Abstract
Macrophages are immune cells of haematopoietic origin that provide crucial innate immune defence and have tissue-specific functions in the regulation and maintenance of organ homeostasis. Recent studies of macrophage ontogeny, as well as transcriptional and epigenetic identity, have started to reveal the decisive role of the tissue stroma in the regulation of macrophage function. These findings suggest that most macrophages seed the tissues during embryonic development and functionally specialize in response to cytokines and metabolites that are released by the stroma and drive the expression of unique transcription factors. In this Review, we discuss how recent insights into macrophage ontogeny and macrophage–stroma interactions contribute to our understanding of the crosstalk that shapes macrophage function and the maintenance of organ integrity.
Macrophages are key components of the innate immune system that reside in tissues, where they function as immune sentinels. They are uniquely equipped to sense and respond to tissue invasion by infectious microorganisms and tissue injury through various scavenger, pattern recognition and phagocytic receptors1–4. Macrophages also have homeostatic functions, such as the clearance of lipoproteins, debris and dead cells using sophisticated phagocytic mechanisms5,6. Accordingly, macrophages are crucial for maintaining a balanced response to homeostatic or tissue-damaging signals and, when this delicate balance is disturbed, inflammatory disease can occur.
Recent studies have revealed the ontogeny and functional diversity of tissue-resident macrophages. These studies have established that tissue-resident macrophages are maintained by distinct precursor populations that can be recruited from either embryonic haematopoietic precursors during fetal development or bone marrow-derived myeloid precursors during adult life7. In addition to developmental diversity, macrophages have unique functions in maintaining homeostasis and exhibit extensive plasticity during disease progression.
Macrophages have classically been defined by their dependence on colony-stimulating factor 1 (CSF1; also known as M-CSF). However, in some tissues, macrophages also depend on other cytokines and meta bolites for their differentiation and maintenance. Recent data acquired by high-throughput sequencing have characterized the transcriptional and epigenetic programmes of tissue-resident macrophages and revealed the extent of diversity in these populations1,8. In addition to differences in ontogeny, locally derived tissue signals can explain some of this diversity, as they drive the expression of unique transcription factors in tissue-resident macrophages, leading to distinct epigenetic profiles, transcriptional programmes and, ultimately, different functions.
In this Review, we discuss the unique ontogeny of tissue-resident macrophages, the interactions of macrophages with their tissue environment and how these interactions shape macrophage function in the steady state and during inflammation.
The mononuclear phagocyte system
A central dogma in immunology posits that monocytes and macrophages are part of a continuum that forms the mononuclear phagocyte system (MPS). According to this system, macrophages are fully differentiated cells that have lost proliferative potential and are constantly repopulated by circulating monocytes produced by bone marrow-derived myeloid progenitors9. The definition of this cellular system stems mostly from studies tracing the differentiation of radiolabelled monocytes in mice with inflammation and thus describes the contri bution of monocytes to inflammatory macrophages that accumulate in injured tissues.
Reinvestigating macrophage ontogeny using congenic parabiotic mice that share the same circulation provided insight into the physiological contribution of circulating monocytes to macrophages residing in healthy tissues. Congenic parabionts have mixed haematopoietic cell precursors in the bone marrow, mixed lymphocytes and monocytes in the blood, and mixed dendritic cells (DCs) in the lymphoid organs10. Therefore, if tissue-resident macrophages were derived from monocytes, they should harbour the same level of chimerism as circulating monocytes. However, the mononuclear phagocytes of the epidermis (known as Langerhans cells)10 and the brain-resident macrophages (known as microglia)11,12 were found not to mix in tissues even after a year of parabiosis, which suggested that they could be maintained independently of circulating precursors in adult mice. More recently, several other tissue-resident macrophages, including alveolar macrophages, spleen red pulp macrophages and Kupffer cells13–17, were also shown to be maintained independently of circulating precursors either through longevity or self-renewal. Several studies in humans were consistent with a circulation-independent maintenance of tissue-resident macrophages: patients with severe monocytopenia have normal numbers of Langerhans cells in the epidermis18,19; donor Langerhans cells can be detected for years in a recipient of a human limb graft20; and host Langerhans cells remained in patients that received sex-mismatched allogeneic bone marrow transplants21,22. Similarly to Langerhans cells, numerous tissue-resident macrophages are present in patients with severe monocytopenia18,19 and in patients who received a sex-mismatched allogeneic bone marrow transplant22.
Together, these studies have challenged the linearity of the MPS, reigniting debate on the contribution of early haematopoiesis to tissue-resident macrophages23, as discussed below.
Origins of tissue-resident macrophages
Embryonic haematopoietic precursors
Early studies reported the presence of a primitive macrophage lineage that has self-renewal capacity and arises before the development of definitive haematopoiesis, without a monocyte intermediate24–26. Primitive macrophages were first identified in the blood islands of the extra-embryonic yolk sac (YS) around embryonic day 8 (E8) and were shown to migrate to various tissues from E8.5 to E10 to give rise to proliferative fetal macrophages independently of monocytes24–26. Similar data were also obtained in zebrafish27, in which the emergence of macrophages was shown to precede the onset of blood circulation. However, whether these primitive macrophages contributed to adult tissue-resident macrophages has never been directly addressed.
In mice, the first haematopoietic cells appear in the YS blood islands around E7.5 and produce primitive erythrocytes and macrophages but not lymphocytes28,29. In humans, haematopoiesis is also initiated in the extra-embryonic YS during the third week of development and is limited to erythromyeloid cells30. YS-derived progenitors migrate and seed the fetal liver through the bloodstream after E8.5, once the circulation is established, to rapidly initiate the first wave of intra-embryonic haematopoiesis31,32. A second wave of haematopoiesis beginning at E10.5 arises in the mouse embryo from major arterial vessels and gives rise to definitive haematopoietic stem cells (HSCs) with multi-lineage potential33. HSC activity subsequently expands in the fetal liver and peaks at E16.5 before transitioning to the bone marrow, which becomes the main site of haematopoiesis in adult life34,35. Although the primitive and definitive haematopoiesis waves have myeloid potential, the contribution of each embryonic wave of haematopoiesis to the adult tissue-resident macrophage pool has not been directly tested.
Experimental models
The first fate mapping model that was used to probe the contribution of embryonic precursors to adult tissue-resident macrophages traced the progeny of YS runt-related transcription factor 1 (RUNX1)+ haematopoietic cells. RUNX1+ haematopoietic precursors are restricted to YS-derived cells between E6.5 and E8, and RUNX1 starts to be expressed by definitive haematopoietic precursors around E8.5 (REFS 11,36). Conditional labelling of RUNX1+ cells in E7–E7.5 embryos allows the contribution of YS haematopoietic cells to fetal and adult macrophages to be traced. RUNX1+ YS-derived macrophages started to infiltrate the whole embryo following the formation of blood circulation around E8.5–E9.5, and a high number of macrophages were labelled in E10 and E13 embryos11,37,38. Strikingly, labelled microglia were retained in adult brains, whereas most tissue macrophages lost their labelling in adult tissues, which suggests that they were being replaced by non-labelled precursors before birth11,37,38. These results led to the hypothesis that microglia that populate adult mouse brains uniquely derive from E7–E7.5 YS-derived cells.
Several studies have since confirmed the embryonic origin of tissue-resident macrophages15,16,38–41. Despite agreeing on a paradigm shift, a controversy arose regarding the exact nature of the embryonic precursor that gives rise to adult tissue-resident macrophages, with some researchers suggesting that YS-derived precursors mainly give rise to microglia, whereas most tissue-resident macrophages derive from fetal liver monocytes15,16,37,39, and other researchers being in favour of a universal YS origin of most adult tissue macrophages including microglia40,41.
The suggestion of a universal YS origin of adult macrophages was based on results showing that mice that lacked the transcription factor MYB, which is required for definitive but not primitive haematopoiesis, retained F4/80hi macrophages in E16.5 embryos40 and that fate mapping of YS macrophages that express the macrophage marker CSF1 receptor (CSF1R) gave rise to these fetal F4/80hi macrophages. However, although fate mapping of E8.5 CSF1R+ YS cells marked a large population of fetal F4/80hi macrophages, a very small population of labelled macrophages remained in adult tissues38,40, with the exception of microglia, which retained maximal labelling after birth38; these results are consistent with those obtained with the RUNX1 fate mapping model. The contribution of YS progenitors to adult tissue macrophages was further substantiated by a novel fate mapping model showing that the angiopoietin 1 receptor TIE2+ YS-derived progenitors with restricted erythroid and myeloid potential — known as erythromyeloid precursors (EMPs) — seed and expand in the fetal liver and give rise to both fetal and adult macrophages41, a result that was not observed with either the RUNX1 or CSF1R fate mapping model described earlier. Pulse labelling of E7.5 TIE2+ cells also labelled definitive HSCs, although at a lower level than YS macrophages41, which raises the possibility that labelled adult macrophages in this model may also derive from definitive HSCs.
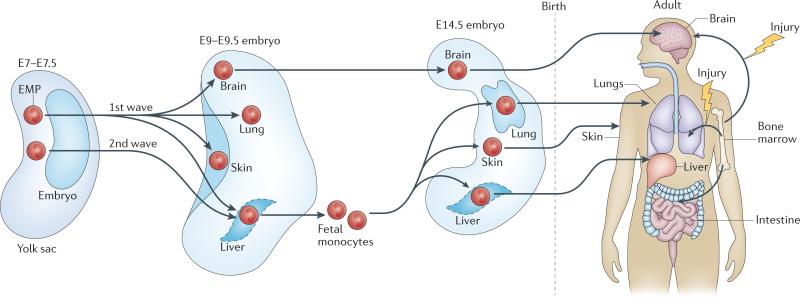
A recent study38 might help to resolve the apparent discrepancy between these studies. Consistent with previous studies42,43, it was shown that YS-derived EMPs are heterogeneous and arise in two waves that differentially contribute to adult microglia and other macrophages38. An early wave of YS-derived EMPs appears around E7.5 and colonizes the brain and other fetal tissues around E9 to give rise to F4/80hi macrophages, as previously described11,40. A later wave of YS-derived EMPs colonizes and expands in the fetal liver to differentiate into fetal liver monocytes, which subsequently replace early F4/80hi macrophages around E14.5 (with the exception of the brain microglia) and maintain macrophages in adult tissues (FIG. 1). Interestingly, whereas the early YS wave lacks the transcription factor MYB40, the second wave of YS-derived haematopoietic cells do express MYB. Fate mapping of MYB-deficient and MYB-sufficient progenitors should help to compare their contribution to tissue-resident macrophages in adult mice.
Figure 1. Origin of tissue-resident macrophages.
Macrophages are maintained in most healthy tissues in mice by embryonic precursors (embryonic macrophages) independently of monocytes, with the exception of intestinal macrophages, dermal macrophages and a subset of cardiac macrophages. Two hypotheses dominate our thinking about the origin of embryonic macrophages. The first hypothesis suggests that all embryonic macrophages derive from yolk sac-derived erythromyeloid precursors (EMPs) that develop around embryonic day 7.5 (E7.5). The second hypothesis suggests that yolk sac-derived EMPs arise in two waves that differentially contribute to adult microglia and other macrophages: an early wave of yolk sac-derived EMPs that appear around E7.5 in the yolk sac colonize the brain and other fetal tissues around E9 to give rise to all tissue macrophages, and a later wave of yolk sac-derived EMPs that colonize and expand in the fetal liver to differentiate into fetal liver monocytes, which subsequently replace fetal macrophages, with the exception of microglia, and maintain them in adulthood.
