Abstract
We previously showed the requirement of both T cells and gamma interferon (IFN-γ)-producing non-T cells for the genetic resistance of BALB/c mice to the development of toxoplasmic encephalitis (TE). In order to define the role of IFN-γ production and the perforin-mediated cytotoxicity of T cells in this resistance, we obtained immune T cells from spleens of infected IFN-γ knockout (IFN-γ−/−), perforin knockout (PO), and wild-type BALB/c mice and transferred them into infected and sulfadiazine-treated athymic nude mice, which lack T cells but have IFN-γ-producing non-T cells. Control nude mice that had not received any T cells developed severe TE and died after discontinuation of sulfadiazine treatment due to the reactivation of infection. Animals that had received immune T cells from either wild-type or PO mice did not develop TE and survived. In contrast, nude mice that had received immune T cells from IFN-γ−/− mice developed severe TE and died as early as control nude mice. T cells obtained from the spleens of animals that had received either PO or wild-type T cells produced large amounts of IFN-γ after stimulation with Toxoplasma gondii antigens in vitro. In addition, the amounts of IFN-γ mRNA expressed in the brains of PO T-cell recipients did not differ from those in wild-type T-cell recipients. Furthermore, PO mice did not develop TE after infection, and their IFN-γ production was equivalent to or higher than that of wild-type animals. These results indicate that IFN-γ production, but not perforin-mediated cytotoxic activity, by T cells is required for the prevention of TE in genetically resistant BALB/c mice.
Toxoplasma gondii, an obligate intracellular protozoan parasite, forms cysts and establishes a latent chronic infection preferentially in the brain after the replication of tachyzoites in various organs during the acute stage of infection. Chronic infection with this parasite is likely the most common infection in humans. The requirement for the immune system to maintain the latency of persistent infection is clearly evident from the reactivation of the infection in immunocompromised individuals, which results in the development of life-threatening toxoplasmic encephalitis (TE) (18, 44). Murine models have been used to analyze the mechanisms of host resistance to TE. C57BL/6 (H-2b) and CBA/Ca (H-2k) mice have often been used for these studies (9, 13, 16, 32, 46). However, these strains of mice are genetically susceptible and spontaneously develop progressive and ultimately fatal TE (4, 33, 34). In contrast to these susceptible strains, genetically resistant strains (e.g., BALB/c [H-2d] mice) are able to control T. gondii infections in their brains and develop a latent chronic infection, as do immunocompetent humans (4, 33, 34). Therefore, these strains of mice appear to be a suitable model for analyzing the mechanisms of host resistance that maintain a chronic infection and prevent TE.
We have developed a murine model of reactivation of T. gondii infection in the brain, using infected, sulfadiazine-treated BALB/c-background gamma interferon (IFN-γ) knockout (IFN-γ−/−) and athymic nude mice (21, 35). Our recent studies with these animal models demonstrated the requirement of both T cells and IFN-γ-producing non-T cells in the brain for prevention of the reactivation of infection and the development of TE (21). However, the function(s) of T cells that is important for this resistance still needs to be defined. In collaboration with IFN-γ-producing non-T cells, a function(s) of T cells other than IFN-γ production may play a critical role in resistance. The cytotoxic activity of T cells (15, 22, 31) may be a function that is crucial for the prevention of TE. In relation to this, Denkers et al. (10) reported that the cytotoxic activity mediated by perforin plays a limited role in the resistance to T. gondii infection in C57BL/6 mice which are genetically susceptible to TE. However, it is possible that the cytotoxic activity of T cells plays a more critical role in the prevention of TE in genetically resistant BALB/c than in susceptible C57BL/6 mice and hence that BALB/c mice are resistant to TE. It is also possible that IFN-γ production by T cells, in addition to IFN-γ production by non-T cells, is required for the prevention of TE since IFN-γ has been shown to play a central role in controlling T. gondii in the brain (12, 32, 35). An unrecognized function(s) of T cells may also be critical for the prevention of TE.
In the present study, we investigated the role of IFN-γ production and the perforin-mediated cytotoxic activity of T cells in the prevention of reactivation of chronic infections with T. gondii in the brains of genetically resistant BALB/c mice. We found that IFN-γ production by T cells is essential for resistance to control the parasite in the brain and that the cytotoxic activity is dispensable for protective activity.
MATERIALS AND METHODS
Mice.
Female BALB/c-background IFN-γ−/−, athymic nude, and wild-type BALB/c mice were obtained from The Jackson Laboratory (Bar Harbor, Maine). Female Swiss-Webster mice were obtained from Taconic (Germantown, N.Y). BALB/c-background perforin knockout (PO) mice (43) were kindly provided by John T. Harty (University of Iowa, Iowa City) and were bred in our animal facility. Females were used for all studies. All mice were housed under specific-pathogen-free conditions and were 8 to 12 weeks old when used. There were four to six mice in each experimental group.
Infection with T. gondii.
Cysts of the T. gondii ME49 strain were obtained from brains of Swiss-Webster mice that had been infected intraperitoneally with 10 cysts for 2 to 3 months. The mice were euthanized by asphyxiation with CO2, and their brains were removed and triturated in phosphate-buffered saline (pH 7.2) (37). An aliquot of the brain suspension was examined for numbers of cysts, and after appropriate dilution of the aliquot in phosphate-buffered saline, all animals were infected with 10 cysts perorally by gavage. Mice were treated with sulfadiazine in their drinking water (400 mg/liter) beginning 4 days (for IFN-γ−/− mice) or 7 days (for nude, PO, and wild-type BALB/c mice) after infection for 3 to 4 weeks. In some experiments, PO and wild-type mice did not receive sulfadiazine after infection.
Purification and transfer of immune T cells.
Four or 5 weeks after infection, spleen cells were obtained from three or four IFN-γ−/−, PO, and wild-type mice, suspended in Hanks' balanced salt solution (Irvine Scientific, Santa Ana, Calif.) with 2% fetal bovine serum (Sigma Chemical Co., St. Louis, Mo.), and pooled within the same groups. The total T-cell population was purified by treating the spleen cells with magnetic bead-conjugated anti-mouse CD4 (GK1.5) and anti-mouse CD8 (53-6.7) monoclonal antibodies (Miltenyi Biotec, Auburn, Calif.) (21). The purity of the T cells in each of the purified preparations was >95%. A total of 107 T cells were injected intravenously into a tail vein in recipient nude mice 9 and 2 days before the discontinuation of treatment with sulfadiazine.
