Abstract
There exists a range of surgical and non-surgical approaches to the treatment of both acute and chronic tendon injuries. Despite surgical advances in the management of acute tears and increasing treatment options for tendinopathies, strategies frequently are unsuccessful, due to impaired mechanical properties of the treated tendon and/or a deficiency in progenitor cell activities. Hence, there is an urgent need for effective therapeutic strategies to augment intrinsic and/or surgical repair. Such approaches can benefit both tendinopathies and tendon tears which, due to their severity, appear to be irreversible or irreparable. Biologic therapies include the utilization of scaffolds as well as gene, growth factor, and cell delivery. These treatment modalities aim to provide mechanical durability or augment the biologic healing potential of the repaired tissue. Here, we review the emerging concepts and scientific evidence which provide a rationale for tissue engineering and regeneration strategies as well as discuss the clinical translation of recent innovations.
Keywords: Tendinopathy, healing, biomechanics, extracellular matrix, therapeutics
Environmental Stressors and Tendon Disease
Tendons are fibrous connective tissues which transmit force between muscle and bone to provide skeletal mobility. Functional impairment of tendons (e.g., rotator cuff, patellar and Achilles) represents a major health care problem. Tendons are composed of a dense, hierarchical organization of a parallel arrangement of type I collagen fibers (~70% of dry weight),1 along with varying, lesser amounts of collagen type III and V. The collagen fibers are embedded within a hydrophilic extracellular matrix consisting of matricellular proteins such as tenascin C, large proteoglycans such as versican and aggrecan, small proteoglycans such as biglycan and decorin, and glycosaminoglycans such as hyaluronan.
Clinically, tendon injuries are typically characterized by pain, swelling, stiffness and immobility. Broadly speaking, they are described as either acute or chronic. Acute injuries are the result of “macro-trauma” to the tendon, such as a laceration or sudden tensile overload, leading to either partial tearing or complete rupture. Chronic injuries, which generally do not involve macro-trauma and present histologically with a conspicuous absence of inflammatory cells,2 can arise from one or more conditions, each of which can either initiate and/or perpetuate the pathology. These stressors can be broadly defined as 1) metabolic (e.g. diabetes),3 2) genetic (e.g. Col5a1 mutations),4 3) vascular imbalance,5 4) hypoxia6 or 5) biomechanical (e.g., overuse injuries).7,8 The attendant cellular responses can include apoptosis, proliferation, migration or differentiation (e.g., adipogenic, chondrogenic, fibrogenic).2 Since such cellular responses often alter anabolic and/or catabolic pathways, they can disrupt collagen organization with a loss of tissue material properties, and they can also interfere with cell–matrix interactions involved in the transduction of mechanical signals to the resident cells.
Histopathology of Tendinopathy
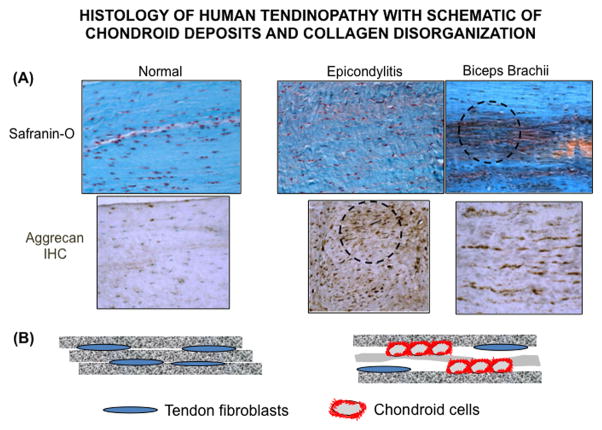
A well-recognized pathognomonic feature of tendinopathy is the presence of multiple small groups of cells with rounded nuclei which are distinct from the spindle-like fibroblasts normally seen on the surface of, or between, collagen fibers.9 These groups of rounded cells (Figure 1) are most often associated with the accumulation of a chondroid (Safranin-O positive) matrix, which is most highly stained near the cell groups and which appears to locally disrupt the normal linear arrangement of distinct collagen fibers.10 Electron microscopic analysis of tendinopathic regions has revealed an abnormal buckling of the collagen fascicles, the tendon cells and their nuclei, and it has been proposed that this is due to “loosening” of a sufficient number of fibers to allow a “cell-induced buckling of the tissue.”11 This raises the possibility that one mechanism by which stressors, such as mechanical overloading or hypoxia, can promote tendinopathic change is via the accumulation of cell clusters in a mechanically stiff chondroid matrix. While tendon loading readily deforms elongated cells in tissue regions of aligned collagen, it has a markedly reduced effect on rounded cells in chondroid regions,12 indicating that cells embedded within tendon chondroid deposits are shielded from tensile strain, which might increase their capacity to disorganize adjacent collagen fibers. Furthermore, a reduction in mechanical stimulation of cells within the chondroid matrix may impede appropriate (e.g., fibrogenic) remodeling of these diseased tendon regions.
Figure 1.
(A) Safranin-O staining and aggrecan immunohistochemical (IHC) localization of human extensor carpi radialis brevis and long head of the biceps tendons. Circled areas highlight chrondroid cell clusters, pericellular aggrecan accumulation, and localized collagen fiber disorganization in tendinopathic tissues. 21 (B) Schematic illustration of tendon fibroblasts within dense, aligned collagen fiber network of uninjured tendon (left panel), in contrast to the accumulation of rounded (chondroid) cells which disrupt the normal collagenous architecture in tendinopathic tissue (right panel)
Aggrecan is a Major Component of Tendinopathic Chondroid Deposits
While the precise structure and cell/matrix composition of chondroid deposits in tendinopathic tissue are largely unknown, they appear to be closely related to the tendon fibrocartilage which normally accumulates as an adaptive response to regions of tendon compression.13,14 Due to its unique intra-tissue osmotic effects, aggrecan is the matrix component which is primarily responsible for the compressive resistance of such normal tendon fibrocartilages.15 The possibility that it also promotes buckling and mechanical weakening in the body of the tendon is suggested by the finding that aggrecan expression in humans,16,17 and its chemical abundance in humans,18,19 horses,20 and mice21 are markedly increased in tendinopathic regions (Figure 1). Moreover, cross-sectional data from asymptomatic tendons found that collagen disorganization occurred only in association with cellular changes and chondroid accumulation.22 Additionally, glycosaminoglycan accumulation, largely due to aggrecan chondroitin sulfate, is strongly correlated with advanced clinical symptoms (pain, tenderness, overall weakness),23 suggesting a direct link of chondroid pathology to mechanical dysfunction and pain.
Hypoxia and Tendinopathy
The histologic appearance of the diseased tendon has also been described as an angio-fibroblastic tendinosis, invading and disrupting normal tissue,24 and it has been suggested that a goal of nonsurgical treatment should be a revascularization of the tissue. Indeed, it has been proposed that hypoxic cell injury is a critical pathophysiological mechanism (primary stressor) responsible for most tendinopathies25 (Figure 2). In support of this idea, increased hypoxia-inducible factor 1 alpha (HIF-1α) protein has been observed in cells within, but not outside, tendinopathic lesions25 and it is also abundant at the edge of torn tendons harvested at the time of rotator cuff surgery.26 Further, there are increased intra-tendinous lactate levels in Achilles tendinopathy,26 consistent with the pathology being associated with hypoxic stress and a switch to anaerobic glucose metabolism. Moreover, in the rotator cuff, a critical zone of hypovascularity (predictive of hypoxia) has been identified histologically27,28 and this coincides with the most common site for cuff tears. Lastly, in vitro studies25 have shown that exposure of tendon cells to hypoxia increases their expression of HIF-1α and collagen type III (Col3a1), both of which are increased in tendinopathic tissues.11,25
Figure 2.
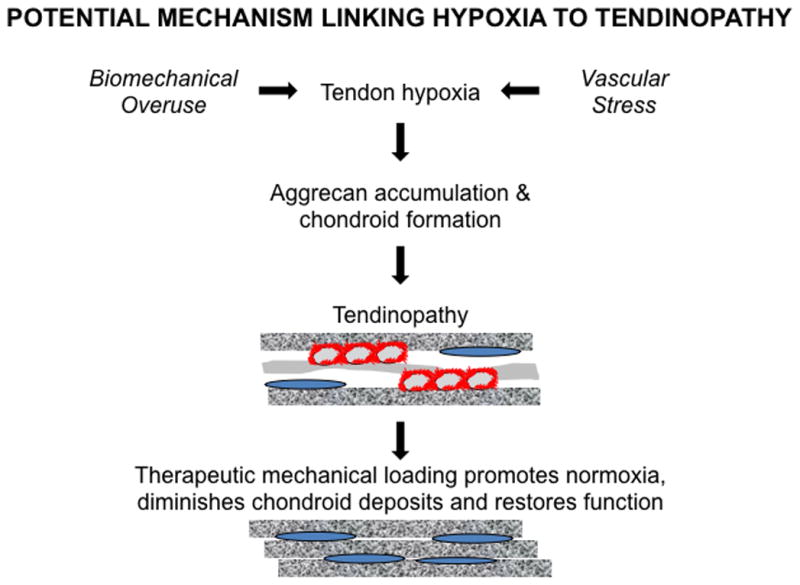
An hypoxic tissue environment (e.g., Hif-1α upregulation)25,236 induced by factors such as overuse or vascular stress may promote chondroid deposition,30–32 leading to collagen disorganization and tendinopathy. Therapeutic approaches such as mechanical loading71 can restore normoxic conditions and eliminate chondroid accumulation21 to restore tendon function.
Does Chondrogenesis Represent a Plausible Link Between Hypoxia and Tendinopathy?
Under normoxic conditions, HIF-1α is maintained at low levels by ubiquitination and proteasomal degradation. However, hypoxic conditions block this degradative pathway leading to high levels of cytoplasmic HIF-1α, which translocates to the nucleus to upregulate a large number of HIF-responsive genes,29 including those required for glucose uptake and anaerobic glycolysis. Such a response maintains high ATP levels in cells and thereby facilitates, among other processes, the synthesis of the UDP-sugar precursors required for synthesis of chondroitin sulfate and hyaluronan. In keeping with this, exposure of adipose-derived stem cells and human chondrocytes to hypoxia stimulates the expression of a wide range of chondrogenic genes,30–32 consistent with the major use of anaerobic glycolysis by chondrocytes in vivo.33 Taken together, these findings appear to provide a direct mechanistic link between hypoxic stress, the appearance of chondroid deposits and the loss of tensile strength in tendons (Figure 2).
Cellular Healing Deficiencies in Tendinopathy
Tendinopathic tendons are characterized by chondroid deposition, collagen disruption and loss of tensile strength. The chronicity of the pathology appears to be another example of that which occurs in any non-healing fibrous tissue wound. Significantly, it has been found that deposition of a chondroid matrix can interfere with dermal healing in an excisional murine model.34 Gene expression analysis of the non-healing dermis showed that it was accompanied by deficiencies in the TGF-β1 pro-fibrogenic pathway. In this regard, it has been shown that a markedly higher number of cells in human patellar tendinopathy express TGF-β1, consistent with an attempted fibrogenic wound healing response.35 Consistent with this notion, studies of tendinopathic tissue have shown an increased expression of pericellular components (often with a short half-life) such as fibronectin, tenascin C, aggrecan and biglycan,19 whereas the expression of constituents more associated with the stable intercellular matrix (decorin and type I collagen), was less altered.17,36,37 When examined from this perspective the healing deficiencies in tendinopathy appear to result from a chronic stimulation of synthesis of pericellular components (within chondroid deposits) without a concomitant increase in the production of stable, long-lived matrix structures (Figure 2).
Experimental Models of Tendinopathy
While changes which follow acute tendon injury (e.g., tendon transection and repair) have been well described in animal models, the cellular and molecular responses to chronic tendinopathies have been less well studied. As described more extensively in other reviews,38–40 tendon injury responses have been characterized both in vivo and in vitro, using various approaches which include: (i) surgical tendon lacerations41–44; (ii) pharmacological induction of tendinopathy (e.g., using bacterial collagenase,45–47 cytokines or prostaglandins48–50; and (iii) in vitro51–53 and in vivo54–59 mechanical loading models (e.g., treadmill overuse, muscle stimulation, and fatigue loading).
In general, animal tendinopathy models are accompanied by an induction of chondrogenic genes and/or chondroid deposits. For example, collagenase injection into horse60 or rat61–63 tendons produced an inflammatory response with tendon collagen bundle disarray, mucoid deposits, and at the molecular level, expression of Col2a1 and Sox9 by fibroblast-like cells and expression of Bmp-4/7 by chondrocytic cells, suggesting that aberrant differentiation of healing tendon progenitor fibroblasts may account for failed healing. Further, when a mixture of cytokines (e.g., IL-1) and growth factors (TGF-β1, FGF2) was injected into rabbit tendons,53 both fibrosis and mucoid accumulation resulted. This consistent finding of a chondroid response has also been described in mechanically-induced (treadmill overuse) models examining rat rotator cuff64,65 and mouse patellar and Achilles tendons.59
Mechanical Loading Can Promote Tendon Healing
Treatment options for tendinopathy patients are limited since corticosteroid injections and surgical debridement show poor long-term outcomes.66 Similarly, effectiveness of modalities such as ultrasound and shock wave therapy has not been proven.67–69 Rehabilitative exercise, specifically eccentric loading (e.g. heel drop exercises) to lengthen the muscle-tendon unit, is currently the most effective long-term therapy for Achilles tendinopathy, as shown by pain reduction and improved tendon function.70–72 However, the mechanisms by which mechanical stimulation contributes to tendon healing and restoration of function are currently unknown. In a recent systematic review focusing predominantly on Achilles tendinopathy, Drew and colleagues73 concluded that the clinical literature does not support the notion that observable structural changes in the tendon can explain the therapeutic efficacy of exercise. Hence, it is likely that other factors (e.g., neurogenic or biochemical) play an integral role in healing of tendinopathies, and strategies to target associated mechanisms are warranted.
There exist numerous reports on the stimulatory effects of mechanical loading on the healing capacity of transected animal tendons.74,75 Killian et al.76 noted that, while controlled mechanical loading is generally beneficial to tendon healing, the magnitude and timing of such loading is dependent on anatomic location (e.g., intrasynovial versus extrasynovial). While these data have important clinical implications on designing rehabilitation protocols following surgical tendon repair, very few models have examined the therapeutic potential of mechanical loading to treat tendinopathy. In a rat Achilles model of collagenase injection,77 early voluntary exercise was found to accelerate collagen deposition but prolonged the inflammatory response and led to decreased tendon biomechanical properties. Tendon healing in rats which initiated voluntary exercise at a later post-injection time point showed minimal differences relative to non-exercised controls. Bell et al.21 developed a TGF-β1 injection model to induce chondroid accumulation, which was associated with a dramatic decrease in the material properties of the murine Achilles tendon. Following injection, 4 weeks of treadmill exercise suppressed chondroid formation, decreased chondrogenic gene expression (relative to mice which did not receive exercise), and stimulated a restoration of mechanical properties to those of uninjured tendons. The latter study thereby lends strong support to the clinical physiotherapy literature (as illustrated in Figure 2).
