Abstract
Purpose
Phase I: to determine the maximum tolerated dose (MTD) of motexafin gadolinium (MGd) given concurrently with temozolomide (TMZ) and radiotherapy (RT) in patients with newly diagnosed supratentorial glioblastoma multiforme (GBM). Phase II: to determine whether this combination improved overall survival (OS) and progression-free survival (PFS) in GBM recursive partitioning analysis (RPA) class III–V patients as compared to recently published historical controls.
Methods and Materials
Dose escalation in phase I progressed through three cohorts until 2 of 6 patients experienced a dose limiting toxicity (DLT) or a dose of 5mg/kg was reached. Once a MTD was established, a one-sided one-sample log-rank test at significance level of 0.1 had 85% power to detect a median survival difference (13.69 vs. 18.48 months) with 60 deaths over a 12 month accrual period and an additional 18 months of follow-up. OS and PFS were estimated using the Kaplan-Meier method.
Results
In phase I, 24 patients were enrolled. The MTD established was 5 mg/kg given intravenously 5 days a week for the first 10 RT fractions then 3 times a week for the duration of RT (1). The 7 patients enrolled to the third dose level and the 94 enrolled to phase II received this dose. Of these 101 patients, 87 were eligible and evaluable. Median survival time (MST) is 15.6 months (95% confidence interval [CI]: 12.9–17.6), not significantly different from the historical control (p=0.36). Median PFS is 7.6 months (95% CI: 5.7–9.6). One patient (1%) experienced a grade 5 possibly related adverse event during the concurrent phase and none during the adjuvant TMZ.
Conclusions
Treatment was well tolerated but median OS did not reach the protocol specified improvement over the historical control, indicating that the combination of standard RT with TMZ and MGd did not achieve a significant survival advantage.
Introduction
Adults with newly diagnosed GBM have a universally poor prognosis with MST of only 14.6 months, and a 2-year survival rate of 26% following fractionated radiotherapy with concurrent followed by adjuvant TMZ (2). Variations in radiation doses and schedules, as well as the addition of chemotherapeutics and biological response modifiers have not significantly changed survival for these patients (3–10). Of interest has been motexafin gadolinium (MGd), an MRI-detectable expanded metalloporphyrin that localizes in tumors with greater affinity than in normal tissues (11). The precise mechanism of action is not fully elucidated but it inhibits oxidative stress-related proteins such as thioredoxin reductase which is believed to lead to a reduced ability to repair radiation-induced oxidative damage (12–14). In 2005 an EORTC phase III clinical trial of concurrent RT and TMZ followed by adjuvant TMZ established a new standard of treatment for newly diagnosed GBM (2, 15). The potential addition of a radiosensitizer such as MGd to this regimen was logical to explore in a clinical trial. In this context, the XXXX initiated a single arm phase I/II study, XXXX, to examine the safety and efficacy of MGd in combination with standard fractionated RT (60Gy) plus concurrent followed by adjuvant TMZ for newly diagnosed GBM.
Methods
Selection Criteria
Adults 18 years or older with adequate performance status (Zubrod 0–1) were eligible for enrollment following biopsy or resective surgery for newly diagnosed, supratentorial, histologically-confirmed GBM or gliosarcoma (GS). Pre-treatment characteristics, including extent of resection and location of lesion, are summarized in supplemental table 1. With the exception of non-melanoma skin cancer, or non-invasive bladder or cervix lesions, no malignancy was allowed within three years prior to the GBM diagnosis.
Additional exclusions included pregnancy, breast feeding, prior treatment with chemotherapy, radiotherapy to the head or neck area, contraindication for MRI with gadolinium, history of porphyria or G6PD deficiency, active inflammatory disorders, or major medical or psychiatric illness.
Treatment Schema
Protocol treatment was mandated to begin within 5 weeks of surgery. Both the phase I and II stages of this study combined fractionated radiation, TMZ, and MGd according to the following specifications.
Radiation Therapy (RT)
RT was delivered using conformal radiotherapy in 2Gy fractions 5 days weekly to a total dose of 60Gy over six weeks. The initial target was the pre-operative T2/axial FLAIR volume plus a tailored 2cm margin to 46Gy, followed by a 14Gy boost to contrast-enhancing tumor plus 2.5cm margin. Standard precautions were taken to limit dose to critical structures, and proton beam, stereotactic radiosurgery, and intensity modulated radiotherapy were not permitted modalities.