Culture of T and spleen cells and detection of IFN-γ in culture supernatants.
T cells were purified (as described above) from the spleens of nude mice that survived for 90 days after the discontinuation of sulfadiazine treatment. T cells were also purified from the spleens of PO and wild-type mice 6 months after infection. Culturing of the purified T and spleen cells with T. gondii antigens (Ags) was performed as previously described (5). Briefly, the cells were suspended in RPMI 1640 (Sigma Chemical Co.) with 10% fetal bovine serum (HyClone, Logan, Utah), penicillin (100 U/ml), and streptomycin (100 μg/ml), placed into flat-bottomed 96-well plates (Costar, Cambridge, Mass.) at a cell density of 4 × 105 per well, and incubated with or without soluble T. gondii lysate Ags (20 μg/ml) in a final volume of 200 μl per well for 72 h. Thereafter, the culture plates were centrifuged and the culture supernatants were collected. The concentration of IFN-γ in the culture supernatants was measured by an enzyme-linked immunosorbent assay using monoclonal antibodies against IFN-γ (R4-6A2 as a capture antibody and XMG1.2 as a secondary antibody) (PharMingen, San Diego, Calif.), as described previously (39).
Histopathology.
When nude mice in the experimental groups developed clinical signs of illness after the discontinuation of sulfadiazine treatment, the animals were euthanized (5 or 6 days after discontinuation of the treatment). Their brains were removed and immediately fixed in a solution containing 10% Formalin, 70% ethanol, and 5% acetic acid. When the nude mice in experimental groups did not develop clinical signs of illness after the discontinuation of treatment with sulfadiazine, the animals were euthanized for a histopathology study of the brains 90 days after the discontinuation of treatment. Two to four 5-μm-thick sagittal sections (50 or 100 μm between sections) of the brain from each mouse were stained with hematoxylin and eosin. Immunoperoxidase staining with rabbit anti-Toxoplasma immunoglobulin G (IgG) was used for the detection of tachyzoites (8). Sections stained with hematoxylin and eosin were evaluated for inflammatory changes. Sections stained by the immunoperoxidase method were evaluated for numbers of inflammatory areas associated with tachyzoites. The sections stained by the immunoperoxidase method were also used to evaluate the numbers of T. gondii cysts in the brain when no acute inflammatory changes were observed. The mean value from these sections for each mouse was calculated as the number per section. These values were used for statistical analyses to compare differences between groups.
Semiquantitative reverse transcription-PCR for detection of mRNA for IFN-γ.
Ninety days after the discontinuation of sulfadiazine treatment, RNA was isolated from the brains of infected nude mice by the use of RNA STAT-60 (TEL-TEST “B,” Inc., Friendswood, Tex.) according to the manufacturer's instructions. cDNA was synthesized from the RNA as described previously (33, 39). PCR for β-actin and IFN-γ was performed with 5 μl of the original cDNA reaction mixture with a Mastercycler 5333 (Eppendorf AG, Hamburg, Germany) for 30 cycles to produce an amount of DNA within a linear range, as described previously (33, 39). This number of cycles was determined by preliminary studies using different amounts of sample cDNA. Specific primers for β-actin and IFN-γ designed to span at least one intron allowed for the differentiation of amplified target DNA derived from either cDNA or genomic DNA in the PCR.
The homology of PCR products to the predicted transcript sequence was examined by Southern blot analysis (33, 39). Ten-microliter aliquots of the final PCR mixtures were electrophoresed at 100 V for 1 h in a 1.5% agarose gel and then were denatured. The DNA was then transferred to a Duralon-UV membrane (Stratagene, La Jolla, Calif.) by a standard blotting procedure (29) and was UV cross-linked. Oligonucleotide probes for β-actin and IFN-γ which hybridized to the PCR products wholly within the region amplified by the primers were end labeled as described for a 3′ end labeling and signal amplification system, and hybridization was detected by scanning of the membranes with an Image Station 440 CF instrument (Eastman Kodak Company, Rochester, N.Y.) as described previously (38). The quantification of mRNA was performed by a densitometry analysis with the Image Station, and values were normalized to the β-actin level.
Statistical analysis.
Levels of significance for numbers of areas associated with tachyzoites, cyst numbers in the brain, and the amounts of IFN-γ in the culture supernatants were determined by Student's t test, the alternate Welch t test, or the Wilcoxon rank sum test. The alternative Welch t test was applied when standard deviations were significantly different between the tested groups. The Wilcoxon rank sum test was applied when the standard deviation was zero. Levels of significance for mortality in mice were determined by Fisher's exact test. Differences which provided P values of <0.05 were considered significant.
RESULTS
Effect of adoptive transfer of T cells from IFN-γ−/− mice on mortality and development of TE in infected athymic nude mice.