Scientific Rationale for New Approaches to Tendon Regeneration
The first-line treatment strategy for tendon tears typically is surgical reattachment of the tendon to its bony insertion site or direct tendon-tendon repair, depending on the injury location and the severity of its impairment on tendon and joint function. In spite of numerous technical advances to date, primary repair in general neither reliably restores pre-injury functional properties nor regenerates the native tendon structure, organization, and composition.78 Biologic therapies represent an experimental strategy for providing mechanical support, augmenting tissue healing, and/or promoting tissue regeneration. Tissue engineering strategies arise from a fundamental understanding of tendon structure, function, composition, and the biologic processes involved in tendon homeostasis and healing. Successful tendon regeneration relies on presence of three critical factors: (1) a scaffold to provide a structural framework for cell attachment and matrix production; (2) progenitor cells, which differentiate into specialized tissue in response to appropriate mechanobiologic signals; (3) growth factors that drive the progenitor cells into a tenogenic differentiation pathway. Biological strategies to augment healing of intrinsic or surgical tendon repair involve utilizing one or more of the aforementioned three factors. Another branch of tendon tissue engineering aims to apply knowledge derived from studies of the embryologic tendon development and postnatal remodeling (e.g., mesenchymal stem cell differentiation pathways and transcription signals) towards strategies for repair after injury.79–82, 83,84
Scaffolds For Use in Tendon Regeneration
Scaffolds provide both mechanical reinforcement and augmentation of healing potential to the injured tissue. Scaffolds come in the form of native tendon matrices85 and collagen-based constructs,86 synthetic polymers,87–89 and engineered forms of endogenous proteins,90 which can facilitate tendon incorporation at the site of injury. These devices should have sufficient mechanical properties to offload and stabilize the repaired tendon during the early postoperative period, and, particularly in the case of ECM-derived scaffolds, should harbor bioactive substances such as growth factors needed to improve the rate and/or effectiveness of tendon healing.91 Importantly, the scaffold should also be amenable to suture retention.91 Among the most common natural biomaterial scaffold materials for tendon repair are porcine small intestine submucosa (SIS), silk, human allograft dermis, and decellularized tendon.92 Once introduced into the body, the scaffold gradually resorbs and is replaced by ECM products synthesized by the scaffold-attached cells.93 Scaffold design should provide mechanical durability, adequate surface area and porosity, and biocompatibility in order to avoid eliciting a host inflammatory response.94 Efforts are underway to engineer new synthetic scaffold designs, such as fibroblast or MSC-seeded polylactic-co-glycolic acid (PLGA) nanofiber95 and MSC seeded collagen sponge scaffolds.96,97
In preclinical large animal models, ECM scaffolds (Restore, Zimmer Collagen Repair, GraftJacket) have shown promising histologic outcomes and tendon-like remodeling but have generally had disappointing biomechanical outcomes with respect to the repaired constructs at 9 to 24 weeks.98–101 In these canine and ovine models there were no unfavorable host inflammatory responses noted, although some degree of transient inflammation lasted longer with the cross-linked porcine dermis scaffold100 than with the non-cross-linked porcine SIS99,101 or non-cross-linked human dermis98 alternatives. In contrast, synthetic scaffolds including Synthasome’s X-Repair (a poly-L-lactide patch) and the Biomerix Rotator Cuff Repair Patch (composed of polycarbonate polyurethane-urea), have proven to be both biocompatible and to significantly enhance the biomechanical function of repaired infraspinatus tendons in canine and ovine models, respectively.102,103
A large number of clinical studies have assessed the therapeutic use of scaffold devices in human subjects (Table 1).104–120 While scaffolds have been among the most studied biologics in tendon repair, the clinical translation of these devices is nuanced. At present there is a paucity of studies with long-term follow-up data on these devices, and their results have been inconsistent with respect to clinical outcomes and adverse effects. Clinically significant aseptic inflammatory reactions have been reported in roughly one-quarter of patients treated with non-cross-linked porcine SIS scaffolds during rotator cuff repair,110,118 and as a result the American Academy of Orthopedic Surgeons (AAOS) does not endorse their use for this clinical indication.121 Variability in clinical outcomes may be attributable to heterogeneity in the design and application of these devices. Studies have variably utilized scaffolds to either augment repair or for interposition between the tendon and bone insertion. Numerous types of materials are available and, although natural ECM scaffolds have been favored initially, preliminary data for synthetic scaffolds are promising,109,120 and it is not clear which type is optimally suited for each clinical application. With regard to regulated clinical use, because synthetic and ECM scaffolds are prepared from non-human sources, they are allowed into the healthcare marketplace by the Food and Drug Administration (FDA) so long as they exhibit substantial equivalence to a reference device in their biocompatibility, sterility, safety, and performance.122 However, manufacturers are not required to demonstrate the efficacy of these nonhuman-derived augmentation devices in preclinical or controlled human clinical testing.122 Notably, the use of human-derived ECM scaffolds as transplantable human tissue is protected under the Code of Federal Regulations and is not subject to FDA approval.91
Table 1.
Clinical studies using scaffolds in tendon repair.
| Study design | Type of scaffold | Study groups | F/U | Outcomes |
|---|---|---|---|---|
|
Metcalf et al114 Case series |
Restore | Open RCR + scaffold (n = 12) | 24 mos | Repair failure in 1/12; UCLA scores doubled |
|
Sclamberg et al116 Case series |
Restore | Open RCR + scaffold (n = 11) | 6 to 10 mos | Repair failure in 10/11; no improvement in ASES scores |
|
Iannotti et al110 RCT |
Restore | Open RCR Scaffold (n = 15) No scaffold (n = 15) |
12 to 26 mos | Scaffold group had lower healing rates; no difference in Penn scores |
|
Malcarney et al113 Case series |
Restore | Open RCR + scaffold (n = 25) | 6 mos | RCT aborted due to postoperative inflammatory reactions in 4/25 causing repair failure and requiring open debridement |
|
Walton et al118 Retrospective case-control |
Restore | Open RCR Scaffold (n = 10) No scaffold (n = 12) |
24 mos | No difference in tendon healing rates, satisfaction scores, or range of motion |
|
Burkhead et al107 Case series |
GraftJacket | Open RCR + scaffold (n = 17) | 14 mos | Repair failure in 3/12; improvement in UCLA scores, strength, and range of motion and satisfaction in 14/17 patients |
|
Dopirak et al108 Bond et al106 Case series |
GraftJacket | Arthroscopic RCR + scaffold (n = 16) | 12 to 38 mos | Incorporation into tendon in 13/16; improvement in UCLA and Constant scores, strength, and range of motion; 15/16 satisfied |
|
Wong et al119 Case series |
GraftJacket | Arthroscopic RCR + scaffold (n = 45) | 24 to 68 mos | Update to above study; continued favorable outcomes |
|
Barber et al105 RCT |
GraftJacket | Open RCR Scaffold (n = 22) No scaffold (n = 20) |
12 to 38 mos | Tendon healing rates and Constant and ASES scores higher in scaffold group; no difference in UCLA scores |
|
Rotini et al115 Case series |
ADM | Open or arthroscopic RCR + scaffold (n = 5) | 12 to 18 mos | Incorporation into tendon in 5/5; improvement in Constant scores |
|
Lee et al111 Lee et al112 Case series |
GraftJacket | Achilles tendon repair + scaffold (n = 9, n = 11) | ≥ 20 mos | All patients able to recover full ROM at 3 months and perform single heel raises at 6 months; improvement in AOFAS scores at 12 months; no re-ruptures, recurrent pain, or reoperations at ≥ 20 months |
|
Soler et al117 Case series |
Zimmer Collagen Repair | Open RCR + scaffold (n = 4) | 3 to 6 mos | All patients had failed incorporation into tendon, inflammatory reaction, graft disintegration, and tissue necrosis at time of revision surgery |
|
Badhe et al104 Case series |
Zimmer Collagen Repair | Open RCR + scaffold (n = 10) | 36 to 60 mos | Incorporation into tendon in 8/10; improvement of Constant scores, pain, ROM, and abduction strength; 9/10 satisfied; no adverse events |
|
Encalada-Diaz et al109 Case series |
Biomerix RCR Patch | Open RCR + scaffold (n = 10) | 12 mos | Intact tendon healing in 9/10; improvement in ASES and SST scores, pain, and ROM; no adverse events |
| Proctor120 | X-Repair | Arthroscopic RCR + scaffold (n = 18) | 42 mos | Intact tendon healing in 14/18; improved ASES scores |
RCT = randomized controlled trial; ADM = acellular dermal matrix (commercial name not specified); RCR = rotator cuff repair; UCLA = University of California, Los Angeles; ASES = American Shoulder and Elbow Surgeons; AOFAS = American Orthopaedic Foot and Ankle Society; SST = Simple Shoulder Test; ROM = range of motion
Gene Therapy Approaches to Tendon Regeneration
Gene therapy is a promising method for facilitating trafficking of growth factors to the injured site,123 potentially allowing sustained temporal delivery.124 This is accomplished using either direct gene transfer or viral or non-viral vectors coding for growth factors, whose delivery can be achieved by local injection. While these in vivo forms of gene therapy allow accurate introduction of genetic products, they are inconsistent in their efficiency of transduction and expression.125 Ex vivo gene therapy relies upon the extraction and viral transduction of autologous cells, cell expansion and antibiotic selection in culture, and reinoculation into the injured tissue.126 While the latter method is more labor intensive and time consuming, it provides a means for quality control and greater efficiency.127
Preclinical models have given credence to this emerging therapeutic modality. Transfection of tenocytes with BMP12 in a chicken flexor profundus tendon transection model significantly improved the tissue tensile strength and failure load at 4 weeks.128 Bone marrow-derived mesenchymal stem cells (BMSCs) transduced with adenovirus carrying human TGF-β1 cDNA accelerated healing of rabbit Achilles tendon defects.129 Implantation of BMP12- and TGF-β1 transfected muscle flaps similarly enhanced healing in transected rat Achilles tendons.130,131 In addition to growth factor-expressing genes, genes involved in tendon cell development and maturation, such as scleraxis,79 tenomodulin,80 tenascin-C,81 and Smad882 have also been highlighted for their tissue engineering potential. The clinical importance of such genes in tendon repair was demonstrated in a murine model of flexor digitorum longus (FDL) transection that showed increased Smad8 expression at 4 weeks after tendon repair, in association with neotendon formation, remodeling of intrasynovial adhesions, and improvement of range of motion.132 Gulotta et al.133 reported that MSCs transduced with adenoviral-mediated scleraxis conferred improved biomechanical properties of repaired rat supraspinatus tendons up to four weeks post-surgery.
Gene therapy remains a highly experimental treatment approach that has yet to be extended to the clinic. To our knowledge, no human studies have employed gene therapy in the treatment of tendon repair. The challenges to implementing gene therapy in human patients include establishing the most efficient method for delivering genetic material (viral or non-viral vectors versus direct gene transfer), selection of candidate genes for enhancement or repression, the timing of such treatments and minimizing off-target effects.
Growth Factor Treatment Strategies for Tendon Healing
Once administered to an injured tendon, selected growth factors have the capacity to promote differentiation of resident stem cells into tendon fibroblasts for augmentation of tendon healing. Successful therapeutic use of growth factors requires optimal targeting to, and mechanical stimulation of, the site of pathology.134 Growth factors shown to contribute to tendon healing include transforming growth factor-β (TGF-β1 and TGF-β3), insulin-like growth factor-1 (IGF-1), platelet-derived growth factor (PDGF-BB), vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), and growth and differentiation factor (GDF)-5, -6, and -7.135,136
While TGF-β has been shown to promote type I and III collagen synthesis, scar formation, and further growth factor elaboration in vitro,137 the endotenon-derived cells appear to down-regulate fibrogenic gene expression in comparison with epitenon and sheath cells.138 Data from in vivo models indicate a positive effect of TGF-β1 on healing of central third patellar tendon defects in rabbits,139 but given that TGF-β1 has been implicated in adhesion formation (fibrosis) of intrasynovial tendons,140 strategies to modulate its expression have recently received attention in the literature.141 IGF-I expression increases during the inflammatory phase of wound healing, and this growth factor also promotes cell proliferation and migration, and subsequently promotes collagen and ECM synthesis during tissue remodeling.142–144 Schnabel et al.,45 in an equine model of collagenase-induced injury of the superficial digital flexor tendon (SDFT), observed that both MSCs and IGF-I gene-enhanced MSCs improved histologic indices of healing, although few differences were noted when comparing these two groups.
PDGF has been shown to contribute to tendon healing through cell proliferation and upregulation of collagen and ECM synthesis,145–147 however it secondarily induces inflammatory cytokine expression which can promote scarring. The extent of scar formation depends upon the timing and duration of PDGF administration.147 Interest in PDGF therapy has been heightened by several animal studies showing a positive effect in aiding the repair of a tendon defect148 as well as ruptured149,150 and collagenase-injected151,152 tendons. Thomopoulos and coworkers146,153 developed a novel fibrin/heparin-based delivery system for controlled release of PDGF-BB. This approach led to increased cell density, proliferation, collagen I gene expression, and reducible collagen crosslinks favorable for improving intrasynovial flexor tendon repair.
In a rat model of Achilles transection and repair, exogenous application of VEGF, a regulator of angiogenesis, improved tensile strength of the healing tendon at one and two weeks post-injury, potentially via an increased expression of TGF-β.154 However, VEGF may contribute to tendon pathology,5 as it has been shown to be increased in ruptured Achilles tendons.155 Acting at the site of injury, bFGF facilitates angiogenesis and mesenchymal cell proliferation.156,157 Also, following transection and repair of rabbit flexor tendons, subcutaneous administration of human recombinant bFGF led to improved collagen indices (fibril diameter and density) and biomechanical properties.158 Central region patellar tendon defects in rats treated with bFGF exhibited a dose-dependent increase in cell proliferation and collagen III expression but did not improve mechanical function.156 bFGF showed promise in the early stages of rat supraspinatus tendon to bone healing via acceleration of bone in growth into the scar tissue interface.159
BMP family members promote cell proliferation, collagen production, and ECM organization,160 and play a role in the transcriptional regulation of tendon repair,135 while also blunting the expression of inflammatory genes.161 Three members of the BMP family (GDF-5,6, and 7) have shown promise in in vivo pre-clinical studies for accelerating tendon repair.128,162–165 In a rat model of Achilles tendon repair, adenovirus-mediated gene therapy with GDF-5 increased the number of tendon progenitor cells at the site of healing and improved the tensile strength of the healed tendon.166 In a murine model using FDL allografts, GDF-5 transduction led to more rapid healing and reduced adhesion formation than controls.167 In two separate rat Achilles tendon repair studies,163,164 GDF5-coated sutures augmented the healing response; however, the authors noted the appearance of chondroid regions at the repair site and speculated that this could be corrected by modifying the GDF5 dosage or the local mechanical loading environment.
