and challenged 16 weeks later by Ixodes scapularis nymphs infected with either the same or the alternative isolate. As controls, groups of infected mice were challenged by uninfected ticks to confirm an absence of reactivation of the original infection or groups of naive mice were fed upon by ticks from cohorts used for an infectious challenge. Xenodiagnostic I. scapularis larvae were fed upon each mouse at 14 and 21 days postchallenge (PCH) and tested for the presence of A. phagocytophilum as freshly molted nymphs. Blood samples for quantitative PCR were collected at 7, 14, 21, and 70 days PCH. Serum samples were collected weekly to monitor development of immune response. The proportion of infected animals, levels of bacteremia, and the prevalence of infection in xenodiagnostic ticks were higher in groups of control mice exposed to A. phagocytophilum for the first time than in mice reinfected with either homologous or heterologous isolates. The presence of antibodies against A. phagocytophilum did not protect mice from a challenge with either homologous or heterologous isolates, however the ensuing reinfection was significantly milder and of a shorter duration than the first infection with either isolate.
Anaplasma phagocytophilum infects a large variety of mammalian species in Europe and North America. The existence of multiple variants of the agent that cause different clinical symptoms and immunologic reactions in different host species was noted early on. Even among strains isolated from the same host species, there is a great deal of variation in virulence as measured by percentage of infected granulocytes, the degree and duration of parasitemia, and the length of the incubation period (12, 14, 16). Biological, clinical, and geographic heterogeneity among A. phagocytophilum isolates is evident from the disparity between seroprevalence and incidence in humans and horses in North America and ruminants in Europe, in clinical severity between geographic locations in North America and Europe, between clinical manifestations of infection in various mammalian hosts (as reviewed by Dumler et al. [8]). Although analyses of the groESL and ank genes, as well as pulsed-field gel electrophoresis analysis, show that North American isolates of A. phagocytophilum are more similar to each other than to European strains (8, 27, 39), they differ antigenically (2, 19). We also found that isolates of A. phagocytophilum vary significantly in infection intensity and longevity in laboratory mice and in the efficiency of transmission to vector ticks (23), which suggests undiscovered differences among these bacteria.
In white-footed mice, infection with A. phagocytophilum is transient and followed by a strong immune response, which results in partial protection against reinfection with the same agent (22). Reactions and responses to an infectious agent in a natural reservoir host (Peromyscus leucopus) may differ from those in accidental hosts like humans and domestic animals. Humans seem to be susceptible to reinfection (17). It is unknown, however, if the preexisting antibodies would affect the severity of clinical symptoms in case of a reinfection, or whether an immune response to one isolate is protective against other isolates circulating in the same geographic region.
The degree of protection against reinfection with A. phagocytophilum varies according to the agent strain, the type and age of the host, and the time and frequency of challenge (13, 36-38, 41, 42, 44). Some researchers reported that under field conditions, one primary infection fails to produce protective immunity in ungulates against challenge. When cattle or sheep were reintroduced into tick-infested areas after a removal for only 3 months they developed febrile illness (18, 20). In one study (41), infection with none of the tested strains resulted in protection against heterologous strains. On the other hand, although animals previously exposed to one strain reacted with fever and parasitemia upon challenge with a heterologous strain, the magnitude and duration of fever and parasitemia were minimal (44). Others reported solid protective immunity that lasted for more than a year (26). It appears that under field conditions, the early breakdown of protective immunity reflects the presence of different strains, while solid immunity may reflect the presence of homologous or cross-protective strains. Experimental studies indicate that sheep may resist homologous challenge for periods that vary from a few months to more than a year, depending on the level of antibodies at the time of challenge or the presence of a carrier state (12, 44).
Unlike white-footed mice (natural reservoirs), which are confined to a relatively small home range, humans and domestic animals (accidental hosts) may visit several adjacent or distant foci and be sequentially exposed to different isolates of the agent circulating in those foci. Using Mus musculus mice (strain BALB/c) as a model, we investigated whether an immune response to a tick-borne infection with a North American isolate of A. phagocytophilum can provide protection against reinfection with the same or a different but sympatric isolate or alters the development of infection in a host and the host's infectivity for ticks.
MATERIALS AND METHODS
BALB/c mice were infected with one of the two sympatric isolates of A. phagocytophilum via tick bite and challenged 15 weeks later by Ixodes scapularis nymphs infected with either the same or the alternative isolate. As a control, groups of infected mice were challenged by uninfected ticks to confirm an absence of reactivation of the original infection, and groups of pathogen-naive mice were fed upon by ticks from cohorts used for an infectious challenge. We used xenodiagnosis to confirm and measure the ability of challenged mice to maintain and transmit the agent to ticks. Dynamics of infection and antibody response in mice were monitored by quantitative real-time PCR and indirect immunofluorescence assay (IFA).
A. phagocytophilum isolates.
Isolates Dawson and Gaillard originated from I. scapularis nymphs collected in Connecticut at Lake Dawson near the town of Woodbridge and at Lake Gaillard in North Branford, respectively. These two sites of endemicity are approximately 18 km apart and are separated by the Quinnipiac River. Both isolates are maintained in our laboratory in separate tick-mouse transmission cycles where infected I. scapularis nymphs are produced by allowing uninfected larval ticks to feed upon BALB/c mice previously exposed to A. phagocytophilum-infected nymphs. A preliminary study showed that an infection with isolate Dawson persists in BALB/c mice up to 9 weeks and infection with isolate Gaillard persists for up to 12 weeks (23). The highest infectivity for xenodiagnostic ticks in BALB/c mice infected with either isolate is observed during the third postinfection week.
Ticks.
Uninfected nymphs and xenodiagnostic larvae were derived from a separate I. scapularis colony. This colony, which originated from adult ticks collected in Connecticut, has been maintained for several generations in our laboratory by feeding on uninfected New Zealand White rabbits. Rabbit sera are routinely screened for antibodies against A. phagocytophilum, and representative samples of reared ticks are regularly tested by PCR to ensure that the colony is free of tick-borne pathogens.
