Abstract
Although CpG oligodeoxynucleotides (CpG ODNs) are known to enhance resistance against infection in a number of animal models, little is known about the CpG-induced protection against acute fatal sepsis such as that associated with the highly virulent bacterium Burkholderia pseudomallei. We previously demonstrated in an in vitro study that immunostimulatory CpG ODN 1826 enhances phagocytosis of B. pseudomallei and induces nitric oxide synthase and nitric oxide production by mouse macrophages. In the present study, CpG ODN 1826 given intramuscularly to BALB/c mice 2 to 10 days prior to B. pseudomallei challenge conferred better than 90% protection. CpG ODN 1826 given 2 days before the bacterial challenge rapidly enhanced the innate immunity of these animals, judging from the elevated serum levels of interleukin-12 (IL-12)p70 and gamma interferon (IFN-γ) over the baseline values. No bacteremia was detected on day 2 in 85 to 90% of the CpG-treated animals, whereas more than 80% of the untreated animals exhibited heavy bacterial loads. Although marked elevation of IFN-γ was found consistently in the infected animals 2 days after the bacterial challenge, it was ameliorated by the CpG ODN 1826 pretreatment (P = 0.0002). Taken together, the kinetics of bacteremia and cytokine profiles presented are compatible with the possibility that protection by CpG ODN 1826 against acute fatal septicemic melioidosis in this animal model is associated with a reduction of bacterial load and interference with the potential detrimental effect of the robust production of proinflammatory cytokines associated with B. pseudomallei multiplication.
Melioidosis is a potentially fatal infectious disease endemic in many parts of the world including northern Australia, China, and several Southeast Asian countries including Thailand (3, 4, 43). The disease is caused by Burkholderia pseudomallei, a facultative intracellular gram-negative bacillus found in soil and water in the areas of endemic infection. The organism is also currently considered a potential biological warfare agent (4, 14). The spectrum of infection varies from asymptomatic to acute fatal septicemia. In the northeastern part of Thailand where infection is endemic, melioidosis is associated with as much as 20% of community-acquired septicemia and relapse after a prolonged latency period or antibiotic treatment is not infrequent (43). No vaccine is currently available for human melioidosis (43).
Patients with septicemic melioidosis and mice experimentally infected with B. pseudomallei have been reported to have elevated levels of several proinflammatory cytokines and mediators, e.g., interleukin-6 (IL-6), IL-8, IL-12, tumor necrosis factor alpha (TNF-α), and gamma interferon-inducible protein 10 (2, 8, 22, 23, 34, 37). In a murine model, exogenous gamma interferon (IFN-γ) could protect the animals against a subsequent fatal challenge with B. pseudomallei, and the administration of IFN-γ monoclonal antibody prior to the bacterial challenge significantly lowered the 50% lethal dose (LD50) and dramatically increased the bacterial burden (30). In a more recent in vitro study, the same group of investigators showed that the γ-irradiated bacteria could also induce a rapid production of IFN-γ by NK cells and bystander cytotoxic CD8+ T lymphocytes (25). However, hyperresponsive production of these mediators may also induce immunopathology (18, 28); therefore, appropriate administration of immunomodulators to regulate these immune aberrations would be beneficial to the host. Unmethylated CpG oligodeoxynucleotides (CpG ODNs) are potent immunomodulators and induce highly effective protective immunity in a number of chronic infectious diseases (6, 19, 20). It was shown recently that the immunostimulatory CpG ODNs could also increase resistance in mice against acute nonspecific polymicrobial sepsis (42). The CpG ODNs can also function as adjuvants to increase the effectiveness of vaccination against a number of microbial infections (7, 19, 20). They are known to be potent stimulators for macrophages, dendritic cells, NK cells, and some subpopulation of lymphocytes and to preferentially induce Th1 development and differentiation (12, 19, 21, 33). However, no published information is currently available on protection against acute fatal infection and severe sepsis such as that caused by B. pseudomallei. We recently demonstrated that CpG ODN could not only rapidly upregulate the production of inducible nitric oxide synthase (iNOS) and NO in mouse macrophages but also enhance the phagocytosis of B. pseudomallei (38, 40). It is therefore logical that we should attempt to verify the effectiveness of immunostimulatory CpG ODN against a fatal infection in BALB/c mice challenged with B. pseudomallei. The results may provide additional insights into the development of a novel approach to enhance the innate immune response to this life-threatening disease.
MATERIALS AND METHODS
Bacterial strain and culture conditions.
The strain of B. pseudomallei used in all experiments (H1038) was a recent isolate from a patient admitted to Srinagarind Hospital in Khon Kaen province, where melioidosis is endemic. The patient presented clinical manifestations compatible with septicemic melioidosis. The organism was isolated and later identified as an arabinose-negative biotype of B. pseudomallei by biochemical tests, antibiotic susceptibility profile, and immunoreactivity with polyclonal and monoclonal antibodies, as described elsewhere (1, 32, 44, 45). For experimental infection of animals, an overnight shaken culture of B. pseudomallei in Trypticase soy broth incubated at 37°C was centrifuged, washed twice with phosphate-buffered saline (pH 7.2), resuspended, and serially diluted in phosphate-buffered saline to a desired concentration. The exact number of viable bacteria in the suspension was retrospectively determined by plating the bacteria on nutrient agar, and the colonies were counted after 30 to 48 h of incubation at 37°C and expressed as CFU. All solutions used were appropriately sterilized, and all procedures described were carried out in a biosafety biohazard hood.
Animals.
BALB/c mice, 6 to 8 weeks old (National Laboratory Animal Center, Nakorn Pathom, Thailand), were used because this inbred strain has been shown to be highly susceptible to B. pseudomallei infection (9, 24, 37). They were housed in polypropylene cages equipped with HEPA-filtered covers and were fed a commercial pellet diet and water ad libitum. The cages were maintained in an isolated animal room. All experimental procedures performed on the animals were approved by the Animal Ethics Research Committee of the Faculty of Medicine, Khon Kaen University.
Determination of the LD50.