An immense degree of clinical interest has been directed in recent years towards the use of autologous platelet-rich plasma (PRP) for healing of skeletal tissues including tendon and ligaments, particularly in the context of sports injuries.168 PRP can be defined as blood plasma containing supra-physiologic levels of platelets, which is rich in numerous growth factors and cytokines considered integral to wound repair,169 and many pre-clinical reports of efficacy are now available. 24 weeks after creating surgical lesions in the equine SDFT, Bosch and coworkers170 observed, relative to saline controls, improved histologic, biochemical, and biomechanical properties following a single intratendinous PRP treatment at 7 days post surgery. Beneficial effects of PRP on tendon healing, including increased collagen deposition and improved collagen fiber orientation and mechanical properties were also reported in rat Achilles tendon defect,75 rabbit patellar tendon defect,171 rat Achilles tendon transection,172 and rabbit chronic rotator cuff tear173 models. In apparent support of these findings, evidence from several in vitro studies174–178 suggests that PRP administration stimulates cell proliferation, increases collagen I and III synthesis, and upregulates the expression of PDGF and TGF-β. While animal models generally support the premise of PRP use for tendon healing in clinical applications, not all studies have shown positive effects. For example, in a sheep model of deep digital flexor tendon injury, addition of PRP to autologous mesenchymal stromal cells did not provide a synergistic regenerative response following injection treatment.179 Furthermore, potential variability in methods of PRP preparation across studies as well as the use of acute tendon injury models should be noted as limitations of such pre-clinical studies.
Clinical outcomes for PRP and its related formulations known as plasma rich in growth factors (PRGF), platelet-rich fibrin matrix (PRFM), and plasma-leukocyte membrane (PLM) have been published, primarily for rotator cuff repair (Table 2).180–198 There remains uncertainty regarding the mechanism of action of PRP and related therapies, as PRP contains a plethora of bioactive growth factors and other compounds. In addition, conflicting results must be reconciled, as these treatments have variably been superior, non-superior, and inferior to controls in different studies, suggesting the need for better patient selection, standardization of treatment protocols, and/or standardization of radiological and clinical outcome measures. Indeed, there is great heterogeneity in commercially available growth-factor preparations.183 Further basic science and clinical investigations will be needed to better elucidate which particular growth factors or combinations of factors provide the greatest therapeutic benefit. Moreover, future clinical trials must clarify not only the optimal dosing and timing of administration but also the most efficient vehicle for delivering growth factors to injured tissue, be it direct injection of soluble factors, introduction of genetic material coding for growth factors, or surgical placement of scaffolds embedded with these factors. A practical challenge is to ensure efficient localization of growth factors to the site of injury as well as adequate retention of the necessary concentration over a managed timeframe.
Table 2.
Clinical studies using growth factor therapies in tendon repair.
| Study design | Type of therapy | Study groups | F/U | Outcomes |
|---|---|---|---|---|
|
Sanchez et al190 Retrospective case control |
PRGF | Open Achilles repair PRGF (n = 6) No PRGF (n = 6) |
50 mos (control) 32 mos (PRGF) |
PRGF group had earlier recovery of ROM and earlier return to activity |
|
Randelli et al187 RCT |
PRP | Arthroscopic RCR PRP (n = 26) No PRP (n = 27) |
3 to 24 mos | PRP group had lower short-term pain scores and significantly higher SST, UCLA, and Constant scores and external rotation strength at 3 months |
|
Jo et al185 RCT |
PRP | Arthroscopic RCR PRP (n = 24) No PRP (n = 24) |
12 mos | PRP group had lower retear rate, larger CSA, and better overall function; no differences in ASES, Constant, UCLA, DASH, SST, or SPADI scores |
|
Malavolta et al186 RCT |
PRP | Arthroscopic RCR PRP (n = 27) No PRP (n = 27) |
24 mos | No difference in retear rates or VAS or Constant scores; PRP group had better UCLA scores at 12 months |
|
Filardo et al197 Case series |
PRP | Chronic Achilles tendinopathy PRP (n = 27; 7 bilateral) |
2, 6, 54.1 mos | Improved VISA-A, Tegner scores |
|
Murawski et al193 Case series |
PRP | Chronic Achilles tendinopathy PRP (n = 32) |
6 mos | 25 of 32 were asymptomatic and returned to sport/activity at 6 mos; 7 required surgery |
|
Kearney et al196 Randomized pilot |
PRP | Chronic Achilles tendinopathy PRP (n = 10) Eccentric loading program (n = 10) |
1.5, 3, 6 mos | No difference in VISA-A scores (attributed to pilot design) |
|
Ferrero et al198 Case series |
PRP | Chronic Achilles (n = 30) or patellar (n = 28) tendinopathy US-guided PRP (n = 58) |
6 mos | Improved VISA scores; reduction of hypoechoic areas in 26 tendons on US |
|
Mishra et al194 RCT |
PRP | Chronic lateral epicondylitis PRP (n = 116) Active control (n = 114) |
12, 24 wks | Better VAS pain improvement and lower rates of elbow tenderness in PRP group at 24 wks, but not 12 wks |
|
Krogh et al195 RCT |
PRP | Chronic lateral epicondylitis PRP (n = 20) CSI (n = 20) Saline (n = 20) |
3 mos | Pain improved in all groups at 3 mos, but no differences; CSI improved pain more than PRP at 1 mo; CSI reduced color Doppler activity and tendon thickness more than PRP |
|
Thanasas et al192 RCT |
PRP | Chronic lateral epicondylitis US-guided PRP (n = 14) Autologous blood (n = 14) |
6 wks, 3 mos, 6 mos | Greater VAS pain improvement with PRP only at 6 wks; no difference in Liverpool elbow scores |
|
Weber et al191 RCT |
PRFM | Arthroscopic RCR PRFM (n = 30) No PRFM (n = 30) |
12 mos | No differences in structural integrity of repair; VAS pain, SST, or ASES scores; or range of motion |
|
Rodeo et al188 RCT |
PRFM | Arthroscopic RCR PRFM (n = 40) No PRFM (n = 39) |
≥ 12 mos | No difference in intact tendon healing rate or ASES or L’Insalata scores; PRFM use predicted tendon defect at 12 weeks |
|
Antuna et al180 Randomized pilot |
PRFM | Arthroscopic RCR PRFM (n = 14) No PRFM (n = 14) |
≥ 24 mos | No difference in retear rates or Constant scores |
|
Barber et al181 Comparative series |
PRFM | Arthroscopic RCR Two sutured PRFM constructs (n = 20) No PRFM (n = 20) |
24 to 44 mos | Persistent tendon defects in more of control than PRFM patients Rowe scores higher in PRFM group, but no differences in ASES, SANE, SST, or Constant scores |
|
Castricini et al183 RCT |
PRFM | Arthroscopic RCR PRFM (n = 43) No PRFM (n = 45) |
16 mos | No differences in repair integrity or Constant scores |
|
Bergeson et al182 Prospective cohort |
PRFM | Arthroscopic RCR | 27 mos (control) 13 mos (PRFM) |
Retears more common in PRFM group No differences in ASES, UCLA, SANE, Constant, or WORC scores |
|
Ruiz-Moneo et al189 RCT |
PRGF | Arthroscopic RCR | 12 mos | No differences in rates of tendon healing, UCLA scores, or satisfaction |
|
Gumina et al184 RCT |
PLM | Arthroscopic RCR PLM (n = 39) No PLM (n = 37) |
13 mos | PLM group had better repair integrity and Constant scores |
RCT = randomized controlled trial; PRP = platelet-rich plasma; PRFM = platelet-rich fibrin matrix; PRGF = plasma rich in growth factors; PLM = plasma-leukocyte membrane; RCR = rotator cuff repair; UCLA = University of California, Los Angeles; ASES = American Shoulder and Elbow Surgeons; SANE = Single Assessment Numeric Evaluation; WORC = Western Ontarior Rotator Cuff Index; SST = Simple Shoulder Test; VAS = visual analog scale; SPADI = Shoulder Pain and Disability Index; DASH = Disabilities of the Arm, Shoulder, and Hand; VISA-A = Victorian Institute of Sport Assessment-Achilles; PRTEE = Patient-Rated Tennis Elbow Evaluation; CSA = cross-sectional area; ROM = range of motion; US = ultrasound; CSI = corticosteroid injection
Use of Mesenchymal Stem Cells for Tendon Healing
Adult stem cells are characterized by their distinct ability for self-renewal. Friedenstein et al. were among the first investigators to demonstrate the presence of fibroblast-like stem cells in the bone marrow that were plastic-adherent and capable of differentiating into multiple phenotypes.199 The fibroblast-like cells were later termed mesenchymal stem (or stromal) cells (MSCs). Two commonly used subtypes of MSCs include bone marrow- and adipose-derived MSCs. Adult stem cells have been harvested from other tissues including muscle, periosteum, adipose tissue, vascular pericytes, dermis and peripheral blood.200 At present the majority of cell therapies focus on the use of MSCs which have multipotent differentiation potential201 and can essentially serve as tissue grafts. Once extracted from the connective tissue or peripheral blood of the patient, undifferentiated MSCs can be injected to give rise to tendon fibroblasts at the site of healing.202 Injected MSCs can replenish and/or augment the reparative cellular pool at the site of tissue repair and also exhibit anti-inflammatory and anti-apoptotic properties.203 Among the disadvantages of BMSCs are the pain associated with bone marrow harvesting and the potential for low cellular yield.201
A number of in vivo studies have explored the potential of BMSCs for tendon regeneration. Transected rat Achilles tendons were treated with MSCs cultured under either hypoxic or normoxic conditions,204 and the failure load of the hypoxia-treated group was significantly higher than that of the normoxic group at both 2 and 4 weeks following injury, although immunostaining of collagen types I and III were similar between the groups. Juncosa-Melvin et al. demonstrated the efficacy of MSC-seeded collagen gel constructs for the treatment of both rabbit Achilles205 and patellar tendon defects.96,206 These studies also highlighted the importance of construct cell density and pre-implantation mechanical stimulation to the biomechanical properties of the healing tissue. Smith and colleagues207 treated tendinopathic horses with an injection of autologous BMSCs suspended in marrow supernatant. Encouragingly, this treatment conferred improvements in tissue structural organization, composition, and vascularity.
Adipose tissue is an attractive site for isolating MSCs due to its abundance and availability, as well as its comparatively minimal harvest-site morbidity.201 In a rabbit model, adipose-derived MSCs (ADSCs) augmented the tensile strength of Achilles tendon repair and increased the expression of type I collagen, FGF, and VEGF.208 Injection of an adipose derived stromal vascular fraction into surgically repaired rabbit flexor tendons enhanced the biomechanical properties of the healing tissue.209 In a rabbit model of chronic subscapularis tendon tears,210 repairs augmented by ADSCs exhibited decreased muscle fatty infiltration and a trend towards improved mechanical properties relative to a saline treatment control group. Encouraging results have also been reported with tissue engineering strategies whereby ADSCs were seeded onto synthetic (PLGA) scaffolds97,211 or acellular tendon ECM.212 However, ADSCs did not improve mechanical properties of healing supraspinatus tendon to bone repairs in rats.213
A cell population described as tendon-derived stem cells (TDSCs) have been isolated and characterized from rotator cuff tendon, patellar tendon, paratenon, subacromial bursa and Achilles tendon.214–217 When TDSCs were isolated and implanted as TDSC-fibrin glue constructs into patellar tendon defects using a rat model,218 the collagen content, fiber orientation, and biomechanical properties were significantly improved in the TDSC-group relative to fibrin only controls at four weeks post-injury. Pietschmann et al.219 created rat Achilles tendon defects which were bridged by PGA and collagen type I scaffolds seeded with either tail tendon-derived cells or BMSCs. Analysis of DNA from the tendon repair site showed that the implanted cells survived at 16 weeks. Consistent with earlier MSC studies, ectopic ossification was found in tendons of both treatment groups. Despite this, the biomechanical properties and collagen alignment of the regenerated tendon were improved relative to those of the MSC-only or scaffold-only groups. Chen et al.220 developed a rabbit model to repair massive rotator cuff tendon defects using an interpositional graft consisting of autologous tendon cells seeded onto either a porcine SIS or a collagen types I/III bioscaffold. An inflammatory reaction was noted at 4 weeks post-surgery, though its severity was diminished at 8 weeks. While histologic features of the repair tissue of both tendon cell-seeded groups was inferior to that of autograft controls, the authors noted a superior healing response of cell-seeded scaffolds relative to that of scaffolds alone.
In comparison to scaffolds and growth factors, there is a paucity of clinical studies using stem cells for augmentation of tendon repair or for treating tendinopathy (Table 3).221–225 Stem cells for clinical use have been harvested from multiple sources including but not limited to bone marrow, adipose tissue, dermis, tendon tissue, periosteum, subacromial bursa.214,215,226,227 The stem cells can be used as a stand-alone therapy or in combination with other tissue engineering approaches (growth factors and scaffolds). Stem cells can be used directly after harvest or can be concentrated to increase the cell count before use at the site of repair. Some sources, such as tendon or dermis, give low cell yields such that the cells need to be expanded in culture prior to use. Culture expansion of stem cells has its own limitations and safety issues. There is a theoretical risk of infection and prolonged ex vivo culture expansion can induce senescence and lead to loss of the multipotent potential of stem cells. Moreover, the culture expansion step may not be cost-effective.228 Although the stem cells can be genetically modified ex vivo using viral gene therapy, there are safety reasons (replication competence of viral vectors, insertional mutagenesis, and immune response) that prohibit the use of this strategy for augmenting tendon repair and healing.229,230
Table 3.