Although the primitive YS-derived versus definitive HSC-derived haematopoiesis model influences many interpretations of haematopoietic cell emergence, it is likely to be an oversimplification. In fact, embryonic haematopoiesis probably occurs in overlapping waves. We currently still know very little about the molecular and cellular steps that control the development of embryonic haematopoietic waves and still lack the tools to genetically trace discrete precursor populations in the embryos. In addition, the use of tamoxifen-dependent Cre recombinase-dependent fate mapping models has caveats that may affect the interpretation of the fate mapping results (BOX 1). Thus, we need to be careful not to over-interpret fate mapping studies, especially when performed in embryos.
Box 1 | Novel tools for macrophage research.
Inducible gene fate mapping models. These models rely on mice expressing an inducible Cre recombinase-encoding gene under the control of endogenous promoters that are expressed by the cell population of choice crossed with mice expressing floxed reporter transgenes that are driven by a ubiquitous reporter such as ROSA26. Upon induction of Cre recombinase expression, the stop-flox cassette is removed, and a reporter transgene is introduced in discrete cell populations at a specific time point. Introduction of the reporter transgene helps to trace the fate of specific populations that originated at a distinct time point during development or adulthood. In these models, the genetic marker that is introduced is irreversible and, therefore, all the progeny of the marked cells express the same genetic marker. However, there are caveats to the floxed-Cre approaches. Expression of the gene expressing Cre recombinase depends on a specific promoter or enhancer, which may be expressed in multiple cell types or expressed at levels that are insufficient to drive the level of Cre recombinase expression necessary for the removal of the loxP site and subsequent cell labelling. Thus, too little Cre expression can result in incomplete labelling of the target cell populations, whereas ‘leaky’ expression can result in undesired cells being labelled.
Mass cytometry (also known as CyTOF). This approach combines mass spectrometry with the principles of flow cytometry and allows samples to be tagged with approximately 40 different antibodies specific for surface markers or intracellular targets145. Compared with standard flow cytometry approaches, it provides a much more detailed analysis of the relative proportions of all immune cells within one sample. It has been used to identify new subpopulations of cells and has also stimulated the development of new tools to analyse the data. Tools such as viSNE and phenograph attempt to cluster populations based on unbiased approaches146–148. Using these improved tools, we now have a better ability to finely distinguish between tissue-resident macrophages and monocytes that accumulate at sites of inflammation and to understand how they change their phenotype in these contexts.
Molecular epigenetics. With improved techniques for high-throughput chromatin immunoprecipitation and sequencing149, cells sorted from mice or humans can now be analysed at the chromatin level8,59. This will continue to expand our understanding of the DNA remodelling that occurs in phagocytes in the steady state and during inflammation. The past and future potential of cells are imprinted on the chromatin, making this dynamic layer of great interest58. Moreover, the role of chromatin modifiers in the context of inflammation has been of increasing interest, as such modifiers have been implicated in different diseases and cell types150. As fewer cells are needed, our understanding of how individual cells respond to a stimulus will continue to improve.
Single-cell genomics. Single-cell RNA sequencing (RNA-seq) is now a well-established technique that provides a snapshot of RNA presence and quantities at a given time151,152. This technique could soon be used to finely map how individual cells respond to a stimulus and to explore to what degree these responses are due to their lineage, epigenetics, cellular interplay or location.
Next-generation histology. With the identification of new cell subpopulations by mass spectrometry and transcriptomics, it is becoming increasingly important to now define their spatial distribution within the tissue. Multiplex immunohistochemistry techniques that allow for high dimensional analysis of tissues while preserving tissue architecture have recently been developed and should help to identify complex cell populations and cellular interactions in tissues. Similarly, in situ RNA-seq that assesses the transcriptome of cells within a structure has an important role in dissecting local interactions153,154, and mass spectrometry-based immunohistochemistry can be used to detect many proteins in a single histological sample using large panels of metal-based antibodies155,156. Determining the effect of localization and local interactions on the range of macrophage phenotypes present in tissues will provide important insight into their function and response to stimuli.
Origin of macrophages in inflamed adult tissues
In contrast to most healthy tissues, in which macrophages are maintained with minimal contribution of adult circulating monocytes, a large influx of monocytes that are produced by adult myeloid progenitors enter injured tissues and differentiate into macrophage-like and DC-like cells. Accumulating evidence suggests that monocyte differentiation into macrophage-like cells in inflamed tissues occurs in parallel with the expansion of tissue-resident macrophages in many tissues13,15,44. In particular, upon nematode infection, the production of interleukin-4 (IL-4) was shown to drive in vivo replication of tissue-resident pleural macrophages as well as resident macrophage populations in the peritoneum, liver and intestine44,46.
The extent and duration of adult monocyte-derived cell engraftment in tissues remains unclear. In the lungs and brain, for example, monocyte-derived macrophages do not seem to substantially contribute to the resident macrophage pool after the infection or injury resolves13,47. However, in some healthy tissues, macrophages are constantly replenished by circulating monocytes, such as mouse intestinal macrophages48,49 and some dermal macrophages50. In other tissues, such as the heart, epidermis and peritoneal cavity, a smaller subset of monocyte-derived macrophages can be found in healthy tissues and may derive from the physiological recruitment of a small monocyte population15,45,51, or they may be remnant monocyte-derived macrophages that have been recruited during a tissue injury52,53.
The contribution of monocyte-derived macrophages to the resident macrophage population may depend both on the organ and the nature of the injury. In the liver, for example, infection with Listeria monocytogenes induces Kupffer cell necrosis and, in this case, monocyte-derived macrophages contribute to repopulating the liver macrophage population54. However, after paracetamol-induced injury, resident Kupffer cells proliferate and expand, and monocytes do not notably contribute to the resident macrophage pool55. It is still unclear to what extent macrophage ontogeny — that is, embryonic versus monocyte-derived — determines macrophage function. Using bone marrow transplant experiments, adult bone marrow-derived macrophages acquire the enhancer profile of the embryonic-derived macrophages that they replace8. However, whether embryonic-derived or adult bone marrow-derived macrophages are functionally identical remains an open question. For example, embryonic and monocyte-derived cardiac macrophage subsets that coexist in the healthy heart have different propensities for promoting tissue repair after cardiomyocyte injury56. Thus, the balance of these different macrophage ontogeny programmes in tissue immunity and homeostasis needs to be addressed in each tissue.
Tissue factors control macrophage identity
In contrast to tissue-resident DCs, which have the same transcriptional programme regardless of the tissue in which they reside57, tissue-resident macrophages share expression of only a few unique transcripts, with most of the transcriptional programme being specific to the tissue of residence1,8. These results suggest that, in addition to potential ontogeny cues, environmental signals contribute to shaping macrophage transcriptional regulation58,59.
Ontogeny and environmental signals can shape cell identity through epigenetic modifications (such as chromatin accessibility) and open chromatin-containing regulatory elements (such as promoters and enhancers)60. During development, pioneer transcription factors open large swaths of chromatin, which allows the binding of other transcription factors that confer cell-type specificity61. In myeloid cells, PU.1 acts as a pioneer transcription factor, binding throughout the genome to both promoter and enhancer regions62,63. The binding of other macrophage-specific transcription factors, together with PU.1, further remodels the chromatin in a cell-specific manner63,64. CCAAT/enhancer-binding protein (CEBP) transcription factors, for example, also have a role in myeloid cell differentiation and act together with PU.1 by binding throughout the genome61,63. In tissue macrophages, CEBPβ may be more important in lung and peritoneal cavity macrophages as these cells are substantially reduced in its absence65. Interestingly, the binding motif of the MAF transcription factor family is enriched in most tissue-resident macrophage enhancers compared with monocytes and neutrophils8. MAF and MAFB are important for macrophage terminal differentiation, and macrophages that lack these transcription factors are immortalized and proliferate indefinitely in the presence of CSF1 (REFS 66,67). Therefore, PU.1, CEBP, MAF and MAFB transcription factors probably work together to shape common tissue-resident macrophage function.
In addition, macrophages receive specific signals from the tissue to drive tissue-specific transcription factor expression. Transcription factors, such as GATA-binding protein 6 (GATA6) in peritoneal cavity macrophages68, peroxisome proliferator-activated receptor-γ (PPARγ) in alveolar macrophages39 and SPIC in spleen red pulp macrophages69, work together with PU.1 to bind enhancers and remodel the chromatin in a tissue-specific manner. Accordingly, whereas promoters are mostly shared between distinct tissue-resident macrophages, open enhancer regions differ between different tissue macrophages8,64. Thus, macrophages are shaped both by common developmental factors and tissue-specific transcription factors triggered by tissue-specific signals — that is, cytokines and metabolites — and together they drive macrophage transcriptional programmes and functional specialization, as discussed below.
The tissue secretome shapes tissue macrophages
Local cytokine and metabolite production in the tissue is important for the regulation, maintenance and functional specialization of macrophages (TABLE 1). Cytokines, such as CSF1, promote macrophage survival and proliferation in many tissues. Other cytokines, such as IL-34 (which also signals through CSF1R) and CSF2 (previously known as GM-CSF), are produced in specific tissues and maintain macrophages locally. Many of these cytokines have been widely explored for their functions in vitro; however, recent work has begun to elucidate their role in the regulation of macrophages in vivo. Together with cytokines, tissue metabolites such as fatty acids and oxysterols have been known to regulate macrophage function70, but recently other metabolites such as haem have also been shown to contribute to shaping tissue-specific macrophage functional identity, as discussed here (FIG. 2).
Table 1.