Groups of mice were inoculated intraperitoneally with different doses of a log-phase culture of B. pseudomallei in a 100-μl volume. The animals were observed on a regular basis during the day, and the time of death was recorded. Deaths that occurred at night were assigned a time of death to coincide with the morning of observation. Under this condition, the 30-day LD50 for this strain as calculated by the method of Reed and Muench (29) was found to be ca. 100 CFU.
ODNs.
The CpG ODN used in this study was 1826 (5′ TCCATGACGTTCCTGACGTT), which was shown previously to have potent immunostimulatory effects on the murine immune system (12). The non-CpG ODN used as control was 1982 (5′TCCAGGACTTCTCTCAGGTT). Both ODNs had a nuclease-resistant phosphorothioate backbone, were provided by Coley Pharmaceutical Group (Wellesley, Mass.), and contained only a trace of endotoxin (less than 7.5 × 10−8 endotoxin unit/ng), as determined by the Limulus assay (Whittaker Bioproducts, Walkersville, Md.). The ODN solutions were stored at 4°C.
Protocols for experimental infection and protection assay.
Unless indicated otherwise, the animals were challenged on day 0 by an intraperitoneal injection of either 1,000 or 5,000 CFU of a log-phase culture of B. pseudomallei H1038 in a 100-μl volume, representing a challenge dose of 10 or 50 LD50, respectively. In an initial series of experiments investigating protection, the CpG ODN was given at different times, varying between 2 and 15 days relative to the time of the B. pseudomallei challenge. In brief, the animals were given an intramuscular injection of 100 μg of either CpG ODN 1826 or non-CpG ODN 1982 in a 100-μl volume as described previously (11). After the preliminary results were obtained, in all subsequent experiments the ODN injections were performed on day −2 relative to the bacterial challenge on day 0. The animals were observed daily as described above, and the survival data were recorded on day 30, when the experiments were terminated. Some of the animals that remained alive on day 30 were kept under further observation for as long as 3 to 4 months in order to observe the possible influence of CpG ODN 1826 on chronic infection or a relapse, while others were sacrificed for bacterial determination.
Cytokine assays.
In some experiments, mice under light anesthesia were serially bled by retroorbital puncture, and plasma cytokine levels from each of these animals were individually determined by sandwich enzyme-linked immunosorbent assay using commercial kits as specified by the manufacturer. Depending on the experimental designs, the cytokines that were assayed included biologically active IL-12 (IL-12p70; R&D Systems, Inc., Minneapolis, Minn.), and IFN-γ and TNF-α (BD Pharmingen, San Diego, Calif.). The detection limits for these assays were 2.5 pg/ml for IL-12p70, 31.3 pg/ml for IFN-γ, and 15.6 pg/ml for TNF-α, but these values could be reduced further without losing much precision by using computer software capable of generating a four-parameter logistic curve-fit.
Determination of bacterial load in blood and tissues of infected animals.
In some experiments, blood was serially collected from each animal via retroorbital puncture with a sterile heparinized capillary tube. The individual blood samples were serially diluted 10-fold, 100-μl volumes were pour plated on nutrient agar, and the number of colonies, expressed as CFU, was counted after 30 to 48 h of incubation at 37°C. The remaining undiluted blood was also streaked directly onto the blood agar plate and similarly incubated. The colonies with appearance typical of B. pseudomallei were biochemically tested (45) and immunologically identified with the latex agglutination test kit specific for the B. pseudomallei 200-kDa exopolysaccharide antigen (1, 32). The spleen, liver, and lungs were aseptically removed from some experimental animals to determine the numbers of residual bacteria in these organs (9). Immediately after removal, these organs were homogenized in sterile normal saline and the presence of B. pseudomallei was determined after serial dilution, plating, and identified as described above.
Statistical analyses.
For comparison of groups, the nonparametric Mann-Whitney U test or unpaired t test were applied. The difference in percent survival between the CpG-treated and control groups was analyzed using the chi-square test. The correlation coefficient was determined using linear regression analysis. The level of significance for all statistical analyses was set at P ≤ 0.05. All data presented as mean ± standard error of the mean (SEM) were analyzed using Stat View version 4.5 (Abacus Concepts, Berkeley, Calif.).
RESULTS
Effect of CpG ODN 1826 treatment on the course of B. pseudomallei infection.
In preliminary experiments, injection of BALB/c mice on day 0 with challenge doses of either 1,000 CFU (10 LD50) or 5,000 CFU (50 LD50) resulted in detectable bacteremia on day 2 (the earliest time point determined) in almost all animals. The infected animals died within 30 days, when the experiment was terminated. The mean time of death varied slightly from experiment to experiment, averaging between 7 and 15 days. With lower challenge doses, e.g., 100 CFU (1 LD50), only a small proportion of animals developed bacteremia, but bacteria could nevertheless be detected in small numbers in the internal organs of all infected animals (data not shown). Initial observations, presented in Table 1, showed that the pretreatment of mice with CpG ODN 1826 between 2 and 10 days prior to the time of bacterial challenge (day 0) conferred between 90 and 100% protection. However, some degree of protection could still be observed when the CpG ODN 1826 was given 15 days before the bacterial challenge. The protection was associated with the CpG motif, since there was no protection in mice receiving the control non-CpG ODN 1982 and all animals in this group died, with a mean survival time not noticeably different from that of the untreated animals. On the other hand, when the CpG ODN 1826 was given at the time of the bacterial challenge, the protection was not as complete, and in this case only one-third of the CpG ODN 1826-treated animals survived. Consistent with this trend, no protection was observed when the CpG was given after the bacterial challenge. These limited preliminary data suggested that for this severe bacterial sepsis model, it takes 48 h for the CpG protection to become effective and that the protection wears off after 10 to 15 days. As a result of this initial observation, the remaining experiments, unless indicated otherwise, were performed using the 2-day pretreatment protocol.
TABLE 1.
