Abstract
We describe the facile synthesis of several two-dimensional covalent–organic frameworks (2D COFs) as films by vapor-assisted conversion at room temperature. High-quality films of benzodithiophene-containing BDT-COF and COF-5 with tunable thickness were synthesized under different conditions on various substrates. BDT-COF films of several micrometer thickness exhibit mesoporosity as well as textural porosity, whereas thinner BDT-COF films materialize as a cohesive dense layer. In addition, we studied the formation of COF-5 films with different solvent mixture compositions serving as vapor source. Room temperature vapor-assisted conversion is an excellent method to form COF films of fragile precursors and on sensitive substrates.
Covalent–organic frameworks (COFs) are crystalline porous polymers formed by a bottom-up approach from molecular building units with a predesigned geometry that are connected through covalent bonds. COFs are typically synthesized as powders through solvothermal synthesis procedures.1−4 Under these conditions, COFs materialize by a condensation reaction in solvent mixtures exhibiting a high boiling point, and usually the reaction is carried out at elevated temperatures to ensure appropriate reaction rates. To shed light on the conditions in which COFs form and to further realize their potential as functional materials,5−7 alternative synthetic routes to form COFs are highly desirable. The preparation of 2D COFs through microwave synthesis, sonication, and attrition has already been discussed.8−11 In those cases, different 2D COFs were realized having different degrees of crystallinity. The main feature shared by those methods is the need for external energy sources to promote the condensation reactions.
The growth of thin COF films with control over the film thickness, morphology, and crystallinity is highly desirable for the utilization of COFs in diverse applications. The known methods used for growing thin COF films are based on reactive precursor solutions. These methods can produce oriented thin COF films on a variety of substrates.12,13 However, challenges such as scalability, yield, and morphology control still remain.
Steam-assisted conversion (SAC) and dry-gel conversion (DGC) methods have recently been developed for the efficient synthesis of bulk and thin film inorganic microporous materials.14−20 To create films, the first step in this approach involves the deposition of the reaction precursors as dry gel on a substrate. Subsequently, the reaction is initiated by exposing the mixture to steam and/or vapor of structure-directing agents at elevated temperatures. Several zeolite structures have been synthesized according to this approach, resulting in highly crystalline and porous materials. Some of us have demonstrated the synthesis of an isoreticular series of single atomic layer 2D COFs on highly ordered graphite (HOPG) using water as a vapor source to promote the condensation reaction, at elevated temperature.21,22 This approach enabled on-surface self-condensation reactions of linear boronic acids to form boroxine rings, eventually resulting in long-range ordered single-layer 2D COFs. The synthesis of imine-based single-layer 2D COFs was also illustrated.23 Very recently, solid-state transformation assisted by vapor source at elevated temperature has been investigated as a route to form an imine-based 2D COF as a bulk powdered material.24
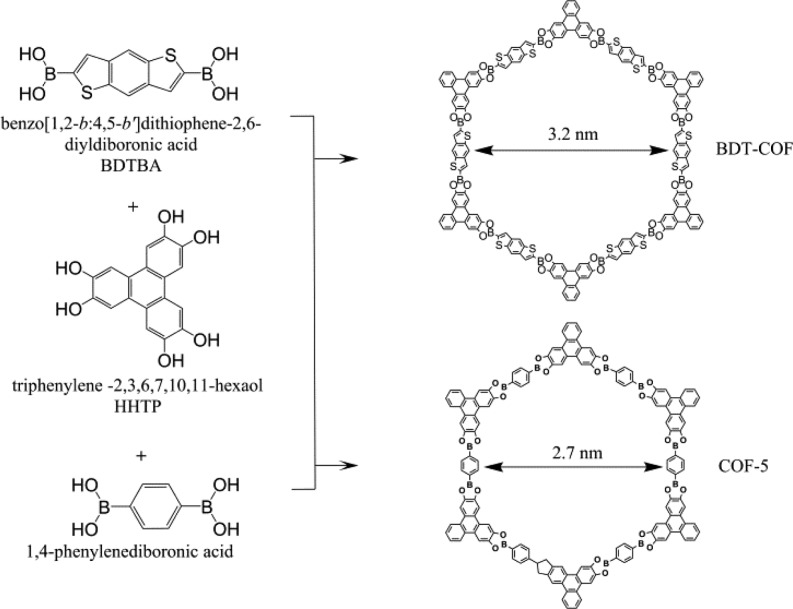