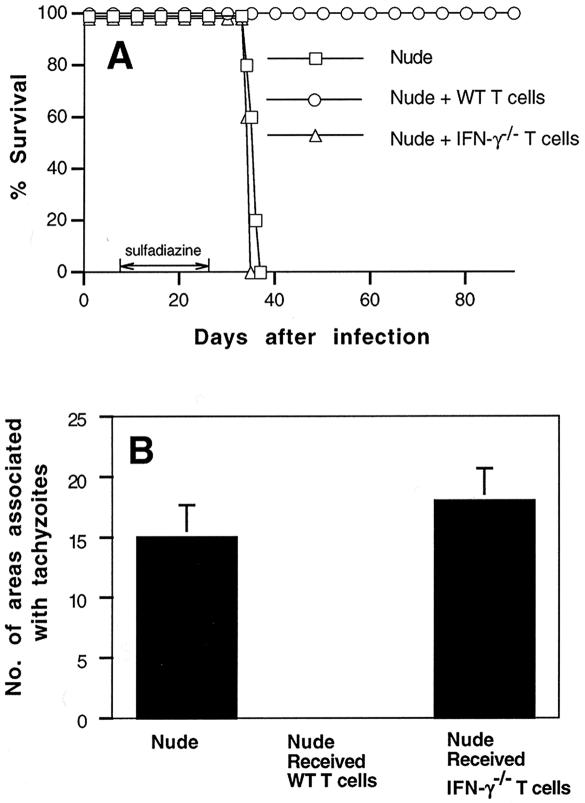
We previously reported that non-T cells which produce IFN-γ and T cells are both required for the genetic resistance of BALB/c mice to prevent the development of TE (21). To analyze whether IFN-γ production by T cells, in addition to that by non-T cells, is required for this resistance, we purified immune T cells from spleens of infected IFN-γ−/− and wild-type mice and injected the purified cells (107) intravenously into infected, sulfadiazine-treated athymic nude mice. As shown previously (21), the infected nude mice had IFN-γ-producing non-T cells in their brains. Adoptive transfer of the immune T cells was performed 9 and 2 days before the discontinuation of sulfadiazine treatment. Control nude mice that had not received the cell transfer all died within 10 days after the discontinuation of sulfadiazine treatment (Fig. 1A). The animals that had received T cells from wild-type donors all survived (Fig. 1A). In contrast, nude mice that had received T cells from IFN-γ−/− mice all died as early as the control animals that had not received the cell transfer (P < 0.01 when compared to wild-type T-cell recipients) (Fig. 1A). Histological studies performed 5 or 6 days after the discontinuation of sulfadiazine treatment revealed large numbers of focal areas associated with tachyzoites in the brains of both control nude mice without cell transfer and those that had received IFN-γ−/− T cells (Fig. 1B and 2A). In contrast, no such areas were observed in the brains of nude mice that had received wild-type T cells when they were examined in the same manner as the animals in the other groups (P < 0.05 when compared to either IFN-γ−/− T-cell recipients or controls without cell transfer) (Fig. 1B).
FIG. 1.
Mortality (A) and development of TE (B) in T. gondii-infected, sulfadiazine-treated athymic nude mice with adoptive transfer of immune T cells from infected IFN-γ−/− or wild-type BALB/c mice. Athymic nude mice were infected with 10 cysts of the ME49 strain perorally and were treated with sulfadiazine for 3 weeks, beginning 7 days after infection. Nine and 2 days before the discontinuation of sulfadiazine treatment, mice received an intravenous injection of 107 immune T cells from infected wild-type or IFN-γ−/− mice (see Materials and Methods). Histological studies were performed 5 or 6 days after the discontinuation of treatment with sulfadiazine. Two to four sagittal sections (distance between sections, 50 μm) were stained with an immunoperoxidase stain by using rabbit anti-T. gondii IgG and were evaluated for the numbers of areas of inflammation associated with tachyzoites. The mean value from these sections for each mouse was calculated as the number per section. These values are shown in the figure and were used for statistical analysis to compare differences between groups. The data shown are representative of two separate experiments. There were four to five mice in each experimental group in each experiment.
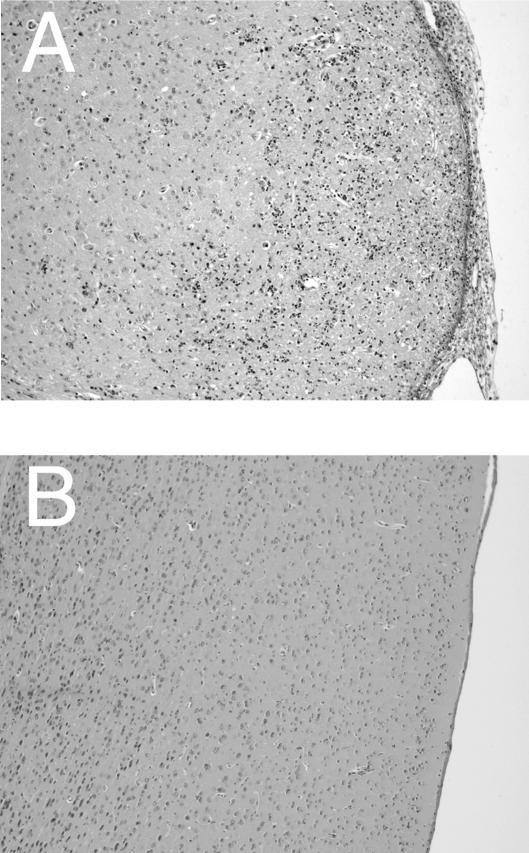
FIG. 2.
Histological changes in brains of T. gondii-infected, sulfadiazine-treated athymic nude mice with adoptive transfer of immune T cells from infected IFN-γ−/− (A) or PO (B) mice. Athymic nude mice were infected with 10 cysts of the ME49 strain perorally and were treated with sulfadiazine for 3 weeks, beginning 7 days after infection. Nine and 2 days before the discontinuation of sulfadiazine treatment, mice received an intravenous injection of 107 immune T cells from the donor animals (see Materials and Methods). Histological studies were performed 6 days (the recipients of IFN-γ−/− T cells) or 90 days (the recipients of PO T cells) after the discontinuation of treatment with sulfadiazine. Sections were stained with hematoxylin and eosin. The experiments were performed twice, and there were four to six mice in each group in each experiment.
Effect of adoptive transfer of T cells from PO mice on mortality and development of TE in infected athymic nude mice.