Experimental design.
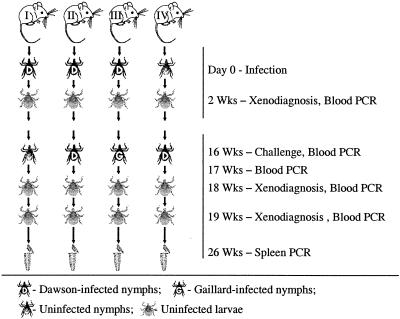
One-month-old tick-naive and specific pathogen-free BALB/c mice were acquired from Harlan and maintained at the Centers for Disease Control and Prevention, Atlanta, Ga., in accordance with an approved Institutional Animal Care and Use Committee protocol. Six mice were randomly assigned to each of eight groups. Four groups of mice (groups 1 to 4) were designated for the primary infection with Dawson isolate of A. phagocytophilum (Tables 1 and 2); the other four groups (groups 5 to 8) were infected with Gaillard isolate (Tables 1 and 2). In each of the two sets, one group received a primary infection and was challenged by uninfected nymphs (unchallenged control); the second group received a primary infection and was challenged by nymphs infected with the same isolate (homologous challenge); the third group received a primary infection and was challenged by nymphs infected with the different isolate (heterologous challenge); and the fourth group was first infested with uninfected nymphs and challenged with infected nymphs from the same cohort as in the homologous challenge group (pathogen-naive control) (Fig. 1). Baseline serum samples were collected from all mice prior to the beginning of the experiment. On day 0, mice in three groups (18 mice total) were each infested with 10 Dawson-infected nymphs, mice in the other three groups (18 mice total) were each infested with 10 Gaillard-infected nymphs, and two groups (12 mice total) with uninfected nymphs. On day 14, blood samples from all 48 mice were tested by PCR to confirm the presence or absence of infection, and mice were infested with uninfected I. scapularis larvae for xenodiagnosis and production of infected and uninfected nymphs used later in challenging infestations (Fig. 1).
TABLE 1.
A. phagocytophilum organisms in BALB/c mouse blood following homologous and heterologous challenge
| Group no. | Original isolate | Challenge isolate | 7 days PCH
|
14 days PCH
|
21 days PCH
|
|||
|---|---|---|---|---|---|---|---|---|
| No. of PCR-positive mice (n = 6) | No. of organismsa | No. of PCR-positive mice (n = 6) | No. of organisms | No. of PCR-positive mice (n = 6) | No. of organisms | |||
| 1 | Dawson | None | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | Dawson | Dawson | 6 | 3,944 ± 3,292 | 0 | 0 | 2 | 93 ± 144 |
| 3 | Dawson | Gaillard | 6 | 2,380 ± 2,626 | 3 | 196 ± 258 | 0 | 0 |
| 4 | None | Dawson | 6 | 11,893 ± 9,251 | 5 | 3,241 ± 6,723 | 6 | 5,150 ± 4,940 |
| 5 | Gaillard | None | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | Gaillard | Gaillard | 5 | 3,572 ± 5,083 | 3 | 213 ± 439 | 0 | 0 |
| 7 | Gaillard | Dawson | 6 | 1,156 ± 672 | 4 | 62 ± 55 | 0 | 0 |
| 8 | None | Gaillard | 6 | 62,563 ± 47,591 | 2 | 73 ± 150 | 5 | 6,409 ± 6,906 |
Average number of A. phagocytophilum organisms per 100 μl of whole blood ± standard deviations.
TABLE 2.
Infectivities of immune and control BALB/c mice for xenodiagnostic ticks following homologous and heterologous challenge
| Group no. | Original isolate | Challenge isolate | 14 days PCH
|
21 days PCH
|
||
|---|---|---|---|---|---|---|
| No. of xenopositive mice (n = 6) | % Ticks infecteda | No. of xenopositive mice (n = 6) | % Ticks infecteda | |||
| 1 | Dawson | None | 0 | 0 | 0 | 0 |
| 2 | Dawson | Dawson | 1 | 0.8 ± 1.6 | 3 | 9.8 ± 19.2 |
| 3 | Dawson | Gaillard | 2 | 11.9 ± 21.5 | 0 | 0 |
| 4 | None | Dawson | 6 | 41.8 ± 12.9 | 6 | 55.2 ± 21.9 |
| 5 | Gaillard | None | 0 | 0 | 0 | 0 |
| 6 | Gaillard | Gaillard | 2 | 9.2 ± 16.1 | 0 | 0 |
| 7 | Gaillard | Dawson | 2 | 6.7 ± 9.8 | 0 | 0 |
| 8 | None | Gaillard | 6 | 40.0 ± 19.8 | 5 | 35.0 ± 20.6 |
Percentage of infected xenodiagnostic ticks ± 95% confidence interval.
FIG. 1.
Scheme of the experiment for groups of mice with primary infection with A. phagocytophilum Dawson isolate. Group I, control without an infectious challenge; group II, mice subjected to a homologous challenge; group III, mice subjected to a heterologous challenge; group IV, control without the primary infection. Four similar groups of mice were designated for primary infection with A. phagocytophilum Gaillard isolate.
On day 112 (16 weeks after infection), mice were tested by blood PCR and infested with infected or uninfected I. scapularis nymphs according to the group designations. Mouse blood samples were tested 7, 14, and 21 days PCH, and I. scapularis larvae were fed upon all mice at 14 and 21 days postchallenge (PCH) for xenodiagnosis (Fig. 1). Larvae were allowed to molt and were tested for the presence of A. phagocytophilum DNA as freshly molted nymphs. Serum samples for IFA were collected weekly throughout the experiment for 26 weeks. Mice were humanely euthanized on day 182 of the experiment (10 weeks PCH), at which time their spleens were also tested by PCR.
Detection of the pathogen by PCR.