CpG ODN 1826 induces protective immunity against challenged with B. pseudomallei
| Treatmenta | Day of adminis- tration | Dose (no. of LD50) | No. that survivedb/ total no. | % Pro- tection | Pc |
|---|---|---|---|---|---|
| CpG ODN 1826 | −15 | 10 | 4/7 | 57 | 0.0125 |
| −10 | 10 | 7/7 | 100 | 0.0001 | |
| −5 | 10 | 7/7 | 100 | 0.0001 | |
| −2 | 10 | 6/6 | 100 | 0.0002 | |
| PBS | −2 | 10 | 0/8 | ||
| CpG ODN 1826d | −2 | 10 | 11/12 | 92 | 0.0001 |
| −2 | 50 | 16/18 | 89 | 0.0001 | |
| Control ODN 1982 | −2 | 10 | 0/12 | 0 | |
| −2 | 50 | 0/12 | 0 | ||
| PBS | −2 | 10 | 0/12 | 0 | |
| −2 | 50 | 0/20 | 0 | ||
| CpG ODN 1826 | 0 | 10 | 2/6 | 33 | 0.1213 |
| 1 | 10 | 0/6 | 0 | ||
| PBS | 0 | 10 | 0/6 | 0 |
A 100-μg amount of CpG ODN 1826, control ODN 1982, or PBS was administered in the tibialis anterior muscle on the day indicated relative to an intraperitoneal challenge with B. pseudomallei on day 0.
As observed on day 30, when the experiments were terminated.
P values were calculated by comparing protection induced by CpG ODN 1826 treatment with that induced by control ODN 1982 or PBS on each of days −15, −10, −5, −2, 0, and 1 relative to the bacterial challenge on day 0, using the chi-square test.
The day −2 protocol was repeated several times with similar results and was used in the remaining part of this study.
The cumulative survival rate of mice challenged with different doses of B. pseudomallei was monitored for up to 30 days. The results showed that with a low challenge dose of 1,000 CFU (10 LD50), 92% of the CpG ODN 1826-treated animals survived until day 30, when the experiments were terminated and data were analyzed. The mean survival period ± was 28.8 ± 1.25 days for CpG ODN 1826, 10.4 ± 2.60 days for the non-CpG ODN 1982, and 6.6 ± 1.33 days for the untreated groups. Similar results were noted when a larger challenge dose of 5,000 CFU (50 LD50) was used; these values were 28.1 ± 1.35, 8.7 ± 1.55, and 7.1 ± 1.48 days, respectively. These data demonstrated clearly that the CpG ODN 1826 treatment significantly prolonged the survival time in B. pseudomallei-infected animals (P = 0.0001 for both). The data shown here for the severe sepsis caused by B. pseudomallei therefore extended the protective efficacy of CpG ODNs reported previously for chronic infections by other investigators (6, 11, 19, 20).
CpG ODN 1826 treatment reduces the bacterial load in blood and averts the robust proinflammatory cytokine responses in infected animals.
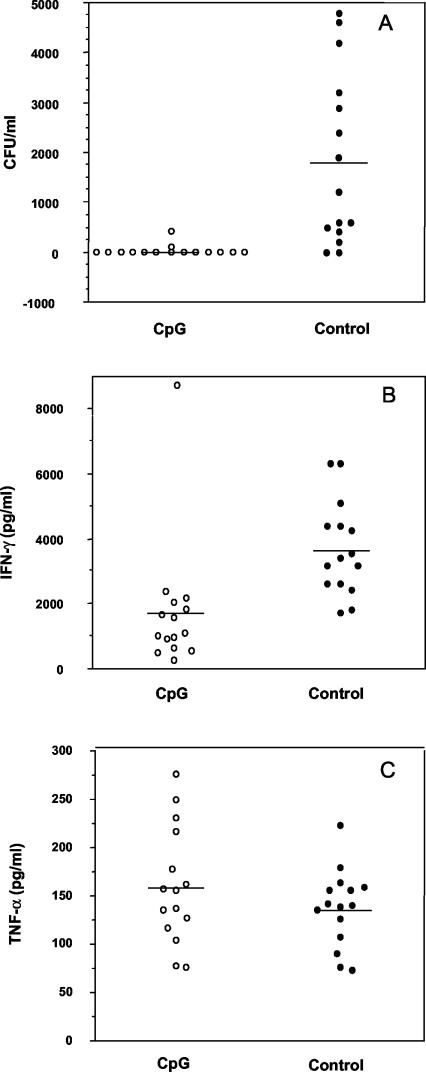
Because the mouse model of melioidosis described in this study resulted in an acute disease, with most deaths occurring within 7 to 10 days, the possible mechanism by which CpG ODN 1826 prevents the rapid death in these animals needs to be examined. Bacterial loads in the day 2 blood specimens of the CpG ODN 1826-pretreated and control (PBS-injected) B. pseudomallei-infected animals were determined. The results presented in Fig. 1A clearly demonstrate that the bacteria could be detected in the day 2 blood in only a small proportion of the CpG-pretreated mice (approximately 13%) and that in those with bacteremia, only a small number of bacteria (less than 400 CFU/ml of blood) were present. In these few bacteremic CpG-treated animals, the bacteria became undetectable in the day 4 blood, but a subsequent breakthrough occasionally occurred during the next few days in a few animals. This was in marked contrast to the untreated B. pseudomallei-infected group (PBS control), in which the bacteremia was detected in almost all animals (approximately 87%), some of which exhibited extremely high blood bacterial counts (several thousand B. pseudomallei bacteria per ml of blood). Moreover, it was observed that various numbers of bacteria were still present in the spleen, liver, and lungs of the untreated, but not the CpG-treated, animals that were sacrificed on day 4, when the bacteria had already disappeared from the circulation (S. Wongratanacheewin, unpublished data).
FIG. 1.
Effect of CpG ODN 1826 pretreatment on bacterial load expressed as CFU (A), IFN-γ levels (B), and TNF-α levels (C). BALB/c mice were injected with either CpG ODN 1826 (○) or PBS (•) on day −2 relative to the time of B. pseudomallei challenge (50 LD50) on day 0. Blood was collected on day 2, and each specimen was used for colony count and cytokine assays. Results from individual mice (n = 15 animals per group) and group means (horizontal line) are shown. Data are representative of three independent experiments. Differences between the CpG and control groups were statistically different for panels A (P = 0.0001) and B (P = 0.0002) by the Mann-Whitney U test.