Clinical studies using stem cell therapies for tendon disorders.
| Study design | Type of stem cell | Study groups | F/U | Outcomes |
|---|---|---|---|---|
|
Hernigou et al223 Retrospective cohort |
BMSCs | 45 pts with SR ARCR treated with BMSCs 45 matched controls treated with ARCR alone |
≥ 120 mos | BMSC group had higher healing rates at 6 mo and 10 yr on MRI compared to matched controls |
|
Wang et al224 Case series |
Patellar tendon derived tenocytes | 17 pts with refractory lateral epidcondylitis were treated with patellar tendon derived tenocytes expanded in vitro | 12 mos | Signficant improvement in pain score, DASH scores, MRI grading of tendinopathy |
|
Gomes et al225 Case series |
BMMC | 14 consecutive pts treated with miniopen transosseous RCR + BMMC | ≥ 12 mos | Significant postoperative increase in UCLA score, Intact repairs on MRI imaging (100%) |
|
Clarke et al221 RCT |
Skin derived tenocyte like cells | 60 pts with refractory patellar tendinopathy were randomized to receive USG guided injections of plasma + autologous dermal fibroblast derived tenocytes (n=33) or plasma alone (n=27) in the patellar tendon | 6 mos | Significant improvement in the VISA scores in the tenocyte group |
|
Connell et al222 Prospective clinical pilot |
Skin derived tenocyte like cells | 12 pts with refractory lateral epicondylitis underwent USG guided injections of | 12 mos | Significant improvement in PRTEE scores, reduction in number of tears, and thickness of tendon |
RCT = randomized-controlled trial; BMMC = mononuclear cell fraction of bone marrow aspirate; USG = ultrasonography; SR = single-row; RCR = rotator cuff repair; ARCR = arthroscopic rotator cuff repair; DASH = Disabilities of the Arm, Shoulder, and Hand; UCLA = University of California, Los Angeles; VISA = Victorian Institute of Sport Assessment; PRTEE = Patient-Rated Tennis Elbow Evaluation
Hernigou et al. retrospectively evaluated a cohort of 45 patients that were treated with concentrated BMSCs as an adjunct to the arthroscopic single row rotator cuff repair (RCR).223 A significant difference between the MRI documented healing rates of RCRs treated with MSCs (100% at 6 months and 87% at minimum 10 years) was noted in comparison to matched controls (67% at 6 months and 44% at minimum 10 years). Two studies demonstrated improved clinical results with the use of dermal fibroblasts (suspended in autologous plasma) for treatment of patellar tendinopathy and lateral epicondylitis (Table 3).221,222 The use of autologous patellar tendon derived tendon cells for the treatment of refractory lateral epicondylitis was demonstrated by Wang et al.224 A significant improvement in the pain scores, DASH score and MRI grading of tendinopathy was present at one-year follow-up after ultrasound guided injection into the lateral epicondyle.
The use of stem cells for tendon repair and regeneration is an intriguing strategy. Preclinical and clinical studies have demonstrated safety and biologic potential for their use. However, the precise mechanisms by which stem cells participate in tendon healing are unknown,214 so that this approach requires further optimization before robust clinical results can be expected. The ideal stem cell type, cell number and ideal scaffold material are topics of ongoing debate and investigation. Improved methods of cell harvest, which can obviate the need for ex vivo expansion is desirable. Strategies for cell harvest which enable their use on the same day and at same time as the index surgical procedure, will facilitate transplantation of highly active cells at the site of repair, and this approach is expected to improve the likelihood of clinical success.
Summary and Future Directions
Despite a myriad of clinical advances in treating tendinopathies, there remains an unmet need for effective therapeutic strategies to augment intrinsic and/or surgical repair. The majority of clinical studies to date have been performed in patients undergoing RCR, and as such these treatment concepts must be recapitulated for other common tendon injuries. The most robustly tested treatment modalities have been PRP and related formulations harboring growth factors as well as ECM-based scaffolds to augment or interpose between the tendon repair. To date, tissue engineering strategies have been harnessed to augment tendon healing in vitro and in vivo with some success. These strategies include the use of scaffolds, cell therapies, gene therapies, and growth factor therapies. Many of these applications have shown early promise in the clinical setting in patients undergoing tendon repair, and merit continued study in prospective randomized trials. Preclinical large-animal models have provided a substantial amount of data to allow extension of nascent biologic therapies into clinical models, though their limitations must be kept in mind, including discrepancies in tendon anatomy and size, age, injury acuity, ability to simulate postoperative rehabilitation protocols within animals as compared to human subjects.231 Moreover, whereas the vast majority of pre-clinical studies utilize acute injury models, more commonly the human tendon disease “phenotype” is chronic and degenerative.
Therapeutic strategies which have received minimal attention include the use of disease-modifying agents such as chondrolytics for targeted removal of mucoid deposits, which are among the most common histopathologic features of tendinopathy (Figure 1). In addition, the further delineation of disease-linked human mutations232–234 and analysis of epigenetic changes in animal models235–237 and human cohorts are expected to facilitate future research into targeted therapeutics and potentially circulating DNA biomarkers of early-stage tendinopathies. Further clinical translation will require improved functional integration of these therapies toward more robust regeneration of native tissue structure, composition, and function. In addition, combination therapies simultaneously employing two or more tissue engineering strategies should be further studied as they may prove to be synergistic in addressing this pathologically complex problem.
Acknowledgments
The authors gratefully acknowledge funding from the NIH (AR063144), Rush Arthritis and Orthopaedic Institute, and American Foundation for Surgery of the Hand.
References
- 1.Rumian AP, Wallace AL, Birch HL. Tendons and ligaments are anatomically distinct but overlap in molecular and morphological features--a comparative study in an ovine model. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2007;25(4):458–464. doi: 10.1002/jor.20218. [DOI] [PubMed] [Google Scholar]
- 2.Khan KM, Cook JL, Bonar F, Harcourt P, Astrom M. Histopathology of common tendinopathies. Update and implications for clinical management. Sports medicine (Auckland, NZ) 1999;27(6):393–408. doi: 10.2165/00007256-199927060-00004. [DOI] [PubMed] [Google Scholar]
- 3.Rechardt M, Shiri R, Karppinen J, Jula A, Heliovaara M, Viikari-Juntura E. Lifestyle and metabolic factors in relation to shoulder pain and rotator cuff tendinitis: a population-based study. BMC musculoskeletal disorders. 2010;11:165. doi: 10.1186/1471-2474-11-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.September AV, Cook J, Handley CJ, van der Merwe L, Schwellnus MP, Collins M. Variants within the COL5A1 gene are associated with Achilles tendinopathy in two populations. British journal of sports medicine. 2009;43(5):357–365. doi: 10.1136/bjsm.2008.048793. [DOI] [PubMed] [Google Scholar]
- 5.Pufe T, Petersen WJ, Mentlein R, Tillmann BN. The role of vasculature and angiogenesis for the pathogenesis of degenerative tendons disease. Scandinavian journal of medicine & science in sports. 2005;15(4):211–222. doi: 10.1111/j.1600-0838.2005.00465.x. [DOI] [PubMed] [Google Scholar]
- 6.Kannus P. Etiology and pathophysiology of chronic tendon disorders in sports. Scandinavian journal of medicine & science in sports. 1997;7(2):78–85. doi: 10.1111/j.1600-0838.1997.tb00123.x. [DOI] [PubMed] [Google Scholar]
- 7.Biundo JJ, Jr, Irwin RW, Umpierre E. Sports and other soft tissue injuries, tendinitis, bursitis, and occupation-related syndromes. Current opinion in rheumatology. 2001;13(2):146–149. doi: 10.1097/00002281-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 8.de Vries AJ, van der Worp H, Diercks RL, van den Akker-Scheek I, Zwerver J. Risk factors for patellar tendinopathy in volleyball and basketball players: A survey-based prospective cohort study. Scandinavian journal of medicine & science in sports. 2014 doi: 10.1111/sms.12294. [DOI] [PubMed] [Google Scholar]
- 9.Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. The Journal of bone and joint surgery. American volume. 1991;73(10):1507–1525. [PubMed] [Google Scholar]
- 10.Jarvinen M, Jozsa L, Kannus P, Jarvinen TL, Kvist M, Leadbetter W. Histopathological findings in chronic tendon disorders. Scandinavian journal of medicine & science in sports. 1997;7(2):86–95. doi: 10.1111/j.1600-0838.1997.tb00124.x. [DOI] [PubMed] [Google Scholar]
- 11.Pingel J, Lu Y, Starborg T, et al. 3-D ultrastructure and collagen composition of healthy and overloaded human tendon: evidence of tenocyte and matrix buckling. Journal of anatomy. 2014;224(5):548–555. doi: 10.1111/joa.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han WM, Heo SJ, Driscoll TP, Smith LJ, Mauck RL, Elliott DM. Macro- to microscale strain transfer in fibrous tissues is heterogeneous and tissue-specific. Biophysical journal. 2013;105(3):807–817. doi: 10.1016/j.bpj.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ralphs JR, Tyers RN, Benjamin M. Development of functionally distinct fibrocartilages at two sites in the quadriceps tendon of the rat: the suprapatella and the attachment to the patella. Anatomy and embryology. 1992;185(2):181–187. doi: 10.1007/BF00185920. [DOI] [PubMed] [Google Scholar]
- 14.Vogel KG, Peters JA. Histochemistry defines a proteoglycan-rich layer in bovine flexor tendon subjected to bending. Journal of musculoskeletal & neuronal interactions. 2005;5(1):64–69. [PubMed] [Google Scholar]
- 15.Benjamin M, Ralphs JR. Fibrocartilage in tendons and ligaments--an adaptation to compressive load. Journal of anatomy. 1998;193( Pt 4):481–494. doi: 10.1046/j.1469-7580.1998.19340481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Mos M, Koevoet W, van Schie HT, et al. In vitro model to study chondrogenic differentiation in tendinopathy. The American journal of sports medicine. 2009;37(6):1214–1222. doi: 10.1177/0363546508331137. [DOI] [PubMed] [Google Scholar]
- 17.Corps AN, Robinson AH, Movin T, Costa ML, Hazleman BL, Riley GP. Increased expression of aggrecan and biglycan mRNA in Achilles tendinopathy. Rheumatology (Oxford, England) 2006;45(3):291–294. doi: 10.1093/rheumatology/kei152. [DOI] [PubMed] [Google Scholar]
- 18.Samiric T, Parkinson J, Ilic MZ, Cook J, Feller JA, Handley CJ. Changes in the composition of the extracellular matrix in patellar tendinopathy. Matrix biology : journal of the International Society for Matrix Biology. 2009;28(4):230–236. doi: 10.1016/j.matbio.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Burssens A, Forsyth R, Bongaerts W, et al. Arguments for an increasing differentiation towards fibrocartilaginous components in midportion Achilles tendinopathy. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2013;21(6):1459–1467. doi: 10.1007/s00167-012-2203-3. [DOI] [PubMed] [Google Scholar]
- 20.Plaas A, Sandy JD, Liu H, et al. Biochemical identification and immunolocalizaton of aggrecan, ADAMTS5 and inter-alpha-trypsin-inhibitor in equine degenerative suspensory ligament desmitis. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2011;29(6):900–906. doi: 10.1002/jor.21332. [DOI] [PubMed] [Google Scholar]
- 21.Bell R, Li J, Gorski DJ, et al. Controlled treadmill exercise eliminates chondroid deposits and restores tensile properties in a new murine tendinopathy model. Journal of biomechanics. 2013;46(3):498–505. doi: 10.1016/j.jbiomech.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 22.Cook JL, Feller JA, Bonar SF, Khan KM. Abnormal tenocyte morphology is more prevalent than collagen disruption in asymptomatic athletes’ patellar tendons. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2004;22(2):334–338. doi: 10.1016/j.orthres.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Attia M, Scott A, Carpentier G, et al. Greater glycosaminoglycan content in human patellar tendon biopsies is associated with more pain and a lower VISA score. British journal of sports medicine. 2014;48(6):469–475. doi: 10.1136/bjsports-2013-092633. [DOI] [PubMed] [Google Scholar]
- 24.Nirschl RP. Elbow tendinosis/tennis elbow. Clinics in sports medicine. 1992;11(4):851–870. [PubMed] [Google Scholar]
- 25.Millar NL, Reilly JH, Kerr SC, et al. Hypoxia: a critical regulator of early human tendinopathy. Annals of the rheumatic diseases. 2012;71(2):302–310. doi: 10.1136/ard.2011.154229. [DOI] [PubMed] [Google Scholar]
- 26.Benson RT, McDonnell SM, Knowles HJ, Rees JL, Carr AJ, Hulley PA. Tendinopathy and tears of the rotator cuff are associated with hypoxia and apoptosis. The Journal of bone and joint surgery. British volume. 2010;92(3):448–453. doi: 10.1302/0301-620X.92B3.23074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lohr JF, Uhthoff HK. The microvascular pattern of the supraspinatus tendon. Clinical orthopaedics and related research. 1990;(254):35–38. [PubMed] [Google Scholar]
- 28.Rathbun JB, Macnab I. The microvascular pattern of the rotator cuff. The Journal of bone and joint surgery. British volume. 1970;52(3):540–553. [PubMed] [Google Scholar]
- 29.Vadlapatla RK, Vadlapudi AD, Mitra AK. Hypoxia-inducible factor-1 (HIF-1): a potential target for intervention in ocular neovascular diseases. Current drug targets. 2013;14(8):919–935. doi: 10.2174/13894501113149990015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Portron S, Merceron C, Gauthier O, et al. Effects of in vitro low oxygen tension preconditioning of adipose stromal cells on their in vivo chondrogenic potential: application in cartilage tissue repair. PloS one. 2013;8(4):e62368. doi: 10.1371/journal.pone.0062368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munir S, Foldager CB, Lind M, Zachar V, Soballe K, Koch TG. Hypoxia enhances chondrogenic differentiation of human adipose tissue-derived stromal cells in scaffold-free and scaffold systems. Cell and tissue research. 2014;355(1):89–102. doi: 10.1007/s00441-013-1732-5. [DOI] [PubMed] [Google Scholar]
