Abstract
Two immortalized human juvenile chondrocyte cell lines, T/C28a2 and C28/I2, were employed to determine the extent to which recombinant human (rh) IL-6 or rh-TNF-α increased the production of matrix metalloproteinase-9 (MMP-9). The effect of rhIL-6 on neutrophil gelatinase-associated lipocalin (NGAL) was also assessed. Although C28/I2 chondrocytes incubated with rhIL-6 (50 ng/ml) increased MMP-9 production which could not be mimicked by the T/C28a2 chondrocyte line, the effect of rhTNF-α on MMP-9 was more robust than with rhIL-6. The combinations of rhIL-6 and soluble IL-6 receptor-α (sIL-6Rα) or rhIL-6 and tocilizumab (TCZ), a fully-humanized recombinant monoclonal antibody that neutralizes the interaction between IL-6 and IL-6R significantly reduced MMP-9 production by C28/I2 chondrocytes. However, TCZ had no effect on rhTNF-α-induced MMP-9 production. By contrast, rhIL-6 did not increase the production of NGAL by C28/I2 chondrocytes although the number of NGAL-positive cells was significantly reduced by sIL-6R compared to its control group, but not by the combination of rhIL-6 plus TCZ compared to rhIL-6. In summary, these results showed that rhIL-6 stimulated the production of MMP-9, but not NGAL, in the C28/I2 chondrocyte line. TCZ or sIL-6Rα suppressed rhIL-6-induced MMP-9 production.
Keywords: IL-6, tocilizumab, matrix, metalloproteinase-9, c28/i2 immortalized human chondrocyte
Introduction
Matrix metalloproteinase-9 (MMP-9; gelatinase B; 92 kDa gelatinase; 92 kDa type IV collagenase) is a critical MMP in mediating the progression of various arthritic conditions [1]. MMP-9 has the capacity to degrade several articular cartilage extracellular matrix (ECM) proteins, including aggrecan, link protein, and type II collagen, all of which help maintain normal cartilage biomechanical functions [1]. Importantly, in various types of arthritis, chondrocyte MMP-9 gene expression is significantly up-regulated in response to the elevated levels of pro-inflammatory cytokines in the synovial fluid milieu, exemplified by intereukin-6 (IL-6), IL-1β, IL-17, and tumor necrosis factor-α (TNF-α) [1-3].
To probe the contribution of each of those cytokines to MMP-9 gene expression by articular chondrocytes in vitro would generally require that specific inhibitors for each of them be individually tested. In that regard, the effect of IL-1β or TNF-α blockade on MMP synthesis was previously reported with the results showing that IL-1 receptor antagonist or TNF-α blocking monoclonal antibodies inhibited MMP production [4]. However, the contribution of IL-6 to MMP-9 production by cultured human chondrocytes remains to be fully elucidated. Therefore, to achieve this objective, the extent to which tocilizumab (TCZ), a recombinant fully humanized IgG1(κ) monoclonal antibody that neutralizes the interaction between IL-6 and the IL-6 receptor-α (IL-6Rα) [5] inhibits recombinant human (rh)-IL-6-mediated MMP-9 production was determined in the immortalized human juvenile T/C28a2 and C28/I2 chondrocyte lines. These human chondrocyte lines were employed for this analysis because they had been previously shown to express cartilage-specific extracellular matrix protein genes [6,7]. T/C28a2 and C28/I2 chondrocytes also expressed several other molecules characteristic of authentic human chondrocytes, most notably the molecular signature SOX9 gene, considered the “master” transcriptional regulator of several cartilage-specific genes as the type II collagen (COL2A1) gene and the aggrecan (AGRN) gene [6-8].
The effect of rhIL-6 on the synthesis of chondrocyte-derived neutrophil gelatinase-associated lipocalin (NGAL) was also evaluated. The rationale for analyzing NGAL production by C28/I2 chondrocytes stemmed from our previously reported finding that chondrocytes obtained from human osteoarthritis knee cartilage synthesized NGAL in response to IL-1β [9]. In addition, we showed that NGAL in synovial fluids obtained from patients with end-stage osteoarthritis was found in a complex with MMP-9. Moreover, we proved that the MMP-9/NGAL complex preserved MMP-9 activity by demonstrating that this complex prevented MMP-9 from being degraded, thus preserving MMP-9 activity [10].
Materials and methods
Human chondrocyte cell lines
The immortalized human juvenile chondrocyte cell lines, T/C28a2 and C28/I2, were obtained from the laboratory of Professor Mary B. Goldring [Hospital for Special Surgery/Weill Medical College of Cornell University (New York, New York.)].
PANC-1
PANC-1, a line of pancreatic carcinoma cells, was obtained from the American Type Culture Collection (Manassas, VA).