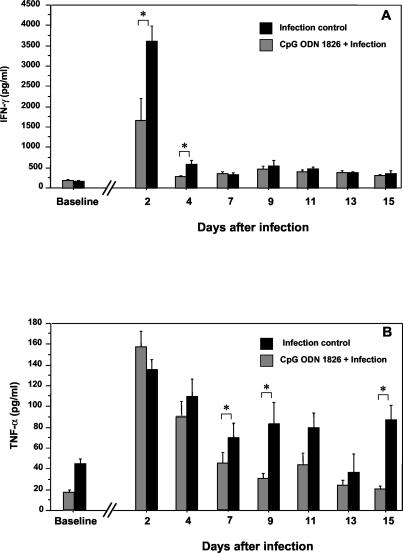
Because different lines of evidence for humans and animals suggest the possible involvement of proinflammatory cytokines in the pathogenesis of septicemic melioidosis (22, 23, 26, 31, 34), we determined the IFN-γ and TNF-α concentrations in the day 2 blood specimens of our experimental animals (Fig. 1B and C). Without the CpG pretreatment, B. pseudomallei infection of the animals was associated with a marked increase in the IFN-γ levels. In some animals, the increased level was many times higher than the corresponding pretreatment levels (approximately 200 pg/ml). These elevated levels were significantly attenuated in the CpG ODN 1826-treated group (P = 0.0002; Fig. 1B). However, regardless of the CpG injection status, the elevation of the TNF-α level on day 2 was not noticeably different between the two groups (Fig. 1C), but these TNF-α levels were higher than the corresponding pretreatment levels in both groups (approximately 25 pg/ml). Thus, unlike IFN-γ, the effect of CpG ODN 1826 treatment on the TNF-α levels was not very striking for the day 2 specimens. However, in a subsequent kinetic study, the effect of CpG ODN administration on TNF-α became noticeable at a later stage of infection, particularly obvious from day 7 onward. Complete profiles of these two proinflammatory cytokines are shown in detail in Fig. 2. While the CpG ODN 1826 interfered with the robust IFN-γ response early during the course of infection (days 2 and 4 in Fig. 2A), its effect on the TNF-α response was noted only during the later stage of infection (Fig. 2B). Within 1 week of infection, the TNF-α levels in the CpG-treated group had already returned to levels approaching that of the baseline, while the levels in animals without the CpG treatment remained significantly elevated until the animals died. However, in this study we did not observe any correlation between the time of death and the bacterial load or the cytokine peak (both the magnitude and the time). Nevertheless, in some animals, a low degree of correlation with the level of TNF-α was noted.
FIG. 2.
Effects of CpG ODN 1826 pretreatment on IFN-γ (A) and TNF-α (B) serum profiles of B. pseudomallei-infected animals. Animals were treated with CpG ODN 1826 and challenged with B. pseudomallei (50 LD50) as described in the legend to Fig. 1. The animals in each group (n = 15) were bled serially as shown (the numbers of specimens at each time point were not equal since some animals in the control group died before day 15). Differences between groups at each time point were calculated by the Mann-Whitney U test, and those indicated by * were statistically significant (P = 0.0002 and P = 0.0047 for IFN-γ on days 2 and 4, respectively, and P = 0.032, P = 0.0058, and P = 0.0087 for TNF-α on days 7, 9, and 15, respectively). Error bars represent SEM.
CpG ODN 1826 pretreatment induces early innate cytokine responses in mice prior to the time of bacterial challenge.
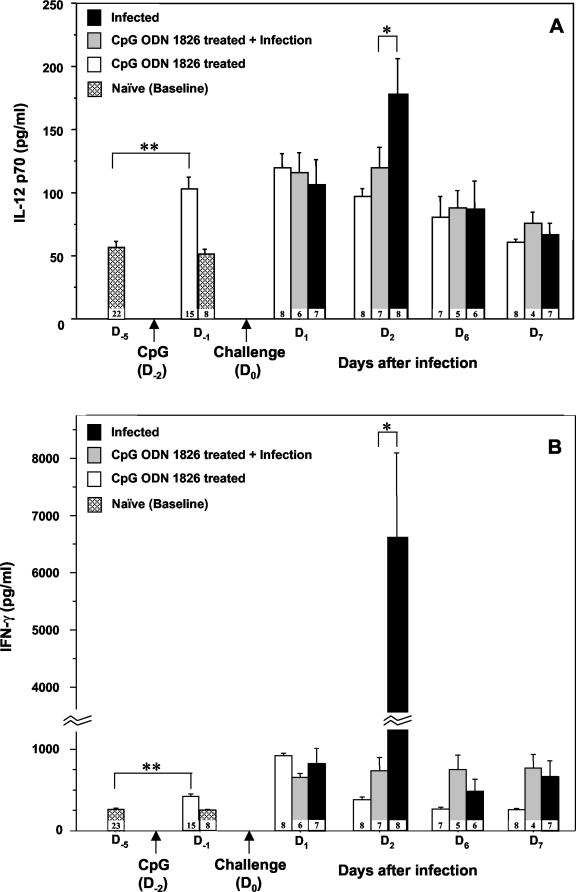
The obligatory role of IFN-γ for host survival has been demonstrated in a murine model of B. pseudomallei infection (30). However, the current data (presented above) clearly demonstrated that the elevated levels of IFN-γ detected in the day 2 specimens in this highly susceptible BALB/c mouse model were not sufficient to protect them from acute lethal melioidosis. One of the protective mechanisms against this bacterial infection is attributable to the ability of the macrophages to produce NO (27), and we recently showed that CpG ODN 1826 could upregulate iNOS and NO production (38). It would be interesting to determine if the pretreatment of mice with CpG ODN 1826, which provided significant protection against a fatal challenge 2 days later, is also associated with the early rise in the levels of IFN-γ-inducing cytokines, such as the biologically active form of IL-12 (IL-12p70) and IL-18. These proinflammatory cytokines may then stimulate the production of IFN-γ and activate other effectors of innate immunity to a level sufficient to control bacterial multiplication. Results presented in Fig. 3 are compatible with our expectation, since the levels of both the IFN-γ-inducing cytokine (i.e., IL-12p70) and IFN-γ in the circulation were found to be significantly elevated (P = 0.0001) within 24 h of the CpG ODN 1826 injection (day −1 in Fig. 3). Whether this small but highly significant early rise in the levels of IL-12p70 and IFN-γ by itself was sufficient to control the growth and survival of the bacteria to a more tolerable level or whether another mechanism, e.g., interference with subsequent bacterium-induced excessive and uncontrolled proinflammatory cytokine production, may be involved remains to be determined. It should be mentioned that we previously presented in vitro evidence indicating that the B. pseudomallei-killing activity of macrophages could be enhanced by pretreatment of these cells with IFN-γ (38, 40). Moreover, we have limited data (unpublished) showing that adherent spleen cells from CpG-pretreated mice had enhanced iNOS production and microbicidal activity against B. pseudomallei compared with those from untreated control animals.