We used conventional PCR to assess the prevalence of A. phagocytophilum infection in xenodiagnostic ticks and quantitative real-time PCR to assess the amount of pathogen in challenging nymphs and in standard 100-μl blood samples. Prior to DNA extraction, individual ticks were frozen in liquid nitrogen, ground with a sterile plastic pestle, and resuspended in 50 μl of Tris-EDTA buffer. DNA was extracted from blood samples and tick suspensions by using the Iso-Quick Nucleic Acid Extraction Kit (ORCA Research, Inc., Bothell, Wash.).
In conventional PCR, primers msp2-3f (5′-CCAGCG TTTAGCAAGATAAGAG) and msp2-3r (5′-GCCCAGTAACATCATAAGC) were used to amplify a 334-bp fragment of the msp2 gene of A. phagocytophilum (28). I. scapularis nymphs infected with A. phagocytophilum from laboratory colonies were used as positive controls and distilled water was used as a negative control for each PCR set. The amplification products were visualized on 2% agarose gels stained with ethidium bromide.
Assessment of A. phagocytophilum copy number in ticks and blood samples was done by quantitative, real-time PCR assay that amplified the spacer region between the 23S and 5S rRNA genes (R. F. Massung, R. A. Priestley, and M. L. Levin, submitted for publication). The primers used were HGE23S-for (5′-TTTTCTGCAATACTTGTCGTGAAAT) and HGE23S-rev (5′-TCGGAATGTTACCGGGTGTT-3′), and the probe was HGE23S-HEX (hexachloro-b-carboxyfluorescein) (5′-HEX-TTTGGCTGGTGGTAATGGCAGGAGTG-dh quenc-her). Quantitative PCR assays were performed on each sample in duplicate, and samples were retested in cases where the duplicate results were discrepant. Results are expressed as the number of A. phagocytophilum organisms per 100 μl of whole blood or per tick.
Serology.
Mouse sera were tested for immunoglobulin G (IgG) antibodies reactive with A. phagocytophilum in IFA as described previously (30). Fluorescein isothiocyanate-labeled, goat anti-mouse IgG (heavy plus light chain) (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) was used at a working dilution 1:100 as determined by checkerboard titration of positive and negative serum. The positive control serum came from a mouse infected with A. phagocytophilum, and the negative control serum came from an uninfected laboratory-reared mouse. Sera were screened at an initial dilution of 1:16, and positive samples were titrated by using a twofold dilution series. Serologic data are reported as the reciprocal of the last dilution showing positive fluorescence.
Statistical analysis.
The significance of differences in mean number of A. phagocytophilum organisms per 100 μl of whole blood was tested using analysis of variance. Differences in the prevalence of infection in xenodiagnostic ticks were analyzed using the χ2 test. A P of <0.05 was considered statistically significant.
RESULTS
Original infection.
When tested on day 14 of the experiment, all mice exposed to either Dawson-infected nymphs or to Gaillard-infected nymphs tested positive for A. phagocytophilum by both blood PCR and xenodiagnosis.
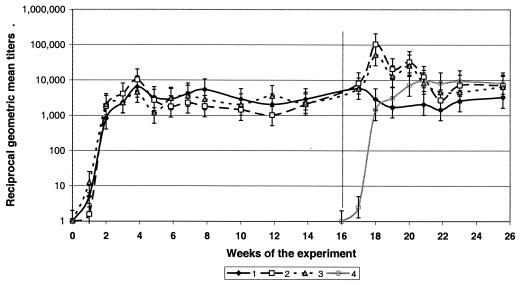
Eight of 18 mice infected with the Dawson isolate started producing IgG antibodies by day 7, with titers ranging from 16 to 128. All 18 mice became seropositive within 2 weeks postinfection, and antibody responses peaked 4 weeks postinfection, with geometric mean titers (GMT) of 6,809 (maximum titer, 16,384). Following the peak, GMT remained relatively stable for the next 12 weeks fluctuating between 1,024 and 8,192 (average, 3,127 ± 1,697) (Fig. 2). Antibody titers did not decline up to 16 weeks postinfection.
FIG. 2.
Dynamics of antibody titers in BALB/c mice originally infected with A. phagocytophilum Dawson isolate and challenged with homologous or heterologous isolates. Group 1, unchallenged control; group 2, homologous challenge (Dawson-Dawson); group 3, heterologous challenge (Dawson-Gaillard); group 4, pathogen-naive control. The vertical line indicates placement of challenging ticks. Error bars show standard deviations.
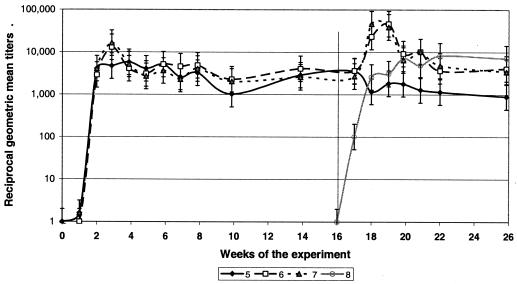
Two of 18 mice infected with the Gaillard isolate seroconverted within 1 week postinfection, with titers not exceeding 16. The other mice developed IgG antibody responses by 2 weeks postinfection. Antibody titers peaked at 3 weeks postinfection, with GMT of 10,809 (maximum titer, 16,384). Following the peak, GMT remained relatively stable for the next 12 weeks fluctuating between 1,024 and 5,161 (average, 3,465 ± 1,117) (see Fig. 3). As in mice infected with the Dawson isolate, antibody titers did not decline up to 16 weeks postinfection.
FIG. 3.
Dynamics of antibody titers in BALB/c mice originally infected with A. phagocytophilum Gaillard isolate and challenged with homologous or heterologous isolates. Group 5, unchallenged control; group 6, homologous challenge (Gaillard-Gaillard); group 7, heterologous challenge (Gaillard-Dawson); group 8, pathogen-naive control. The vertical line indicates placement of challenging ticks. Error bars show standard deviations.
None of the mice exposed only to uninfected nymphs yielded positive blood samples or xenodiagnostic ticks, and they remained seronegative until the challenge. At 16 weeks postinfection, all 48 mice were negative by blood PCR.
Unchallenged control.