Here we translate the concept of steam-assisted conversion to the synthesis of a series of 2D COF films based on the co-condensation reaction of diboronic acids and hexahydroxytriphenylene (HHTP). Strikingly, this process can be performed at room temperature, thus establishing that highly ordered 2D COF structures based on boronic ester linkages can be created under very mild conditions. Furthermore, the novel room temperature vapor-assisted conversion approach provides a scalable film synthesis route with excellent control of film thickness. To illustrate the generality of this strategy, we demonstrate the synthesis of a benzodithiophene-based COF (BDT-COF) as well as the well-known COF-5 and the boroxine-based pyrene-COF by room temperature vapor-assisted conversion in the form of films (Figure 1 and Supporting Information, SI).1,13,25
Figure 1.
Schematic representation of BDT-COF and COF-5.
In a typical experiment, COF precursors were dissolved in a polar solvent mixture of acetone and ethanol, and 150–200 μL of the precursor solution was drop-cast on a clean 15 mm × 20 mm glass substrate. Five such wet glass substrates were immediately placed into a desiccator along with a small vessel containing mesitylene and dioxane at a volume ratio of 1:1. To achieve complete conversion of the drop-cast precursor solution into the final COF structure, the desiccator was stored for up to 72 h at room temperature (for more information see the SI file). At the end of the conversion reaction, the glass substrates were evenly covered with a colored layer of COF material. The structural analysis of the resulting films was performed without further purification unless mentioned otherwise.
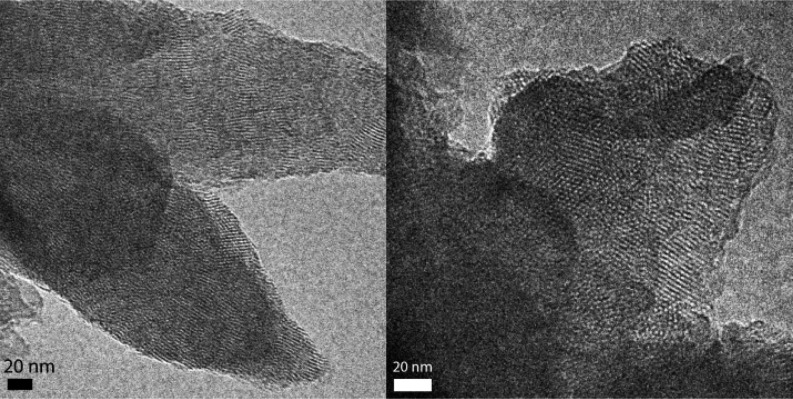
The synthesis of BDT-COF via room temperature vapor-assisted conversion results in a dark-green organic layer on the glass substrate. Top-view scanning electron microscopy (SEM) studies reveal a homogeneous surface consisting of small intergrown particles forming a continuous coverage on the substrate (Figure 2a). Furthermore, the surface is characterized by submicron voids between the intergrown particles, which endow the films with textural porosity. SEM cross-sectional analysis reveals a cohesive film of about 7.5 μm in thickness, composed of small randomly intergrown particles that form a porous film (Figure 2b). Powder X-ray diffraction (PXRD) confirms the existence of BDT-COF as expected from the condensation reaction of the linear diboronic acid and the triangular HHTP building units. The diffraction pattern is in very good agreement with the PXRD we recently reported for BDT-COF synthesized under solvothermal conditions (Figure 2c).13 The BDT-COF synthesized under room temperature vapor-assisted conversion conditions exhibits an eclipsed AA packing arrangement rather than a staggered AB packing arrangement as depicted in Figure 2c. Permanent porosity and pore accessibility are important properties of COF materials; therefore, we studied the porosity of freshly prepared BDT-COF films by means of krypton sorption at 77.3 K. To remove possible guest molecules from the COF pores, prior to the sorption the BDT-COF films were heated to 150 °C under vacuum. A characteristic type IV adsorption isotherm is obtained for the BDT-COF films, verifying the successful synthesis of a mesoporous BDT-COF by room temperature vapor-assisted conversion (Figure 2d). The sharp rise of the krypton uptake at about p/p0 = 0.2 illustrates the pore regularity and the narrow pore size distribution. The Brunauer–Emmett–Teller (BET) surface area is calculated (p/p0 = 0.05–0.11 in the adsorption branch) to be as high as 990 m2/g. Transmission electron microscopy (TEM) analysis of material removed from the film reveals a polycrystalline BDT-COF material with domain sizes of about 40 ± 3 nm and random crystallite orientation; projections along the c-axis show the typical hexagonal pattern with pore width of approximately 3 nm (Figure S1, SI).