FIG.3.
Innate cytokine profiles in the serum of infected BALB/c mice with or without a prior CpG ODN 1826 pretreatment. The animals were injected with 100 μg of CpG ODN 1826 on day −2 (arrow) relative to the challenge on day 0 (arrow) with B. pseudomallei (50 LD50), as described in the legend to Fig. 1. The animals were bled serially on day −5, day −1 (1 day after either CpG ODN 1826 or PBS injection), day 1 (1 day after the B. pseudomallei challenge), day 2, day 6, and day 7. It should be noted that compared with day −5 (**), CpG ODN 1826 induced a significant elevation of both IL-12p70 (A) and IFN-γ (B) cytokines on day −1 (P = 0.0001 for both by the unpaired t test). The CpG ODN treatment was associated with a marked attenuation of the IFN-γ (P = 0.0002) and, to a lesser extent, IL-12p70 (P = 0.0486) production on day 2 (*) following a bacterial challenge. The number at the bottom of each bar indicates the number of specimens available for analysis at each time point. Data are representative results of two independent experiments. Error bars indicate SEM.
DISCUSSION
B. pseudomallei infection exhibits broad clinical manifestations in humans and animals. The mechanisms underlying the development of different forms of disease remain poorly understood, but there is no doubt that the degree of virulence of the organisms and host factors are important in pathogenesis and disease progression (4, 9, 24-27, 30, 36, 37). The results of the present study reveal new information not only about the more complete bacterially induced proinflammatory-cytokine profiles and development of bacteremia in this animal model of melioidosis sepsis but also about the way in which these could be averted by appropriate administration of an immunomodulating agent. The levels of a number of proinflammatory cytokines and other mediators have been reported to be elevated, and this has been correlated to some extent with disease severity in patients with severe melioidosis (2, 8, 22, 23, 34). However, some of these cytokines, particularly IFN-γ and, to a lesser extent, TNF-α and IL-12p70, have been shown to provide protection against melioidosis in experimental animals (30). On the other hand, it is well documented that excessive and unregulated production of proinflammatory cytokines may be detrimental to the host and can result in septic shock and death, depending on the bacterial burden and time of cytokine production relative to the time of bacterial exposure (13, 18, 28). The data presented in this study showed for the first time that without CpG intervention, practically all animals died and that the time of death for each animal correlated with neither the time of development and magnitude of bacteremia nor the peak levels of cytokines. Some untreated B. pseudomallei-infected (control) animals with heavy bacterial loads and extremely high IFN-γ levels survived for several more days without clinical signs of infection when the bacteria had already disappeared from the blood and the elevated level of IFN-γ had returned to a baseline value. However, almost all of these animals died, mostly before day 30, when the experiment was terminated. In the experiment whose results are shown in Fig. 1 and 2, the presence of bacteria in the internal organs was not determined. However, we have limited data from a parallel experiment demonstrating that B. pseudomallei could still be detected in the spleen, liver, and lungs of the untreated animals, but not the CpG-treated animals, that were sacrificed on day 4 when the bacteria had already disappeared from the circulation (Wongratanacheewin, unpublished).
The CpG ODN 1826 given to the animals 2 days before the bacterial challenge dramatically reduced bacteremia, prolonged the survival time, and conferred to these animals a significant degree of protection against lethal sepsis. No bacteremia could be detected in a majority of CpG-treated animals (Fig. 1A), and in the few animals in which bacteremia was detected, the bacterial load was considerably smaller than what was normally found in untreated animals. It appears, therefore, that CpG could not only favorably affect the outcome of acute infection, as shown here, but could also reduce the chance of the infection progressing to a chronic phase, since the CpG-treated animals sacrificed after day 30 had no demonstrable B. pseudomallei in their internal organs (data not shown). Interference with bacterial multiplication and prolongation of the survival time in the CpG-treated group must be related to enhancing innate immunity immediately prior to the time of bacterial challenge (Fig. 3), since little or no protection was observed when the same quantity of CpG ODN 1826 was given at the same time as or after the bacterial challenge (Table 1). The rapid induction of the IFN-γ-inducing cytokine IL-12p70 by CpG ODN, which induced IFN-γ immediately thereafter, would preactivate the effector cells of innate immunity, thus enhancing their antimicrobial power at the time of bacterial challenge 2 days later. Although an array of other cytokines, including IL-18 and IL-27, are known to synergistically play a role in inducing IFN-γ production (5, 35), in the present study only the IFN-γ-inducing cytokine IL-12p70 was analyzed, because it appears to act upstream in the induction of IFN-γ synthesis. The protective role of an early IFN-γ response in a murine melioidosis model had been demonstrated using other approaches, e.g., neutralizing monoclonal antibody to IFN-γ and IFN-γ receptor knockout animals (30). We previously demonstrated in an in vitro study that the CpG ODN 1826 not only could upregulate the expression and production of iNOS and NO but also could enhance the uptake of B. pseudomallei (38, 40) by mouse macrophages. These in vitro data are in accord with our more recent in vivo observations that the adherent spleen cells of mice injected with CpG 2 days earlier could upregulate the production of iNOS and enhance both the uptake and intracellular killing of B. pseudomallei (data not shown). However, the possibility that the CpG might also enhance killing by neutrophils cannot be ruled out, since it was demonstrated previously that the CpG could increase phagocytosis and production of reactive oxygen intermediates of the neutrophils (42).