Temozolomide (TMZ)
Concurrent treatment
Oral TMZ was dosed at 75mg/m2 /day during radiation therapy for a maximum of 42 consecutive days, beginning the night before the first radiation treatment and ending the night before the last radiation treatment. Adjuvant treatment: Oral TMZ was administered in adjuvant cycles of 5 days every 28 days, beginning 28 days after completion of concurrent chemoradiotherapy. All adjuvant cycles were administered at 200mg/m2 /day. Six adjuvant cycles of TMZ were planned per protocol, but patients were allowed to continue TMZ for up to twelve cycles. Dose escalations, reductions, and treatment delays were based on hematologic and non-hematologic adverse events. Prophylactic treatment for Pneumocystis Pneumonia (PCP) was required with standard agents within 48 hours of initiating TMZ.
Motexafin Gadolinium (MGd)
To determine the MTD in the phase I study three treatment arms were designed to escalate the dose of MGd, as previously reported (1). Starting the first day of RT, MGd was given intravenously over 30 minutes on Monday through Friday during weeks 1 and 2 of RT/TMZ, then three times weekly (Monday, Wednesday, Friday) during weeks 3 through 6 for a total of 22 doses. MGd infusion was to be given between 2 to 5 hours prior to RT. Arm 1 dose was 3mg/kg/day, Arm 2 4mg/kg/day, and Arm 3 5mg/kg/day. The phase II study used the MTD MGd dose determined in the phase I study (5mg/kg/day) duplicating the same infusion schedule.
Patient Assessments
Pre-treatment evaluation included history, physical with skin assessment, neurological examination with mini mental status examination (MMSE), complete blood count (CBC), transaminases, serum phosphorus and neuroimaging with MRI. During concurrent RT/TMZ/MGd clinical assessment and laboratory evaluations were performed weekly. Clinical assessment was performed monthly during adjuvant cycles of TMZ, while neurological imaging, neurological examinations, MMSE and Zubrod scoring were performed every two months during year one, every three months through year three, every 6 months through year 6, then annually thereafter.
Statistical Methodology
The phase I portion of this study was to determine the MTD of MGd when given concurrently with RT/TMZ. DLT’s for the phase I study were defined as grade ≥4 neurological adverse events (AEs) that were considered to be related to the RT/TMZ/MGd combination treatment occurring within 21 days of conclusion of RT as measured by CTCAE v3. A dose level of MGd was considered acceptable if no more than 1 patient in 6 experienced a DLT. If the current level was acceptable, then dose escalation would occur; otherwise the preceding dose level was declared the MTD. A maximum of two dose level escalations were planned and a maximum sample size for the phase I portion of the study was determined to be 21 patients. Seven patients were enrolled per dose level to account for potential ineligibility and/or patients receiving no protocol treatment. The primary objective of the phase II study was to determine whether the addition of MGd to standard RT/TMZ would improve OS compared to published survival results from the EORTC clinical trial of RT vs. RT/TMZ (2). The secondary objectives were to determine any short or long term adverse effects of combination therapy and to estimate the PFS of patients treated with this protocol. Assuming an exponential survival with a MST of 13.69 months for the historical control arm, a 35% improvement in MST for the experimental arm corresponds to a MST of 18.48 months. This corresponds to a 26% relative reduction in the monthly hazard rate from 0.0506 (historical control) to 0.0375 (experimental arm), resulting in a hazard ratio of 0.74. A one-sided log-rank test at significance level of 0.1 has 85% power to detect a survival difference with 60 observed deaths. This required 94 patients accrued over 12 months and at least 18 months follow-up. Adjusting for 95% eligibility, phase II required a total sample size of 99 patients. OS and PFS were estimated using the Kaplan-Meier method. An event for OS was death due to any cause, for PFS an event was the first report of disease progression or death. All eligible patients starting protocol treatment were included in the analysis.