- 32.Markway BD, Cho H, Johnstone B. Hypoxia promotes redifferentiation and suppresses markers of hypertrophy and degeneration in both healthy and osteoarthritic chondrocytes. Arthritis research & therapy. 2013;15(4):R92. doi: 10.1186/ar4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heywood HK, Knight MM, Lee DA. Both superficial and deep zone articular chondrocyte subpopulations exhibit the Crabtree effect but have different basal oxygen consumption rates. Journal of cellular physiology. 2010;223(3):630–639. doi: 10.1002/jcp.22061. [DOI] [PubMed] [Google Scholar]
- 34.Velasco J, Li J, DiPietro L, Stepp MA, Sandy JD, Plaas A. Adamts5 deletion blocks murine dermal repair through CD44-mediated aggrecan accumulation and modulation of transforming growth factor beta1 (TGFbeta1) signaling. The Journal of biological chemistry. 2011;286(29):26016–26027. doi: 10.1074/jbc.M110.208694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu SC, Wang W, Pau HM, Wong YP, Chan KM, Rolf CG. Increased expression of transforming growth factor-beta1 in patellar tendinosis. Clinical orthopaedics and related research. 2002;(400):174–183. doi: 10.1097/00003086-200207000-00022. [DOI] [PubMed] [Google Scholar]
- 36.Alfredson H, Lorentzon M, Backman S, Backman A, Lerner UH. cDNA-arrays and real-time quantitative PCR techniques in the investigation of chronic Achilles tendinosis. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2003;21(6):970–975. doi: 10.1016/S0736-0266(03)00107-4. [DOI] [PubMed] [Google Scholar]
- 37.Lundin AC, Aspenberg P, Eliasson P. Trigger finger, tendinosis, and intratendinous gene expression. Scandinavian journal of medicine & science in sports. 2014;24(2):363–368. doi: 10.1111/j.1600-0838.2012.01514.x. [DOI] [PubMed] [Google Scholar]
- 38.Dirks RC, Warden SJ. Models for the study of tendinopathy. Journal of musculoskeletal & neuronal interactions. 2011;11(2):141–149. [PubMed] [Google Scholar]
- 39.Lui PP, Maffulli N, Rolf C, Smith RK. What are the validated animal models for tendinopathy? Scandinavian journal of medicine & science in sports. 2011;21(1):3–17. doi: 10.1111/j.1600-0838.2010.01164.x. [DOI] [PubMed] [Google Scholar]
- 40.Voleti PB, Buckley MR, Soslowsky LJ. Tendon healing: repair and regeneration. Annual review of biomedical engineering. 2012;14:47–71. doi: 10.1146/annurev-bioeng-071811-150122. [DOI] [PubMed] [Google Scholar]
- 41.Murrell GA, Lilly EG, 3rd, Goldner RD, Seaber AV, Best TM. Effects of immobilization on Achilles tendon healing in a rat model. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 1994;12(4):582–591. doi: 10.1002/jor.1100120415. [DOI] [PubMed] [Google Scholar]
- 42.Silva MJ, Brodt MD, Boyer MI, et al. Effects of increased in vivo excursion on digital range of motion and tendon strength following flexor tendon repair. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 1999;17(5):777–783. doi: 10.1002/jor.1100170524. [DOI] [PubMed] [Google Scholar]
- 43.Eliasson P, Andersson T, Aspenberg P. Achilles tendon healing in rats is improved by intermittent mechanical loading during the inflammatory phase. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2012;30(2):274–279. doi: 10.1002/jor.21511. [DOI] [PubMed] [Google Scholar]
- 44.Friel NA, Wang VM, Slabaugh MA, Wang F, Chubinskaya S, Cole BJ. Rotator cuff healing after continuous subacromial bupivacaine infusion: an in vivo rabbit study. Journal of shoulder and elbow surgery/American Shoulder and Elbow Surgeons ... [et al. ] 2013;22(4):489–499. doi: 10.1016/j.jse.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schnabel LV, Lynch ME, van der Meulen MC, Yeager AE, Kornatowski MA, Nixon AJ. Mesenchymal stem cells and insulin-like growth factor-I gene-enhanced mesenchymal stem cells improve structural aspects of healing in equine flexor digitorum superficialis tendons. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2009;27(10):1392–1398. doi: 10.1002/jor.20887. [DOI] [PubMed] [Google Scholar]
- 46.Chen J, Yu Q, Wu B, et al. Autologous tenocyte therapy for experimental Achilles tendinopathy in a rabbit model. Tissue engineering. Part A. 2011;17(15–16):2037–2048. doi: 10.1089/ten.TEA.2010.0492. [DOI] [PubMed] [Google Scholar]
- 47.Rui YF, Lui PP, Wong YM, Tan Q, Chan KM. Altered fate of tendon-derived stem cells isolated from a failed tendon-healing animal model of tendinopathy. Stem cells and development. 2013;22(7):1076–1085. doi: 10.1089/scd.2012.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Archambault J, Tsuzaki M, Herzog W, Banes AJ. Stretch and interleukin-1beta induce matrix metalloproteinases in rabbit tendon cells in vitro. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2002;20(1):36–39. doi: 10.1016/S0736-0266(01)00075-4. [DOI] [PubMed] [Google Scholar]
- 49.Khan MH, Li Z, Wang JH. Repeated exposure of tendon to prostaglandin-E2 leads to localized tendon degeneration. Clinical journal of sport medicine : official journal of the Canadian Academy of Sport Medicine. 2005;15(1):27–33. doi: 10.1097/00042752-200501000-00006. [DOI] [PubMed] [Google Scholar]
- 50.Stone D, Green C, Rao U, et al. Cytokine-induced tendinitis: a preliminary study in rabbits. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 1999;17(2):168–177. doi: 10.1002/jor.1100170204. [DOI] [PubMed] [Google Scholar]
- 51.Banes AJ, Horesovsky G, Larson C, et al. Mechanical load stimulates expression of novel genes in vivo and in vitro in avian flexor tendon cells. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 1999;7(1):141–153. doi: 10.1053/joca.1998.0169. [DOI] [PubMed] [Google Scholar]
- 52.Jones ER, Jones GC, Legerlotz K, Riley GP. Cyclical strain modulates metalloprotease and matrix gene expression in human tenocytes via activation of TGFbeta. Biochimica et biophysica acta. 2013;1833(12):2596–2607. doi: 10.1016/j.bbamcr.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Legerlotz K, Jones GC, Screen HR, Riley GP. Cyclic loading of tendon fascicles using a novel fatigue loading system increases interleukin-6 expression by tenocytes. Scandinavian journal of medicine & science in sports. 2013;23(1):31–37. doi: 10.1111/j.1600-0838.2011.01410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Archambault JM, Hart DA, Herzog W. Response of rabbit Achilles tendon to chronic repetitive loading. Connective tissue research. 2001;42(1):13–23. doi: 10.3109/03008200109014245. [DOI] [PubMed] [Google Scholar]
- 55.Fung DT, Wang VM, Andarawis-Puri N, et al. Early response to tendon fatigue damage accumulation in a novel in vivo model. Journal of biomechanics. 2010;43(2):274–279. doi: 10.1016/j.jbiomech.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Glazebrook MA, Wright JR, Jr, Langman M, Stanish WD, Lee JM. Histological analysis of achilles tendons in an overuse rat model. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2008;26(6):840–846. doi: 10.1002/jor.20546. [DOI] [PubMed] [Google Scholar]
- 57.Kietrys DM, Barr-Gillespie AE, Amin M, Wade CK, Popoff SN, Barbe MF. Aging contributes to inflammation in upper extremity tendons and declines in forelimb agility in a rat model of upper extremity overuse. PloS one. 2012;7(10):e46954. doi: 10.1371/journal.pone.0046954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soslowsky LJ, Thomopoulos S, Tun S, et al. Neer Award 1999. Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. Journal of shoulder and elbow surgery/American Shoulder and Elbow Surgeons ... [et al. ] 2000;9(2):79–84. [PubMed] [Google Scholar]
- 59.Zhang J, Wang JH. Production of PGE(2) increases in tendons subjected to repetitive mechanical loading and induces differentiation of tendon stem cells into non-tenocytes. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2010;28(2):198–203. doi: 10.1002/jor.20962. [DOI] [PubMed] [Google Scholar]
- 60.Dahlgren LA, van der Meulen MC, Bertram JE, Starrak GS, Nixon AJ. Insulin-like growth factor-I improves cellular and molecular aspects of healing in a collagenase-induced model of flexor tendinitis. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2002;20(5):910–919. doi: 10.1016/S0736-0266(02)00009-8. [DOI] [PubMed] [Google Scholar]
- 61.Lui PP, Chan LS, Lee YW, Fu SC, Chan KM. Sustained expression of proteoglycans and collagen type III/type I ratio in a calcified tendinopathy model. Rheumatology (Oxford, England) 2010;49(2):231–239. doi: 10.1093/rheumatology/kep384. [DOI] [PubMed] [Google Scholar]
- 62.Lui PP, Fu SC, Chan LS, Hung LK, Chan KM. Chondrocyte phenotype and ectopic ossification in collagenase-induced tendon degeneration. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2009;57(2):91–100. doi: 10.1369/jhc.2008.952143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yee Lui PP, Wong YM, Rui YF, Lee YW, Chan LS, Chan KM. Expression of chondro-osteogenic BMPs in ossified failed tendon healing model of tendinopathy. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2011;29(6):816–821. doi: 10.1002/jor.21313. [DOI] [PubMed] [Google Scholar]
- 64.Archambault JM, Jelinsky SA, Lake SP, Hill AA, Glaser DL, Soslowsky LJ. Rat supraspinatus tendon expresses cartilage markers with overuse. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2007;25(5):617–624. doi: 10.1002/jor.20347. [DOI] [PubMed] [Google Scholar]
- 65.Attia M, Scott A, Duchesnay A, et al. Alterations of overused supraspinatus tendon: a possible role of glycosaminoglycans and HARP/pleiotrophin in early tendon pathology. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2012;30(1):61–71. doi: 10.1002/jor.21479. [DOI] [PubMed] [Google Scholar]
- 66.Maffulli N, Longo UG, Denaro V. Novel approaches for the management of tendinopathy. The Journal of bone and joint surgery. American volume. 2010;92(15):2604–2613. doi: 10.2106/JBJS.I.01744. [DOI] [PubMed] [Google Scholar]
- 67.Chester R, Costa ML, Shepstone L, Cooper A, Donell ST. Eccentric calf muscle training compared with therapeutic ultrasound for chronic Achilles tendon pain--a pilot study. Manual therapy. 2008;13(6):484–491. doi: 10.1016/j.math.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 68.Lebrun CM. Low-dose extracorporeal shock wave therapy for previously untreated lateral epicondylitis. Clinical journal of sport medicine : official journal of the Canadian Academy of Sport Medicine. 2005;15(5):401–402. doi: 10.1097/01.jsm.0000180017.37265.ae. [DOI] [PubMed] [Google Scholar]
- 69.Schofer MD, Hinrichs F, Peterlein CD, Arendt M, Schmitt J. High- versus low-energy extracorporeal shock wave therapy of rotator cuff tendinopathy: a prospective, randomised, controlled study. Acta orthopaedica Belgica. 2009;75(4):452–458. [PubMed] [Google Scholar]
- 70.Rees JD, Lichtwark GA, Wolman RL, Wilson AM. The mechanism for efficacy of eccentric loading in Achilles tendon injury; an in vivo study in humans. Rheumatology (Oxford, England) 2008;47(10):1493–1497. doi: 10.1093/rheumatology/ken262. [DOI] [PubMed] [Google Scholar]
- 71.Silbernagel KG, Brorsson A, Lundberg M. The majority of patients with Achilles tendinopathy recover fully when treated with exercise alone: a 5-year follow-up. The American journal of sports medicine. 2011;39(3):607–613. doi: 10.1177/0363546510384789. [DOI] [PubMed] [Google Scholar]
- 72.Yu J, Park D, Lee G. Effect of eccentric strengthening on pain, muscle strength, endurance, and functional fitness factors in male patients with achilles tendinopathy. American journal of physical medicine & rehabilitation/Association of Academic Physiatrists. 2013;92(1):68–76. doi: 10.1097/PHM.0b013e31826eda63. [DOI] [PubMed] [Google Scholar]
- 73.Drew BT, Smith TO, Littlewood C, Sturrock B. Do structural changes (eg, collagen/matrix) explain the response to therapeutic exercises in tendinopathy: a systematic review. British journal of sports medicine. 2014;48(12):966–972. doi: 10.1136/bjsports-2012-091285. [DOI] [PubMed] [Google Scholar]
- 74.Palmes D, Spiegel HU, Schneider TO, et al. Achilles tendon healing: long-term biomechanical effects of postoperative mobilization and immobilization in a new mouse model. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2002;20(5):939–946. doi: 10.1016/S0736-0266(02)00032-3. [DOI] [PubMed] [Google Scholar]
- 75.Virchenko O, Aspenberg P. How can one platelet injection after tendon injury lead to a stronger tendon after 4 weeks? Interplay between early regeneration and mechanical stimulation. Acta orthopaedica. 2006;77(5):806–812. doi: 10.1080/17453670610013033. [DOI] [PubMed] [Google Scholar]
- 76.Killian ML, Cavinatto L, Galatz LM, Thomopoulos S. The role of mechanobiology in tendon healing. Journal of shoulder and elbow surgery/American Shoulder and Elbow Surgeons ... [et al. ] 2012;21(2):228–237. doi: 10.1016/j.jse.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Godbout C, Ang O, Frenette J. Early voluntary exercise does not promote healing in a rat model of Achilles tendon injury. Journal of applied physiology (Bethesda, Md. : 1985) 2006;101(6):1720–1726. doi: 10.1152/japplphysiol.00301.2006. [DOI] [PubMed] [Google Scholar]
- 78.Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. The Journal of bone and joint surgery. American volume. 2005;87(1):187–202. doi: 10.2106/JBJS.D.01850. [DOI] [PubMed] [Google Scholar]
- 79.Schweitzer R, Chyung JH, Murtaugh LC, et al. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development (Cambridge, England) 2001;128(19):3855–3866. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- 80.Shukunami C, Takimoto A, Oro M, Hiraki Y. Scleraxis positively regulates the expression of tenomodulin, a differentiation marker of tenocytes. Developmental biology. 2006;298(1):234–247. doi: 10.1016/j.ydbio.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 81.Aslan H, Kimelman-Bleich N, Pelled G, Gazit D. Molecular targets for tendon neoformation. The Journal of clinical investigation. 2008;118(2):439–444. doi: 10.1172/JCI33944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hoffmann A, Pelled G, Turgeman G, et al. Neotendon formation induced by manipulation of the Smad8 signalling pathway in mesenchymal stem cells. The Journal of clinical investigation. 2006;116(4):940–952. doi: 10.1172/JCI22689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guerquin MJ, Charvet B, Nourissat G, et al. Transcription factor EGR1 directs tendon differentiation and promotes tendon repair. The Journal of clinical investigation. 2013;123(8):3564–3576. doi: 10.1172/JCI67521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cserjesi P, Brown D, Ligon KL, et al. Scleraxis: a basic helix-loop-helix protein that prefigures skeletal formation during mouse embryogenesis. Development (Cambridge, England) 1995;121(4):1099–1110. doi: 10.1242/dev.121.4.1099. [DOI] [PubMed] [Google Scholar]