Figure 2.
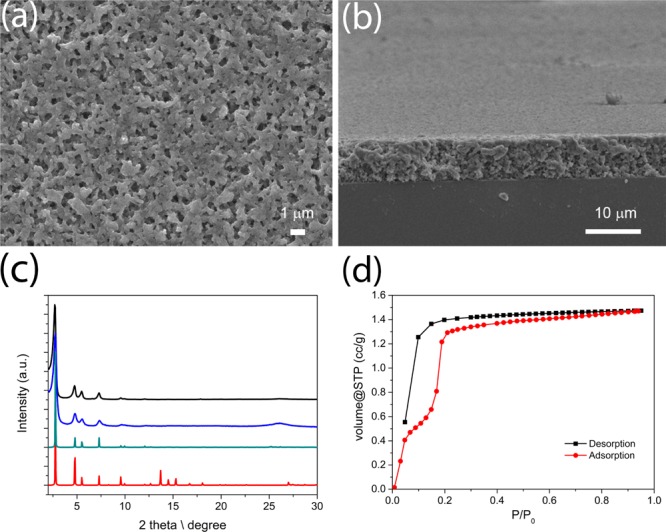
(a) Top view SEM micrograph of BDT-COF film synthesized by room temperature vapor-assisted conversion, representing the surface morphology. (b) Cross-section SEM micrograph shows a uniform film thickness. (c) Experimental PXRD pattern of a BDT-COF film prepared by room temperature vapor-assisted conversion (black), solvothermal synthesis (blue), simulated PXRD patterns based on an eclipsed AA arrangement (green), and a staggered AB arrangement (red). (d) Krypton sorption isotherm of degassed BDT-COF thin film on glass substrate measured at 77.3 K.
Next, we addressed the control of film thickness. To decrease the film thickness using the vapor-assisted conversion approach, we first reduced the droplet volume for the drop-casting experiments (see SI file). In these experiments the volume of the drop-cast precursor solution was reduced to 60 μL, which is the amount required for complete wetting of the glass surface. After 72 h in a room temperature vapor-assisted conversion reaction, a green overlayer is obtained on the glass substrate, similar to the previously synthesized BDT-COF films. The SEM cross-section reveals a 2 μm film consisting of intergrown small particles. The general morphology of these films is similar to the observed morphology of the above thicker BDT-COF films (Figure S2b, SI), and the XRD pattern confirms the formation of BDT-COF.
To further reduce the film thickness, we investigated the condensation reaction of drop-cast diluted BDT-COF precursor solution. To obtain the thin BDT-COF films, a diluted precursor solution, up to 1/3 of the initial concentration, was prepared. Subsequently, 60 μL of that solution was drop-cast on the glass substrate. At the end of the reaction, the glass substrate was covered with a yellow transparent layer. The SEM cross-section shows a dense homogeneous organic layer of approximately 300 nm thickness. The textural porosity observed for the above thicker BDT-COF films is not obtained with diluted precursor solution. X-ray diffraction confirms the formation of BDT-COF in the form of a thin film having random crystal orientation (Figure S2, SI).