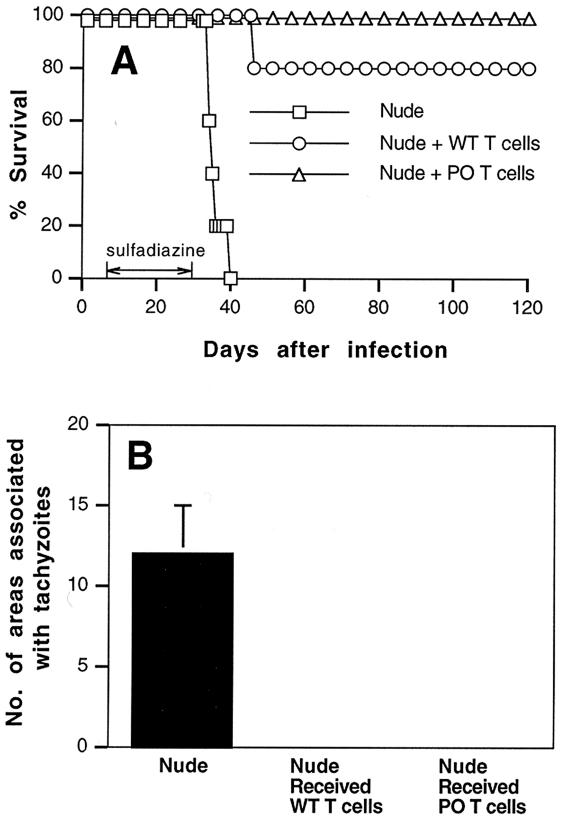
Next, we examined whether the perforin-mediated cytotoxic activity of T cells also plays an important role in the prevention of TE. For this purpose, purified immune T cells (107) from infected PO and wild-type mice were transferred to infected, sulfadiazine-treated nude mice. Most animals that had received T cells from wild-type mice survived until the end of the observation period (90 days after the discontinuation of sulfadiazine treatment) (Fig. 3A). Nude mice that had received T cells from PO animals also all survived after the discontinuation of sulfadiazine treatment, whereas control nude mice that had not received any T cells all died within 10 days after the discontinuation of sulfadiazine (P < 0.01) (Fig. 3A). Histological studies performed at the end of the observation period revealed no inflammatory changes or only a mild infiltration of inflammatory cells in limited areas in the brains of nude mice that had received immune T cells from either PO or wild-type animals (Fig. 2B and 3B). No inflammatory areas associated with tachyzoites were observed in the brains from these groups of mice. In contrast, large numbers of areas associated with tachyzoites were observed in the brains of control nude mice without cell transfer when they developed clinical signs of illness (P < 0.05 when compared to animals that received either PO T cells or wild-type T cells).
FIG. 3.
Mortality (A) and development of TE (B) in T. gondii-infected, sulfadiazine-treated athymic nude mice with adoptive transfer of immune T cells from infected PO or wild-type BALB/c mice. Athymic nude mice were infected with 10 cysts of the ME49 strain perorally and were treated with sulfadiazine for 3 weeks, beginning 7 days after infection. Nine and 2 days before the discontinuation of sulfadiazine treatment, mice received an intravenous injection of 107 immune T cells from infected wild-type or PO mice (see Materials and Methods). Histological studies were performed 90 days after the discontinuation of treatment with sulfadiazine. For control animals without cell transfer, the histological studies were done 6 or 7 days after discontinuation of the treatment. Two to four sagittal sections (distance between sections, 50 μm) were stained with an immunoperoxidase stain by using rabbit anti-Toxoplasma IgG and were evaluated for the numbers of areas of inflammation associated with tachyzoites. The mean value from these sections for each mouse was calculated as the number per section. These values are shown in the figure and were used for statistical analysis to compare differences between groups. The data shown are representative of two separate experiments. There were four to six mice in each experimental group in each experiment.
Comparison of IFN-γ production between splenic T cells obtained from nude mice that received immune T cells from PO or wild-type donors.
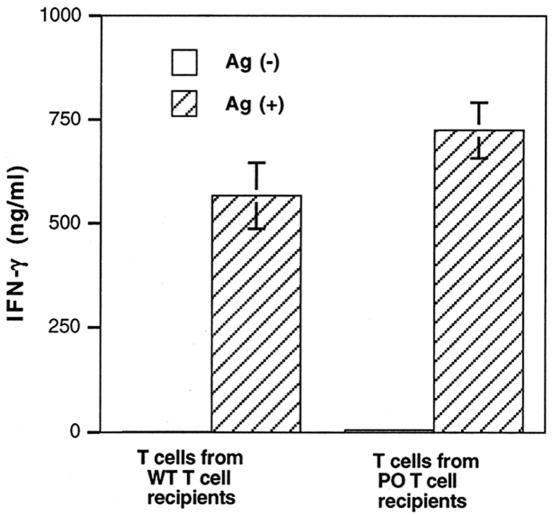
Ninety days after the discontinuation of sulfadiazine treatment, T cells were purified from the spleens of nude mice that had received immune T cells from PO or wild-type animals. These were the groups of animals that prevented TE and survived until the time that the study was performed (Fig. 3A). The T cells were then stimulated with tachyzoite Ags in vitro to evaluate their IFN-γ production. Because nude mice originally lacked T cells before the cell transfer, the T cells purified from the recipient animals were all of donor origin. T cells obtained from the animals that had received PO T cells produced large amounts of IFN-γ when they were stimulated with Ags (Fig. 4). The amounts of IFN-γ produced by these T cells did not differ from those produced by T cells obtained from nude mice that had received immune T cells from wild-type animals (Fig. 4).
FIG. 4.
Production of IFN-γ by T cells purified from spleens of nude mice that had received immune T cells from PO or wild-type BALB/c mice. Athymic nude mice were infected with 10 cysts of the ME49 strain perorally and were treated with sulfadiazine for 3 weeks, beginning 7 days after infection. Nine and 2 days before the discontinuation of sulfadiazine treatment, mice received an intravenous injection of 107 immune T cells from infected wild-type or PO mice (see Materials and Methods). Ninety days after the discontinuation of sulfadiazine, T cells were purified from the spleens of the recipient animals and then stimulated with tachyzoite lysate Ags (20 μl/ml) in the presence of antigen-presenting cells (plastic-adherent cells from spleens of normal BALB/c mice) (see Materials and Methods). The experiment shows means ± standard deviations of triplicate cultures. The data shown are representative of two separate experiments.
Comparison of IFN-γ expression in brains of nude mice that received immune T cells from PO and wild-type donors.