Results
Phase I
The phase I trial opened 2/9/2006 and closed 10/15/2007, accruing 24 patients (Table 1). Seven enrolled at dose level 1; one experienced a DLT. At dose level 2, 10 patients enrolled, one was ineligible and none had a DLT. Dose level 3 accrued seven patients, one of whom did not initiate therapy. No DLT’s were noted in this cohort and the MTD of MGd was determined to be 5mg/kg when administered as 22 doses over 6 weeks with concurrent TMZ and 60Gy fractionated radiation.
Table 1. Patient Accrual.
| Phase I component | |
| Study sample size | 21 |
| Patients required per dose level | 7 |
| Total patients entered | 24 |
| Phase II component | |
| Study sample size | 92 |
| Total patients entered | 94 |
| Average monthly accrual for the study | 6.0 |
Phase II
Phase II enrolled 94 patients between 3/14/2008 and 7/1/2009 with patients to receive 22 doses of 5mg/kg MGd given over 6 weeks with concurrent TMZ and fractionated RT followed by adjuvant TMZ (Table 1). The 7 patients enrolled to the third dose level of phase I and 94 patients enrolled to phase II component received the same protocol treatment. Among these 101 patients, 87 were eligible and started protocol treatment (7 were ineligible; 7 did not start treatment). The most common side-effects of MGd in combination with TMZ and RT were out of field dermatologic (especially acral blisters, Supplemental figure 1), asymptomatic hypophosphatemia, GI upset, and fatigue (supplemental table 2).
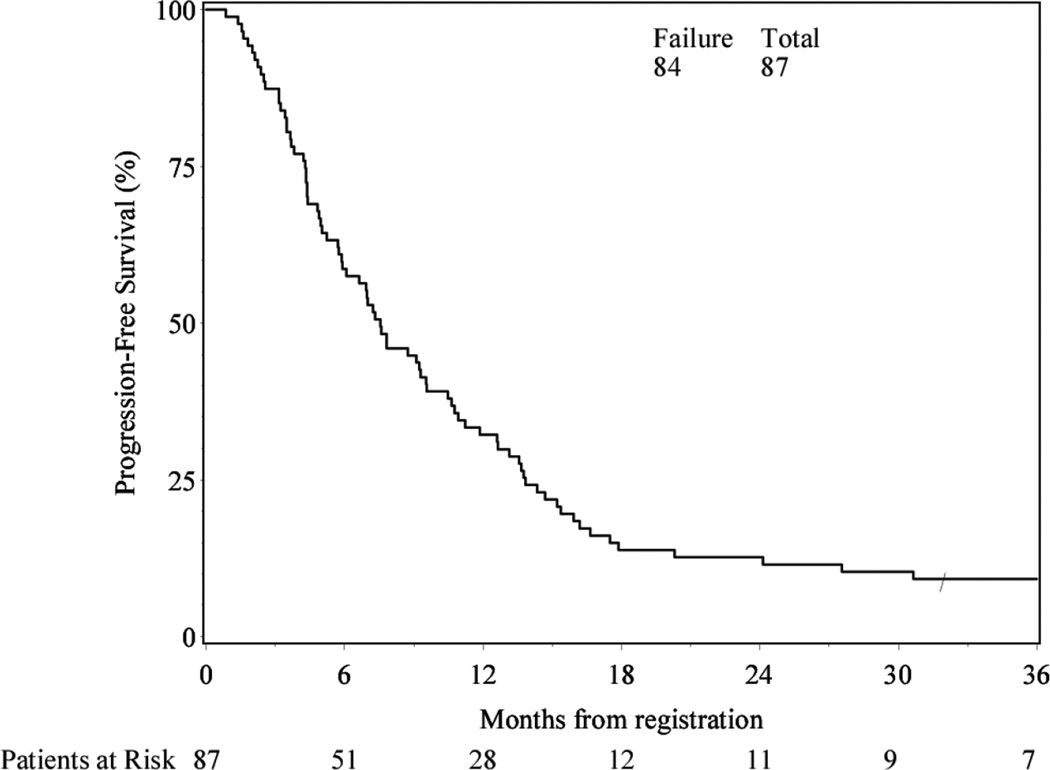
Among these 87 patients, 79 deaths occurred and 8 patients were alive at latest follow-up (Table 2). Median follow-up time for those still alive was 46.1 months (range: 31.9–54.3). Median PFS for the experimental arm was 7.6 months (95% confidence interval [CI]: 5.7–9.6) (Figure 1).