The feasibility of growing COFs as films on different substrates enables their incorporation into devices. Thus, BDT-COF was grown on gold, silicon, and fluorine-doped tin oxide (FTO) by the room temperature vapor-assisted conversion method. In all cases total surface coverage was obtained, the films appeared homogeneous, and the SEM analysis revealed a uniform COF film. Furthermore, the BDT-COF films are crystalline and exhibit random crystallite orientations on the different surfaces (Figure S3, SI).
Reaction parameters such as the solvent composition can have a strong influence on the condensation reactions of the COF building blocks. To study this issue, we turned to the well-known COF-5 structure formed in the reaction of benzene 1,4-diboronic acid (BDBA) and HHTP.1 First, we allowed the COF-5 precursors to react under the same reaction conditions as for the synthesis of BDT-COF but without a vapor source in the desiccator. At the end of the process we obtained a grayish colored organic deposit on the glass substrate. Strikingly, the X-ray diffraction pattern revealed that the condensation reaction was initiated at room temperature solely by depositing the COF precursor solution on a glass substrate (Figure S5, SI). Small COF domains are formed in this process, indicated by the broad (110) and the interlayer (001) reflections in the diffraction pattern. The presence of the interlayer reflection suggests that the COF-5 crystallites preferably grow in the c-direction (Figure S5, SI).13 Additionally, we synthesized a series of COF-5 films where the solvent composition in the vessel was modified. The X-ray diffraction patterns indicate the formation of COF-5 material with the solvent composition of mesitylene/dioxane 1:4 and 4:1 (v/v), respectively (Figure S6, SI). Further, COF-5 films were synthesized following the same reaction procedure as for the synthesis of BDT-COF films, namely, with a composition of mesitylene/dioxane 1:1 (v/v) in the vessel. The SEM cross-section analysis of the obtained films reveals the formation of an approximately 10 μm thick COF-5 film having a similar morphology as the thick BDT-COF films (Figure S4, SI). The X-ray diffraction pattern of the as-synthesized COF-5 film is in good agreement with the X-ray diffraction pattern reported for the solvothermal synthesis of COF-5 powder (Figure S5, SI), thus indicating the formation of a highly crystalline COF-5.1 FT-IR spectroscopy of the obtained films supports the formation of boronic esters in the process (Figure S7, SI). TEM analysis reveals a polycrystalline COF-5 with crystal domain sizes of about 20 nm (Figure 3). In addition, we monitored the COF-5 growth with time by X-ray diffraction. In this experiment the drop-cast surfaces were exposed to the mesitylene/dioxane 1:1 (v/v) vapor for limited times (Figure S8, SI). After 1 h of vapor exposure, a periodic phase starts to evolve and a gradual increase in periodicity is observed over 8 h. The formation of COF-5 through vapor-assisted conversion at room temperature exhibits slower rates compared to the growth under solvothermal conditions.5 We conclude that the mesitylene/dioxane solvent mixture of 1:1 (v/v) provides a suitable vapor atmosphere for the room temperature conversion of monomeric COF building units to an ordered mesoporous framework.
Figure 3.
TEM micrographs of polycrystalline COF-5 removed from a film showing the general morphology (right). Projections along the hexagonal c-axis show the COF-5 honeycomb structure (the COF-5 films were synthesized in the presence of mesitylene / dioxane 1:1 (v/v) solvent mixture for 72 h).