- 85.Derwin KA, Baker AR, Spragg RK, Leigh DR, Farhat W, Iannotti JP. Regional variability, processing methods, and biophysical properties of human fascia lata extracellular matrix. Journal of biomedical materials research. Part A. 2008;84(2):500–507. doi: 10.1002/jbm.a.31455. [DOI] [PubMed] [Google Scholar]
- 86.Cheng X, Gurkan UA, Dehen CJ, et al. An electrochemical fabrication process for the assembly of anisotropically oriented collagen bundles. Biomaterials. 2008;29(22):3278–3288. doi: 10.1016/j.biomaterials.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 87.Barber JG, Handorf AM, Allee TJ, Li WJ. Braided nanofibrous scaffold for tendon and ligament tissue engineering. Tissue engineering. Part A. 2013;19(11–12):1265–1274. doi: 10.1089/ten.tea.2010.0538. [DOI] [PubMed] [Google Scholar]
- 88.Chainani A, Hippensteel KJ, Kishan A, et al. Multilayered electrospun scaffolds for tendon tissue engineering. Tissue engineering. Part A. 2013;19(23–24):2594–2604. doi: 10.1089/ten.tea.2013.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kolluru PV, Lipner J, Liu W, et al. Strong and tough mineralized PLGA nanofibers for tendon-to-bone scaffolds. Acta biomaterialia. 2013;9(12):9442–9450. doi: 10.1016/j.actbio.2013.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fessel G, Gerber C, Snedeker JG. Potential of collagen cross-linking therapies to mediate tendon mechanical properties. Journal of shoulder and elbow surgery/American Shoulder and Elbow Surgeons ... [et al. ] 2012;21(2):209–217. doi: 10.1016/j.jse.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 91.Ricchetti ET, Aurora A, Iannotti JP, Derwin KA. Scaffold devices for rotator cuff repair. Journal of shoulder and elbow surgery/American Shoulder and Elbow Surgeons ... [et al. ] 2012;21(2):251–265. doi: 10.1016/j.jse.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 92.Longo UG, Lamberti A, Petrillo S, Maffulli N, Denaro V. Scaffolds in tendon tissue engineering. Stem cells international. 2012;2012:517165. doi: 10.1155/2012/517165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zantop T, Gilbert TW, Yoder MC, Badylak SF. Extracellular matrix scaffolds are repopulated by bone marrow-derived cells in a mouse model of achilles tendon reconstruction. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2006;24(6):1299–1309. doi: 10.1002/jor.20071. [DOI] [PubMed] [Google Scholar]
- 94.Turner NJ, Badylak SF. Biologic scaffolds for musculotendinous tissue repair. European cells & materials. 2013;25:130–143. doi: 10.22203/ecm.v025a09. [DOI] [PubMed] [Google Scholar]
- 95.Moffat KL, Kwei AS, Spalazzi JP, Doty SB, Levine WN, Lu HH. Novel nanofiber-based scaffold for rotator cuff repair and augmentation. Tissue engineering. Part A. 2009;15(1):115–126. doi: 10.1089/ten.tea.2008.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Juncosa-Melvin N, Boivin GP, Gooch C, et al. The effect of autologous mesenchymal stem cells on the biomechanics and histology of gel-collagen sponge constructs used for rabbit patellar tendon repair. Tissue engineering. 2006;12(2):369–379. doi: 10.1089/ten.2006.12.369. [DOI] [PubMed] [Google Scholar]
- 97.Manning CN, Schwartz AG, Liu W, et al. Controlled delivery of mesenchymal stem cells and growth factors using a nanofiber scaffold for tendon repair. Acta biomaterialia. 2013;9(6):6905–6914. doi: 10.1016/j.actbio.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Adams JE, Zobitz ME, Reach JS, Jr, An KN, Steinmann SP. Rotator cuff repair using an acellular dermal matrix graft: an in vivo study in a canine model. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2006;22(7):700–709. doi: 10.1016/j.arthro.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 99.Dejardin LM, Arnoczky SP, Ewers BJ, Haut RC, Clarke RB. Tissue-engineered rotator cuff tendon using porcine small intestine submucosa. Histologic and mechanical evaluation in dogs. The American journal of sports medicine. 2001;29(2):175–184. doi: 10.1177/03635465010290021001. [DOI] [PubMed] [Google Scholar]
- 100.Nicholson GP, Breur GJ, Van Sickle D, Yao JQ, Kim J, Blanchard CR. Evaluation of a cross-linked acellular porcine dermal patch for rotator cuff repair augmentation in an ovine model. Journal of shoulder and elbow surgery/American Shoulder and Elbow Surgeons ... [et al. ] 2007;16(5 Suppl):S184–190. doi: 10.1016/j.jse.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 101.Schlegel TF, Hawkins RJ, Lewis CW, Motta T, Turner AS. The effects of augmentation with Swine small intestine submucosa on tendon healing under tension: histologic and mechanical evaluations in sheep. The American journal of sports medicine. 2006;34(2):275–280. doi: 10.1177/0363546505279912. [DOI] [PubMed] [Google Scholar]
- 102.Derwin KA, Codsi MJ, Milks RA, Baker AR, McCarron JA, Iannotti JP. Rotator cuff repair augmentation in a canine model with use of a woven poly-L-lactide device. The Journal of bone and joint surgery. American volume. 2009;91(5):1159–1171. doi: 10.2106/JBJS.H.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Santoni BG, McGilvray KC, Lyons AS, et al. Biomechanical analysis of an ovine rotator cuff repair via porous patch augmentation in a chronic rupture model. The American journal of sports medicine. 2010;38(4):679–686. doi: 10.1177/0363546510366866. [DOI] [PubMed] [Google Scholar]
- 104.Badhe SP, Lawrence TM, Smith FD, Lunn PG. An assessment of porcine dermal xenograft as an augmentation graft in the treatment of extensive rotator cuff tears. Journal of shoulder and elbow surgery/American Shoulder and Elbow Surgeons ... [et al. ] 2008;17(1 Suppl):35s–39s. doi: 10.1016/j.jse.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 105.Barber FA, Burns JP, Deutsch A, Labbe MR, Litchfield RB. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2012;28(1):8–15. doi: 10.1016/j.arthro.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 106.Bond JL, Dopirak RM, Higgins J, Burns J, Snyder SJ. Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2008;24(4):403–409.e401. doi: 10.1016/j.arthro.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 107.Burkhead WZ, Jr, Schiffern SC, Krishnan SG. Use of Graft Jacket as an augmentation for massive rotator cuff tears. Paper presented at: Seminars in Arthroplasty; 2007. [Google Scholar]
- 108.Dopirak R, Bond JL, Snyder SJ. Arthroscopic total rotator cuff replacement with an acellular human dermal allograft matrix. International Journal of Shoulder Surgery. 2007;1(1):7. [Google Scholar]
- 109.Encalada-Diaz I, Cole BJ, Macgillivray JD, et al. Rotator cuff repair augmentation using a novel polycarbonate polyurethane patch: preliminary results at 12 months’ follow-up. Journal of shoulder and elbow surgery/American Shoulder and Elbow Surgeons ... [et al. ] 2011;20(5):788–794. doi: 10.1016/j.jse.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Iannotti JP, Codsi MJ, Kwon YW, Derwin K, Ciccone J, Brems JJ. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears. A randomized, controlled trial. The Journal of bone and joint surgery. American volume. 2006;88(6):1238–1244. doi: 10.2106/JBJS.E.00524. [DOI] [PubMed] [Google Scholar]
- 111.Lee DK. Achilles tendon repair with acellular tissue graft augmentation in neglected ruptures. The Journal of foot and ankle surgery : official publication of the American College of Foot and Ankle Surgeons. 2007;46(6):451–455. doi: 10.1053/j.jfas.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 112.Lee DK. A preliminary study on the effects of acellular tissue graft augmentation in acute Achilles tendon ruptures. The Journal of foot and ankle surgery : official publication of the American College of Foot and Ankle Surgeons. 2008;47(1):8–12. doi: 10.1053/j.jfas.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 113.Malcarney HL, Bonar F, Murrell GA. Early inflammatory reaction after rotator cuff repair with a porcine small intestine submucosal implant: a report of 4 cases. The American journal of sports medicine. 2005;33(6):907–911. doi: 10.1177/0363546504271500. [DOI] [PubMed] [Google Scholar]
- 114.Metcalf MH, Savoie FH, III, Kellum B. Surgical technique for xenograft (SIS) augmentation of rotator-cuff repairs. Operative Techniques in Orthopaedics. 2002;12(3):204–208. [Google Scholar]
- 115.Rotini R, Marinelli A, Guerra E, et al. Human dermal matrix scaffold augmentation for large and massive rotator cuff repairs: preliminary clinical and MRI results at 1-year follow-up. Musculoskeletal surgery. 2011;95(Suppl 1):S13–23. doi: 10.1007/s12306-011-0141-8. [DOI] [PubMed] [Google Scholar]
- 116.Sclamberg SG, Tibone JE, Itamura JM, Kasraeian S. Six-month magnetic resonance imaging follow-up of large and massive rotator cuff repairs reinforced with porcine small intestinal submucosa. Journal of shoulder and elbow surgery/American Shoulder and Elbow Surgeons ... [et al. ] 2004;13(5):538–541. doi: 10.1016/j.jse.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 117.Soler JA, Gidwani S, Curtis MJ. Early complications from the use of porcine dermal collagen implants (Permacol) as bridging constructs in the repair of massive rotator cuff tears. A report of 4 cases. Acta orthopaedica Belgica. 2007;73(4):432–436. [PubMed] [Google Scholar]
- 118.Walton JR, Bowman NK, Khatib Y, Linklater J, Murrell GA. Restore orthobiologic implant: not recommended for augmentation of rotator cuff repairs. The Journal of bone and joint surgery. American volume. 2007;89(4):786–791. doi: 10.2106/JBJS.F.00315. [DOI] [PubMed] [Google Scholar]
- 119.Wong I, Burns J, Snyder S. Arthroscopic GraftJacket repair of rotator cuff tears. Journal of shoulder and elbow surgery/American Shoulder and Elbow Surgeons ... [et al. ] 2010;19(2 Suppl):104–109. doi: 10.1016/j.jse.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 120.Proctor CS. Long-term successful arthroscopic repair of large and massive rotator cuff tears with a functional and degradable reinforcement device. Journal of shoulder and elbow surgery/American Shoulder and Elbow Surgeons ... [et al. ] 2014;23(10):1508–1513. doi: 10.1016/j.jse.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 121.Pedowitz RA, Yamaguchi K, Ahmad CS, et al. Optimizing the management of rotator cuff problems. The Journal of the American Academy of Orthopaedic Surgeons. 2011;19(6):368–379. doi: 10.5435/00124635-201106000-00007. [DOI] [PubMed] [Google Scholar]
- 122.De Deyne PG, Dauria RE, Bruder SP. The introduction of an extracellular matrix-based scaffold to the marketplace. Journal of shoulder and elbow surgery/American Shoulder and Elbow Surgeons ... [et al. ] 2007;16(5 Suppl):S164–170. doi: 10.1016/j.jse.2007.02.111. [DOI] [PubMed] [Google Scholar]
- 123.Evans CH, Ghivizzani SC, Herndon JH, Robbins PD. Gene therapy for the treatment of musculoskeletal diseases. The Journal of the American Academy of Orthopaedic Surgeons. 2005;13(4):230–242. doi: 10.5435/00124635-200507000-00003. [DOI] [PubMed] [Google Scholar]
- 124.Manning CN, Kim HM, Sakiyama-Elbert S, Galatz LM, Havlioglu N, Thomopoulos S. Sustained delivery of transforming growth factor beta three enhances tendon-to-bone healing in a rat model. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2011;29(7):1099–1105. doi: 10.1002/jor.21301. [DOI] [PubMed] [Google Scholar]
- 125.Eming SA, Krieg T, Davidson JM. Gene transfer in tissue repair: status, challenges and future directions. Expert opinion on biological therapy. 2004;4(9):1373–1386. doi: 10.1517/14712598.4.9.1373. [DOI] [PubMed] [Google Scholar]
- 126.Isaac C, Gharaibeh B, Witt M, Wright VJ, Huard J. Biologic approaches to enhance rotator cuff healing after injury. Journal of shoulder and elbow surgery/American Shoulder and Elbow Surgeons ... [et al. ] 2012;21(2):181–190. doi: 10.1016/j.jse.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 127.Pelinkovic D, Lee JY, Engelhardt M, et al. Muscle cell-mediated gene delivery to the rotator cuff. Tissue engineering. 2003;9(1):143–151. doi: 10.1089/107632703762687627. [DOI] [PubMed] [Google Scholar]
- 128.Lou J, Tu Y, Burns M, Silva MJ, Manske P. BMP-12 gene transfer augmentation of lacerated tendon repair. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2001;19(6):1199–1202. doi: 10.1016/S0736-0266(01)00042-0. [DOI] [PubMed] [Google Scholar]
- 129.Hou Y, Mao Z, Wei X, et al. Effects of transforming growth factor-beta1 and vascular endothelial growth factor 165 gene transfer on Achilles tendon healing. Matrix biology : journal of the International Society for Matrix Biology. 2009;28(6):324–335. doi: 10.1016/j.matbio.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 130.Majewski M, Porter RM, Betz OB, et al. Improvement of tendon repair using muscle grafts transduced with TGF-beta1 cDNA. European cells & materials. 2012;23:94–101. doi: 10.22203/ecm.v023a07. discussion 101–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Majewski M, Betz O, Ochsner PE, Liu F, Porter RM, Evans CH. Ex vivo adenoviral transfer of bone morphogenetic protein 12 (BMP-12) cDNA improves Achilles tendon healing in a rat model. Gene therapy. 2008;15(16):1139–1146. doi: 10.1038/gt.2008.48. [DOI] [PubMed] [Google Scholar]
- 132.Loiselle AE, Bragdon GA, Jacobson JA, et al. Remodeling of murine intrasynovial tendon adhesions following injury: MMP and neotendon gene expression. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2009;27(6):833–840. doi: 10.1002/jor.20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gulotta LV, Kovacevic D, Packer JD, Deng XH, Rodeo SA. Bone marrow-derived mesenchymal stem cells transduced with scleraxis improve rotator cuff healing in a rat model. The American journal of sports medicine. 2011;39(6):1282–1289. doi: 10.1177/0363546510395485. [DOI] [PubMed] [Google Scholar]