Tissue-resident macrophage phenotypes in healthy tissues
| Macrophage subset | Tissue | Homeostatic function | Specific surface markers | Specific transcription factors | Cytokine and metabolite regulation |
|---|---|---|---|---|---|
| Alveolar macrophages |
Lung, alveoli | Surfactant clearance | F4/80, Siglec-F, CD11chi, CD169 |
PPARγ, BACH2 (REF. 105) |
CSF2 (REFS 16,115) |
| Red pulp macrophages |
Spleen red pulp | Erythrocyte clearance, iron recycling |
F4/80, VCAM1 | SPIC6,69, PPARγ? | Haem69, CSF2?, CSF1 (REF. 71) |
| Marginal zone macrophages |
Spleen marginal zone |
Trap circulating particulates, marginal zone B cell maintenance |
SIGN-R1, MARCO | LXRα134 | CSF1 (REF. 72)? sterols134? |
| Metallophilic macrophages |
Spleen, adjacent to white pulp and marginal sinus |
Sample the circulation | CD169, MOMA1 | LXRα134 | CSF1 (REF. 71), sterols134? |
| Kupffer cells | Liver, sinusoids | Erythrocyte clearance, bilirubin metabolism, particulate antigen clearance from portal circulation |
F4/80, CD169, CLEC4F | LXRα135? SPIC69? | CSF1 (REF. 71), haem? |
| Microglial cells | Brain, CNS | Synaptic pruning, learning-dependent synapse formation |
CX3CR1, CD45mid, FCRLS, Siglec-H |
SMADs64,87? SALL1 (REF. 84)? MEF2C8? IRF8 (REF. 165) |
IL-34 (REFS 79,83), TGFβ84,87 |
| Peritoneal cavity macrophages |
Peritoneum | Maintain B-1 cell-derived IgA production |
ICAM2, F4/80hi | GATA6 (REFS 68, 120,121) |
CSF1 (REF. 73), retinoic acid, omental factors68 |
| Langerhans cells | Epidermis | Skin tolerance and immunity | CD11chi, MHC class IIhi, EPCAM |
ID2, RUNX3 (REFS 90,91) |
IL-34 (REF. 79,83), TGFβ85,89 |
| Lamina propria intestinal macrophages |
Large and small intestinal lamina propria |
Gut tolerance and immunity | F4/80, CX3CR1 | RUNX3 (REF. 8)? | CSF1 (REF. 71), CSF2 (REF. 93), IL-10 (REF. 108), microbial products166 |
| Intestinal muscularis macrophages |
Muscularis layer of intestine |
Maintain normal peristalsis | CX3CR1, MHC class IIhi | ND | CSF1, microbial products78 |
| Bone marrow macrophages |
Bone marrow | Regulate retention factors on nestin+ HSC niche cells, erythroblast development and release, remove aged neutrophils |
VCAM1, CD169 | SPIC69, LXRα123 | CSF1 (REF 122), sterols123? |
| Subcapsular sinus macrophages |
Lymph node | Trap particulate antigens | CD169 | ND | CSF1 (REF. 71), LTα1β2 (REF. 128) |
| Cardiac macrophages |
Heart | Phagocytose dying cardiomyocytes |
Subpopulations distinguished by: CX3CR1, MHC class II, CCR2 |
ND | CSF1 (REF. 80) |
CCR2, CC-chemokine receptor 2; CLEC4F, C-type lectin domain family 4 member F; CNS, central nervous system; CSF, colony-stimulating factor; CX3CR1, CX3C-chemokine receptor 1; EPCAM, epithelial cell adhesion molecule; FCRLS, Fc receptor-like S; GATA6, GATA-binding protein 6; HSC, haematopoietic stem cell; ICAM2, intercellular adhesion molecule 2; ID2, inhibitor of DNA binding 2; IL, interleukin; IRF8, interferon-regulatory factor 8; LTα1β2, lymphotoxin α1β2; LXRα, liver X receptor-α; ND, not determined; MEF2C, monocyte enhancer factor 2C; PPARγ, peroxisome proliferator-activated receptor-γ; RUNX3, runt-related transcription factor 3; SALL1, Sal-like protein 1; Siglec, sialic acid-binding immunoglobulin-like lectin; TGFβ, transforming growth factor-β; VCAM1, vascular cell adhesion molecule 1.
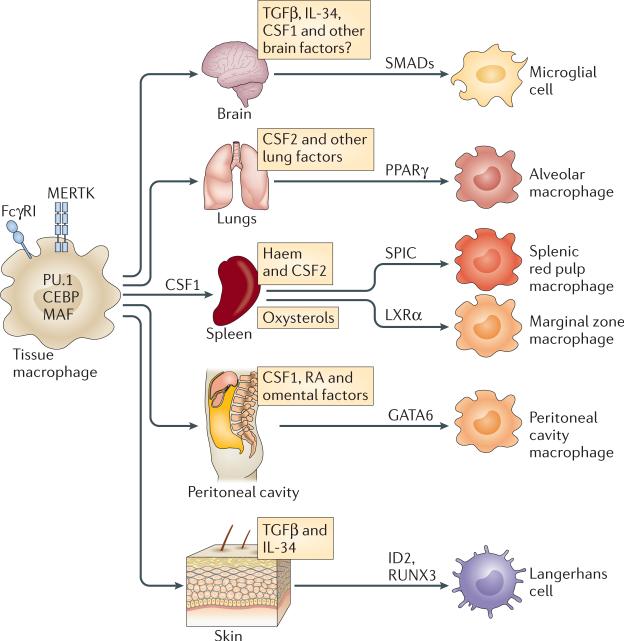
Figure 2. The tissue microenvironment determines macrophage differentiation cues.
During embryonic development, macrophages enter the tissues where they self-renew and proliferate. Macrophages in all tissues are characterized by expression of the cell surface marker FcγRI (also known as CD64), tyrosine-protein kinase MER (MERTK) and the transcription factors PU.1, CCAAT/enhancer-binding protein (CEBP) family members, MAF and MAFB. In the tissues, macrophage identity and functions are shaped by cytokines and metabolites that are produced in the local environment and drive specific transcription factor expression. In the brain, incoming yolk sac-derived cells are exposed to locally expressed transforming growth factor-β (TGFβ), which drives SMAD phosphorylation and the expression of genes that are unique to microglia. In the lungs, fetal monocytes that are exposed to colony-stimulating factor 2 (CSF2) express peroxisome proliferator-activated receptor-γ (PPARγ), which drives their differentiation into alveolar macrophages. In the spleen, haem drives SPIC expression, which controls the differentiation and maintenance of red pulp macrophages and the expression of key splenic red pulp macrophage-specific molecules, including vascular cell adhesion molecule 1 (VCAM1). In the marginal zone of the spleen, macrophage maintenance depends on liver X receptor-α (LXRα)-mediated signals. Retinoic acid (RA) and omental factors induce the expression of GATA-binding protein 6 (GATA6), which promotes the differentiation of peritoneal cavity macrophages. ID2, inhibitor of DNA binding 2; IL-34, interleukin-34; RUNX3, runt-related transcription factor 3.
Local production of CSF1 controls macrophage homeostasis in tissues
Most macrophages express high levels of CSF1R. The production of CSF1 in peripheral tissues is required for macrophage maintenance as mice that have a spontaneous null mutation in Csf1 (Csf1op/op mice) have a broad reduction of tissue-resident macrophage populations. Transgenic local expression of CSF1 but not intravascular injection rescues macrophage development in Csf1op/op mice71,72, and transgenic expression of cell surface CSF1 is sufficient to restore many tissue-resident macrophage populations73, which indicates the importance of local CSF1 production in macrophage homeostasis. In Csf1op/op mice, as well as upon antibody-mediated blockade of CSF1R, numbers of LY6Chi inflammatory monocytes are only slightly reduced14,74–76, whereas LY6Clow monocytes are almost undetectable owing to defective differentiation of LY6Chi monocytes into LY6Clow monocytes14,77. These results further indicate the distinct regulation and maintenance mechanisms of LY6Chi monocytes and resident macrophages, and support the importance of local CSF1 production for macrophage maintenance in vivo. Studies using Csf1 reporter mice identified CSF1 production in macrophage-enriched sites, such as the marginal zone and red pulp of the spleen, the bone marrow, the base of the crypts in the intestine, and the cortex and medulla of the lymph node72. However, the exact mechanisms and sources that produce CSF1 in most tissues remain unclear. In the muscularis layer of the gut, microbial signals drive enteric neurons to produce CSF1, which is required to maintain muscularis macrophage homeostasis78. Inflammatory signals can also drive high levels of CSF1 production, even in tissues that do not produce it in the steady state, such as the epidermis, indicating that CSF1 may have distinct roles in healthy and injured tissues79.
IL-34 production by neurons and keratinocytes shapes microglia and Langerhans cells, respectively
IL-34 is an alternative ligand for CSF1R; it has a very restricted tissue expression pattern (mainly in the brain and epidermis)79–83. IL-34 has very little homology with CSF1 and binds to CSF1R with higher affinity than CSF1 (REF. 81). In the central nervous system, IL-34 and CSF1 are produced in non-overlapping regions of the brain79,82,83: IL-34 is produced mainly by neurons in the cortex, striatum, olfactory bulb and hippocampus, whereas CSF1 is highly expressed in the cerebellum79–83. IL-34-deficient mice have fewer microglia in the brain regions in which IL-34 is normally produced79,83. In Csf1op/op mice, microglia numbers are only slightly reduced11, suggesting that IL-34 may compensate for the loss of CSF1, although regional microglial loss has not been precisely quantified in these mice (FIG. 3a).
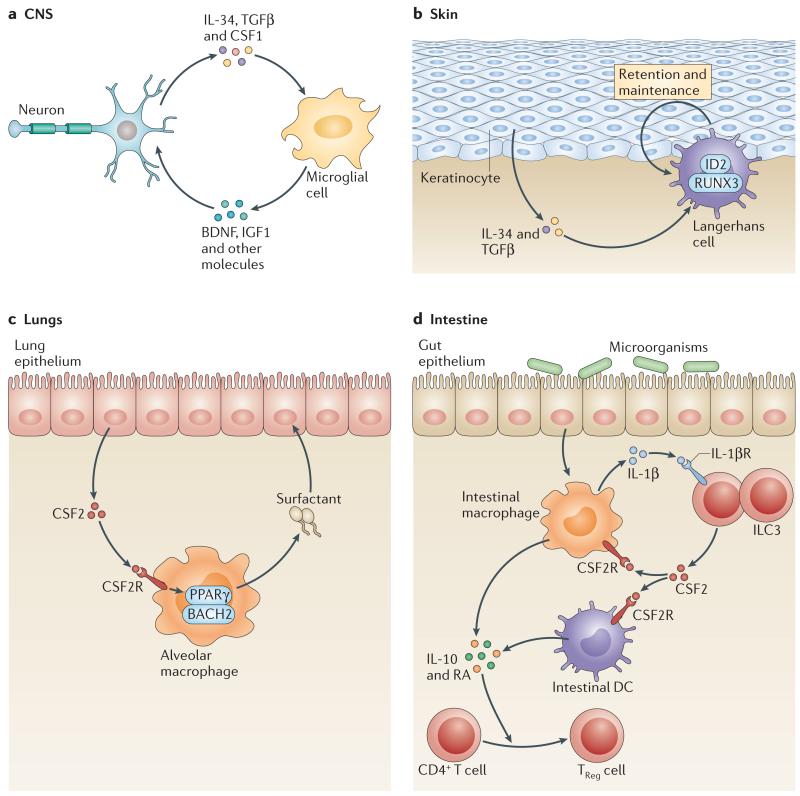
Figure 3. IL-34 and CSF2 regulate specific tissue macrophage maintenance.
a | In the brain, interleukin-34 (IL-34) is produced by neurons in the cortex, hippocampus and striatum and is necessary for microglial cell maintenance. Transforming growth factor-β (TGFβ) may be important for microglia maintenance, but it is still unclear which cells produce it in the brain. Conversely, microglia produce brain-derived neurotrophic factor (BDNF), which is important for learning-dependent synapse formation, and insulin-like growth factor 1 (IGF1), which is crucial for survival of layer V cortical neurons. b | In the skin, keratinocytes produce IL-34 and TGFβ, which are required for Langerhans cell homeostasis in the epithelium. TGFβ drives the expression of the transcription factors inhibitor of DNA binding 2 (ID2) and runt-related transcription factor 3 (RUNX3) in Langerhans cells and promotes their retention in the epidermis. The exact mechanism by which IL-34 drives Langerhans cell maintenance in the epidermis is unclear. c | In the lungs, epithelial cell-derived colony-stimulating factor 2 (CSF2) promotes peroxisome proliferator-activated receptor-γ (PPARγ) expression, and final maturation of alveolar macrophages is required for surfactant catabolism, which clears excess surfactant and maintains lung homeostasis. d | In the intestine, microbial products drive macrophage production of IL-1β that stimulates group 3 innate lymphoid cells (ILC3s) to produce CSF2. ILC3-mediated CSF2 production promotes the survival of intestinal macrophages and dendritic cells (DCs) and their production of IL-10 and retinoic acid (RA), which are required for the maintenance of regulatory T(TReg) cell homeostasis in the intestine. CSF2R, CSF2 receptor.