To study the role of the solvent vapor in this process, we examined the impact of different vapor compositions of the different mesitylene/dioxane mixtures, that is, 1:4, 4:1, and 1:1 (v/v), respectively. As mesitylene and dioxane exhibit different vapor pressures at room temperature, the vapor composition in the desiccator can be substantially different from the composition of the solvent mixtures in the vessel. We therefore determined the vapor compositions by gas chromatography (Figure S9, SI). The vapor of all solvent mixtures was found to be highly enriched with dioxane. Thus, we studied the growth of COF-5 in pure dioxane vapor; however, this did not result in a highly periodic material. The growth of COF-5 in pure mesitylene atmosphere shows the evolution of COF-5 material (Figure S10, SI). We therefore conclude that the presence of mesitylene in the vapor mixture is crucial for the formation of a highly regular COF-5 structure. To further investigate the role of the vapor in the process, we synthesized COF-5 films with a different vapor source. We chose anisole, an aromatic ether, as a substitution for the mesitylene/dioxane mixture. The X-ray diffraction pattern of the obtained film reveals a highly periodic COF-5 material (Figure S10, SI). To extend the vapor conversion method to different COF linkages, we synthesized boroxine-based pyrene-COF25 films under anisole vapor. The X-ray diffraction pattern reveals the existence of a periodic structure (Figure S11, SI). The above observations emphasize the versatility of constructing COF materials by means of the room temperature vapor-assisted conversion method.
Summarizing, BDT-COF, COF-5, and pyrene-COF films could be synthesized through a vapor-assisted conversion procedure at room temperature. This novel method provides precise control of the film thickness ranging from a few hundred nanometers to several microns and allows for the realization of smooth and homogeneous coatings over large sample areas and on different substrates. We demonstrated the synthesis of BDT-COF films of 7.5 μm as well as thin films of 300 nm in thickness. BDT-COF films of larger thickness exhibit an additional textural porosity, whereas the thin films form dense layers consisting of small intergrown COF crystallites. In both cases, the BDT-COF crystals in the films exhibit random orientation on the surfaces. The vapor source composition plays an important role regarding the formation of highly periodic structures. In conclusion, we have established that room temperature vapor-assisted conversion synthesis is a powerful method for the generation of COF-based thin porous films. It is anticipated that the method will be of particular interest for fragile building blocks that do not survive the vigorous conditions typically used in solvothermal synthesis procedures.
Acknowledgments
The authors gratefully acknowledge funding from the NIM excellence cluster (DFG) and from the Bavarian SolTech research network. The research leading to these results received funding from the European Research Council under the European Union’s Seventh Framework Programme (FP7/2007-2013)/ERC Grant Agreement 321339 (ECOF project, T.B.). D.M. and J.R. acknowledge Alesja Ivanova and Peter Dowling for analytical support.
Supporting Information Available
Materials and methods section; structural, microscopic and spectroscopic characterization of different COF films synthesized by vapor-assisted conversion on various substrates. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