MMP-9 ELISA/NGAL ELISA, anti-MMP-9/anti-NGAL Immunocytochemistry (ICC)
An ELISA kit containing anti-MMP-9 antibody was purchased from Aviva Systems (San Diego, CA). An ELISA kit for the detection of NGAL was purchased from Pierce Biotechnology (Rockford, IL). The anti-MMP-9 antibody and anti-NGAL antibody employed for ICC were purchased from Abcam (Cambridge, MA). The human anti-MMP-9 antibody used for ICC was a rabbit polyclonal antibody produced against a rat recombinant fragment of the MMP-9 catalytic domain. According to the manufacturer, this anti-MMP-9 antibody is reactive with both pro-MMP-9 and activated MMP-9. For the NGAL ICC, the immunogen used to produce the anti-NGAL antibody was a synthetic peptide corresponding to the 38-53 amino acid sequence, LQPGFWTERFQGRWFV located at the N-terminus of rat NGAL.
Recombinant human (rh) IL-6, rhTNF-α and soluble IL-6 Receptor-α (sIL-6Rα)
The cytokines, rhIL-6, and rhTNF-α, and soluble IL-6Rα (sIL-6R) were purchased from various commercial vendors.
Tocilizumab (TCZ)
Tocilizumab (TCZ) was obtained through a research contract between Genentech/Roche Group (South San Francisco, CA) and Case Western Reserve University.
Human T/C28a2, C28/I2 Chondrocytes and PANC-1
T/C28a2 or C28/I2 chondrocytes were maintained in Dulbecco's Modified Eagle's Medium (DMEM)/F12 medium (1:1 ratio) supplemented with 10% (v/v) fetal bovine serum (FBS). PANC-1 was maintained in continuous culture according to instructions provided by the American Tissue Culture Collection.
Experimental Conditions for MMP-9/NGAL ELISA and MMP-9/NGAL ICC
TC28a2 or C28/I2 chondrocytes (3 × 105 cells/ml, ELISA; 105 cells/ ml, ICC) were incubated in DMEM/F12 (1:1) containing 0.5% FBS under the following conditions: “No additions” (control), rhIL-6 (50 ng/ml), sIL-6R (30 ng/ml); rhTNF-α (20 ng/ml), TCZ (200-800 ng/ml), rhIL-6 plus TCZ, rhTNF-α plus TCZ, or rhIL-6 plus sIL-6R each for 60 min and 4 hrs. The concentrations of rhIL-6 and sIL-6R used in this study were based on previously published data, which showed 1) that IL-6 (50 ng/ml) activated chondrocyte JAK/STAT and ERK-MAPK signaling [11]; and 2) that the concentration of sIL-6R (30 ng/ml) approximated the maximal concentration of sIL-6R reportedly found in RA synovial fluid (range 10-40 ng/ml) [12].
PANC-1 was employed as the positive control for MMP-9 production because PANC-1 had previously been shown to over-produce MMP-9 [13]. In addition, MMP-9 was found to be substantially increased over control levels in response to phorbol myristate acetate (PMA) [13]. Thus, PANC-1, T/C28a2 and C28/I2 chondrocytes were employed for the MMP-9 ELISA and PANC-1 and C28/I2 chondrocytes for the anti-MMP-9 antibody ICC. For the MMP-9 ELISA, the concentration of MMP-9 in T/C28a2, C28/I2 or PANC-1 was determined from serial dilutions of the protein lysate. The average of duplicate ELISA determinations was used to calculate the amount of MMP-9 (pg/ml) produced by the cells.
For ICC, the number of anti-MMP-9-negative or anti-MMP-9-positive PANC-1 or C28/I2 chondrocytes and NGAL-negative or NGAL-positive chondrocytes were enumerated by quantifying the mean intensity of 6 sampled areas in photomicrographs from each of the control and treatment groups using the Metamorph® software program. The mean intensities were then normalized to the negative control to determine the number of MMP-9 or NGAL-positive cells reported in arbitrary units.
Data analysis
ELISA Analysis
The amount of MMP-9 or NGAL (pg/ml) produced by C28/I2 chondrocytes was calculated using the average of duplicate determinations from their respective ELISA. Chi-square (www.socscistatistics.com/tests/chisquare/default2.aspx) was used to calculate the significance of differences between the amount of MMP-9 produced by C28/I2 chondrocytes treated with rhIL-6 alone (50 ng/ml) and rhIL-6 plus TCZ, at various concentrations with an expected difference of Δ=+/−20% between the groups employed in the chi-square 2 × 2 table.
ICC Analysis
The frequency of MMP-9-positive or NGAL-positive cells in the control groups (n=5) or the various treatment groups (n=5) was calculated from the computer-generated anti-MMP-9 antibody/anti-NGAL antibody ICC results. The results shown as “arbitrary units” was then analyzed by determining whether the comparison groups had equal variances. Then the F-value was calculated using the one-way ANOVA calculator (turner.faculty.swau.edu/mathematics/math241/materials/anova). To calculate a p-value, the Student's t-test was employed for comparison of groups with equal variances where p<0.05 was considered significant.