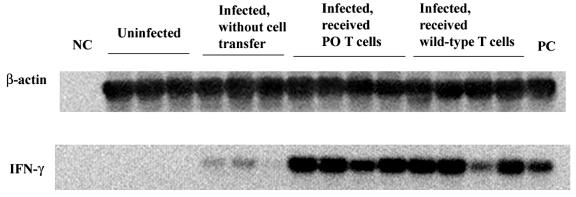
The amounts of mRNA for IFN-γ in the total RNA fractions obtained from the brains of nude mice that had received immune T cells from PO mice and from those that had received wild-type T cells were measured by reverse transcription-PCR 90 days after the discontinuation of sulfadiazine. Large amounts of IFN-γ mRNA were detected in the brains for these two groups of animals (Fig. 5), and the amounts of mRNA did not differ between these animals (IFN-γ/β-actin ratio, 0.271 ± 0.077 for PO T-cell recipients and 0.183 ± 0.041 for wild-type T-cell recipients; P = 0.091). Without cell transfer, only small amounts of mRNA for this cytokine were detectable in the brains of infected nude mice when they were treated with sulfadiazine (Fig. 5) (IFN-γ/β-actin ratio, 0.040 ± 0.012; P < 0.001 when compared to PO T-cell recipients and P < 0.005 when compared to wild-type T-cell recipients).
FIG. 5.
Expression of IFN-γ mRNA in brains of T. gondii-infected, sulfadiazine-treated nude mice after adoptive transfer of immune T cells from infected PO or wild-type BALB/c mice. Athymic nude mice were infected with 10 cysts of the ME49 strain perorally and were treated with sulfadiazine for 3 weeks, beginning 7 days after infection. Nine and 2 days before the discontinuation of sulfadiazine treatment, mice received an intravenous injection of 107 immune T cells from donor animals (see Materials and Methods). Ninety days after the discontinuation of sulfadiazine treatment, their brains were analyzed for the amounts of mRNAs for β-actin and IFN-γ (see Materials and Methods). NC, negative control; PC, positive control. There were three or four mice in each experimental group.
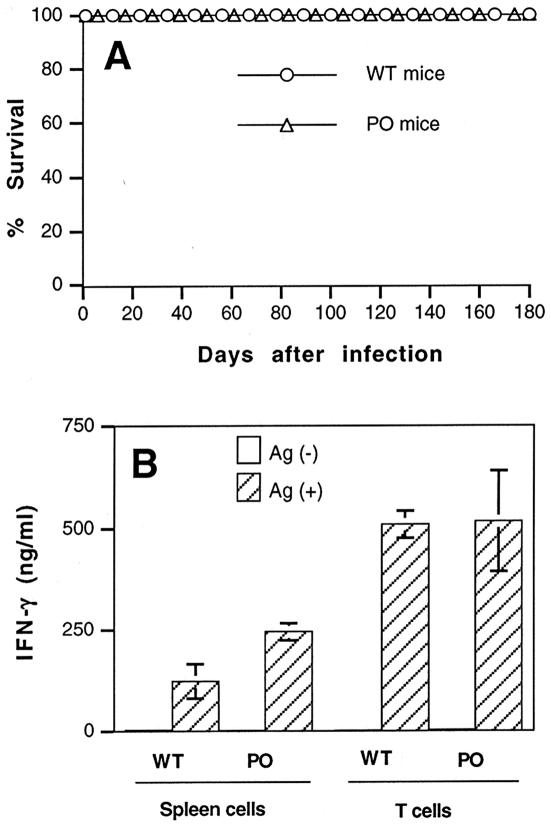
Mortality and histological changes in the brain in PO mice after infection without treatment with sulfadiazine.
PO and wild-type mice were infected with 10 cysts, and their mortality was monitored for 6 months. All animals in both groups survived until the end of the observation period (Fig. 6A). Histological studies performed at the end of the observation period revealed no inflammatory changes or only a mild infiltration of inflammatory cells in limited areas of the brain, mainly the meninges, for both PO and wild-type animals. In addition, there were no inflammatory areas associated with tachyzoites in the brains of both groups of animals. The numbers of cysts observed in the brains were small for both groups of animals, and there were no differences in cyst numbers (number of cysts/sagittal section, 0.33 ± 0.47 for wild-type controls [n = 4] and 0.58 ± 0.56 for PO mice [n = 4]).
FIG. 6.
Mortality (A) and production of IFN-γ by T and spleen cells (B) in infected PO and wild-type BALB/c mice. Mice were infected with 10 cysts of the ME49 strain perorally. T cells were purified from the spleens of the animals 6 months after infection and then stimulated with tachyzoite lysate Ags (20 μl/ml) in the presence of antigen-presenting cells (plastic-adherent cells from spleens of normal BALB/c mice) (see Materials and Methods). Total spleen cells were also stimulated with the lysate Ags. The experiment shows means ± standard deviations of triplicate cultures. The data shown are representative of two separate experiments.
Comparison of IFN-γ production by T cells and spleen cells in infected PO and wild-type mice.
T cells and spleen cells were obtained from PO and wild-type mice 6 months after infection and then were stimulated with tachyzoite Ags in vitro for an evaluation of their IFN-γ production. Large amounts of IFN-γ were detected in the culture supernatants of T cells from both groups of animals, and there were no differences in the amounts of cytokine detected between these groups (Fig. 6B). In contrast, the amounts of IFN-γ in the culture supernatants of spleen cells were twice as large in the cells obtained from PO mice than in those obtained from wild-type animals (P < 0.02) (Fig. 6B). The relative percentages of T cells in total spleen cells, calculated by the numbers of T cells purified, did not differ between these groups of mice (data not shown).
DISCUSSION
We investigated the functions of T cells that are required for the genetic resistance of BALB/c mice against the reactivation of chronic infection with T. gondii in the brain. Our previous studies demonstrated the requirement of both T cells and IFN-γ-producing non-T cells for this resistance (21). The present study reveals that IFN-γ production by T cells, in addition to non-T cells, is essential for maintaining the latency of chronic infection and for preventing TE. The adoptive transfer of immune T cells from infected IFN-γ−/− mice failed to prevent the development of TE and mortality in infected, sulfadiazine-treated athymic nude mice which originally had IFN-γ-producing non-T cells. Their development of the disease and mortality occurred as early as 1 week after the discontinuation of sulfadiazine treatment. In contrast to the lack of protective activity of IFN-γ−/− immune T cells, the adoptive transfer of immune T cells from infected PO mice prevented TE and mortality in recipient nude mice, as did the transfer of immune T cells from wild-type BALB/c mice. It has been reported that T. gondii infection induces cytotoxic T cells that lyse infected host cells (15, 22, 31). However, the results of the present study clearly demonstrate that the perforin-mediated cytotoxic activity of T cells is not required to prevent the reactivation of a chronic infection with this parasite in the brain.