Langerhans cells are the only mononuclear phagocytes that populate the epidermis in mice and humans. CSF1 is not produced in the healthy epidermis and, accordingly, Langerhans cells are maintained independently of CSF1 (REF. 74). By contrast, IL-34 is constitutively produced by keratinocytes, and IL-34-deficient animals lack Langerhans cells whereas dermal DCs and other macrophages remain unaffected in these mice79,83 (FIG. 3b).
Langerhans cells and microglia also depend on transforming growth factor-β (TGFβ), as both of these populations are reduced in the absence of TGFβ84,85. The receptor for TGFβ, TGFβR1, is highly expressed by microglial cells compared with other myeloid cells8. In the brain, TGFβ production by astrocytes drives synaptic pruning by microglia through C1q production by neurons86. TGFβ signals through the phosphorylation of SMAD transcription factors downstream of TGFβR1 (REF. 87), and a SMAD-binding motif is enriched in PU.1-bound enhancer sites in microglial cells64. TGFβR1 is also highly expressed by Langerhans cells, and TGFβ is required to retain Langerhans cells in the epidermis85,88. Interestingly, TGFβ works both in a paracrine and autocrine loop to retain Langerhans cells in the skin and prevent them from upregulating maturation markers that mediate trafficking to the lymph nodes88,89. TGFβ induces the expression of inhibitor of DNA binding 2 (ID2) and RUNX3, which are required for Langerhans cell maintenance in the skin90,91 (FIG. 3b). By contrast, inflammatory monocytes that are recruited to the epidermis upon exposure to ultraviolet light are independent of ID2 (REFS 90,91) and infiltrate the skin independently of IL-34 (REF. 79).
These studies established that local production of both IL-34 and TGFβ is important for the maintenance and regulation of microglia and Langerhans cells. In vitro, monocytes cultured with CSF1 and TGFβ express microglia-specific genes84, whereas they exhibit Langerhans cell-like characteristics when cultured with CSF2 and TGFβ90. Signalling through CSF1R promotes the expression of TGFβR1 as peritoneal macrophages cultured with either CSF1 or IL-34 start to upregulate this receptor64. However, how IL-34 and TGFβ cooperate to shape microglia and Langerhans cell function in vivo remains to be explored.
CSF2 produced by local radio-resistant cells shapes mucosal macrophages
CSF2 is an important myeloid growth factor with a key role in the maintenance and homeostasis of lung and intestinal tissue macrophages. In the absence of functional CSF2 or its receptor, the homeostasis of lung and intestinal macrophages is specifically altered, which results in an increased susceptibility to infection and impaired tissue repair13,94–96. CSF2 is produced by radio-resistant cell types that require IL-1-mediated activation93,97,98; it is produced mostly by epithelial cells in the lungs and by retinoic acid-related orphan receptor-γt (RORγt)-expressing innate lymphocytes in the intestine93,97. Interestingly, CSF2 promotes the survival of macrophages through signal transducer and activator of transcription 5 (STAT5)-mediated anti-apoptotic gene regulation99. Similar findings are documented for DCs, in which CSF2-mediated STAT5 phosphorylation leads to the expression of anti-apoptotic genes through the exchange of STAT3 at gene regulatory elements100. Thus, the balance of phosphorylated STATs is likely to drive gene expression in macrophages and thus allows for a distinction between steady state and activated state.
In addition, CSF2 promotes the differentiation of alveolar macrophage precursors from the fetal liver into mature alveolar macrophages through the induction of PPARγ expression16,39,101. PPARγ is highly expressed by lung alveolar macrophages and regulates their development and function, partly through the induction of a specific transcriptional programme that involves the expression of genes related to lipid degradation and fatty acid oxidation required for surfactant catabolism39,101,102. Besides acting as sentinels for bacterial infiltration, alveolar macrophages are crucial for maintaining lung homeostasis through the clearance of excess phospholipid surfactant. Mice that lack CSF2 or CSF2R, and patients with defective CSF2R signalling, develop severe lung inflammatory disease known as pulmonary alveolar proteinosis owing to accumulation of surfactant in the lung tissues103,104. The transcriptional repressor BACH2 has also been implicated as a regulator of alveolar macrophages and in effective clearance of surfactant105, suggesting that additional factors may be involved in the regulation of alveolar macrophages (FIG. 3c).
Similarly, CSF2 has an important role in maintaining homeostasis in intestinal macrophages. Macrophages in the lamina propria of the small and large bowel are permanently exposed to a very large number of commensal microorganisms and to ingested antigens and potential pathogens. Therefore, the mechanisms that help to distinguish between harmful or innocuous antigens are vital for macrophages of the gut mucosa. Macrophages actively sample the intestinal lumen and undergo functional changes in response to signals induced by intestinal microorganisms or their metabolites106,107. Recognition of luminal microorganisms by macrophages promotes CSF2 release by group 3 innate lymphoid cells. In turn, this helps to maintain DC and macrophage numbers and imprints their regulatory function that supports the induction and local expansion of regulatory T cells93 (FIG. 3d). Regulatory T cells are the dominant producers of IL-10, which acts through a feedback loop on macrophages to dampen their inflammatory phenotype and prevent excessive immune activation and tissue damage108,109. Indeed, deletion of IL-10 receptor, but not the cytokine IL-10, from intestinal macrophages leads to the development of spontaneous colitis in mice108. Thus, microbial stimuli trigger CSF2- and IL-10-dependent dampening of macrophage stimulation and drive a tolerogenic programme required for tissue homeostasis. PPARγ expression also helps to dampen tissue damage and modulate the expression of inflammatory cytokines, ultimately contributing to the maintenance of intestinal homeostasis110,111. Thus, in both the lungs and intestine, CSF2 is required to maintain essential macrophage functions and ensure appropriate organ homeostasis (FIG. 3c,d).
The homeostatic role of cytokines in the maintenance of macrophage survival and function may depend on the location, the dose and the context in which the cytokine is produced. For example, CSF2 may promote macrophage survival and/or tolerogenic functions when expressed at low doses in healthy intestinal tissues93, but at higher doses, concomitantly with injury signals, CSF2 may contribute to inflammatory disease112.
It is unclear why different cytokines are required to maintain macrophage homeostasis. In particular, it is surprising that both CSF1 and IL-34, two cytokines that signal through the same receptor, have been evolutionary conserved in two restricted sites — the epidermis and specific regions of the brain, in mice, birds and humans79,83,113. It is also intriguing that both CSF1 and CSF2 are required to maintain two distinct macrophage populations in the lungs16,114. Macrophage dependency on distinct cytokines may reflect, in addition to viability requirements, additional genetic imprinting that confers functional specificity. CSF2 production by lung epithelial cells specifically confers alveolar macrophages with the ability to clear lung surfactant115, whereas lung interstitial macrophages, which require CSF1 but not CSF2 for their homeostasis, are unable to clear surfactant but instead modulate overt inflammatory responses to innocuous airborne antigens116.
Splenic red pulp macrophages are shaped by CSF1 and haem
In the red pulp of the spleen, macrophages phagocytose senescent erythrocytes and specialize in the recycling of iron stores117. CSF1 is expressed in the splenic red pulp71 and is required for the maintenance of red pulp macrophages. Red pulp macrophages are constantly exposed to senescent red blood cells that contain high levels of haemoglobin and its iron- containing moiety haem. Haem, together with CSF1, acts on red pulp macrophages to induce the expression of SPIC69, a transcription factor that drives the expression of key red pulp macrophage genes, such as vascular cell adhesion molecule 1 (VCAM1), and is required for macrophage maintenance6,69.
Interestingly, high levels of intracellular haem are toxic to macrophages118, and thus SPIC-driven signals may be crucial for expanding the macrophage pool to respond to increased levels of senescent red blood cells that may occur under conditions such as haemolysis. SPIC is also expressed by other macrophages that interact with erythrocytes, such as VCAM1+ bone marrow macrophages and a subset of liver macrophages, implicating a role for haem in regulating macrophages that are exposed to erythrocytes69,119.
Retinoic acid controls the function of peritoneal cavity macrophages
Macrophages and B-1 cells are the main immune cell populations that reside in the peritoneum. Peritoneal cavity macrophages express CSF1R and depend on CSF1 for their maintenance73, whereas retinoic acid signalling is required for the specialization of peritoneal cavity macrophages68. In the omentum, high levels of retinoic acid-converting enzymes allow for macrophage exposure to retinoic acid, which stimulates the expression of GATA6, probably through retinoic acid response elements in the GATA6 promoter68. The GATA motif is enriched in open enhancers of peritoneal cavity macrophage-specific genes, such as TGFB2, suggesting that retinoic acid-induced GATA6 contributes to shaping the chromatin state of peritoneal cavity macrophages8. Retinoic acid-induced GATA6 expression promotes macrophage accumulation in the peritoneal cavity, macrophage self-renewal potential and the expression of many peritoneal cavity macrophage-specific genes, including TGFB2 and ASPA68,120,121. TGFβ2 production by peritoneal macrophages drives peritoneal B-1 cell class switching and IgA production68, and GATA6-deficient mice, which have reduced numbers of peritoneal cavity macrophages and can no longer make TGFβ2, have lower levels of IgA in the intestine68.
Macrophages control organ homeostasis
Shaped by their environment, macrophages are key sensors of tissue signals and are crucial for the maintenance of organ functionality and immune homeostasis. Here, we discuss the crucial role of macrophage–tissue interactions in tissue homeostasis (FIG. 4).
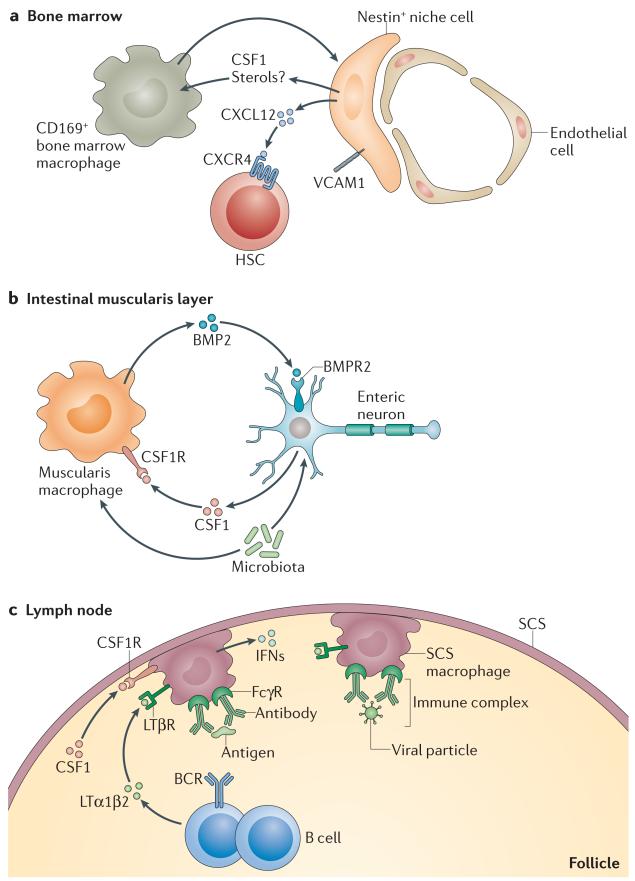
Figure 4. Macrophage–tissue crosstalk.
a | Bone marrow macrophages are important for stimulating nestin+ niche cells to produce haematopoietic stem cell (HSC) retention factors, such as CXC-chemokine ligand 12 (CXCL12) and vascular cell adhesion molecule 1 (VCAM1). In turn, niche cells may produce colony-stimulating factor 1 (CSF1) to help to maintain macrophages in the niche. b | Intestinal muscularis macrophages produce bone morphogenetic protein 2 (BMP2), which signals through BMP receptor type 2 (BMPR2) expressed on enteric neurons and promotes neuronal control of peristaltic activity of the gut muscularis layer. In turn, enteric neurons, in response to microbial signals, produce CSF1, which is required to maintain muscularis macrophage homeostasis in vivo. c | In the lymph nodes, macrophages are situated directly beneath the subcapsular sinus (SCS) surrounding the B cell zone. They present large particulate antigen to B cells in the form of immune complexes and are important for trapping viral particles. In turn, B cells help to retain the SCS macrophage layer through the secretion of lymphotoxin α1β2 (LTα1β2). SCS macrophages also depend on local production of CSF1, although its source is unknown. BCR, B cell receptor; CSF1R, CSF1 receptor; FcγR, Fc receptor for IgG; IFNs, interferons; LTβR, lymphotoxin-β receptor.