It is possible that in the absence of CpG ODN 1826, the B. pseudomallei used in our model is allowed to replicate rapidly and unopposed to a level that cannot be successfully controlled by the IFN-γ that is subsequently induced by the bacteria. This speculation is consistent with our previous findings that, compared with other gram-negative bacteria, not only was B. pseudomallei inherently less potent in stimulating the effectors of innate immunity, e.g., TNF-α, IFN-β, iNOS, and NO, but also it did so with slower kinetics (39, 41). The robust production of IFN-γ noted on day 2 in the untreated B. pseudomallei-infected animals was most probably related to the presence of a very large bacterial burden, and we theorize that it was then too late to control the ongoing infection. At this time, the macrophages might also be so severely damaged that they are unable to mount an effective antimicrobial response against the bacteria. It has been observed that patients with septicemic melioidosis not only have higher blood bacterial counts but also have a higher mortality rate than those infected with other gram-negative bacteria (31). It should be mentioned that both the bacterial load and the proinflammatory cytokine levels noted in the present study were considerably greater than those found in the patients. It is unlikely, however, that in our murine model, the transient elevation of the IFN-γ level in the untreated B. pseudomallei-infected animals by itself (Fig. 2 and 3) is the primary cause of death, since some of the animals survived for many more days after the IFN-γ level returned to normal. It is possible that other factors, e.g., TNF-α, might contribute to fatal sepsis in this animal model. Our data on TNF-α suggested that the elevated level of this cytokine during the late stage of infection (e.g., from day 7 onward) might be more closely related to the outcome of infection. For example, the results presented in Fig. 2 showed that from day 7 onward, the TNF-α levels in the untreated, B. pseudomallei-infected mice remained elevated until the time of death compared with the levels in the CpG-treated animals. This was particularly obvious on days 9 (P = 0.0058) and 15 (P = 0.0087), when the difference between the two groups became highly statistically significant. In accord with this observation, it was shown previously that the more resistant C57BL/6 mice exhibited a considerably lower level of TNF-α than did the more susceptible BALB/c mice, which succumbed to fatal sepsis following B. pseudomallei infection (26). These observations support the previous results of studies with humans, showing that the levels of TNF-α in melioidosis patients correlated with the severity of the disease and could be considered a predictive marker for the outcome of infection (22, 34). Since B. pseudomallei itself is a poor stimulator of the innate immune response (39, 41), it is possible that the initially weak innate immune response allows a rapid multiplication of the bacteria, resulting in an excessive and uncontrolled cytokine production, which could lead to shock and death. Taken together, these data are compatible with the notion that the enhanced innate immune response induced by CpG administration would avert this process. On the other hand, one should also keep in mind the possible direct action of CpG ODN 1826 administration in ameliorating the potential detrimental effects of a subsequent bacterially induced cytokine production, similar to the endotoxin tolerance which is known to protect the host against infections (15-17, 46). In the present study, we found no direct evidence that CpG ODN 1826 could negatively regulate the proinflammatory cytokine response in this experimental model of melioidosis. However, it is a question that should receive further attention, particularly in view of the more recent report showing that CpG differed from lipopolysaccharide in inducing IFN-γ production (10, 18).
Taken together, the data presented here suggest that a rapid induction of innate cytokines by CpG treatment before the time of bacterial challenge would determine whether the disease is obligatory and will proceed to an acute lethal outcome or to full recovery without going through a chronic stage or latency phase. We feel that the time when the proinflammatory cytokines are induced by immunomodulating agents is highly critical, and this window is rather narrow in melioidosis, requiring at least 48 h prior to the infectious challenge but lasting only 10 to 15 days. It should be interesting to determine whether the protective window for this potentially fatal acute infection could be increased if a larger dose of CpG is used. Our proposal based on the B. pseudomallei model presented in this communication is in accord with the prediction of the occurrence of sepsis and the outcome of infection proposed previously by other investigators (18, 28).
In conclusion, the results presented in this study showed that CpG ODN 1826 is a potentially useful immunomodulating agent that is as effective in preventing an acute and lethal sepsis in animals experimentally infected with B. pseudomallei as it is in preventing chronic infections in other experimental models. It provides new insights for future investigation if one is to develop a novel approach to the management of patients with severe melioidosis. Lastly, it should be mentioned that in the absence of vaccine and in the setting of ineffective antibiotic therapy and the potential danger of B. pseudomallei as a biological warfare agent, the beneficial effect offered by immunomodulating agent such as CpG ODN should provide an alternative approach to preventing fatal sepsis.
Acknowledgments
This work was supported by Thailand Research Fund (RDG 4530209) and Chulabhorn Research Institute, Bangkok, Thailand.
We appreciate the kind comments and suggestions from Nicholus J. White (University of Oxford, Oxford, United Kingdom). We also thank Bryan R. Hamman for assistance during the preparation of the manuscript.