Mice originally infected with either Dawson or Gaillard isolates and only infested with uninfected nymphs at 16 weeks postinfection remained negative by both blood PCR and xenodiagnosis for the rest of the study (Tables 1 and 2, groups 1 and 5). GMT in the unchallenged mice declined somewhat by 20 to 21 weeks postinfection, although this decline was not statistically significant (Fig. 2 and 3). Spleen samples from all 12 mice tested by PCR yielded negative results at 26 weeks postinfection.
Homologous challenges.
When mice previously infected with the Dawson isolate were challenged by nymphs infected with the same isolate, all six became blood PCR positive at day 7 PCH. Bacteremia ranged from 450 to 9,350 (average, 3,944) organisms per 100 μl of blood (Table 1, group 2). At 2 weeks PCH, all mice were blood PCR negative, but two of them again became PCR positive one week later, each carrying approximately 280 A. phagocytophilum organisms per 100 μl of blood. During the third week PCH, one of six mice transmitted the pathogen to approximately 8% of xenodiagnostic ticks; the average prevalence of infection in xenodiagnostic ticks fed upon this group of mice was less than 1% (Table 2, group 2). During week 4 PCH, three mice were infectious for xenodiagnostic ticks (two were blood PCR positive), and as many as 29.4% of ticks acquiring infection from one of the mice (average for the group, 9.8%). All six mice responded to the challenge by a rapid rise in IgG titers to a new high of 32,768 to 131,072 (GMT, 104,032) within only 1 week PCH (Fig. 2). However, this increase was short-lived, and GMT returned to the prechallenge level of approximately 6,020 within the next 3 to 4 weeks or by 5 weeks after the homologous challenge. Spleen samples tested by PCR at 10 weeks PCH yielded negative results.
Similarly, all mice previously exposed to the Gaillard isolate became blood PCR positive (at different time points) when challenged by Gaillard-infected ticks. Five of the six mice were PCR positive at 1 week PCH, with bacteremia levels of 280 to 8,238 (average, 3,572) A. phagocytophilum organisms per 100 μl of blood (Table 1, group 6). At week 2 PCH, the sixth mouse became PCR positive with a bacteremia level of 1,096 A. phagocytophilum organisms per 100 μl of blood; another mouse remained blood PCR positive with low bacteremia (180 organisms per 100 μl of blood). All six mice tested negative by blood PCR at 3 weeks PCH. At 2 weeks PCH, two of the six mice were infectious to xenodiagnostic ticks, with 5 and 48% of ticks acquiring infection from individual hosts, and the average prevalence of infection was 9.2% (Table 2, group 6). At 3 weeks PCH, none of the mice were infectious for xenodiagnostic ticks. Mice responded to the homologous Gaillard challenge by an interim increase in IgG titers up to 8,192 to 131,072 (GMT, 46,341) within 1 to 2 weeks PCH (Fig. 3). GMT returned to the prechallenge level of 3,649 within the next 3 or 4 weeks (i.e., by 5 weeks after the homologous challenge). Spleen samples tested by PCR at 10 weeks PCH yielded negative results.
Heterologous challenges.
All mice previously exposed to the Dawson isolate became blood PCR positive when challenged by Gaillard-infected nymphs. At 1 week PCH, six of six were positive, with bacteremia of 128 to 5,712 (average, 2,380) organisms per 100 μl of blood (Table 1, group 3). At 2 weeks PCH, three remained PCR positive, each carrying 160 to 614 (average, 196) A. phagocytophilum organisms per 100 μl of blood. All mice were blood PCR negative by week 3 PCH. During week 3 PCH, two of the six mice were infectious to xenodiagnostic ticks: 8 and 68% of ticks acquired infection from individual hosts (average prevalence of infection, 11.9% [Table 2, group 6]). During week 4 PCH, none of the mice were infectious for xenodiagnostic ticks. Mice responded to the challenge by an increase in IgG titers to a new high of 16,384 to 131,072 within 1 (three mice) or 3 (three mice) weeks PCH, and titers returned to the prechallenge levels of 4,096 to 8,192 within 1 week after the peak. The highest GMT (52,016) was recorded at 1 week PCH (Fig. 2). Spleen samples tested by PCR at 10 weeks PCH yielded negative results.
Similarly, all mice previously exposed to Gaillard isolate became blood PCR positive when challenged by Dawson-infected nymphs. At 1 week PCH, six out of six were positive, with the bacteremia ranging from 104 to 1,949 (average, 1,156) organisms per 100 μl of blood (Table 1, group 7). At week 2 PCH, four remained PCR positive, each carrying 74 to 132 (average, 62) A. phagocytophilum organisms per 100 μl of blood. All mice turned blood PCR negative by week 3 PCH. During week 3 PCH, two of the six mice were infectious to xenodiagnostic ticks, with 20% of ticks acquiring infection from each host. The average prevalence of infection in xenodiagnostic ticks fed upon this group of mice was 6.7% (Table 2, group 7). During week 4 PCH, none of the mice were infectious for xenodiagnostic ticks. Mice responded to the challenge by an increase in IgG titers to a new high of 8,192 to 131,072 within 1 to 2 weeks PCH, and titers returned to the prechallenge level of 4,096 to 8,192 within 1 week after the peak. The highest GMT (64,341) was recorded at 1 week PCH (Fig. 2). Spleen samples tested by PCR at 10 weeks PCH yielded negative results.
Pathogen-naive control.
All mice that were exposed only to uninfected ticks at the beginning of the study acquired infection from challenging infected nymphs as determined by blood PCR and xenodiagnosis. Both the bacteremia levels and prevalence of infection in xenodiagnostic ticks were much higher than in previously infected mice.