Table 2. Overall Survival.
| Month | Estimate (%) | 95% CI (%) | Cumulative Failures |
At Risk |
|---|---|---|---|---|
| 0 | 100.0 | -- | 0 | 87 |
| 6 | 82.8 | 74.8, 90.7 | 15 | 72 |
| 12 | 65.5 | 55.5, 75.5 | 30 | 57 |
| 18 | 37.9 | 27.7, 48.1 | 54 | 33 |
| 24 | 24.1 | 15.1, 33.1 | 66 | 21 |
| 30 | 20.7 | 12.2, 29.2 | 69 | 18 |
| 36 | 17.1 | 9.1, 25.0 | 72 | 13 |
| Total | 79 | |||
| Median survival time (95%CI) | 15.6 months (12.9,17.6) | |||
Figure 1.
Progression-free Survival
Median OS for the experimental arm was 15.6 months (95% CI: 12.9–17.6), not significantly different from the historical control of 13.69 months (p=0.36) (Table 2, Figure 2). Because the distribution of patients by RPA class (14%, 60% and 26% for III, IV and V, respectively) was different from those assumed in the original study design (17%, 45%, 38% for III, IV and V, respectively), the protocol-specified fixed control monthly hazard rate was re-calculated to be 0.0495, corresponding to a median OS of 13.99 months. The comparison between this and the observed monthly hazard rate is also not statistically different (p=0.43).
Figure 2.
Overall survival: 0513 vs. historical control: EORTC
Protocol Compliance
Radiotherapy and chemotherapy treatment reviews were conducted on all patients. Radiation treatment was found to be per protocol or within an acceptable variation in 95% of phase I patients and 97% of phase II patients. The median number of MGd doses received in phase I for the 3 mg/kg cohort was 22 (range 22-22), for the 4 mg/kg cohort 20 (range 13–22) and for the 5 mg/kg cohort 20.5 (range 9–22). In phase II, the median number of MGd doses was 22 (range 6–22). Chemotherapy review was conducted on all evaluable and eligible cases. 74 cases receiving 5mg/kg MGd had their chemotherapy administered per protocol with or without protocol specific required modifications or delays. Ten cases had chemotherapy administered with dose violations and 3 were not evaluable due to the data submitted. The most common deviation was administration of 150 instead of 200mg/m2/day for 5 consecutive days as the first adjuvant cycle.
Discussion
We report the mature results of a phase I/II study utilizing the novel combination of concurrent MGd, TMZ, and fractionated RT in the treatment of newly diagnosed supratentorial GBM. The first in man use of this combination was the 24 patient phase I run-in designed to establish the MTD of MGd for this combination through stepwise dose escalation of MGd. The phase II trial used the MTD from phase I to determine if a pre-specified survival advantage for the combination therapy could be achieved in newly diagnosed supratentorial GBM when compared to historical controls. The phase I portion was successful in establishing an MTD value for the novel combination. Nevertheless, this combination as prescribed in the phase II study resulted in a 15.6 month median survival, failing to reach a pre-determined statistical significance of a 35% improvement in the historical survival of 13.69 months to 18.48 months.