Editor: J. D. Clements
REFERENCES
- 1.Anuntagool, N., P. Naigowit, V. Petkanchanapong, P. Aramsri, T. Panichakul, and S. Sirisinha. 2000. Monoclonal antibody-based rapid identification of Burkholderia pseudomallei in blood culture fluid from patients with community-acquired septicaemia. J. Med. Microbiol. 49:1075-1078. [DOI] [PubMed] [Google Scholar]
- 2.Brown, A. E., D. A. Dance, Y. Suputtamongkol, W. Chaowagul, S. Kongchareon, H. K. Webster, and N. J. White. 1991. Immune cell activation in melioidosis: increased serum levels of interferon-gamma and soluble interleukin-2 receptors without change in soluble CD8 protein. J. Infect. Dis. 163:1145-1148. [DOI] [PubMed] [Google Scholar]
- 3.Dance, D. A. 2000. Melioidosis as an emerging global problem. Acta Trop. 74:115-119. [DOI] [PubMed] [Google Scholar]
- 4.Dharakul, T., and S. Songsivilai. 1999. The many facets of melioidosis. Trends Microbiol. 7:138-140. [DOI] [PubMed] [Google Scholar]
- 5.Dinarello, C. A., and G. Fantuzzi. 2003. Interleukin-18 and host defense against infection. J. Infect. Dis. 187:S370-S384. [DOI] [PubMed] [Google Scholar]
- 6.Elkins, K. L., T. R. Rhinehart-Jones, S. Stibitz, J. S. Conover, and D. M. Klinman. 1999. Bacterial DNA containing CpG motifs stimulates lymphocyte-dependent protection of mice against lethal infection with intracellular bacteria. J. Immunol. 162:2291-2298. [PubMed] [Google Scholar]
- 7.Freidag, B. L., G. B. Melton, F. Collins, D. M. Klinman, A. Cheever, L. Stobie, W. Suen, and R. A. Seder. 2000. CpG oligodeoxynucleotides and interleukin-12 improve the efficacy of Mycobacterium bovis BCG vaccination in mice challenged with M. tuberculosis. Infect. Immun. 68:2948-2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedland, J. S., Y. Suputtamongkol, D. G. Remick, W. Chaowagul, R. M. Strieter, S. L. Kunkel, N. J. White, and G. E. Griffin. 1992. Prolonged elevation of interleukin-8 and interleukin-6 concentrations in plasma and of leukocyte interleukin-8 mRNA levels during septicemic and localized Pseudomonas pseudomallei infection. Infect. Immun. 60:2402-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gauthier, Y. P., R. M. Hagen, G. S. Brochier, H. Neubauer, W. D. Splettstoesser, E. Finke, and D. R. Vidal. 2001. Study on the pathophysiology of experimental Burkholderia pseudomallei infection in mice. FEMS. Immunol. Med. Microbiol. 30:53-63. [DOI] [PubMed] [Google Scholar]
- 10.Gould, M. P., J. A. Greene, V. Bhoj, J. L. DeVecchio, and F. P. Heinzel. 2004. Distinct modulatory effects of LPS and CpG on IL-18-dependent IFN-gamma synthesis. J. Immunol. 172:1754-1762. [DOI] [PubMed] [Google Scholar]
- 11.Gramzinski, R. A., D. L. Doolan, M. Sedegah, H. L. Davis, A. M. Krieg, and S. L. Hoffman. 2001. Interleukin-12- and gamma interferon-dependent protection against malaria conferred by CpG oligodeoxynucleotide in mice. Infect. Immun. 69:1643-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartmann, G., R. D. Weeratna, Z. K. Ballas, P. Payette, S. Blackwell, I. Suparto, W. L. Rasmussen, M. Waldschmidt, D. Sajuthi, R. H. Purcell, H. L. Davis, and A. M. Krieg. 2000. Delineation of a CpG phosphorothioate oligodeoxynucleotide for activating primate immune responses in vitro and in vivo. J. Immunol. 164:1617-1624. [DOI] [PubMed] [Google Scholar]
- 13.Hotchkiss, R. S., and I. E. Karl. 2003. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 348:138-150. [DOI] [PubMed] [Google Scholar]
- 14.Jeddeloh, J. A., D. L. Fritz, D. M. Waag, J. M. Hartings, and G. P. Andrews. 2003. Biodefense-driven murine model of pneumonic melioidosis. Infect. Immun. 71:584-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston, J. A., and J. J. O'Shea. 2003. Matching SOCS with function. Nat. Immunol. 4:507-509. [DOI] [PubMed] [Google Scholar]
- 16.Klinman, D. M., J. Conover, and C. Coban. 1999. Repeated administration of synthetic oligodeoxynucleotides expressing CpG motifs provides long-term protection against bacterial infection. Infect. Immun. 67:5658-5663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi, K., L. D. Hernandez, J. E. Galan, C. A. Janeway, Jr., R. Medzhitov, and R. A. Flavell. 2002. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell 110:191-202. [DOI] [PubMed] [Google Scholar]
- 18.Krieg, A. M. 2003. CpG DNA: trigger of sepsis, mediator of protection, or both? Scand. J. Infect. Dis. 35:653-659. [DOI] [PubMed] [Google Scholar]
- 19.Krieg, A. M. 2002. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 20:709-760. [DOI] [PubMed] [Google Scholar]
- 20.Krieg, A. M., and H. L. Davis. 2001. Enhancing vaccines with immune stimulatory CpG DNA. Curr. Opin. Mol. Ther. 3:15-24. [PubMed] [Google Scholar]
- 21.Krug, A., S. Rothenfusser, V. Hornung, B. Jahrsdorfer, S. Blackwell, Z. K. Ballas, S. Endres, A. M. Krieg, and G. Hartmann. 2001. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur. J. Immunol. 31:2154-2163. [DOI] [PubMed] [Google Scholar]
- 22.Lauw, F. N., A. J. Simpson, J. M. Prins, M. D. Smith, M. Kurimoto, S. J. van Deventer, P. Speelman, W. Chaowagul, N. J. White, and T. van der Poll. 1999. Elevated plasma concentrations of interferon (IFN)-gamma and the IFN-gamma-inducing cytokines interleukin (IL)-18, IL-12, and IL-15 in severe melioidosis. J. Infect. Dis. 180:1878-1885. [DOI] [PubMed] [Google Scholar]
- 23.Lauw, F. N., A. J. Simpson, J. M. Prins, S. J. van Deventer, W. Chaowagul, N. J. White, and T. van Der Poll. 2000. The CXC chemokines gamma interferon (IFN-γ)-inducible protein 10 and monokine induced by IFN-γ are released during severe melioidosis. Infect. Immun. 68:3888-3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leakey, A. K., G. C. Ulett, and R. G. Hirst. 1998. BALB/c and C57BL/6 mice infected with virulent Burkholderia pseudomallei provide contrasting animal models for the acute and chronic forms of human melioidosis. Microb. Pathog. 24:269-275. [DOI] [PubMed] [Google Scholar]