When pathogen-naive mice were challenged with Dawson-infected nymphs, all six became blood PCR positive at 1 week PCH, with bacteremia levels of 781 to 26,775 (average, 11,893) organisms per 100 μl of blood (Table 1, group 4). These bacteremia levels were significantly higher than those observed in mice previously infected with either Dawson or Gaillard isolates (P = 0.0455 and P = 0.0177 compared to groups 2 and 7, respectively). Five of the six mice were positive at week 2 PCH, and all six were positive at week 3 PCH. Bacteremia levels at 2 and 3 weeks PCH were not significantly different between groups of control and previously infected mice due to high variability within the groups. All previously naive mice were infectious for xenodiagnostic ticks after being challenged with Dawson isolate. The resulting prevalence of infection among ticks fed upon individual animals was 24 to 65% (average, 41.8%) at 2 weeks PCH and 35 to 85% (average, 55.2%) at 3 weeks PCH (Table 2, group 4). These prevalence indices were significantly higher (P << 0.00001) than those obtained in groups of mice challenged with Dawson isolate following recovery from infection with either Dawson (group 2) or Gaillard (group 7) isolates.
In pathogen-naive mice challenged with Gaillard-infected nymphs, all six became blood PCR positive at 1 week PCH, with bacteremia levels of 29,625 to 151,125 (average, 62,563) organisms per 100 μl of blood (Table 1, group 8). These bacteremia levels were significantly higher than in mice previously infected with either Dawson or Gaillard isolates (P = 0.0114 and P = 0.0127 compared to groups 3 and 6, respectively). Only two mice were positive at 2 weeks PCH, with low amounts of pathogen (63 and 374 organisms per 100 μl), and five were positive at 3 weeks PCH, with bacteremia ranging from 333 to 15,338 (average, 6,409) organisms per 100 μl of blood (Table 1, group 8). Despite relatively low levels of bacteremia; all six mice were infectious to xenodiagnostic ticks at 2 weeks PCH, with the resulting prevalence of infection among ticks fed upon individual animals ranging from 20 to 75% (average, 40.0%). Five mice (the same as those that were PCR positive) transmitted the Gaillard isolate to 25 to 60% (average, 35%) of ticks during week 4 PCH (Table 2, group 8). These prevalence indices were significantly higher (P << 0.00001) than those obtained in groups of mice challenged with Gaillard isolate following recovery from infection with either Dawson (group 3) or Gaillard (group 6) isolates.
Seroconversion and dynamics of antibody titers in pathogen-naive control mice following the challenge were similar to those observed in mice infected with Dawson and Gaillard isolates at the beginning of this study. All seroconverted within two weeks after challenge; GMT peaked at week 4 PCH and remained relatively stable for the following 8-week duration of observation (Fig. 2 and 3). Spleen samples from all 12 mice tested by PCR at 10 weeks PCH yielded negative results.
DISCUSSION
A number of studies have assessed reinfection with and cross-protection between different strains and variants of A. phagocytophilum in ruminants, especially in Europe. That animals previously exposed to the pathogen acquire some degree of resistance is undoubted. What is not clear is whether an immune response to previous infection is fully protective against a reinfection or just decreases its severity. Different researchers reported different degrees and durations of cross-protection. Some animals are immune for a few months while others may resist reinfection (i.e., a symptomatic disease) for over a year (44). Hudson (18) claimed there was considerable cross-protection between bovine and ovine strains, while Foggie and Allison (13) found no evidence of immunity to ovine strains following infections with bovine strains and Tuomi (41) found that Finnish bovine strains did not confer protections against Scottish ovine strains and vice versa.
Stuen et al. (38) reported that sheep previously infected with one variant of A. phagocytophilum were to some degree protected against the other variant, while in a reverse challenge, all of the lambs developed high temperatures and displayed hematological reactions similar to those seen following primary infection. These results seem to demonstrate an inadequate protection against reinfection with heterologous isolates. Unfortunately, the authors did not confirm complete recovery from the primary infection before the challenge and challenged lambs only 6 weeks postinfection, when the original isolate may have still persisted. It is hard, therefore, to determine if the reported results were due to an immune response to the original infection or to persistence of the original strain.
It is also noteworthy that in most of the cited studies both the primary and the challenging exposures were instituted via needle inoculation of either infected blood or a cultured pathogen and not via the natural route of a tick bite. This is important because it has been repeatedly demonstrated that development and the severity of infection strongly depend both on the pathogen dose and on the inoculation route (7, 15, 32; Massung et al., submitted). Moreover, in the case of Ehrlichia (formerly Cowdria) ruminantium, the efficiency of immune protection against either homologous or heterologous reinfection varies significantly depending on the mode of the challenge. A larger proportion of immunized animals were susceptible to tick-borne reinfection with E. ruminantium than to inoculation of infectious blood (9). Therefore, protectiveness of naturally occurring immune response against a naturally occurring challenge may not be directly inferred from studies employing artificial routs of infection.
In this study, we sought to approximate a natural situation that may arise if humans or domestic animals previously infected with A. phagocytophilum again become exposed to infected ticks at the same or a different focus of infection. Therefore, we used naturally infected I. scapularis ticks as the source of both the original infection and the challenge instead of needle inoculation, even though this limited our ability to standardize the administered pathogen dose. The dose of A. phagocytophilum inoculated by an infected tick into the attachment site over 3 to 5 days of feeding on a host remains unknown. Thus, we were only able ensure that all experimental and control animals were exposed to a standard number of infected ticks from the same cohort. Variation of challenging doses presumably resulted in the wide variability in quantitative results of both blood PCR and xenodiagnosis. In nature, though, animals and humans are challenged by ticks that also administer unknown and unstandardized amounts of pathogen. In this way, our experimental design was a closer approximation to natural circumstance than a needle inoculation of a standard but arbitrary chosen amount of the pathogen.