- 134.Aspenberg P. Stimulation of tendon repair: mechanical loading, GDFs and platelets. A mini-review. International orthopaedics. 2007;31(6):783–789. doi: 10.1007/s00264-007-0398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.James R, Kesturu G, Balian G, Chhabra AB. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. The Journal of hand surgery. 2008;33(1):102–112. doi: 10.1016/j.jhsa.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 136.Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament healing. Sports medicine (Auckland, NZ) 2003;33(5):381–394. doi: 10.2165/00007256-200333050-00004. [DOI] [PubMed] [Google Scholar]
- 137.Klein MB, Yalamanchi N, Pham H, Longaker MT, Chang J. Flexor tendon healing in vitro: effects of TGF-beta on tendon cell collagen production. The Journal of hand surgery. 2002;27(4):615–620. doi: 10.1053/jhsu.2002.34004. [DOI] [PubMed] [Google Scholar]
- 138.Klass BR, Rolfe KJ, Grobbelaar AO. In vitro flexor tendon cell response to TGF-beta1: a gene expression study. The Journal of hand surgery. 2009;34(3):495–503. doi: 10.1016/j.jhsa.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 139.Anaguchi Y, Yasuda K, Majima T, Tohyama H, Minami A, Hayashi K. The effect of transforming growth factor-beta on mechanical properties of the fibrous tissue regenerated in the patellar tendon after resecting the central portion. Clinical biomechanics (Bristol, Avon) 2005;20(9):959–965. doi: 10.1016/j.clinbiomech.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 140.Chang J, Most D, Stelnicki E, et al. Gene expression of transforming growth factor beta-1 in rabbit zone II flexor tendon wound healing: evidence for dual mechanisms of repair. Plastic and reconstructive surgery. 1997;100(4):937–944. doi: 10.1097/00006534-199709001-00016. [DOI] [PubMed] [Google Scholar]
- 141.Tsubone T, Moran SL, Subramaniam M, Amadio PC, Spelsberg TC, An KN. Effect of TGF-beta inducible early gene deficiency on flexor tendon healing. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2006;24(3):569–575. doi: 10.1002/jor.20101. [DOI] [PubMed] [Google Scholar]
- 142.Abrahamsson SO, Lohmander S. Differential effects of insulin-like growth factor-I on matrix and DNA synthesis in various regions and types of rabbit tendons. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 1996;14(3):370–376. doi: 10.1002/jor.1100140305. [DOI] [PubMed] [Google Scholar]
- 143.Hansson HA, Dahlin LB, Lundborg G, Lowenadler B, Paleus S, Skottner A. Transiently increased insulin-like growth factor I immunoreactivity in tendons after vibration trauma. An immunohistochemical study on rats. Scandinavian journal of plastic and reconstructive surgery and hand surgery/Nordisk plastikkirurgisk forening [and] Nordisk klubb for handkirurgi. 1988;22(1):1–6. doi: 10.3109/02844318809097928. [DOI] [PubMed] [Google Scholar]
- 144.Sciore P, Boykiw R, Hart DA. Semiquantitative reverse transcription-polymerase chain reaction analysis of mRNA for growth factors and growth factor receptors from normal and healing rabbit medial collateral ligament tissue. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 1998;16(4):429–437. doi: 10.1002/jor.1100160406. [DOI] [PubMed] [Google Scholar]
- 145.Nakamura N, Shino K, Natsuume T, et al. Early biological effect of in vivo gene transfer of platelet-derived growth factor (PDGF)-B into healing patellar ligament. Gene therapy. 1998;5(9):1165–1170. doi: 10.1038/sj.gt.3300712. [DOI] [PubMed] [Google Scholar]
- 146.Thomopoulos S, Zaegel M, Das R, et al. PDGF-BB released in tendon repair using a novel delivery system promotes cell proliferation and collagen remodeling. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2007;25(10):1358–1368. doi: 10.1002/jor.20444. [DOI] [PubMed] [Google Scholar]
- 147.Wang XT, Liu PY, Tang JB. Tendon healing in vitro: genetic modification of tenocytes with exogenous PDGF gene and promotion of collagen gene expression. The Journal of hand surgery. 2004;29(5):884–890. doi: 10.1016/j.jhsa.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 148.Chan BP, Fu SC, Qin L, Rolf C, Chan KM. Supplementation-time dependence of growth factors in promoting tendon healing. Clinical orthopaedics and related research. 2006;448:240–247. doi: 10.1097/01.blo.0000205875.97468.e4. [DOI] [PubMed] [Google Scholar]
- 149.Cummings SH, Grande DA, Hee CK, et al. Effect of recombinant human platelet-derived growth factor-BB-coated sutures on Achilles tendon healing in a rat model: A histological and biomechanical study. Journal of tissue engineering. 2012;3(1):2041731412453577. doi: 10.1177/2041731412453577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hee CK, Dines JS, Dines DM, et al. Augmentation of a rotator cuff suture repair using rhPDGF-BB and a type I bovine collagen matrix in an ovine model. The American journal of sports medicine. 2011;39(8):1630–1639. doi: 10.1177/0363546511404942. [DOI] [PubMed] [Google Scholar]
- 151.Shah V, Bendele A, Dines JS, et al. Dose-response effect of an intra-tendon application of recombinant human platelet-derived growth factor-BB (rhPDGF-BB) in a rat Achilles tendinopathy model. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2013;31(3):413–420. doi: 10.1002/jor.22222. [DOI] [PubMed] [Google Scholar]
- 152.Solchaga LA, Bendele A, Shah V, et al. Comparison of the effect of intra-tendon applications of recombinant human platelet-derived growth factor-BB, platelet-rich plasma, steroids in a rat achilles tendon collagenase model. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2014;32(1):145–150. doi: 10.1002/jor.22483. [DOI] [PubMed] [Google Scholar]
- 153.Thomopoulos S, Das R, Silva MJ, et al. Enhanced flexor tendon healing through controlled delivery of PDGF-BB. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2009;27(9):1209–1215. doi: 10.1002/jor.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang F, Liu H, Stile F, et al. Effect of vascular endothelial growth factor on rat Achilles tendon healing. Plastic and reconstructive surgery. 2003;112(6):1613–1619. doi: 10.1097/01.PRS.0000086772.72535.A4. [DOI] [PubMed] [Google Scholar]
- 155.Pufe T, Petersen W, Tillmann B, Mentlein R. The angiogenic peptide vascular endothelial growth factor is expressed in foetal and ruptured tendons. Virchows Archiv : an international journal of pathology. 2001;439(4):579–585. doi: 10.1007/s004280100422. [DOI] [PubMed] [Google Scholar]
- 156.Chan BP, Fu S, Qin L, Lee K, Rolf CG, Chan K. Effects of basic fibroblast growth factor (bFGF) on early stages of tendon healing: a rat patellar tendon model. Acta orthopaedica Scandinavica. 2000;71(5):513–518. doi: 10.1080/000164700317381234. [DOI] [PubMed] [Google Scholar]
- 157.Itoh N, Ornitz DM. Evolution of the Fgf and Fgfr gene families. Trends in genetics : TIG. 2004;20(11):563–569. doi: 10.1016/j.tig.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 158.Oryan A, Moshiri A. A long term study on the role of exogenous human recombinant basic fibroblast growth factor on the superficial digital flexor tendon healing in rabbits. Journal of musculoskeletal & neuronal interactions. 2011;11(2):185–195. [PubMed] [Google Scholar]
- 159.Ide J, Kikukawa K, Hirose J, et al. The effect of a local application of fibroblast growth factor-2 on tendon-to-bone remodeling in rats with acute injury and repair of the supraspinatus tendon. Journal of shoulder and elbow surgery/American Shoulder and Elbow Surgeons ... [et al. ] 2009;18(3):391–398. doi: 10.1016/j.jse.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 160.Wolfman NM, Hattersley G, Cox K, et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. The Journal of clinical investigation. 1997;100(2):321–330. doi: 10.1172/JCI119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hogan M, Girish K, James R, Balian G, Hurwitz S, Chhabra AB. Growth differentiation factor-5 regulation of extracellular matrix gene expression in murine tendon fibroblasts. Journal of tissue engineering and regenerative medicine. 2011;5(3):191–200. doi: 10.1002/term.304. [DOI] [PubMed] [Google Scholar]
- 162.Aspenberg P, Forslund C. Enhanced tendon healing with GDF 5 and 6. Acta orthopaedica Scandinavica. 1999;70(1):51–54. doi: 10.3109/17453679909000958. [DOI] [PubMed] [Google Scholar]
- 163.Dines JS, Weber L, Razzano P, et al. The effect of growth differentiation factor-5-coated sutures on tendon repair in a rat model. Journal of shoulder and elbow surgery/American Shoulder and Elbow Surgeons ... [et al. ] 2007;16(5 Suppl):S215–221. doi: 10.1016/j.jse.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 164.Rickert M, Jung M, Adiyaman M, Richter W, Simank HG. A growth and differentiation factor-5 (GDF-5)-coated suture stimulates tendon healing in an Achilles tendon model in rats. Growth factors (Chur, Switzerland) 2001;19(2):115–126. doi: 10.3109/08977190109001080. [DOI] [PubMed] [Google Scholar]
- 165.Rickert M, Wang H, Wieloch P, et al. Adenovirus-mediated gene transfer of growth and differentiation factor-5 into tenocytes and the healing rat Achilles tendon. Connective tissue research. 2005;46(4–5):175–183. doi: 10.1080/03008200500237120. [DOI] [PubMed] [Google Scholar]
- 166.Bolt P, Clerk AN, Luu HH, et al. BMP-14 gene therapy increases tendon tensile strength in a rat model of Achilles tendon injury. The Journal of bone and joint surgery. American volume. 2007;89(6):1315–1320. doi: 10.2106/JBJS.F.00257. [DOI] [PubMed] [Google Scholar]
- 167.Basile P, Dadali T, Jacobson J, et al. Freeze-dried tendon allografts as tissue-engineering scaffolds for Gdf5 gene delivery. Molecular therapy : the journal of the American Society of Gene Therapy. 2008;16(3):466–473. doi: 10.1038/sj.mt.6300395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma: from basic science to clinical applications. The American journal of sports medicine. 2009;37(11):2259–2272. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 169.Weibrich G, Kleis WK, Hafner G, Hitzler WE. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. Journal of cranio-maxillo-facial surgery : official publication of the European Association for Cranio-Maxillo-Facial Surgery. 2002;30(2):97–102. doi: 10.1054/jcms.2002.0285. [DOI] [PubMed] [Google Scholar]
- 170.Bosch G, van Schie HT, de Groot MW, et al. Effects of platelet-rich plasma on the quality of repair of mechanically induced core lesions in equine superficial digital flexor tendons: A placebo-controlled experimental study. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2010;28(2):211–217. doi: 10.1002/jor.20980. [DOI] [PubMed] [Google Scholar]
- 171.Lyras DN, Kazakos K, Verettas D, et al. The effect of platelet-rich plasma gel in the early phase of patellar tendon healing. Archives of orthopaedic and trauma surgery. 2009;129(11):1577–1582. doi: 10.1007/s00402-009-0935-4. [DOI] [PubMed] [Google Scholar]
- 172.Kaux JF, Drion PV, Colige A, et al. Effects of platelet-rich plasma (PRP) on the healing of Achilles tendons of rats. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2012;20(5):748–756. doi: 10.1111/j.1524-475X.2012.00826.x. [DOI] [PubMed] [Google Scholar]
- 173.Chung SW, Song BW, Kim YH, Park KU, Oh JH. Effect of platelet-rich plasma and porcine dermal collagen graft augmentation for rotator cuff healing in a rabbit model. The American journal of sports medicine. 2013;41(12):2909–2918. doi: 10.1177/0363546513503810. [DOI] [PubMed] [Google Scholar]
- 174.de Mos M, van der Windt AE, Jahr H, et al. Can platelet-rich plasma enhance tendon repair? A cell culture study. The American journal of sports medicine. 2008;36(6):1171–1178. doi: 10.1177/0363546508314430. [DOI] [PubMed] [Google Scholar]
- 175.McCarrel T, Fortier L. Temporal growth factor release from platelet-rich plasma, trehalose lyophilized platelets, and bone marrow aspirate and their effect on tendon and ligament gene expression. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2009;27(8):1033–1042. doi: 10.1002/jor.20853. [DOI] [PubMed] [Google Scholar]
- 176.Schnabel LV, Mohammed HO, Miller BJ, et al. Platelet rich plasma (PRP) enhances anabolic gene expression patterns in flexor digitorum superficialis tendons. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2007;25(2):230–240. doi: 10.1002/jor.20278. [DOI] [PubMed] [Google Scholar]
- 177.Visser LC, Arnoczky SP, Caballero O, Kern A, Ratcliffe A, Gardner KL. Growth factor-rich plasma increases tendon cell proliferation and matrix synthesis on a synthetic scaffold: an in vitro study. Tissue engineering. Part A. 2010;16(3):1021–1029. doi: 10.1089/ten.TEA.2009.0254. [DOI] [PubMed] [Google Scholar]
- 178.Zhang J, Wang JH. Platelet-rich plasma releasate promotes differentiation of tendon stem cells into active tenocytes. The American journal of sports medicine. 2010;38(12):2477–2486. doi: 10.1177/0363546510376750. [DOI] [PubMed] [Google Scholar]
- 179.Martinello T, Bronzini I, Perazzi A, et al. Effects of in vivo applications of peripheral blood-derived mesenchymal stromal cells (PB-MSCs) and platlet-rich plasma (PRP) on experimentally injured deep digital flexor tendons of sheep. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2013;31(2):306–314. doi: 10.1002/jor.22205. [DOI] [PubMed] [Google Scholar]
- 180.Antuna S, Barco R, Martinez Diez JM, Sanchez Marquez JM. Platelet-rich fibrin in arthroscopic repair of massive rotator cuff tears: a prospective randomized pilot clinical trial. Acta orthopaedica Belgica. 2013;79(1):25–30. [PubMed] [Google Scholar]
- 181.Barber FA, Hrnack SA, Snyder SJ, Hapa O. Rotator cuff repair healing influenced by platelet-rich plasma construct augmentation. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2011;27(8):1029–1035. doi: 10.1016/j.arthro.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 182.Bergeson AG, Tashjian RZ, Greis PE, Crim J, Stoddard GJ, Burks RT. Effects of platelet-rich fibrin matrix on repair integrity of at-risk rotator cuff tears. The American journal of sports medicine. 2012;40(2):286–293. doi: 10.1177/0363546511424402. [DOI] [PubMed] [Google Scholar]
- 183.Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. The American journal of sports medicine. 2011;39(2):258–265. doi: 10.1177/0363546510390780. [DOI] [PubMed] [Google Scholar]