- 25.Lertmemongkolchai, G., G. Cai, C. A. Hunter, and G. J. Bancroft. 2001. Bystander activation of CD8+ T cells contributes to the rapid production of IFN-gamma in response to bacterial pathogens. J. Immunol. 166:1097-1105. [DOI] [PubMed] [Google Scholar]
- 26.Liu, B., G. C. Koo, E. H. Yap, K. L. Chua, and Y. H. Gan. 2002. Model of differential susceptibility to mucosal Burkholderia pseudomallei infection. Infect. Immun. 70:504-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyagi, K., K. Kawakami, and A. Saito. 1997. Role of reactive nitrogen and oxygen intermediates in gamma interferon-stimulated murine macrophage bactericidal activity against Burkholderia pseudomallei. Infect. Immun. 65:4108-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Netea, M. G., J. W. van der Meer, M. van Deuren, and B. J. Kullberg. 2003. Proinflammatory cytokines and sepsis syndrome: not enough, or too much of a good thing? Trends Immunol. 24:254-258. [DOI] [PubMed] [Google Scholar]
- 29.Reed, L. J., and H. Muench. 1938. A simple method for estimating fifty percent end points. Am. J. Hyg. 27:493-497. [Google Scholar]
- 30.Santanirand, P., V. S. Harley, D. A. Dance, B. S. Drasar, and G. J. Bancroft. 1999. Obligatory role of gamma interferon for host survival in a murine model of infection with Burkholderia pseudomallei. Infect. Immun. 67:3593-3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simpson, A. J., M. D. Smith, G. J. Weverling, Y. Suputtamongkol, B. J. Angus, W. Chaowagul, N. J. White, S. J. van Deventer, and J. M. Prins. 2000. Prognostic value of cytokine concentrations (tumor necrosis factor-alpha, interleukin-6, and interleukin-10) and clinical parameters in severe melioidosis. J. Infect. Dis. 181:621-625. [DOI] [PubMed] [Google Scholar]
- 32.Sirisinha, S., N. Anuntagool, P. Intachote, V. Wuthiekanun, S. D. Puthucheary, J. Vadivelu, and N. J. White. 1998. Antigenic differences between clinical and environmental isolates of Burkholderia pseudomallei. Microbiol. Immunol. 42:731-737. [DOI] [PubMed] [Google Scholar]
- 33.Sparwasser, T., E. S. Koch, R. M. Vabulas, K. Heeg, G. B. Lipford, J. W. Ellwart, and H. Wagner. 1998. Bacterial DNA and immunostimulatory CpG oligonucleotides trigger maturation and activation of murine dendritic cells. Eur. J. Immunol. 28:2045-2054. [DOI] [PubMed] [Google Scholar]
- 34.Suputtamongkol, Y., D. Kwiatkowski, D. A. Dance, W. Chaowagul, and N. J. White. 1992. Tumor necrosis factor in septicemic melioidosis. J. Infect. Dis. 165:561-564. [DOI] [PubMed] [Google Scholar]
- 35.Trinchieri, G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3:133-146. [DOI] [PubMed] [Google Scholar]
- 36.Ulett, G. C., B. J. Currie, T. W. Clair, M. Mayo, N. Ketheesan, J. Labrooy, D. Gal, R. Norton, C. A. Smith, J. Barnes, J. Warner, and R. G. Hirst. 2001. Burkholderia pseudomallei virulence: definition, stability and association with clonality. Microbes Infect. 3:621-631. [DOI] [PubMed] [Google Scholar]
- 37.Ulett, G. C., N. Ketheesan, and R. G. Hirst. 2000. Cytokine gene expression in innately susceptible BALB/c mice and relatively resistant C57BL/6 mice during infection with virulent Burkholderia pseudomallei. Infect. Immun. 68:2034-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Utaisincharoen, P., N. Anuntagool, P. Chaisuriya, S. Pichyangkul, and S. Sirisinha. 2002. CpG ODN activates NO and iNOS production in mouse macrophage cell line (RAW 264.7) Clin. Exp. Immunol. 128:467-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Utaisincharoen, P., N. Anuntagool, K. Limposuwan, P. Chaisuriya, and S. Sirisinha. 2003. Involvement of beta interferon in enhancing inducible nitric oxide synthase production and antimicrobial activity of Burkholderia pseudomallei-infected macrophages. Infect. Immun. 71:3053-3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Utaisincharoen, P., W. Kespichayawattana, N. Anuntagool, P. Chaisuriya, S. Pichyangkul, A. M. Krieg, and S. Sirisinha. 2003. CpG ODN enhances uptake of bacteria by mouse macrophages. Clin. Exp. Immunol. 132:70-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Utaisincharoen, P., N. Tangthawornchaikul, W. Kespichayawattana, P. Chaisuriya, and S. Sirisinha. 2001. Burkholderia pseudomallei interferes with inducible nitric oxide synthase (iNOS) production: a possible mechanism of evading macrophage killing. Microbiol. Immunol. 45:307-313. [DOI] [PubMed] [Google Scholar]
- 42.Weighardt, H., C. Feterowski, M. Veit, M. Rump, H. Wagner, and B. Holzmann. 2000. Increased resistance against acute polymicrobial sepsis in mice challenged with immunostimulatory CpG oligodeoxynucleotides is related to an enhanced innate effector cell response. J. Immunol. 165:4537-4543. [DOI] [PubMed] [Google Scholar]
- 43.White, N. J. 2003. Melioidosis. Lancet 361:1715-1722. [DOI] [PubMed] [Google Scholar]
- 44.Wuthiekanun, V., N. Anuntagool, N. J. White, and S. Sirisinha. 2002. Short report: a rapid method for the differentiation of Burkholderia pseudomallei and Burkholderia thailandensis Am. J. Trop. Med. Hyg. 66:759-761. [DOI] [PubMed] [Google Scholar]
- 45.Wuthiekanun, V., M. D. Smith, D. A. Dance, A. L. Walsh, T. L. Pitt, and N. J. White. 1996. Biochemical characteristics of clinical and environmental isolates of Burkholderia pseudomallei. J. Med. Microbiol. 45:408-412. [DOI] [PubMed] [Google Scholar]
- 46.Yeo, S. J., J. G. Yoon, S. C. Hong, and A. K. Yi. 2003. CpG DNA induces self and cross-hyporesponsiveness of RAW264.7 cells in response to CpG DNA and lipopolysaccharide: alterations in IL-1 receptor-associated kinase expression. J. Immunol. 170:1052-1061. [DOI] [PubMed] [Google Scholar]