The decision of the group to study this therapeutic combination was firmly grounded. For decades, outside of highly specialized brain tumor treatment centers involved in clinical research programs, the standard of care remained surgery followed by postoperative radiotherapy (9). In 2005, two companion papers reported the findings of the EORTC trial that tested the addition of TMZ for the treatment of newly diagnosed GBM (2, 15). In this trial treatment was randomized between the control group: surgery followed by radiotherapy alone; and the study group: surgery followed by radiotherapy with concurrently administered TMZ chemotherapy followed by six adjuvant TMZ cycles (2). The advantage achieved by adding TMZ to standard treatment extended MST from less than one year to nearly 15 months. Two-year OS reached 26% in a subgroup of patients with a molecular profile characterized by methylation (and inactivation) of the promoter region of the MGMT (methylguanine methyl transferase) gene, responsible for expressing a DNA repair protein contributing, at least in part, to treatment resistance to alkylating agents (15). Nevertheless, the improvement in survival with the addition of TMZ still left over half the patients with newly diagnosed GBM dead at two years. The efficacy, ease of administration, and well-tolerated side-effect profile of TMZ make it an ideal agent for multi-modality combinations, especially with agents that are radiosensitizers, including MGd (10). Parallel in time with the TMZ work, Motexafin gadolinium (Xcytrin®, Pharmacyclics) was being investigated for its potential therapeutic benefits as a radiosensitizer (11). This interest was because of its properties as a metallotexaphyrin redox agent that selectively targets tumor cells, inhibits cellular respiration, and possibly reduced radiation-induced oxidative damage repair (12, 13, 14, 16). The first large clinical study to use MGd as a radiosensitizer was by Mehta et al in a phase III trial of 401 patients with brain metastases (17). Overall MST for the entire cohort was 5 months, with patients in the WBRT alone group versus WBRT plus MGd group surviving a median of 4.9 months and 5.2 months, respectively. Although this study was negative for achieving additional OS, it demonstrated the potential value of MGd as a well-tolerated agent with certain real benefits for a subgroup of patients, specifically the non-small cell lung cancer group. Ford et al conducted a phase I clinical trial treating a total of 33 patients with GBM with MGd dose escalation to determine safety, tolerability, tumor uptake, tissue distribution, and to establish the MTD of MGd when combined with fractionated RT to 59.4Gy (11). Although this was not an efficacy study, the MST for all 33 subjects was 17.6 months suggesting a survival advantage over historic controls treated with RT alone. Of note, hematological side effects were mild, positioning MGd to be an ideal agent to be combined with cytotoxic agents such as TMZ. With evidence suggesting MGd was a well-tolerated potential radiosensitizer in the treatment of brain metastases and GBM, there was sound rationale to undertake this trial to study the use of this agent in the treatment of primary brain tumors as an addition to involved field RT plus TMZ as per the newly published EORTC regimen.
Many aspects of the brain tumor clinical trial landscape have changed since this study was conceived, and a discussion of three of these issues is important in the context of the data presented. First, we did not mandate tissue collection, and therefore no stratification for MGMT methylation status or other molecular correlative studies were done. Subsequent evidence has established this as a critical tumor-related prognostic variable in GBM survival outcomes (6). Molecular analyses are important even in the post-trial setting to stratify results and identify potential subgroups that may have true benefit from treatments otherwise deemed to have no advantage. A recent example of the importance of applying new tissue analyses in the post-trial setting is the observation of clear therapeutic benefit of chemotherapy in the treatment of anaplastic oligodendroglioma when 1p19q analysis was performed retrospectively on samples from RTOG 9402 (18). These results have had a pivotal impact on redesigning ongoing and future clinical trials for this disease, as well as on the current standard of care. In hindsight, a clear shortcoming of this study was the lack of translational endpoints. For possible future reference, 10 patients in the phase I and 30 patients in the phase II had blocks and H&E slides submitted, and one phase I patient and 19 phase II patients had just H&E slides submitted to the central tissue bank
Second, when this trial was designed classic Macdonald criteria were used to evaluate radiographic response, defining disease progression as a >25% increase in tumor area (two dimensions) on post-contrast images (19). During the time of active enrollment, treatment, and assessment of patients on XXXX the concept of pseudoprogression had not yet been well-defined. We now appreciate that pronounced enhancement and vasogenic edema resulting from increased vascular permeability may be accompanied by worsening of clinical symptoms in the first weeks following completion of concurrent chemoradiotherapy and be misinterpreted as treatment failure. This phenomenon has been well described by Wen et. al. (20) and new criteria for treatment response address this potential pitfall and redefine disease progression based on if these findings occur within or beyond 12 weeks of completing concurrent treatment (21). At the time of our study, it is likely that individual treating physicians did not consider these observations in day to day protocol decisions for study patients. This could have been of particular importance in this trial which utilized MGd as an additional radiosensitizing agent to the RT/TMZ combination making study patients even more likely to experience this phenomenon. Another variable that possibly affected data reporting in this group is the nature of MGd itself as a substance taken up by tumor cells resulting in the appearance of avid enhancement completely unrelated to pseudo or real tumor progression, potentially confounding MRI interpretation even further (Figure 3, a, b)(12).