We attempted to assess the degree of protection against a reinfection with homologous and heterologous isolates of A. phagocytophilum afforded by an immune response to a previous tick-borne infection. Although the two sympatric isolates clearly differ physiologically (23), distinctions between them on the molecular level are unknown. Thus, there are no direct methods to prove that the isolate detected in challenged animals is truly heterologous and not recrudescence of the primary isolate. It is especially important in view of the fact that the IgG titers do not significantly drop following primary infection, which may perhaps be consistent with persistence of the pathogen. In order to attribute differences observed between seropositive and naive animals to effects of the immune response and not to possible interaction between preexisting and challenging infections, we ensured that all animals completely recovered from the primary infection before the challenge. We previously observed that Dawson and Gaillard isolates of A. phagocytophilum persist in BALB/c mice for up to 9 and 12 weeks, respectively (23). Therefore, an infectious challenge in the present study was administered at 16 weeks postinfection—at least 1 month after the expected complete recovery of all animals from the primary infection. Recovery of all mice from the original infection and an absence of reactivation were further confirmed by the lack of A. phagocytophilum DNA in both the blood of unchallenged control mice and xenodiagnostic ticks feeding upon them at 16 to 26 weeks postinfection.
Our control groups of both unchallenged and pathogen-naive mice had a tick infestation schedule identical to that in the experimental groups but were fed upon by uninfected ticks. This ensured that the differences noted were due to the pathogen exposure and not to the tick exposure.
BALB/c mice originally exposed to either isolate seroconverted within two weeks and maintained surprisingly high titers of IgG antibodies (GMT > 3,000) for the following 14 weeks, until the challenge. In unchallenged mice, mean antibody titers began declining slowly at approximately 20 to 21 weeks postinfection. As stimuli that elicit antibody can also elicit cell-mediated immunity, it is difficult to determine the relative contributions of antibody- and cell-mediated immunity in protection of previously infected hosts against reinfection. Such determination was not an aim of this study, and we interpret titers of specific antibody simply as a measure of the total immune response to a natural infection with A. phagocytophilum.
In spite of a robust immune response to the primary infection and high titers of antibodies present in all mice at the time of challenge, the majority of the seropositive mice were susceptible to reinfection with either the same or a different isolate of A. phagocytophilum. However, both the levels of bacteremia and the prevalence of infection in xenodiagnostic ticks were much lower in seropositive mice than in control mice exposed to A. phagocytophilum for the first time.
Another study (Massung et al., submitted) has shown that amounts of A. phagocytophilum DNA in the circulating blood of nonimmune white-footed mice remain bellow detection levels during the feeding period of infecting nymphs (3 to 4 days). The amount of pathogen reaches and exceeds the threshold of PCR sensitivity only a few days after tick detachment, indicating that proliferation of the pathogen in a susceptible host is required for its successful detection by blood PCR, while the original amount of A. phagocytophilum inoculated by feeding ticks is bellow the threshold of blood PCR sensitivity. The fact that the amount of A. phagocytophilum DNA in the blood of most seropositive mice in our study surpassed the PCR sensitivity threshold following either homologous or heterologous challenges indicates that the agent actually proliferated in their blood in spite of the presence of antibodies in high titers.
An infectious challenge, either homologous or heterologous, elicited a quick memory response in all previously infected mice with antibody titers rising up to 4 logs above the prechallenge level within 1 to 2 weeks after placement of infected ticks. Within the next 2 to 4 weeks, however, antibody titers declined again to the prechallenge level. Thus, not only the immune response to a primary A. phagocytophilum infection did not protect mice from a challenge with either homologous or heterologous isolates, but also a rapid decline of the postchallenge IgG titers to the unprotective prechallenge level suggests the possibility of multiple reinfection events.
Nonetheless, the ensuing reinfection seemed to be significantly milder and of a shorter duration than the first infection with either isolate. These results are in agreement with a previous report that white-footed mice once recovered from A. phagocytophilum infection are only partially protected against reinfection with the same agent, yet become reservoir incompetent (unable to transmit the agent to uninfected ticks) for at least 3 to 4 months (22).
Robust antibody responses are typically generated against intracellular bacterial pathogens. In several cases, humoral responses have been reported to be partially or fully protective at least against a homologous challenge, notwithstanding artificial routes of inoculation in these studies (10, 11, 21, 25, 29, 31, 34). Humoral immunity during intracellular bacterial infections has often been considered paradoxical, because the infected host cells are thought to provide a protective niche for the pathogens.
Because there is no evidence that antibodies have direct access to the pathogens inside the host cell, it has been reasoned that they may be exposed to antibodies in the host extracellular milieu, perhaps during intercellular spreading (24). Such a mechanism would not necessarily prevent a reinfection per se but may limit the severity and longevity of ensuing disease. For example, persistent antibody titers against Ehrlichia canis in dogs do not infer protection against homologous or heterologous challenge (4, 5); however, immunized dogs become refractory to disease symptoms following challenge (35). Immunization of cattle with antigens derived from vaccine isolates of Babesia divergens or E. ruminantium resulted only in reduction of the severity of disease following a needle- or tick-borne challenge with heterologous isolates, but not in immunity against a reinfection (8, 40). Woldehiwet and Scott (44) found that sheep challenged when their antibody titers against A. phagocytophilum were above 16 did not develop clinical symptoms, irrespective of the time between first exposure and challenge, while those challenged after titers dropped below 16 reacted with parasitemia with or without fever. Results of our study are in agreement with these findings.
On the other hand, antibodies enhance the uptake of various rickettsiae by macrophages and granulocytes in vitro (43). Whether antibodies against A. phagocytophilum play the role of the Trojan horse in a seropositive animal remains to be ascertained.
Apparently, immune responses elicited in BALB/c mice by previous infection do not prevent a new transmission of A. phagocytophilum by ticks into the host and infection of neutrophils sequestered to the tick attachment site with ensuing proliferation of the pathogen inside infected cells. However, further intercellular spread of the pathogen in an immune organism seems impaired, which results in an abbreviated course of infection.
It is not clear whether the immune response to A. phagocytophilum in humans or domestic animals is similarly inefficient. GMT reported for humans in the United States usually do not exceed 2,560 (1, 3, 6, 33) and are lower than those reported in the present study. If austerity of antibody response corresponds with the degree of protection against a reinfection, humans may be even more susceptible to reinfection with A. phagocytophilum than the BALB/c mice in our study.
Acknowledgments
This study was supported in part by the James A. Ferguson Emerging Infectious Diseases Fellowship Program.