Bone marrow-resident macrophages
Bone marrow-resident macrophages contribute to the maintenance of bone marrow HSCs by regulating the expression of key HSC retention factors, such as CXC-chemokine ligand 12 (CXCL12) and VCAM1, by nestin+ stromal cells122. Following the depletion of macrophages, nestin+ stromal cells lose expression of these retention factors, and HSCs are released into the bloodstream at significantly higher rates than in macrophage-sufficient controls122. Bone marrow macrophages also regulate the circadian release of haematopoietic progenitor cells into the bloodstream by downregulating CXCL12 levels through phagocytosis of aged neutrophils123. Stimulation of liver X receptor-α (LXRα) controls macro phage ability to retain haematopoietic progenitors in the bone marrow94. Accordingly, absence of LXRα and LXRβ impairs the phagocytic potential of macrophages through the dysregulation of phagocytic receptors, such as tyrosine-protein kinase MER (MERTK)92,93, and disrupts normal circadian fluctuations in the HSC niche upon engulfment of aged neutrophils95. In turn, nestin+ niche cells express high levels of CSF1, although CSF1 deletion in these cellsdoes not significantly reduce bone marrow macrophage numbers122,124, indicating that there are probably other regulators of macrophage homeostasis in the bone marrow such as agonists of LXRs123 (FIG. 4a).
Bone marrow macrophages are also crucial for the production of erythroblasts in the bone marrow, and depletion of macrophages can help to control malignant proliferation of erythroblasts in vivo119. Together, these results suggest a crucial role for macrophages in the regulation of HSC release into the bloodstream.
Intestinal muscularis macrophages
Macrophages in the intestinal muscularis layer also engage in crosstalk with their surrounding cells. The surrounding muscle cells, enteric neurons and tissue-resident macrophages all contribute to ensuring normal intestinal peristalsis. Intestinal muscularis macrophages produce bone morphogenetic protein 2 (BMP2), which signals through the BMP receptor type 2 expressed by enteric neurons, promoting SMAD translocation to the nucleus and neuronal control of peristaltic activity of the gut muscularis layer78. In turn, enteric neurons in response to microbial signals produce CSF1, which is required to maintain muscularis macrophage homeostasis in vivo. Alterations in the gut flora, the intestinal macrophages or BMP2 production affect peristaltic activity and subsequently alter colonic transit time78 (FIG. 4b).
Lymph node subcapsular sinus macrophages
Subcapsular sinus (SCS) macrophages are located in the floor of the SCS and are directly exposed to the afferent lymphatic vessels, allowing them to trap particulate tissue antigens from the lymph and limit systemic dissemination of virus. SCS macrophages present lymph-derived antigens to follicular B cells, probably through immune complexes, to promote the induction of antiviral humoral immunity125–127. These macrophages interact closely with B cells and depend on B cell production of lymphotoxin α1β2 (LTα1β2) for their maintenance, differentiation and function128,129. Upon viral infection, B cell production of LTα1β2 drives permissive viral replication within SCS macrophages, which in turn promotes macrophage production of type I interferons required for viral clearance129,130. Conversely, the positioning of macrophages in the SCS is required for effective B cell responses, and disruption of SCS macrophage positioning alters B cell responses131. The close interaction of B cells and SCS macrophages is therefore crucial for the induction of innate and adaptive immunity to tissue antigens that travel via lymphatic vessels (FIG. 4c).
Splenic marginal zone macrophages
Marginal zone (MZ) macrophages are important for the capture of blood-borne antigens and for the proper positioning of MZ B cells132,133. Absence of MZ macrophages in LXRα-deficient mice impairs the capture of blood-borne antigens134 and affects B cell positioning in the MZ, reducing antibody responses to thymus-independent antigens134. However, the exact source and contribution of CSF1 and LXRα agonists to macrophage maintenance remain to be established.
Kupffer cells
Embedded in the hepatic sinusoids of the liver, Kupffer cells are well positioned to capture blood antigens. They express the transcription factor LXRα, and the LXR motif is enriched in the enhancers of Kupffer cell-specific genes8,135. Kupffer cells are also involved in recycling erythrocytes and therefore, similar to red pulp macrophages, they express haem-related genes such as haem oxygenase 1 (HMOX1) and may be partially dependent on the transcription factor SPIC8,69,136. The direct role of hepatocytes in Kupffer cell maintenance is unclear but, during infection, hepatocyte-derived IL-33 leads to the release by basophils of IL-4 and promotes the proliferation of monocyte-derived macrophages54. Local CSF1 production also contributes to Kupffer cell maintenance71, but the exact source of CSF1 in the liver is unknown.
Cardiac macrophages
Cardiac macrophages are in close contact with cardiomyocytes throughout the myocardium where they have important homeostatic roles through the phagocytosis of dying cardiomyocytes and debris, and the production of pro-angiogenic and tissue-protective molecules137. CSF1 is highly expressed in the heart tissue in comparison to other organs, probably contributing to the maintenance of macrophages80.
Cardiac monocytes are heterogeneous in origin and derive from embryonic and adult haematopoiesis (as discussed earlier), with adult monocyte-derived macrophages increasing with age as embryonic macrophages lose their capacity for self-renewal15,51. Embryonic and adult monocyte-derived macrophages have different propensities for phagocytosis and inflammasome activation15. Monocyte-derived macrophages have pro-inflammatory potential and lack reparative activities, and inhibition of monocyte recruitment to the adult heart preserves embryonic-derived macrophage subsets, reduces inflammation and enhances tissue repair56. The mechanisms that regulate the maintenance and proliferation of these distinct populations remain to be analysed.
Macrophages and tissue cells thus engage in crosstalk to modulate both immune and tissue homeostasis. Distinguishing between monocytes and resident macrophages has provided further clarity in establishing the role of local tissue signals that regulate the maintenance and proliferation of tissue-resident macrophages. These local communication networks are being elucidated in both the steady state and inflammation and provide important insights into the mechanisms by which macrophages contribute to tissue maintenance.
Tissue control of macrophage plasticity
The plasticity of macrophages in inflammatory settings has been well described and extensively studied in the setting of tumours (BOX 2), in which macrophages can alternatively express pro-inflammatory or immunemodulatory cytokines and other molecules138,139. More recently, it has been appreciated that tissue-specific regulation provides an additional layer that contributes to macrophage plasticity.
Box 2 | Macrophages in tumours.
Similarly to chronically injured tissues, tumour lesions are heavily populated by monocytes and macrophages. The quantification and prognostic value of macrophage accumulation in human tumours have mainly been assessed by histological staining for CD68 (also known as macrosialin)+ cells. However, staining for CD68 does not distinguish between tissue-resident macrophages, monocytes and monocyte-derived cells and does not assess the functional state of the macrophages, which has led to considerable confusion regarding the prognostic value of tumour-associated macrophages.
Tumour-associated macrophages are highly heterogeneous, with a mixture of pro-inflammatory and anti-inflammatory gene expression signatures157,158 that may reflect a range of macrophage activation pathways and/or responses by different macrophage populations. Indeed, macrophages at different stages in tumour development may function differently; the resident macrophages and monocyte-derived inflammatory macrophages may have distinct functions, and different macrophage effector functions are likely to be induced depending on the nature of the tumour tissue. This may explain why, in some tumours, the accumulation of CD68+ cells correlates with tumour growth and decreased survival159,160, whereas in other tumours (such as non-small cell lung carcinoma and colorectal cancer) the presence of CD68+ cells correlates with prolonged survival161,162. Better characterization of macrophages in tumours through the use of multiple surface markers that define ontogeny and functional diversity should help to clarify the roles of macrophages in cancer.
Similar to healthy tissues, crosstalk between the tumour tissue and macrophages can benefit both tumour and macrophage homeostasis. One of the best examples of this crosstalk has been identified in mammary tumour lesions, in which mammary carcinoma cells produce colony-stimulating factor 1 (CSF1) to promote the recruitment and survival of macrophages, as well as the induction of epidermal growth factor (EGF) production by macrophages. Macrophage production of EGF promotes invasion of the tumour by blood cells and induces further CSF1 production by carcinoma cells, thereby generating a positive feedback loop that enhances both tumour spread and macrophage survival163,164.
Our improved understanding of the distinction between tissue-resident macrophages and monocyte-derived cells has raised the question of whether they may have distinct roles in the context of tumours. Moreover, as the role of the environment and tissue–macrophage crosstalk has been shown to shape steady state macrophages, understanding the contribution of tumour-specific environmental signals to macrophage phenotype and function will become more important. Exploring how distinct tumour environments shape the function of resident macrophages and monocytes will provide important insights into the role of phagocytes in cancer and how they might be best modulated by immunotherapies.
Bone marrow chimeric mice have been used to explore the contribution of tissue factors to reprogramming macrophage identity. Exposure to a lethal dose of ionizing radiation (approximately 12 Gy in C57BL/6 mice) eliminates most embryonic-derived tissue-resident macrophages — with the exception of microglia11,140, Langerhans cells10 and potentially Kupffer cells141— and promotes their replacement by donor-derived adult bone marrow progenitors. These studies revealed that macrophages that derive from donor bone marrow cells almost entirely recapitulate the enhancer profile and transcriptional programme of the embryonic-derived macrophages that they replace8,142. Moreover, in mice with impaired development of lung tissue-resident macrophages, bone marrow-derived cells can restore the function of alveolar macrophages, clear surfactant and protect from lung inflammatory diseases142–144.
Recent results have also revealed that even terminally differentiated peritoneal macrophages can retain some levels of plasticity and adapt to new environmental cues. For example, peritoneal cavity macrophages, when adoptively transferred into the lung microenvironment, down-regulate GATA6 and upregulate PPARG expression, along with other lung macrophage-specific gene transcripts, although some transcripts remained fixed and retained the signature of the tissue of origin8. Accordingly, in vitro exposure to TGFβ alters the enhancer profile of differentiated peritoneal macrophages64, and a vitamin A-deficient diet dramatically alters peritoneal macrophage expression of GATA6, which in turn compromises macrophage homing and function in the peritoneum68. Therefore, although tissue-derived signals have a key role in shaping macrophage functional identity, tissue-resident macrophages retain the ability to adapt to new environments. Determining the genes that can be remodelled and those that are fixed may help to identify new physiological cues and novel potential targets that modulate macrophage cell fate and differentiation in tissues.