Figure 3.
Finally, the lack of a formal quality of life assessment was a shortcoming, and the impact of omission would be magnified had this been a positive study. The 24 patient phase I run-in study defined the MTD for this combination through stepwise dose escalation of MGd with the DLT defined as a grade 4 neurologic event considered related to the combination occurring within a 21 day window from the end of RT. In this regard the study combination was well tolerated as only 1 of 24 patients (4%) experienced such an event, and grade 4–5 non-hematologic events were infrequent (6%, Table 3). However, 91% of patients (79/87) in the 5 mg /kg cohort experienced a non-hematologic grade 2 or 3 toxicity during concurrent treatment with a combination of fatigue, nausea, and pyrexia being particularly common. These events contributed to investigators’ perception of a diminished sense of well-being on the part of the trial subjects and may have led to a potentially premature discontinuation of therapy in the full dose cohorts. That this “malaise”-type syndrome was not more apparent in the initial dose cohorts raises the possibility that it was dose dependent, either based on daily dose or cumulative dose. This is a somewhat intriguing question as the average survival from registration of patients 1,2,4,5, and 6 is 37 months, all having received the 3mg/kg MGd dose and all completed 22 infusions concurrently with RT and TMZ (personal communication, XXX). If efficacy were actually a function of duration of MGd exposure or duration of concurrence with TMZ, RT, or the combination of agents rather than dose intensity then MGd discontinuation for self-limited events may have impacted the chance of finding a positive outcome. The median number of MGd doses received in the phase I 5 mg/kg arm was 20.1 (range, 9–22) and 22 in the 5 mg/kg cohort in phase II (range 6–22) but currently available data do not allow a breakdown of outcomes based on MGd dose and/or number of concurrent RT/TMZ/MGd treatments received.
Table 3. Summary of Worst Adverse Event per Patient - Concurrent RT+MGd+TMZ.
Adverse Events: (Definitely, Probably, or Possibly Related to Treatment)
| Grade | Phase I: MGd 3mg/kg (n=7) |
Phase I: MGd 4mg/kg (n=9) |
Phase I: MGd 5mg/kg (n=6) |
Phase II: MGd 5mg/kg (n=81) |
|
|---|---|---|---|---|---|
| Worst non-hematologic | 1 | 2 (27%) | 0 (0%) | 0 (0%) | 2 (3%) |
| 2–3 | 4 (57%) | 9 (100%) | 6 (100%) | 73 (90%) | |
| 4–5 | 1 (14%) | 0 (0%) | 0 (0%) | 6 (6%) | |
| Worst overall (includes hematologic) | 1 | 2 (29%) | 0 (0%) | 0 (0%) | 2 (2%) |
| 2–3 | 4 (57%) | 5 (56%) | 6 (100%) | 62 (76%) | |
| 4–5 | 1 (14%) | 4 (44%) | 0 (0%) | 16 (20%) |
Includes adverse events where relationship to protocol treatment is missing
Conclusion
Treatment was generally well tolerated and few significant untoward events were noted. The phase I portion was successful in establishing an MTD value for the novel combination. Nevertheless, this combination as prescribed in the phase II study resulted in a 15.6 month median survival, failing to reach a pre-determined statistical significance of a 35% improvement over the historical control.
Supplementary Material
Summary.
The addition of MGd to RT and TMZ does not appear to improve outcome in the general population of newly diagnosed GBM patients.
Acknowledgments
This trial was conducted by the Radiation Therapy Oncology Group (RTOG), and was supported by RTOG grant U10 CA21661 and CCOP grant U10 CA37422 from the National Cancer Institute (NCI). This manuscript’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: none.