T cells obtained from the spleens of nude mice that had received PO immune T cells produced large amounts of IFN-γ which were equivalent to those produced by T cells obtained from animals that had received wild-type immune T cells. These results indicate that a lack of perforin-mediated cytotoxicity in T cells did not affect their IFN-γ production in response to T. gondii. This was further supported by the evidence that IFN-γ mRNA expression did not differ in brains from the two groups of mice that had received either PO or wild-type immune T cells. This unimpaired ability of PO T cells to produce IFN-γ is likely the reason for their potent protective activity for the prevention of TE. Our findings are consistent with the results of others showing an unimpaired production of IFN-γ by PO lymphocytes when they are stimulated by allogeneic spleen cells (23). We previously reported that the adoptive transfer of either CD4+ or CD8+ T cells prevented the development of TE in infected, sulfadiazine-treated nude mice (21). Therefore, IFN-γ production by both CD4+ and CD8+ T cells likely contributes to this resistance. The contribution of both CD4+ and CD8+ T cells to resistance against T. gondii in the brain was also suggested in a T-cell depletion study in C57BL/6 mice that were genetically susceptible to TE (12).
Nude mice that had received T cells from PO mice lacked perforin-mediated cytotoxic activity of T cells, but not that of NK cells. In the present study, in addition to nude mice with cell transfer, PO mice did not develop TE and all survived, as did wild-type animals, for 6 months after infection. PO mice lack perforin-mediated cytotoxic activity in both T and NK cells. These results indicate that the perforin-mediated cytotoxic activity of not only T, but also NK, cells is not required for the genetic resistance of BALB/c mice against the development of TE. This is in contrast to the results with C57BL/6 (susceptible to TE)-background PO mice that were previously reported by Denkers et al. (10), in which accelerated mortality was observed during the chronic stage of infection. In addition, the number of T. gondii cysts in the brain did not differ between BALB/c-background PO and wild-type mice in the present study, whereas a three- to fourfold increase in cyst numbers was reported for C57BL/6-background PO mice (10). Therefore, it appears that the role of perforin in resistance against T. gondii differs between these two strains of mice. Furthermore, because differences in mortality between these two strains of mice were notable for both PO and wild-type animals, it is likely that perforin-mediated cytotoxic activity does not play a major role in regulating the genetic resistance of mice to chronic infection with T. gondii and the development of TE.
In regard to the increased number of brain cysts in C57BL/6-background PO mice (10), it would be possible that perforin-mediated cytotoxicity played an important protective role during the acute stage of infection, although no mortality in mice was noted during that period of time. The lack of perforin-mediated cytotoxicity during the acute stage may have resulted in an accelerated proliferation of tachyzoites in various organs and the formation of larger numbers of cysts in the brain. Such increased parasite loading in the brain could have contributed to the accelerated mortality in the later stage of infection, since C57BL/6 mice are genetically susceptible to the development of TE during this stage. In relation to a possible role of the cytotoxic activity of T cells during the acute stage of infection, it has been shown that the perforin-mediated lysis of tachyzoite-infected cells in vitro does not kill the intracellular parasite and results in the release of viable tachyzoites (45). It is possible that these released tachyzoites are effectively killed, at least in part, by antibody-dependent complement-mediated cytolysis (36) and antibody-mediated phagocytosis by macrophages (2).
In the present study, IFN-γ production by T cells did not differ between infected BALB/c-background PO and wild-type mice during the late stage of infection. These results are consistent with our findings for T cells obtained from nude mice that received T cells from infected PO and wild-type animals. In contrast, IFN-γ production by spleen cells was significantly higher in infected PO than wild-type mice, although the relative percentages of T cells in total spleen cells did not differ between these mice. These results strongly suggest that the lack of perforin-mediated cytotoxic activity resulted in the upregulation of IFN-γ production by non-T cells in the PO mice, whereas the ability of T cells to produce IFN-γ was not altered by the deficiency. Since NK cells have been shown to produce this cytokine in response to T. gondii infection (14, 17), it is possible that NK cells contribute to the upregulated production of IFN-γ by total spleen cells in PO mice. This upregulated IFN-γ production by non-T cells might have played an important role in compensating for the lack of perforin-mediated cytotoxic activity in controlling T. gondii in these animals. Interestingly, it was reported that in C57BL/6-backround PO mice, IFN-γ production by spleen cells does not differ from that of wild-type mice (10). As mentioned earlier, accelerated mortality and increased numbers of T. gondii cysts were observed in these PO mice (10). Therefore, the presence or absence of upregulation of IFN-γ production by non-T cells may have contributed to the differences in the outcome of infection in PO mice from these two different genetic backgrounds.
With regard to the effector mechanisms of IFN-γ-mediated resistance to control T. gondii during the chronic stage of infection, an involvement of tumor necrosis factor (TNF) receptor p55, inducible nitric oxide synthase, and IFN-γ-induced GTPase has been demonstrated in C57BL/6-background mice (6, 30). It is still unknown whether the effector mechanisms of IFN-γ-mediated resistance in genetically resistant BALB/c mice differ from those in susceptible C57BL/6 mice.
The perforin-mediated cytotoxic activity of T cells is known to play important roles in resistance to many intracellular pathogens (7, 20, 24, 28, 41, 43). On the other hand, it has been shown that the protective activity of T cells for clearing out viruses from the brain is noncytolytic and IFN-γ dependent (25). This strategy for the clearance of virus infections without lysing infected cells is probably due to the limited capability for tissue renewal in the brain (26, 27). The present study demonstrated that the protective activity of T cells to control T. gondii in the brain in genetically resistant BALB/c mice is noncytolytic and IFN-γ dependent. IFN-γ has been shown to play an important role in the resistance of the brain against infections with many intracellular microorganisms, such as bacteria (19, 42), a fungus (1), and a protozoan parasite (11) other than T. gondii, in addition to viruses (3, 25, 40). Our results for T. gondii-infected mice may suggest that IFN-γ-mediated, noncytolytic mechanisms are a strategy of T cells that is widely applied for controlling both viral and nonviral intracellular pathogens in the brain without developing tissue damage.
Acknowledgments
We thank Laurel Rodgers for her assistance in preparing the manuscript.