We extend our gratitude to Amanda Loftis and Alana Aisthorpe for much-appreciated assistance in various parts of the study. We also thank William Nicholson for providing A. phagocytophilum antigen for our serological assays.
Editor: D. L. Burns
REFERENCES
- 1.Aguero-Rosenfeld, M. E., F. Kalantarpour, M. Baluch, H. W. Horowitz, D. F. McKenna, J. T. Raffalli, T. C. Hsieh, J. Wu, J. S. Dumler, and G. P. Wormser. 2000. Serology of culture-confirmed cases of human granulocytic ehrlichiosis. J. Clin. Microbiol. 38:635-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asanovich, K. M., J. S. Bakken, J. E. Madigan, M. Aguero-Rosenfeld, G. P. Wormser, and J. S. Dumler. 1997. Antigenic diversity of granulocytic Ehrlichia isolates from humans in Wisconsin and New York and a horse in California. J. Infect. Dis. 176:1029-1034. [DOI] [PubMed] [Google Scholar]
- 3.Bakken, J. S., I. Haller, D. Riddell, J. J. Walls, and J. S. Dumler. 2002. The serological response of patients infected with the agent of human granulocytic ehrlichiosis. Clin. Infect. Dis. 34:22-27. [DOI] [PubMed] [Google Scholar]
- 4.Breitschwerdt, E. B., B. C. Hegarty, and S. I. Hancock. 1998. Doxycycline hyclate treatment of experimental canine ehrlichiosis followed by challenge inoculation with two Ehrlichia canis strains. Antimicrob. Agents Chemother. 42:362-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buhles, W. C., D. L. Huxsoll, and M. Ristic. 1974. Tropical canine pancytopenia: Clinical, hematologic, and serologic response of dogs to Ehrlichia canis infection, tetracycline therapy, and challenge inoculation. J. Infect. Dis. 130:357-67. [DOI] [PubMed] [Google Scholar]
- 6.Comer, J. A., W. L. Nicholson, J. G. Olson, and J. E. Childs. 1999. Serologic testing for human granulocytic ehrlichiosis at a national referral center. J. Clin. Microbiol. 37:558-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Souza, M. S., A. L. Smith, D. S. Beck, L. J. Kim, G. M. Hansen, Jr., and S. W. Barthold. 1993. Variant responses of mice to Borrelia burgdorferi depending on the site of intradermal inoculation. Infect. Immun. 61:4493-4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dumler, J. S., K. M. Asanovich, and J. S. Bakken. 2003. Analysis of genetic identity of North American Anaplasma phagocytophilum strains by pulsed-field gel electrophoresis. J. Clin. Microbiol. 41:3392-3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du Plessis, J. L., and L. Van Gas. 1989. Immunity of tick-exposed seronegative and seropositive small stock challenged with two stocks of Cowdria ruminantium. Onderstepoort J. Vet. Res. 56:185-188. [PubMed] [Google Scholar]
- 10.Edelson, B. T., P. Cossart, and E. R. Unanue. 1999. Cutting edge: paradigm revisited: antibody provides resistance to Listeria infection. J. Immunol. 163:4087-4090. [PubMed] [Google Scholar]
- 11.Eisenstein, T. K., L. M. Killar, and B. M. Sultzer. 1984. Immunity to infection with Salmonella typhimurium: mouse-strain differences in vaccine- and serum-mediated protection. J. Infect. Dis. 150:425-435. [DOI] [PubMed] [Google Scholar]
- 12.Foggie, A. 1951. Studies on the infectious agent of tick-borne fever in sheep. J. Pathol. Bacteriol. 63:1-15. [DOI] [PubMed] [Google Scholar]
- 13.Foggie, A., and A. J. Allison. 1960. A note on the occurrence of tick-borne fever in cattle in Scotland with comparative studies of bovine and ovine strains of the organism. Vet. Rec. 72:767-770. [Google Scholar]
- 14.Foster, W. N., and A. E. Cameron. 1970. Observations on ovine strains of tick-borne fever. J. Comp. Pathol. 80:429-436. [DOI] [PubMed] [Google Scholar]
- 15.Gern, L., U. E. Schaible, and M. M. Simon. 1993. Mode of inoculation of the Lyme disease agent Borrelia burgdorferi influences infection and immune responses in inbred strains of mice. J. Infect. Dis. 167:971-975. [DOI] [PubMed] [Google Scholar]
- 16.Gordon, W. S., A. Brownlee, D. R. Wilson, and J. MacLeod. 1932. “Tick-borne fever” (a hitherto undescribed disease of sheep). J. Comp. Pathol. Ther. 45:301-312. [Google Scholar]
- 17.Horowitz, H. W., M. Aguero-Rosenfeld, J. S. Dumler, D. F. McKena, T. C. Hsieh, J. Wu, I. Schwartz, and G. P. Wormser. 1998. Reinfection with the agent of human granulocytic ehrlichiosis. Ann. Intern. Med. 129:461-463. [DOI] [PubMed] [Google Scholar]
- 18.Hudson, J. R. 1950. The recognition of tick-borne fever as a disease in cattle. Br. Vet. J. 106:3-17. [Google Scholar]
- 19.Inokuma, H., P. Brouqui, J. S. Dumler, and D. Raoult. 2003. Serotyping isolates of Anaplasma phagocytophilum by using monoclonal antibodies. Clin. Diagn. Lab. Immunol. 10:969-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jamieson, S. 1947. Some aspects of immunity to tick-borne fever in hoggs. Vet. Rec. 59:201-202. [PubMed] [Google Scholar]
- 21.Kosoy, M. Y., R. L. Regnery, O. I. Kosaya, and J. E. Childs. 1999. Experimental infection of cotton rats with three naturally occurring Bartonella species. J. Wildl. Dis. 35:275-84. [DOI] [PubMed] [Google Scholar]
- 22.Levin, M. L., and D. Fish. 2000. Immunity reduces reservoir host competence of Peromyscus leucopus for Ehrlichia phagocytophila. Infect. Immun. 68:1514-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levin, M. L., and D. E. Ross. 2004. Acquisition of different isolates of Anaplasma phagocytophilum by Ixodes scapularis from a model animal. Vector Borne Zoonotic Dis. 4:53-59. [DOI] [PubMed] [Google Scholar]