- 184.Gumina S, Campagna V, Ferrazza G, et al. Use of platelet-leukocyte membrane in arthroscopic repair of large rotator cuff tears: a prospective randomized study. The Journal of bone and joint surgery. American volume. 2012;94(15):1345–1352. doi: 10.2106/JBJS.K.00394. [DOI] [PubMed] [Google Scholar]
- 185.Jo CH, Shin JS, Lee YG, et al. Platelet-rich plasma for arthroscopic repair of large to massive rotator cuff tears: a randomized, single-blind, parallel-group trial. The American journal of sports medicine. 2013;41(10):2240–2248. doi: 10.1177/0363546513497925. [DOI] [PubMed] [Google Scholar]
- 186.Malavolta EA, Gracitelli ME, Ferreira Neto AA, Assuncao JH, Bordalo-Rodrigues M, de Camargo OP. Platelet-rich plasma in rotator cuff repair: a prospective randomized study. The American journal of sports medicine. 2014;42(10):2446–2454. doi: 10.1177/0363546514541777. [DOI] [PubMed] [Google Scholar]
- 187.Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. Journal of shoulder and elbow surgery/American Shoulder and Elbow Surgeons ... [et al. ] 2011;20(4):518–528. doi: 10.1016/j.jse.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 188.Rodeo SA, Delos D, Williams RJ, Adler RS, Pearle A, Warren RF. The effect of platelet-rich fibrin matrix on rotator cuff tendon healing: a prospective, randomized clinical study. The American journal of sports medicine. 2012;40(6):1234–1241. doi: 10.1177/0363546512442924. [DOI] [PubMed] [Google Scholar]
- 189.Ruiz-Moneo P, Molano-Munoz J, Prieto E, Algorta J. Plasma rich in growth factors in arthroscopic rotator cuff repair: a randomized, double-blind, controlled clinical trial. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2013;29(1):2–9. doi: 10.1016/j.arthro.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 190.Sanchez M, Anitua E, Azofra J, Andia I, Padilla S, Mujika I. Comparison of surgically repaired Achilles tendon tears using platelet-rich fibrin matrices. The American journal of sports medicine. 2007;35(2):245–251. doi: 10.1177/0363546506294078. [DOI] [PubMed] [Google Scholar]
- 191.Weber SC, Kauffman JI, Parise C, Weber SJ, Katz SD. Platelet-rich fibrin matrix in the management of arthroscopic repair of the rotator cuff: a prospective, randomized, double-blinded study. The American journal of sports medicine. 2013;41(2):263–270. doi: 10.1177/0363546512467621. [DOI] [PubMed] [Google Scholar]
- 192.Thanasas C, Papadimitriou G, Charalambidis C, Paraskevopoulos I, Papanikolaou A. Platelet-rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: a randomized controlled clinical trial. The American journal of sports medicine. 2011;39(10):2130–2134. doi: 10.1177/0363546511417113. [DOI] [PubMed] [Google Scholar]
- 193.Murawski CD, Smyth NA, Newman H, Kennedy JG. A single platelet-rich plasma injection for chronic midsubstance achilles tendinopathy: a retrospective preliminary analysis. Foot & ankle specialist. 2014;7(5):372–376. doi: 10.1177/1938640014532129. [DOI] [PubMed] [Google Scholar]
- 194.Mishra AK, Skrepnik NV, Edwards SG, et al. Efficacy of platelet-rich plasma for chronic tennis elbow: a double-blind, prospective, multicenter, randomized controlled trial of 230 patients. The American journal of sports medicine. 2014;42(2):463–471. doi: 10.1177/0363546513494359. [DOI] [PubMed] [Google Scholar]
- 195.Krogh TP, Fredberg U, Stengaard-Pedersen K, Christensen R, Jensen P, Ellingsen T. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. The American journal of sports medicine. 2013;41(3):625–635. doi: 10.1177/0363546512472975. [DOI] [PubMed] [Google Scholar]
- 196.Kearney RS, Parsons N, Costa ML. Achilles tendinopathy management: A pilot randomised controlled trial comparing platelet-richplasma injection with an eccentric loading programme. Bone & joint research. 2013;2(10):227–232. doi: 10.1302/2046-3758.210.2000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Filardo G, Kon E, Di Matteo B, et al. Platelet-rich plasma injections for the treatment of refractory Achilles tendinopathy: results at 4 years. Blood transfusion = Trasfusione del sangue. 2014;12(4):533–540. doi: 10.2450/2014.0289-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ferrero G, Fabbro E, Orlandi D, et al. Ultrasound-guided injection of platelet-rich plasma in chronic Achilles and patellar tendinopathy. Journal of ultrasound. 2012;15(4):260–266. doi: 10.1016/j.jus.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6(2):230–247. [PubMed] [Google Scholar]
- 200.Mafi R, Hindocha S, Mafi P, Griffin M, Khan WS. Sources of adult mesenchymal stem cells applicable for musculoskeletal applications - a systematic review of the literature. The open orthopaedics journal. 2011;5(Suppl 2):242–248. doi: 10.2174/1874325001105010242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Hogan MV, Bagayoko N, James R, Starnes T, Katz A, Chhabra AB. Tissue engineering solutions for tendon repair. The Journal of the American Academy of Orthopaedic Surgeons. 2011;19(3):134–142. doi: 10.5435/00124635-201103000-00002. [DOI] [PubMed] [Google Scholar]
- 202.Chong AK, Chang J, Go JC. Mesenchymal stem cells and tendon healing. Frontiers in bioscience (Landmark edition) 2009;14:4598–4605. doi: 10.2741/3552. [DOI] [PubMed] [Google Scholar]
- 203.da Meirelles LS, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine & growth factor reviews. 2009;20(5–6):419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 204.Huang TF, Yew TL, Chiang ER, et al. Mesenchymal stem cells from a hypoxic culture improve and engraft Achilles tendon repair. The American journal of sports medicine. 2013;41(5):1117–1125. doi: 10.1177/0363546513480786. [DOI] [PubMed] [Google Scholar]
- 205.Juncosa-Melvin N, Boivin GP, Galloway MT, Gooch C, West JR, Butler DL. Effects of cell-to-collagen ratio in stem cell-seeded constructs for Achilles tendon repair. Tissue engineering. 2006;12(4):681–689. doi: 10.1089/ten.2006.12.681. [DOI] [PubMed] [Google Scholar]
- 206.Juncosa-Melvin N, Shearn JT, Boivin GP, et al. Effects of mechanical stimulation on the biomechanics and histology of stem cell-collagen sponge constructs for rabbit patellar tendon repair. Tissue engineering. 2006;12(8):2291–2300. doi: 10.1089/ten.2006.12.2291. [DOI] [PubMed] [Google Scholar]
- 207.Smith RK, Werling NJ, Dakin SG, Alam R, Goodship AE, Dudhia J. Beneficial effects of autologous bone marrow-derived mesenchymal stem cells in naturally occurring tendinopathy. PloS one. 2013;8(9):e75697. doi: 10.1371/journal.pone.0075697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Uysal CA, Tobita M, Hyakusoku H, Mizuno H. Adipose-derived stem cells enhance primary tendon repair: biomechanical and immunohistochemical evaluation. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2012;65(12):1712–1719. doi: 10.1016/j.bjps.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 209.Behfar M, Sarrafzadeh-Rezaei F, Hobbenaghi R, Delirezh N, Dalir-Naghadeh B. Enhanced mechanical properties of rabbit flexor tendons in response to intratendinous injection of adipose derived stromal vascular fraction. Current stem cell research & therapy. 2012;7(3):173–178. doi: 10.2174/157488812799859874. [DOI] [PubMed] [Google Scholar]
- 210.Oh JH, Chung SW, Kim SH, Chung JY, Kim JY. 2013 Neer Award: Effect of the adipose-derived stem cell for the improvement of fatty degeneration and rotator cuff healing in rabbit model. Journal of shoulder and elbow surgery/American Shoulder and Elbow Surgeons ... [et al. ] 2014;23(4):445–455. doi: 10.1016/j.jse.2013.07.054. [DOI] [PubMed] [Google Scholar]
- 211.Deng D, Wang W, Wang B, et al. Repair of Achilles tendon defect with autologous ASCs engineered tendon in a rabbit model. Biomaterials. 2014;35(31):8801–8809. doi: 10.1016/j.biomaterials.2014.06.058. [DOI] [PubMed] [Google Scholar]
- 212.Yang G, Rothrauff BB, Lin H, Gottardi R, Alexander PG, Tuan RS. Enhancement of tenogenic differentiation of human adipose stem cells by tendon-derived extracellular matrix. Biomaterials. 2013;34(37):9295–9306. doi: 10.1016/j.biomaterials.2013.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Valencia Mora M, Antuna Antuna S, Garcia Arranz M, Carrascal MT, Barco R. Application of adipose tissue-derived stem cells in a rat rotator cuff repair model. Injury. 2014;45(Suppl 4):S22–27. doi: 10.1016/S0020-1383(14)70006-3. [DOI] [PubMed] [Google Scholar]
- 214.Lui PP. Identity of tendon stem cells--how much do we know? Journal of cellular and molecular medicine. 2013;17(1):55–64. doi: 10.1111/jcmm.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Tsai CC, Huang TF, Ma HL, Chiang ER, Hung SC. Isolation of mesenchymal stem cells from shoulder rotator cuff: a potential source for muscle and tendon repair. Cell transplantation. 2013;22(3):413–422. doi: 10.3727/096368912X656090. [DOI] [PubMed] [Google Scholar]
- 216.Zhang Q, Cheng B. Tendon-derived stem cells as a new cell source for tendon tissue engineering. Frontiers in bioscience (Landmark edition) 2013;18:756–764. doi: 10.2741/4138. [DOI] [PubMed] [Google Scholar]
- 217.Bi Y, Ehirchiou D, Kilts TM, et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nature medicine. 2007;13(10):1219–1227. doi: 10.1038/nm1630. [DOI] [PubMed] [Google Scholar]
- 218.Ni M, Lui PP, Rui YF, et al. Tendon-derived stem cells (TDSCs) promote tendon repair in a rat patellar tendon window defect model. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2012;30(4):613–619. doi: 10.1002/jor.21559. [DOI] [PubMed] [Google Scholar]
- 219.Pietschmann MF, Frankewycz B, Schmitz P, et al. Comparison of tenocytes and mesenchymal stem cells seeded on biodegradable scaffolds in a full-size tendon defect model. Journal of materials science. Materials in medicine. 2013;24(1):211–220. doi: 10.1007/s10856-012-4791-3. [DOI] [PubMed] [Google Scholar]
- 220.Chen JM, Willers C, Xu J, Wang A, Zheng MH. Autologous tenocyte therapy using porcine-derived bioscaffolds for massive rotator cuff defect in rabbits. Tissue engineering. 2007;13(7):1479–1491. doi: 10.1089/ten.2006.0266. [DOI] [PubMed] [Google Scholar]
- 221.Clarke AW, Alyas F, Morris T, Robertson CJ, Bell J, Connell DA. Skin-derived tenocyte-like cells for the treatment of patellar tendinopathy. The American journal of sports medicine. 2011;39(3):614–623. doi: 10.1177/0363546510387095. [DOI] [PubMed] [Google Scholar]
- 222.Connell D, Datir A, Alyas F, Curtis M. Treatment of lateral epicondylitis using skin-derived tenocyte-like cells. British journal of sports medicine. 2009;43(4):293–298. doi: 10.1136/bjsm.2008.056457. [DOI] [PubMed] [Google Scholar]
- 223.Hernigou P, Flouzat Lachaniette CH, Delambre J, et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. International orthopaedics. 2014;38(9):1811–1818. doi: 10.1007/s00264-014-2391-1. [DOI] [PubMed] [Google Scholar]
- 224.Wang A, Breidahl W, Mackie KE, et al. Autologous tenocyte injection for the treatment of severe, chronic resistant lateral epicondylitis: a pilot study. The American journal of sports medicine. 2013;41(12):2925–2932. doi: 10.1177/0363546513504285. [DOI] [PubMed] [Google Scholar]
- 225.Ellera Gomes JL, da Silva RC, Silla LM, Abreu MR, Pellanda R. Conventional rotator cuff repair complemented by the aid of mononuclear autologous stem cells. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2012;20(2):373–377. doi: 10.1007/s00167-011-1607-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Song N, Armstrong AD, Li F, Ouyang H, Niyibizi C. Multipotent mesenchymal stem cells from human subacromial bursa: potential for cell based tendon tissue engineering. Tissue engineering. Part A. 2014;20(1–2):239–249. doi: 10.1089/ten.tea.2013.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Mazzocca AD, McCarthy MB, Chowaniec DM, Cote MP, Arciero RA, Drissi H. Rapid isolation of human stem cells (connective tissue progenitor cells) from the proximal humerus during arthroscopic rotator cuff surgery. The American journal of sports medicine. 2010;38(7):1438–1447. doi: 10.1177/0363546509360924. [DOI] [PubMed] [Google Scholar]
- 228.Virk MS, Lieberman JR. Biologic adjuvants for fracture healing. Arthritis research & therapy. 2012;14(6):225. doi: 10.1186/ar4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Hannallah D, Peterson B, Lieberman JR, Fu FH, Huard J. Gene therapy in orthopaedic surgery. The Journal of Bone & Joint Surgery. 2002;84(6):1046–1061. [Google Scholar]
- 230.Virk MS, Sugiyama O, Park SH, et al. “Same day” ex-vivo regional gene therapy: a novel strategy to enhance bone repair. Molecular Therapy. 2011;19(5):960–968. doi: 10.1038/mt.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Derwin KA, Baker AR, Iannotti JP, McCarron JA. Preclinical models for translating regenerative medicine therapies for rotator cuff repair. Tissue engineering. Part B, Reviews. 2010;16(1):21–30. doi: 10.1089/ten.teb.2009.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hay M, Patricios J, Collins R, et al. Association of type XI collagen genes with chronic Achilles tendinopathy in independent populations from South Africa and Australia. British journal of sports medicine. 2013;47(9):569–574. doi: 10.1136/bjsports-2013-092379. [DOI] [PubMed] [Google Scholar]
- 233.Longo UG, Loppini M, Margiotti K, et al. Unravelling the genetic susceptibility to develop ligament and tendon injuries. Current stem cell research & therapy. 2014;10(1):56–63. doi: 10.2174/1574888x09666140710112535. [DOI] [PubMed] [Google Scholar]
- 234.Mokone GG, Schwellnus MP, Noakes TD, Collins M. The COL5A1 gene and Achilles tendon pathology. Scandinavian journal of medicine & science in sports. 2006;16(1):19–26. doi: 10.1111/j.1600-0838.2005.00439.x. [DOI] [PubMed] [Google Scholar]
- 235.Farthing CR, Ficz G, Ng RK, et al. Global mapping of DNA methylation in mouse promoters reveals epigenetic reprogramming of pluripotency genes. PLoS genetics. 2008;4(6):e1000116. doi: 10.1371/journal.pgen.1000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Trella KJ, Frank JM, Mikecz K, Galante JO, Sandy JD, Plaas A, Wang VM, Wysocki RW. Epigenetic changes in a murine model of tendinopathy. Transactions of the Orthopaedic Research Society. 2015;40:1355. [Google Scholar]
- 237.Trella KJ, Frank JM, Galante JO, Sandy JD, Plaas A, Wysocki RW, Wang VM. Does hypoxia contribute to tendinopathy? Evidence from an in-vivo murine model. Paper presented at: 14th International Symposium on Ligaments and Tendons; March 27, 2015; Las Vegas, NV. [Google Scholar]