This work was supported by Public Health Service grants AI 47730 and AI04717 from the National Institutes of Health and by a grant from the universitywide AIDS research program of the University of California (R00-PAM-015).
Editor: S. H. E. Kaufmann
REFERENCES
- 1.Aguirre, K., E. A. Havell, G. W. Gibson, and L. L. Johnson. 1995. Role of tumor necrosis factor and gamma interferon in acquired resistance to Cryptococcus neoformans in the central nervous system of mice. Infect. Immun. 63:1725-1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, S. E., Jr., S. C. Bautista, and J. S. Remington. 1976. Specific antibody-dependent killing of Toxoplasma gondii by normal macrophages. Clin. Exp. Immunol. 26:375-380. [PMC free article] [PubMed] [Google Scholar]
- 3.Binder, G. K., and D. E. Griffin. 2001. Interferon-γ-mediated site-specific clearance of alphavirus from CNS neurons. Science 293:303-306. [DOI] [PubMed] [Google Scholar]
- 4.Brown, C. R., C. A. Hunter, R. G. Estes, E. Beckmann, J. Forman, C. David, J. S. Remington, and R. McLeod. 1995. Definitive identification of a gene that confers resistance against Toxoplasma cyst burden and encephalitis. Immunology 85:419-428. [PMC free article] [PubMed] [Google Scholar]
- 5.Candolfi, E., C. A. Hunter, and J. S. Remington. 1994. Mitogen- and antigen-specific proliferation of T cells in murine toxoplasmosis is inhibited by reactive nitrogen intermediates. Infect. Immun. 62:1995-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collazo, C. M., G. S. Yap, S. Hieny, P. Caspar, C. G. Feng, G. A. Taylor, and A. Sher. 2002. The function of gamma interferon-inducible GTP-binding protein IGTP in host resistance to Toxoplasma gondii is Stat1 dependent and requires expression in both hematopoietic and nonhematopoietic cellular compartments. Infect. Immun. 70:6933-6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colmenares, M., P. E. Kima, E. Samoff, L. Soong, and D. McMahon-Pratt. 2003. Perforin and gamma interferon are critical CD8+-T-cell-mediated responses in vaccine-induced immunity against Leishmania amazonensis infection. Infect. Immun. 71:3172-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conley, F. K., K. A. Jenkins, and J. S. Remington. 1981. Toxoplasma gondii infection of the central nervous system. Use of the peroxidase-antiperoxidase method to demonstrate toxoplasma in formalin fixed, paraffin embedded tissue sections. Hum. Pathol. 12:690-698. [DOI] [PubMed] [Google Scholar]
- 9.Deckert-Schluter, M., H. Bluethmann, A. Rang, H. Hof, and D. Schluter. 1998. Crucial role of TNF receptor type 1 (p55), but not of TNF receptor type 2 (p75), in murine toxoplasmosis. J. Immunol. 160:3427-3436. [PubMed] [Google Scholar]
- 10.Denkers, E. Y., G. Yap, T. Scharton-Kersten, H. Charest, B. A. Butcher, P. Caspar, S. Heiny, and A. Sher. 1997. Perforin-mediated cytolysis plays a limited role in host resistance to Toxoplasma gondii. J. Immunol. 159:1903-1908. [PubMed] [Google Scholar]
- 11.Fritz, D. L., and J. P. Dubey. 2002. Pathology of Sarcocystis neurona in interferon-gamma gene knockout mice. Vet. Pathol. 39:137-140. [DOI] [PubMed] [Google Scholar]
- 12.Gazzinelli, R., Y. Xu, S. Hieny, A. Cheever, and A. Sher. 1992. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J. Immunol. 149:175-180. [PubMed] [Google Scholar]
- 13.Gazzinelli, R. T., I. Eltoum, T. A. Wynn, and A. Sher. 1993. Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-alpha and correlates with the down-regulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J. Immunol. 151:3672-3681. [PubMed] [Google Scholar]
- 14.Gazzinelli, R. T., S. Hieny, T. A. Wynn, S. Wolf, and A. Sher. 1993. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc. Natl. Acad. Sci. USA 90:6115-6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hakim, F. T., R. T. Gazzinelli, E. Denkers, S. Hieny, G. M. Shearer, and A. Sher. 1991. CD8+ T cells from mice vaccinated against Toxoplasma gondii are cytotoxic for parasite-infected or antigen-pulsed host cells. J. Immunol. 147:2310-2316. [PubMed] [Google Scholar]
- 16.Hayashi, S., C. C. Chan, R. Gazzinelli, and F. G. Roberge. 1996. Contribution of nitric oxide to the host parasite equilibrium in toxoplasmosis. J. Immunol. 156:1476-1481. [PubMed] [Google Scholar]
- 17.Hunter, C. A., C. S. Subauste, V. H. Van Cleave, and J. S. Remington. 1994. Production of gamma interferon by natural killer cells from Toxoplasma gondii-infected SCID mice: regulation by interleukin-10, interleukin-12, and tumor necrosis factor alpha. Infect. Immun. 62:2818-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Israelski, D. M., and J. S. Remington. 1993. Toxoplasmosis in the non-AIDS immunocompromised host. Curr. Clin. Top. Infect. Dis. 13:322-356. [PubMed] [Google Scholar]
- 19.Jin, Y., L. Dons, K. Kristensson, and M. E. Rottenberg. 2001. Neural route of cerebral Listeria monocytogenes murine infection: role of immune response mechanisms in controlling bacterial neuroinvasion. Infect. Immun. 69:1093-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kagi, D., B. Ledermann, K. Burki, H. Hengartner, and R. M. Zinkernagel. 1994. CD8+ T cell-mediated protection against an intracellular bacterium by perforin-dependent cytotoxicity. Eur. J. Immunol. 24:3068-3072. [DOI] [PubMed] [Google Scholar]
- 21.Kang, H., and Y. Suzuki. 2001. Requirement of non-T cells that produce gamma interferon for prevention of reactivation of Toxoplasma gondii infection in the brain. Infect. Immun. 69:2920-2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan, I. A., K. A. Smith, and L. H. Kasper. 1988. Induction of antigen-specific parasiticidal cytotoxic T cell splenocytes by a major membrane protein (P30) of Toxoplasma gondii. J. Immunol. 141:3600-3605. [PubMed] [Google Scholar]