Conclusion
Macrophages are an essential part of the tissue immune compartment. They are in close contact with the surrounding stroma where they constantly sense environmental cues. Tissue-specific cytokines and metabolites contribute to shaping the local steady-state programme of tissue-resident macrophages. In turn, macrophages are important regulators of tissue homeostasis during the steady state and inflammatory settings. The crucial nature of macrophage interactions with the local microenvironment adds another layer of complexity to macro phage functional plasticity. More work is needed to better define the tissue-specific metabolites and regulators of macrophage function in different tissues and how they are affected during inflammation and disease. It is also important to further probe whether the tissue regulators and macrophage transcription factors that have been defined in mice have equally important roles in tissue-resident macrophages in humans.
Mononuclear phagocyte system.
(MPS). A group of bone marrow-derived cells (monocytes, macrophages and dendritic cells) with different morphologies. These cells are mainly responsible for phagocytosis, cytokine secretion and antigen presentation.
Parabiotic mice.
Mice in which the blood circulation has been joined surgically. Parabiotic mice share the same blood circulation and exchange blood precursor cells, thereby providing a model to trace the physiological contribution of circulating precursors to tissue-resident cells.
B-1 cells.
An innate-like population of B cells found mainly in the peritoneal and pleural cavities. B-1 cell precursors develop in the fetal liver and omentum. B-1 cells recognize self-antigens as well as common bacterial antigens and secrete antibodies of low affinity and broad specificity.
Omentum.
A fatty tissue in the peritoneum that connects the spleen, stomach, pancreas and colon.
Acknowledgements
The authors thank M. Acebes-Casanova, V. Kana and A. Chudnovsky for critical review of the manuscript. This work was supported by the following grants awarded to M.M.: R01CA154947A, R01CA190400, R01CA173861, U01AI095611 and R01AI104848.
Footnotes
Competing interests statement
The authors declare no competing interests.
References
- 1.Gautier EL, et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol. 2012;13:1118–1128. doi: 10.1038/ni.2419. [This ImmGen consortia study shows that tissue macrophages have a distinct transcriptional profile that depends on the tissue in which they reside, highlighting the potential contribution of tissue imprinting to macrophage identity.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zagorska A, Traves PG, Lew ED, Dransfield I, Lemke G. Diversification of TAM receptor tyrosine kinase function. Nat. Immunol. 2014;15:920–928. doi: 10.1038/ni.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canton J, Neculai D, Grinstein S. Scavenger receptors in homeostasis and immunity. Nat. Rev. Immunol. 2013;13:621–634. doi: 10.1038/nri3515. [DOI] [PubMed] [Google Scholar]
- 4.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 5.Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nat. Rev. Immunol. 2014;14:81–93. doi: 10.1038/nri3600. [DOI] [PubMed] [Google Scholar]
- 6.Kohyama M, et al. Role for Spi-C in the development of red pulp macrophages and splenic iron homeostasis. Nature. 2009;457:318–321. doi: 10.1038/nature07472. [This study reveals the contribution of tissue-derived factors in establishing macrophage functional identity.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014;14:392–404. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- 8.Lavin Y, et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159:1312–1326. doi: 10.1016/j.cell.2014.11.018. [This is the first study to establish the epigenetic profile of tissue-resident macrophages.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J. Exp. Med. 1968;128:415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merad M, et al. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat. Immunol. 2002;3:1135–1141. doi: 10.1038/ni852. [This is the first study that shows that adult mononuclear phagocytes of the epidermis are renewed locally and independently of circulating monocytes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ginhoux F, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [This report describes the first fate mapping model that shows that YS-derived cells can contribute to adult microglia.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 13.Hashimoto D, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yona S, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epelman S, et al. Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guilliams M, et al. Alveolar macrophages develop from fetal monocytes that differentiate into long-lived cells in the first week of life via GM-CSF. J. Exp. Med. 2013;210:1977–1992. doi: 10.1084/jem.20131199. [This study establishes that fetal monocytes differentiate into alveolar macrophages in response to local CFS2.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jakubzick C, et al. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39:599–610. doi: 10.1016/j.immuni.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bigley V, et al. The human syndrome of dendritic cell, monocyte, B and NK lymphoid deficiency. J. Exp. Med. 2011;208:227–234. doi: 10.1084/jem.20101459. [This study shows that macrophages are present in normal numbers in patients that lack monocytes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hambleton S, et al. IRF8 mutations and human dendritic-cell immunodeficiency. N. Engl. J. Med. 2011;365:127–138. doi: 10.1056/NEJMoa1100066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanitakis J, Petruzzo P, Dubernard JM. Turnover of epidermal Langerhans’ cells. N. Engl. J. Med. 2004;351:2661–2662. doi: 10.1056/NEJM200412163512523. [This elegant study reports that, in a patient that received a hand allograft, grafted epidermal mononuclear phagocytes (also known as Langerhans cells) remain of donor origin for at least 5 years after transplant, which suggests that, similar to findings in mice, Langerhans cells can renew locally and independently of circulating precursors.] [DOI] [PubMed] [Google Scholar]
- 21.Collin MP, et al. The fate of human Langerhans cells in hematopoietic stem cell transplantation. J. Exp. Med. 2006;203:27–33. doi: 10.1084/jem.20051787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mielcarek M, et al. Langerhans cell homeostasis and turnover after nonmyeloablative and myeloablative allogeneic hematopoietic cell transplantation. Transplantation. 2014;98:563–568. doi: 10.1097/TP.0000000000000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lichanska AM, Hume DA. Origins and functions of phagocytes in the embryo. Exp. Hematol. 2000;28:601–611. doi: 10.1016/s0301-472x(00)00157-0. [DOI] [PubMed] [Google Scholar]
- 24.Naito M, et al. Development, differentiation, and phenotypic heterogeneity of murine tissue macrophages. J. Leukoc. Biol. 1996;59:133–138. doi: 10.1002/jlb.59.2.133. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi K, Yamamura F, Naito M. Differentiation, maturation, and proliferation of macrophages in the mouse yolk sac: a light-microscopic, enzyme-cytochemical, immunohistochemical, and ultrastructural study. J. Leukoc. Biol. 1989;45:87–96. doi: 10.1002/jlb.45.2.87. [This study shows that YS-derived primitive macrophages actively proliferate and colonize the embryonic tissues, where they differentiate into fetal macrophages before the development of monocytes.] [DOI] [PubMed] [Google Scholar]
- 26.Naito M, Takahashi K, Nishikawa S. Development, differentiation, and maturation of macrophages in the fetal mouse liver. J. Leukoc. Biol. 1990;48:27–37. doi: 10.1002/jlb.48.1.27. [DOI] [PubMed] [Google Scholar]
- 27.Herbomel P, Thisse B, Thisse C. Zebrafish early macrophages colonize cephalic mesenchyme and developing brain, retina, and epidermis through a M-CSF receptor-dependent invasive process. Dev. Biol. 2001;238:274–288. doi: 10.1006/dbio.2001.0393. [This study shows that YS-derived macrophages colonize zebrafish embryos before the onset of blood circulation.] [DOI] [PubMed] [Google Scholar]
- 28.Bertrand JY, et al. Three pathways to mature macrophages in the early mouse yolk sac. Blood. 2005;106:3004–3011. doi: 10.1182/blood-2005-02-0461. [This study describes the different waves of myelopoiesis in the mouse YS.] [DOI] [PubMed] [Google Scholar]
- 29.Palis J, Robertson S, Kennedy M, Wall C, Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126:5073–5084. doi: 10.1242/dev.126.22.5073. [This study identifies YS-derived cells with erythroid and myeloid potential.] [DOI] [PubMed] [Google Scholar]
- 30.Tavian M, Péault B. Embryonic development of the human hematopoietic system. Int. J. Dev. Biol. 2005;49:243–250. doi: 10.1387/ijdb.041957mt. [DOI] [PubMed] [Google Scholar]
- 31.McGrath KE, Koniski AD, Malik J, Palis J. Circulation is established in a stepwise pattern in the mammalian embryo. Blood. 2003;101:1669–1676. doi: 10.1182/blood-2002-08-2531. [DOI] [PubMed] [Google Scholar]
- 32.Palis J, Yoder MC. Yolk-sac hematopoiesis: the first blood cells of mouse and man. Exp. Hematol. 2001;29:927–936. doi: 10.1016/s0301-472x(01)00669-5. [DOI] [PubMed] [Google Scholar]
- 33.Kumaravelu P, et al. Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development. 2002;129:4891–4899. doi: 10.1242/dev.129.21.4891. [DOI] [PubMed] [Google Scholar]
- 34.Orkin SH, Zon LI. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 2008;132:631–644. doi: 10.1016/j.cell.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cumano A, Godin I. Ontogeny of the hematopoietic system. Annu. Rev. Immunol. 2007;25:745–785. doi: 10.1146/annurev.immunol.25.022106.141538. [DOI] [PubMed] [Google Scholar]
- 36.Samokhvalov IM, Samokhvalova NI, Nishikawa S. Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature. 2007;446:1056–1061. doi: 10.1038/nature05725. [This is the first fate mapping model to trace the progeny of YS-derived cells.] [DOI] [PubMed] [Google Scholar]
- 37.Hoeffel G, et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J. Exp. Med. 2012;209:1167–1181. doi: 10.1084/jem.20120340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoeffel G, et al. C-myb+ erythro-myeloid progenitor-derived fetal monocytes give rise to adult tissue-resident macrophages. Immunity. 2015;42:665–678. doi: 10.1016/j.immuni.2015.03.011. [This study reveals the contribution of the distinct YS haematopoiesis waves to microglia versus other tissue-resident macrophages.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schneider C, et al. Induction of the nuclear receptor PPAR-γ by the cytokine GM-CSF is critical for the differentiation of fetal monocytes into alveolar macrophages. Nat. Immunol. 2014;15:1026–1037. doi: 10.1038/ni.3005. [DOI] [PubMed] [Google Scholar]
- 40.Schulz C, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [This study shows that MYB-deficient mice retain fetal F4/80hi macrophages.] [DOI] [PubMed] [Google Scholar]
- 41.Gomez Perdiguero E, et al. Tissue-resident macrophages originate from yolk-sac-derived erythromyeloid progenitors. Nature. 2015;518:547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGrath KE, et al. Distinct sources of hematopoietic progenitors emerge before HSCs and provide functional blood cells in the mammalian embryo. Cell Rep. 2015;11:1892–1904. doi: 10.1016/j.celrep.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bertrand JY, et al. Definitive hematopoiesis initiates through a committed erythromyeloid progenitor in the zebrafish embryo. Development. 2007;134:4147–4156. doi: 10.1242/dev.012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jenkins SJ, et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [This study indicates that, upon helminth infection, the T helper 2-type cytokine IL-4 drives the expansion of pleural tissue-resident macrophages without any contribution from monocyte-derived macrophages.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghosn EE, et al. Two physically, functionally, and developmentally distinct peritoneal macrophage subsets. Proc. Natl Acad. Sci. USA. 2010;107:2568–2573. doi: 10.1073/pnas.0915000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jenkins SJ, et al. IL-4 directly signals tissue-resident macrophages to proliferate beyond homeostatic levels controlled by CSF-1. J. Exp. Med. 2013;210:2477–2491. doi: 10.1084/jem.20121999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ajami B, Bennett JL, Krieger C, McNagny KM, Rossi FM. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat. Neurosci. 2011;14:1142–1149. doi: 10.1038/nn.2887. [This study shows that monocyte-derived macrophages infiltrate, but do not engraft long term in, the inflamed brain.] [DOI] [PubMed] [Google Scholar]