‡ These authors contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Côté A. P.; Benin A. I.; Ockwig N. W.; O’Keeffe M.; Matzger A. J.; Yaghi O. M. Science 2005, 310, 1166. [DOI] [PubMed] [Google Scholar]
- Colson J. W.; Dichtel W. R. Nat. Chem. 2013, 5, 453. [DOI] [PubMed] [Google Scholar]
- Feng X.; Ding X. S.; Jiang D. L. Chem. Soc. Rev. 2012, 41, 6010. [DOI] [PubMed] [Google Scholar]
- El-Kaderi H. M.; Hunt J. R.; Mendoza-Cortes J. L.; Cote A. P.; Taylor R. E.; O’Keeffe M.; Yaghi O. M. Science 2007, 316, 268. [DOI] [PubMed] [Google Scholar]
- Smith B. J.; Dichtel W. R. J. Am. Chem. Soc. 2014, 136, 8783. [DOI] [PubMed] [Google Scholar]
- a Dogru M.; Handloser M.; Auras F.; Kunz T.; Medina D.; Hartschuh A.; Knochel P.; Bein T. Angew. Chem., Int. Ed. 2013, 52, 2920. [DOI] [PubMed] [Google Scholar]; b Calik M.; Auras F.; Salonen M. L.; Bader K.; Grill I.; Handloser M.; Medina D. D.; Dogru M.; Löbermann F.; Trauner D.; Hartschuh A.; Bein T. J. Am. Chem. Soc. 2014, 136, 17802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H.; Chen X.; Gao J.; Lin J.; Addicoat M.; Irle S.; Jiang D. Chem. Commun. 2014, 50, 1292. [DOI] [PubMed] [Google Scholar]
- Dogru M.; Sonnauer A.; Zimdars S.; Doblinger M.; Knochel P.; Bein T. CrystEngComm 2013, 15, 1500. [Google Scholar]
- Yang S.-T.; Kim J.; Cho H.-Y.; Kim S.; Ahn W.-S. RSC Adv. 2012, 2, 10179. [Google Scholar]
- Biswal B. P.; Chandra S.; Kandambeth S.; Lukose B.; Heine T.; Banerjee R. J. Am. Chem. Soc. 2013, 135, 5328. [DOI] [PubMed] [Google Scholar]
- Gobinda Das; D. B S.; Sharath K.; Bishnu B.; Banerjee R. Chem. Commun. 2014, 50, 12615. [DOI] [PubMed] [Google Scholar]
- Colson J. W.; Woll A. R.; Mukherjee A.; Levendorf M. P.; Spitler E. L.; Shields V. B.; Spencer M. G.; Park J.; Dichtel W. R. Science 2011, 332, 228. [DOI] [PubMed] [Google Scholar]
- Medina D. D.; Werner V.; Auras F.; Tautz R.; Dogru M.; Schuster J.; Linke S.; Doeblinger M.; Feldmann J.; Knochel P.; Bein T. ACS Nano 2014, 8, 4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukata M.; Ogura M.; Osaki T.; Rao P.; Nomura M.; Kikuchi E. Top. Catal. 1999, 9, 77. [Google Scholar]
- Rao P.; Matsukata M. Chem. Commun. 1996, 1441. [Google Scholar]
- Tatsumi T.; Jappar N. J. Phys. Chem. B 1998, 102, 7126. [Google Scholar]
- Majano G.; Mintova S.; Ovsitser O.; Mihailova B.; Bein T. Microporous Mesoporous Mater. 2005, 80, 227. [Google Scholar]
- Moeller K.; Yilmaz B.; Jacubinas R. M.; Mueller U.; Bein T. J. Am. Chem. Soc. 2011, 133, 5284. [DOI] [PubMed] [Google Scholar]
- Shi Q.; Chen Z.; Song Z.; Li J.; Dong J. Angew. Chem., Int. Ed. 2011, 50, 672. [DOI] [PubMed] [Google Scholar]
- Changjean W.-C.; Chiang A. S. T.; Tsai T.-C. Thin Solid Films 2013, 529, 327. [Google Scholar]
- Dienstmaier J. F.; Gigler A. M.; Goetz A. J.; Knochel P.; Bein T.; Lyapin A.; Reichlmaier S.; Heckl W. M.; Lackinger M. ACS Nano 2011, 5, 9737. [DOI] [PubMed] [Google Scholar]
- Dienstmaier J. F.; Medina D. D.; Dogru M.; Knochel P.; Bein T.; Heckl W. M.; Lackinger M. ACS Nano 2012, 6, 7234. [DOI] [PubMed] [Google Scholar]
- Xu L. R.; Zhou X.; Yu Y. X.; Tian W. Q.; Ma J.; Lei S. B. ACS Nano 2013, 7, 8066. [DOI] [PubMed] [Google Scholar]
- Jiang Y.; Huang W.; Wang J.; Wu Q.; Wang H.; Pan L.; Liu X. J. Mater. Chem. A 2014, 2, 8201. [Google Scholar]
- Wan S.; Guo J.; Kim J.; Ihee H.; Jiang D. Angew. Chem., Int. Ed. 2009, 48, 5439. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.