- 23.Lowin, B., F. Beermann, A. Schmidt, and J. Tschopp. 1994. A null mutation in the perforin gene impairs cytolytic T lymphocyte- and natural killer cell-mediated cytotoxicity. Proc. Natl. Acad. Sci. USA 91:11571-11575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marten, N. W., S. A. Stohlman, and C. C. Bergmann. 2001. MHV infection of the CNS: mechanisms of immune-mediated control. Viral Immunol. 14:1-18. [DOI] [PubMed] [Google Scholar]
- 25.Patterson, C. E., D. M. Lawrence, L. A. Echols, and G. F. Rall. 2002. Immune-mediated protection from measles virus-induced central nervous system disease is noncytolytic and gamma interferon dependent. J. Virol. 76:4497-4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rall, G. F. 1998. CNS neurons: the basis and benefits of low class I major histocompatibility complex expression. Curr. Top. Microbiol. Immunol. 232:115-134. [DOI] [PubMed] [Google Scholar]
- 27.Rall, G. F., and M. B. Oldstone. 1995. Virus-neuron-cytotoxic T lymphocyte interactions. Curr. Top. Microbiol. Immunol. 202:261-273. [DOI] [PubMed] [Google Scholar]
- 28.Rossi, C. P., A. McAllister, M. Tanguy, D. Kagi, and M. Brahic. 1998. Theiler's virus infection of perforin-deficient mice. J. Virol. 72:4515-4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 30.Silva, N. M., W. L. Tafuri, J. I. Alvarez-Leite, J. R. Mineo, and R. T. Gazzinelli. 2002. Toxoplasma gondii: in vivo expression of BAG-5 and cyst formation is independent of TNF p55 receptor and inducible nitric oxide synthase functions. Microbes Infect. 4:261-270. [DOI] [PubMed] [Google Scholar]
- 31.Subauste, C. S., A. H. Koniaris, and J. S. Remington. 1991. Murine CD8+ cytotoxic T lymphocytes lyse Toxoplasma gondii-infected cells. J. Immunol. 147:3955-3959. [PubMed] [Google Scholar]
- 32.Suzuki, Y., F. K. Conley, and J. S. Remington. 1989. Importance of endogenous IFN-gamma for prevention of toxoplasmic encephalitis in mice. J. Immunol. 143:2045-2050. [PubMed] [Google Scholar]
- 33.Suzuki, Y., K. Joh, O. C. Kwon, Q. Yang, F. K. Conley, and J. S. Remington. 1994. MHC class I gene(s) in the D/L region but not the TNF-alpha gene determines development of toxoplasmic encephalitis in mice. J. Immunol. 153:4649-4654. [PubMed] [Google Scholar]
- 34.Suzuki, Y., K. Joh, M. A. Orellana, F. K. Conley, and J. S. Remington. 1991. A gene(s) within the H-2D region determines the development of toxoplasmic encephalitis in mice. Immunology 74:732-739. [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki, Y., H. Kang, S. Parmley, S. Lim, and D. Park. 2000. Induction of tumor necrosis factor-alpha and inducible nitric oxide synthase fails to prevent toxoplasmic encephalitis in the absence of interferon-gamma in genetically resistant BALB/c mice. Microbes Infect. 2:455-462. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki, Y., and A. Kobayashi. 1985. Requirement for calcium ions in antibody-dependent complement-mediated cytolysis of Toxoplasma gondii. Zentbl. Bakteriol. Mikrobiol. Hyg. A 259:426-431. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki, Y., M. A. Orellana, S. Y. Wong, F. K. Conley, and J. S. Remington. 1993. Susceptibility to chronic infection with Toxoplasma gondii does not correlate with susceptibility to acute infection in mice. Infect. Immun. 61:2284-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suzuki, Y., S. Rani, O. Liesenfeld, T. Kojima, S. Lim, T. A. Nguyen, S. A. Dalrymple, R. Murray, and J. S. Remington. 1997. Impaired resistance to the development of toxoplasmic encephalitis in interleukin-6-deficient mice. Infect. Immun. 65:2339-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki, Y., Q. Yang, S. Yang, N. Nguyen, S. Lim, O. Liesenfeld, T. Kojima, and J. S. Remington. 1996. IL-4 is protective against development of toxoplasmic encephalitis. J. Immunol. 157:2564-2569. [PubMed] [Google Scholar]
- 40.Tishon, A., H. Lewicki, G. Rall, M. Von Herrath, and M. B. Oldstone. 1995. An essential role for type 1 interferon-gamma in terminating persistent viral infection. Virology 212:244-250. [DOI] [PubMed] [Google Scholar]
- 41.Topham, D. J., R. A. Tripp, and P. C. Doherty. 1997. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J. Immunol. 159:5197-5200. [PubMed] [Google Scholar]
- 42.Walker, D. H., V. L. Popov, and H. M. Feng. 2000. Establishment of a novel endothelial target mouse model of a typhus group rickettsiosis: evidence for critical roles for gamma interferon and CD8 T lymphocytes. Lab. Investig. 80:1361-1372. [DOI] [PubMed] [Google Scholar]
- 43.White, D. W., A. MacNeil, D. H. Busch, I. M. Pilip, E. G. Pamer, and J. T. Harty. 1999. Perforin-deficient CD8+ T cells: in vivo priming and antigen-specific immunity against Listeria monocytogenes. J. Immunol. 162:980-988. [PubMed] [Google Scholar]
- 44.Wong, S. Y., and J. S. Remington. 1994. Toxoplasmosis in the setting of AIDS. Williams & Wilkins, Baltimore, Md.
- 45.Yamashita, K., K. Yui, M. Ueda, and A. Yano. 1998. Cytotoxic T-lymphocyte-mediated lysis of Toxoplasma gondii-infected target cells does not lead to death of intracellular parasites. Infect. Immun. 66:4651-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yap, G. S., T. Scharton-Kersten, H. Charest, and A. Sher. 1998. Decreased resistance of TNF receptor p55- and p75-deficient mice to chronic toxoplasmosis despite normal activation of inducible nitric oxide synthase in vivo. J. Immunol. 160:1340-1345. [PubMed] [Google Scholar]