- 48.Bogunovic M, et al. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Varol C, et al. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 50.Tamoutounour S, et al. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity. 2013;39:925–938. doi: 10.1016/j.immuni.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 51.Molawi K, et al. Progressive replacement of embryo-derived cardiac macrophages with age. J. Exp. Med. 2014;211:2151–2158. doi: 10.1084/jem.20140639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sere K, et al. Two distinct types of Langerhans cells populate the skin during steady state and inflammation. Immunity. 2012;37:905–916. doi: 10.1016/j.immuni.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 53.Nagao K, et al. Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nat. Immunol. 2012;13:744–752. doi: 10.1038/ni.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blériot C, et al. Liver-resident macrophage necroptosis orchestrates type 1 microbicidal inflammation and type-2-mediated tissue repair during bacterial infection. Immunity. 2015;42:145–158. doi: 10.1016/j.immuni.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 55.Zigmond E, et al. Infiltrating monocyte-derived macrophages and resident kupffer cells display different ontogeny and functions in acute liver injury. J. Immunol. 2014;193:344–353. doi: 10.4049/jimmunol.1400574. [DOI] [PubMed] [Google Scholar]
- 56.Lavine KJ, et al. Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart. Proc. Natl Acad. Sci. USA. 2014;111:16029–16034. doi: 10.1073/pnas.1406508111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller JC, et al. Deciphering the transcriptional network of the dendritic cell lineage. Nat. Immunol. 2012;13:888–899. doi: 10.1038/ni.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Winter DR, Amit I. The role of chromatin dynamics in immune cell development. Immunol. Rev. 2014;261:9–22. doi: 10.1111/imr.12200. [DOI] [PubMed] [Google Scholar]
- 59.Lara-Astiaso D, et al. Immunogenetics. Chromatin state dynamics during blood formation. Science. 2014;345:943–949. doi: 10.1126/science.1256271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gross DS, Garrard WT. Nuclease hypersensitive sites in chromatin. Annu. Rev. Biochem. 1988;57:159–197. doi: 10.1146/annurev.bi.57.070188.001111. [DOI] [PubMed] [Google Scholar]
- 61.Garber M, et al. A high-throughput chromatin immunoprecipitation approach reveals principles of dynamic gene regulation in mammals. Mol. Cell. 2012;47:810–822. doi: 10.1016/j.molcel.2012.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ghisletti S, et al. Identification and characterization of enhancers controlling the inflammatory gene expression program in macrophages. Immunity. 2010;32:317–328. doi: 10.1016/j.immuni.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 63.Heinz S, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell. 2010;38:576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gosselin D, et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell. 2014;159:1327–1340. doi: 10.1016/j.cell.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cain DW, et al. Identification of a tissue-specific, C/EBPβ-dependent pathway of differentiation for murine peritoneal macrophages. J. Immunol. 2013;191:4665–4675. doi: 10.4049/jimmunol.1300581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aziz A, Soucie E, Sarrazin S, Sieweke MH. MafB/c-Maf deficiency enables self-renewal of differentiated functional macrophages. Science. 2009;326:867–871. doi: 10.1126/science.1176056. [DOI] [PubMed] [Google Scholar]
- 67.Kelly LM, Englmeier U, Lafon I, Sieweke MH, Graf T. MafB is an inducer of monocytic differentiation. EMBO J. 2000;19:1987–1997. doi: 10.1093/emboj/19.9.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Okabe Y, Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157:832–844. doi: 10.1016/j.cell.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haldar M, et al. Heme-mediated SPI-C induction promotes monocyte differentiation into iron-recycling macrophages. Cell. 2014;156:1223–1234. doi: 10.1016/j.cell.2014.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Spann NJ, Glass CK. Sterols and oxysterols in immune cell function. Nat. Immunol. 2013;14:893–900. doi: 10.1038/ni.2681. [DOI] [PubMed] [Google Scholar]
- 71.Cecchini MG, et al. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development. 1994;120:1357–1372. doi: 10.1242/dev.120.6.1357. [DOI] [PubMed] [Google Scholar]
- 72.Ryan GR, et al. Rescue of the colony-stimulating factor 1 (CSF-1)-nullizygous mouse (Csf1op/Csf1op) phenotype with a CSF-1 transgene and identification of sites of local CSF-1 synthesis. Blood. 2001;98:74–84. doi: 10.1182/blood.v98.1.74. [DOI] [PubMed] [Google Scholar]
- 73.Dai XM, Zong XH, Sylvestre V, Stanley ER. Incomplete restoration of colony-stimulating factor 1 (CSF-1) function in CSF-1-deficient Csf1op/Csf1op mice by transgenic expression of cell surface CSF-1. Blood. 2004;103:1114–1123. doi: 10.1182/blood-2003-08-2739. [DOI] [PubMed] [Google Scholar]
- 74.Ginhoux F, et al. Langerhans cells arise from monocytes in vivo. Nat. Immunol. 2006;7:265–273. doi: 10.1038/ni1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hashimoto D, et al. Pretransplant CSF-1 therapy expands recipient macrophages and ameliorates GVHD after allogeneic hematopoietic cell transplantation. J. Exp. Med. 2011;208:1069–1082. doi: 10.1084/jem.20101709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.MacDonald KP, et al. An antibody against the colony-stimulating factor 1 receptor depletes the resident subset of monocytes and tissue- and tumor-associated macrophages but does not inhibit inflammation. Blood. 2010;116:3955–3963. doi: 10.1182/blood-2010-02-266296. [DOI] [PubMed] [Google Scholar]
- 77.Varol C, et al. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J. Exp. Med. 2007;204:171–180. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Muller PA, et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158:300–313. doi: 10.1016/j.cell.2014.04.050. [This study shows that macrophages promote neuronal control of gut peristalsis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Greter M, et al. Stroma-derived interleukin-34 controls the development and maintenance of langerhans cells and the maintenance of microglia. Immunity. 2012;37:1050–1060. doi: 10.1016/j.immuni.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wei S, et al. Functional overlap but differential expression of CSF-1 and IL-34 in their CSF-1 receptor-mediated regulation of myeloid cells. J. Leukoc. Biol. 2010;88:495–505. doi: 10.1189/jlb.1209822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lin H, et al. Discovery of a cytokine and its receptor by functional screening of the extracellular proteome. Science. 2008;320:807–811. doi: 10.1126/science.1154370. [This study led to the identification of IL-34 in human blood, a novel cytokine that shares a receptor with CSF1.] [DOI] [PubMed] [Google Scholar]
- 82.Nandi S, et al. The CSF-1 receptor ligands IL-34 and CSF-1 exhibit distinct developmental brain expression patterns and regulate neural progenitor cell maintenance and maturation. Dev. Biol. 2012;367:100–113. doi: 10.1016/j.ydbio.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang Y, et al. IL-34 is a tissue-restricted ligand of CSF1R required for the development of Langerhans cells and microglia. Nat. Immunol. 2012;13:753–760. doi: 10.1038/ni.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Butovsky O, et al. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat. Neurosci. 2014;17:131–143. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Borkowski TA, Letterio JJ, Farr AG, Udey MC. A role for endogenous transforming growth factor β1 in Langerhans cell biology: the skin of transforming growth factor β1 null mice is devoid of epidermal Langerhans cells. J. Exp. Med. 1996;184:2417–2422. doi: 10.1084/jem.184.6.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bialas AR, Stevens B. TGF-β signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat. Neurosci. 2013;16:1773–1782. doi: 10.1038/nn.3560. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 87.Abutbul S, et al. TGF-β signaling through SMAD2/3 induces the quiescent microglial phenotype within the CNS environment. Glia. 2012;60:1160–1171. doi: 10.1002/glia.22343. [DOI] [PubMed] [Google Scholar]
- 88.Bobr A, et al. Autocrine/paracrine TGF-β1 inhibits Langerhans cell migration. Proc. Natl Acad. Sci. USA. 2012;109:10492–10497. doi: 10.1073/pnas.1119178109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kaplan DH, et al. Autocrine/paracrine TGFβ1 is required for the development of epidermal Langerhans cells. J. Exp. Med. 2007;204:2545–2552. doi: 10.1084/jem.20071401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chopin M, et al. Langerhans cells are generated by two distinct PU.1-dependent transcriptional networks. J. Exp. Med. 2013;210:2967–2980. doi: 10.1084/jem.20130930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hacker C, et al. Transcriptional profiling identifies Id2 function in dendritic cell development. Nat. Immunol. 2003;4:380–386. doi: 10.1038/ni903. [DOI] [PubMed] [Google Scholar]
- 92.Hashimoto D, Miller J, Merad M. Dendritic cell and macrophage heterogeneity in vivo. Immunity. 2011;35:323–335. doi: 10.1016/j.immuni.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mortha A, et al. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science. 2014;343:1249288. doi: 10.1126/science.1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schneider C, et al. Alveolar macrophages are essential for protection from respiratory failure and associated morbidity following influenza virus infection. PLoS Pathog. 2014;10:e1004053. doi: 10.1371/journal.ppat.1004053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sainathan SK, et al. Granulocyte macrophage colony-stimulating factor ameliorates DSS-induced experimental colitis. Inflamm. Bowel Dis. 2008;14:88–99. doi: 10.1002/ibd.20279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hirata Y, Egea L, Dann SM, Eckmann L, Kagnoff MF. GM-CSF-facilitated dendritic cell recruitment and survival govern the intestinal mucosal response to a mouse enteric bacterial pathogen. Cell Host Microbe. 2010;7:151–163. doi: 10.1016/j.chom.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Marini M, Soloperto M, Mezzetti M, Fasoli A, Mattoli S. Interleukin-1 binds to specific receptors on human bronchial epithelial cells and upregulates granulocyte/macrophage colony-stimulating factor synthesis and release. Am. J. Respir. Cell. Mol. Biol. 1991;4:519–524. doi: 10.1165/ajrcmb/4.6.519. [DOI] [PubMed] [Google Scholar]
- 98.Egea L, et al. GM-CSF produced by nonhematopoietic cells is required for early epithelial cell proliferation and repair of injured colonic mucosa. J. Immunol. 2013;190:1702–1713. doi: 10.4049/jimmunol.1202368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.de Groot RP, Coffer PJ, Koenderman L. Regulation of proliferation, differentiation and survival by the IL-3/IL-5/GM-CSF receptor family. Cell. Signal. 1998;10:619–628. doi: 10.1016/s0898-6568(98)00023-0. [DOI] [PubMed] [Google Scholar]
- 100.Wan CK, et al. The cytokines IL-21 and GM-CSF have opposing regulatory roles in the apoptosis of conventional dendritic cells. Immunity. 2013;38:514–527. doi: 10.1016/j.immuni.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bonfield TL, et al. Peroxisome proliferator-activated receptor-γ is deficient in alveolar macrophages from patients with alveolar proteinosis. Am. J. Respir. Cell. Mol. Biol. 2003;29:677–682. doi: 10.1165/rcmb.2003-0148OC. [DOI] [PubMed] [Google Scholar]
- 102.Gautier EL, et al. Systemic analysis of PPARγ in mouse macrophage populations reveals marked diversity in expression with critical roles in resolution of inflammation and airway immunity. J. Immunol. 2012;189:2614–2624. doi: 10.4049/jimmunol.1200495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Trapnell BC, Whitsett JA, Nakata K. Pulmonary alveolar proteinosis. New Engl. J. Med. 2003;349:2527–2539. doi: 10.1056/NEJMra023226. [DOI] [PubMed] [Google Scholar]