References
- 1.Brachman DG, Ashby LS, Wang M, et al. Phase I/II Trial of Temozolomide (TMZ), Motexafin Gadolinium (MGd), and 60GY Fractionated Radiation for Newly Diagnosed Supratentorial Glioblastoma Multiforme (GBM): Results of RTOG 0513 Phase I Dose Escalation Toxicity Analysis [Abstract] J Society for NeuroOncology. 2008;10(5):889. [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;32(8):699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckner JC, Ballman KV, Michalak JC, et al. Phase III trial of carmustine and cisplatin compared with carmustine alone and standard radiation therapy or accelerated radiation therapy in patients with glioblastoma multiforme: North Central Cancer Treatment Group 93-72-52 and Southwest Oncology Group 9503 Trials. J Clin Oncol. 2006;24:3871–3879. doi: 10.1200/JCO.2005.04.6979. [DOI] [PubMed] [Google Scholar]
- 5.Pichlmeier U, Bink A, Schackert G, et al. Resection and survival in glioblastoma multiforme: an RTOG recursive partitioning analysis of ALA study patients. Neuro Oncol. 2008;10(6):1025–1034. doi: 10.1215/15228517-2008-052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 7.Keime-Guibert F, Chinot O, Taillandier L, et al. Radiotherapy for glioblastoma in the elderly. N Engl J Med. 2007;356(15):1527–1535. doi: 10.1056/NEJMoa065901. [DOI] [PubMed] [Google Scholar]
- 8.Taal W, Segers-van Rijn JMW, Kros JM, et al. Dose dense 1 week on/1 week off temozolomide in recurrent glioma: a retrospective study. J Neuro-Oncol. 2012;108(1):195–200. doi: 10.1007/s11060-012-0832-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahluwalia MS. American Society of Clinical Oncology 2011 CNS tumor update. Expert Rev Anticancer Ther. 2011;11:1495–1497. doi: 10.1586/era.11.151. [DOI] [PubMed] [Google Scholar]
- 10.Chang JE, Khuntia D, Robins HI, et al. Radiotherapy and radiosensitizers in the treatment of glioblastoma multiforme. Clin Adv Hematol Oncol. 2007;5:894–915. [PubMed] [Google Scholar]
- 11.Ford JM, Seiferheld W, Alger JR, et al. Results of the phase I dose-escalating study of motexafin gadolinium with standard radiotherapy in patients with glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2007;69(3):831–838. doi: 10.1016/j.ijrobp.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 12.Hashemy SI, Ungerstedt JS, Zahedi Avval F, et al. Motexafin gadolinium, a tumor-selective drug targeting thioredoxin reductase and ribonucleotide reductase. J Biol Chem. 2006;281:10691–10697. doi: 10.1074/jbc.M511373200. [DOI] [PubMed] [Google Scholar]
- 13.Lecane PS, Karaman MW, Sirisawad M, et al. Motexafin gadolinium and zinc induce oxidative stress responses and apoptosis in B-cell lymphoma lines. Cancer Res. 2005;65:11676–11688. doi: 10.1158/0008-5472.CAN-05-2754. [DOI] [PubMed] [Google Scholar]
- 14.Magda D, Lecane P, Miller RA, et al. Motexafin gadolinium disrupts zinc metabolism in human cancer cell lines. Cancer Res. 2005;65:3837–3845. doi: 10.1158/0008-5472.CAN-04-4099. [DOI] [PubMed] [Google Scholar]
- 15.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 16.De Stasio G, Rajesh D, Ford JM, et al. Motexafin-gadolinium taken up in-vitro by at least 90% of glioblastoma cell nuclei. Clin Cancer Res. 2006;12(1):206–213. doi: 10.1158/1078-0432.CCR-05-0743. [DOI] [PubMed] [Google Scholar]
- 17.Mehta MP, Rodrigus P, Terhaard CHJ, et al. Survival and neurologic outcomes in a randomized trial of Motexafin Gadolinium and whole-brain radiation therapy in brain metastases. J Clin Oncol. 2003;21:2529–2536. doi: 10.1200/JCO.2003.12.122. [DOI] [PubMed] [Google Scholar]
- 18.Cairncross G, Wang M, Shaw E, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma : Long-term results of RTOG 9402. J Clin Oncol. 2013;31:337–343. doi: 10.1200/JCO.2012.43.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macdonald DR, Cascino T, Schold SC, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 20.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas : Response Assessment in Neuro-Oncology Working Group. J Clin Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 21.Vogelbaum MA, Jost S, Aghi MK, et al. Application of novel response/progression measures for surgically delivered therapies for gliomas: Response Assessment in Neuro-Oncology (RANO) Working Group. Neurosurgery. 2012;70:234–243. doi: 10.1227/NEU.0b013e318223f5a7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.