- 24.Li, J. S. Y., and G. M. Winslow. 2003. Survival, replication, and antibody susceptibility of Ehrlichia chaffeensis outside of host cells. Infect. Immun. 71:4229-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li, J. S. Y., E. Yager, M. Reilly, C. Freeman, G. R. Reddy, A. A. Reilly, F. K. Chu, and G. M. Winslow. 2001. Outer membrane protein-specific monoclonal antibodies protect SCID mice from fatal infection by the obligate intracellular bacterial pathogen Ehrlichia chaffeensis. J. Immunol. 166:1855-1862. [DOI] [PubMed] [Google Scholar]
- 26.Littlejohn, A. I. 1950. Tick-borne fever as a cause of abortion in sheep. Vet. Rec. 62:577-579. [DOI] [PubMed] [Google Scholar]
- 27.Massung, R. F., J. H. Owens, D. Ross, K. D. Reed, M. Petrovec, A. Bjoersdorff, R. T. Coughlin, G. A. Beltz, and C. I. Murphy. 2000. Sequence analysis of the ank gene of granulocytic ehrlichiae. J. Clin. Microbiol. 38:2917-2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Massung, R. F., and K. G. Slater. 2003. Comparison of PCR assays for detection of the agent of human granulocytic ehrlichiosis, Anaplasma phagocytophilum. J. Clin. Microbiol. 41:717-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McSorley, S. J., and M. K. Jenkins. 2000. Antibody is required for protection against virulent but not attenuated Salmonella enterica serovar Typhimurium. Infect. Immun. 68:3344-3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicholson, W. L., J. A. Comer, J. W. Sumner, C. Gingrich-Baker, R. T. Coughlin, L. A. Magnarelli, J. G. Olson, and J. E. Childs. 1997. An indirect immunofluorescence assay using a cell culture-derived antigen for detection of antibodies to the agent of human granulocytic ehrlichiosis. J. Clin. Microbiol. 35:1510-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nyindo, M. B., M. Ristic, G. E. Lewis, Jr., D. L. Huxsoll, and E. H. Stephenson. 1978. Immune response of ponies to experimental infection with Ehrlichia equi. Am. J. Vet. Res. 39:15-18. [PubMed] [Google Scholar]
- 32.Pusterla, N., C. M. Leutenegger, J. S. Chae, H. Lutz, R. B. Kimsey, J. S. Dumler, and J. E. Madigan. 1999. Quantitative evaluation of ehrlichial burden in horses after experimental transmission of human granulocytic Ehrlichia agent by intravenous inoculation with infected leukocytes and by infected ticks. J. Clin. Microbiol. 37:4042-4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ravyn, M. D., J. L. Goodman, C. B. Kodner, D. K. Westad, L. A. Coleman, S. M. Engstrom, C. M. Nelson, and R. C. Johnson. 1998. Immunodiagnosis of human granulocytic ehrlichiosis by using culture-derived human isolates. J. Clin. Microbiol. 36:1480-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Regnery, R. L., J. A. Rooney, A. M. Johnson, S. L. Nesby, P. Manzewitsch, K. Beaver, and J. G. Olson. 1996. Experimentally induced Bartonella henselae infections followed by challenge exposure and antimicrobial therapy in cats. Am. J. Vet. Res. 57:1714-1719. (Erratum, 58:803, 1997.) [PubMed] [Google Scholar]
- 35.Ristic, M., and C. J. Holland. 1993. Canine ehrlichiosis, p. 169-186. In Z. Woldehiwet and M. Ristic (ed.), Rickettsial and chlamydial diseases of domestic animals. Pergamon Press, Oxford, United Kingdom.
- 36.Snodgrass, D. R., and S. Ramachandran. 1971. A complement fixation test for tick-borne fever of sheep. Br. Vet. J. 127(9):44-46. [DOI] [PubMed] [Google Scholar]
- 37.Stuen, S., K. Artursson, and E. O. Engvall. 1998. Experimental infection of lambs with an equine granulocytic Ehrlichia species resembling the agent that causes human granulocytic ehrlichiosis (HGE). Acta. Vet. Scand. 39:491-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stuen, S., K. Bergstrom, M. Petrovec, I. Van de Pol, and L. M. Schouls. 2003. Differences in clinical manifestations and hematological and serological responses after experimental infection with genetic variants of Anaplasma phagocytophilum in sheep. Clin. Diagn. Lab. Immunol. 10:692-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sumner, J. W., W. L. Nicholson, and R. F. Massung. 1997. PCR amplification and comparison of nucleotide sequences from the groESL heat shock operon of Ehrlichia species. J. Clin. Microbiol. 35:2087-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor, S. M., C. T. Elliott, and J. Kenny. 1986. Babesia divergens: sequential exposure to heterologous tick-borne challenge of cattle immunized with a fraction of parasitized erythrocytes. J. Comp. Pathol. 96:101-107. [DOI] [PubMed] [Google Scholar]
- 41.Tuomi, J. 1967. Experimental studies on bovine tick-borne fever. 3. Immunological strain difference. Acta Pathol. Microbiol. Scand. 71:89-100. [PubMed] [Google Scholar]
- 42.Tuomi, J. 1966. Studies in epidemiology of bovine tick-borne fever in Finland and a clinical description of field cases. Ann. Med. Exp. Biol. Fenn. 44(Suppl. 6):61-62. [PubMed] [Google Scholar]
- 43.Woldehiwet, Z., and M. Ristic (ed.). 1993. Rickettsial and chlamydial diseases of domestic animals, p. 1-26. Pergamon Press, Oxford, United Kingdom.
- 44.Woldehiwet, Z., and G. R. Scott. 1982. Immunological studies on tick-borne fever in sheep. J. Comp. Pathol. 92:457-467. [DOI] [PubMed] [Google Scholar]