- 104.Dranoff G, et al. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science. 1994;264:713–716. doi: 10.1126/science.8171324. [This report demonstrates that the absence of local production of CSF2 leads to an accumulation of surfactant in the alveolar space of lung tissue.] [DOI] [PubMed] [Google Scholar]
- 105.Nakamura A, et al. Transcription repressor Bach2 is required for pulmonary surfactant homeostasis and alveolar macrophage function. J. Exp. Med. 2013;210:2191–2204. doi: 10.1084/jem.20130028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Niess JH, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 107.Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl Acad. Sci. USA. 2014;111:2247–2252. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zigmond E, et al. Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity. 2014;40:720–733. doi: 10.1016/j.immuni.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 109.Shouval DS, et al. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity. 2014;40:706–719. doi: 10.1016/j.immuni.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shah YM, Morimura K, Gonzalez FJ. Expression of peroxisome proliferator-activated receptor-γ in macrophage suppresses experimentally induced colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G657–G666. doi: 10.1152/ajpgi.00381.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jiang C, Ting AT, Seed B. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 112.Griseri T, McKenzie BS, Schiering C, Powrie F. Dysregulated hematopoietic stem and progenitor cell activity promotes interleukin-23-driven chronic intestinal inflammation. Immunity. 2012;37:1116–1129. doi: 10.1016/j.immuni.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Garceau V, et al. Pivotal advance: avian colony-stimulating factor 1 (CSF-1), interleukin-34 (IL-34), and CSF-1 receptor genes and gene products. J. Leukoc. Biol. 2010;87:753–764. doi: 10.1189/jlb.0909624. [This study shows that, similar to findings in mice and humans, IL-34 and CSF1 are present and use the same receptor in chickens.] [DOI] [PubMed] [Google Scholar]
- 114.Schlitzer A, et al. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity. 2013;38:970–983. doi: 10.1016/j.immuni.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Trapnell BC, Whitsett JA. GM-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage-mediated innate host defense. Annu. Rev. Physiol. 2002;64:775–802. doi: 10.1146/annurev.physiol.64.090601.113847. [DOI] [PubMed] [Google Scholar]
- 116.Bedoret D, et al. Lung interstitial macrophages alter dendritic cell functions to prevent airway allergy in mice. J. Clin. Invest. 2009;119:3723–3738. doi: 10.1172/JCI39717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ganz T. Macrophages and systemic iron homeostasis. J. Innate Immun. 2012;4:446–453. doi: 10.1159/000336423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cambos M, Scorza T. Robust erythrophagocytosis leads to macrophage apoptosis via a hemin-mediated redox imbalance: role in hemolytic disorders. J. Leukoc. Biol. 2011;89:159–171. doi: 10.1189/jlb.0510249. [DOI] [PubMed] [Google Scholar]
- 119.Chow A, et al. CD169+ macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nat. Med. 2013;19:429–436. doi: 10.1038/nm.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rosas M, et al. The transcription factor Gata6 links tissue macrophage phenotype and proliferative renewal. Science. 2014;344:645–648. doi: 10.1126/science.1251414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gautier EL, et al. Gata6 regulates aspartoacylase expression in resident peritoneal macrophages and controls their survival. J. Exp. Med. 2014;211:1525–1531. doi: 10.1084/jem.20140570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chow A, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J. Exp. Med. 2011;208:261–271. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Casanova-Acebes M, et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell. 2013;153:1025–1035. doi: 10.1016/j.cell.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mendez-Ferrer S, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Junt T, et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- 126.Carrasco YR, Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity. 2007;27:160–171. doi: 10.1016/j.immuni.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 127.Phan TG, Grigorova I, Okada T, Cyster JG. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat. Immunol. 2007;8:992–1000. doi: 10.1038/ni1494. [DOI] [PubMed] [Google Scholar]
- 128.Phan TG, Green JA, Gray EE, Xu Y, Cyster JG. Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nat. Immunol. 2009;10:786–793. doi: 10.1038/ni.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Moseman EA, et al. B cell maintenance of subcapsular sinus macrophages protects against a fatal viral infection independent of adaptive immunity. Immunity. 2012;36:415–426. doi: 10.1016/j.immuni.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Iannacone M, et al. Subcapsular sinus macrophages prevent CNS invasion on peripheral infection with a neurotropic virus. Nature. 2010;465:1079–1083. doi: 10.1038/nature09118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gaya M, et al. Host response. Inflammation-induced disruption of SCS macrophages impairs B cell responses to secondary infection. Science. 2015;347:667–672. doi: 10.1126/science.aaa1300. [DOI] [PubMed] [Google Scholar]
- 132.Karlsson MC, et al. Macrophages control the retention and trafficking of B lymphocytes in the splenic marginal zone. J. Exp. Med. 2003;198:333–340. doi: 10.1084/jem.20030684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Aichele P, et al. Macrophages of the splenic marginal zone are essential for trapping of blood-borne particulate antigen but dispensable for induction of specific T cell responses. J. Immunol. 2003;171:1148–1155. doi: 10.4049/jimmunol.171.3.1148. [DOI] [PubMed] [Google Scholar]
- 134.A.-Gonzalez N, et al. The nuclear receptor LXRα controls the functional specialization of splenic macrophages. Nat. Immunol. 2013;14:831–839. doi: 10.1038/ni.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Joseph SB, et al. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell. 2004;119:299–309. doi: 10.1016/j.cell.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 136.Kondo H, Saito K, Grasso JP, Aisen P. Iron metabolism in the erythrophagocytosing Kupffer cell. Hepatology. 1988;8:32–38. doi: 10.1002/hep.1840080108. [DOI] [PubMed] [Google Scholar]
- 137.Pinto AR, et al. An abundant tissue macrophage population in the adult murine heart with a distinct alternatively-activated macrophage profile. PLoS ONE. 2012;7:e36814. doi: 10.1371/journal.pone.0036814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat. Rev. Immunol. 2011;11:750–761. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 140.Priller J, et al. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat. Med. 2001;7:1356–1361. doi: 10.1038/nm1201-1356. [DOI] [PubMed] [Google Scholar]
- 141.Klein I, et al. Kupffer cell heterogeneity: functional properties of bone marrow derived and sessile hepatic macrophages. Blood. 2007;110:4077–4085. doi: 10.1182/blood-2007-02-073841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Suzuki T, et al. Pulmonary macrophage transplantation therapy. Nature. 2014;514:450–454. doi: 10.1038/nature13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Godleski JJ, Brain JD. The origin of alveolar macrophages in mouse radiation chimeras. J. Exp. Med. 1972;136:630–643. doi: 10.1084/jem.136.3.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Happle C, et al. Pulmonary transplantation of macrophage progenitors as effective and long-lasting therapy for hereditary pulmonary alveolar proteinosis. Sci. Transl Med. 2014;6:250ra113. doi: 10.1126/scitranslmed.3009750. [DOI] [PubMed] [Google Scholar]
- 145.Bendall SC, et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science. 2011;332:687–696. doi: 10.1126/science.1198704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Becher B, et al. High-dimensional analysis of the murine myeloid cell system. Nat. Immunol. 2014;15:1181–1189. doi: 10.1038/ni.3006. [DOI] [PubMed] [Google Scholar]
- 147.Amir el AD, et al. viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia. Nat. Biotech. 2013;31:545–552. doi: 10.1038/nbt.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Levine JH, et al. Data-driven phenotypic dissection of AML reveals progenitor-like cells that correlate with prognosis. Cell. 2015;162:184–197. doi: 10.1016/j.cell.2015.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Blecher-Gonen R, et al. High-throughput chromatin immunoprecipitation for genome-wide mapping of in vivo protein-DNA interactions and epigenomic states. Nat. Protoc. 2013;8:539–554. doi: 10.1038/nprot.2013.023. [DOI] [PubMed] [Google Scholar]
- 150.Alvarez-Errico D, Vento-Tormo R, Sieweke M, Ballestar E. Epigenetic control of myeloid cell differentiation, identity and function. Nat. Rev. Immunol. 2014;15:7–17. doi: 10.1038/nri3777. [DOI] [PubMed] [Google Scholar]
- 151.Jaitin DA, et al. Massively parallel single-cell RNA-seq for marker-free decomposition of tissues into cell types. Science. 2014;343:776–779. doi: 10.1126/science.1247651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shalek AK, et al. Single-cell RNA-seq reveals dynamic paracrine control of cellular variation. Nature. 2014;510:363–369. doi: 10.1038/nature13437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ke R, et al. In situ sequencing for RNA analysis in preserved tissue and cells. Nat. Methods. 2013;10:857–860. doi: 10.1038/nmeth.2563. [DOI] [PubMed] [Google Scholar]
- 154.Lee JH, et al. Highly multiplexed subcellular RNA sequencing in situ. Science. 2014;343:1360–1363. doi: 10.1126/science.1250212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Angelo M, et al. Multiplexed ion beam imaging of human breast tumors. Nat. Med. 2014;20:436–442. doi: 10.1038/nm.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Giesen C, et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nat. Methods. 2014;11:417–422. doi: 10.1038/nmeth.2869. [DOI] [PubMed] [Google Scholar]
- 157.Biswas SK, et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-κB and enhanced IRF-3/STAT1 activation). Blood. 2006;107:2112–2122. doi: 10.1182/blood-2005-01-0428. [DOI] [PubMed] [Google Scholar]
- 158.Movahedi K, et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6Chigh monocytes. Cancer Res. 2010;70:5728–5739. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 159.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J. Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 160.Steidl C, et al. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. N. Engl. J. Med. 2010;362:875–885. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Forssell J, et al. High macrophage infiltration along the tumor front correlate with improved survival in colon cancer. Clin. Cancer Res. 2007;13:1472–1479. doi: 10.1158/1078-0432.CCR-06-2073. [DOI] [PubMed] [Google Scholar]
- 162.Kim DW, et al. High tumour islet macrophage infiltration correlates with improved patient survival but not with EGFR mutations, gene copy number or protein expression in resected non-small cell lung cancer. Br. J. Cancer. 2008;98:1118–1124. doi: 10.1038/sj.bjc.6604256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Goswami S, et al. Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop. Cancer Res. 2005;65:5278–5283. doi: 10.1158/0008-5472.CAN-04-1853. [This study describes the crosstalk between macrophages and tumour cells that promotes tumour cell invasion.] [DOI] [PubMed] [Google Scholar]
- 164.Patsialou A, et al. Invasion of human breast cancer cells in vivo requires both paracrine and autocrine loops involving the colony-stimulating factor-1 receptor. Cancer Res. 2009;69:9498–9506. doi: 10.1158/0008-5472.CAN-09-1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kierdorf K, et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 2013;16:273–280. doi: 10.1038/nn.3318. [This study shows that microglia derive from an early wave of YS-derived EMPs.] [DOI] [PubMed] [Google Scholar]
- 166.Bain CC, et al. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat. Immunol. 2014;15:929–937. doi: 10.1038/ni.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]