Results
MMP-9 production is increased by rhIL-6 and rhTNF-α: Effect of sIL-6R and TCZ
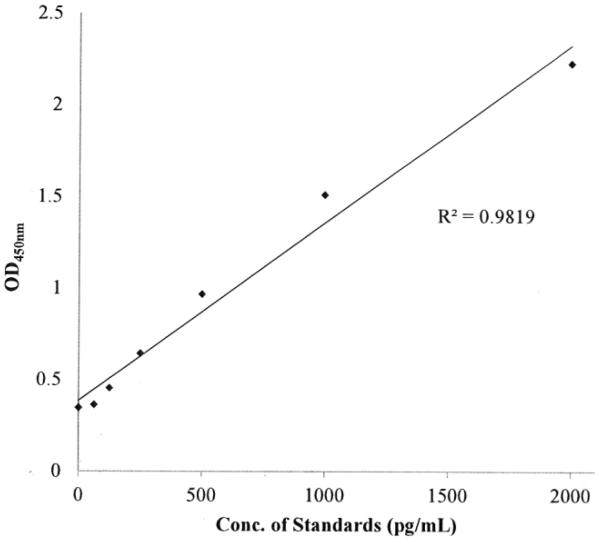
A standard curve was produced for the MMP-9 ELISA which showed that the concentration of MMP-9 as a function of OD450nm was linear (Figure 1). This standard curve was used to calculate the amount of MMP-9 (pg/ml) produced by T/C28a2 or C28/I2 chondrocytes or by PANC-1 cells using direct interpolation of the OD450nm measurements performed on the various cell lysates.
Figure 1.
MMP-9 ELISA Standard Curve.
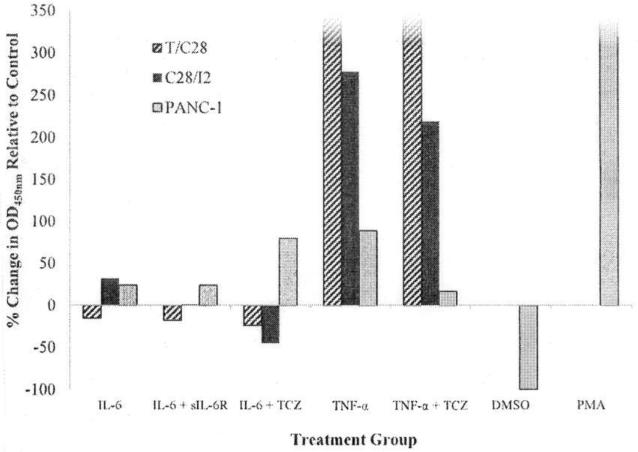
Incubation of C28/I2 chondrocytes, but not T/C28a2 chondrocytes, with rhIL-6 increased MMP-9 production. MMP-9 production was reduced when rhIL-6 was combined with either sIL-6R or TCZ (Figure 2). Although rhIL-6 also increased MMP-9 in PANC-1 cells, the combination of rhIL-6 plus TCZ did not inhibit MMP-9. Of note, the effect of rhTNF-α on MMP-9 production by T/C28a2 or C28/I2 chondrocytes was far more robust than with rhIL-6 (Figure 2). However, the combination of rhTNF-α and TCZ had no effect on MMP-9 production by T/C28a2 chondrocytes whereas the combination of rhTNF-α and TCZ reduced MMP-9 production by Δ=−10% in C28/I2 chondrocytes.
Figure 2.
MMP-9 Production by T/C28a2, C28/I2 Chondrocytes and PANC-1 Cells Measured by an MMP-9 ELISA. MMP-9 Production: % Change OD450nm Relative to the “No Additions” Control Group. The concentration of cytokines, inhibitors or other factors for each group were rhIL-6-50 ng/ml; sIL-6R-30 ng/ml; TCZ-200 ng/ml; rhTNF-α −20 ng/ml; PMA-30.7 ng/ml. Values shown for MMP-9 produced by T/C28a2 chondrocytes incubated with either rhTNF-α or rhTNF-α plus TCZ or PANC-1 cells incubated with PMA exceeded 350 pg/ml.
Incubation of PANC-1 cells with rhIL-6 increased MMP-9 to a similar extent as was found for C28/I2 chondrocytes (Figure 2). However, whereas the combination of rhIL-6 plus sIL-6R reduced MMP-9 to an undetectable level in C28/I2 chondrocytes, the combination of rhIL-6 plus sIL-6R had no effect on MMP-9 produced by PANC-1.
In contrast, PANC-1 cells incubated with PMA (30.7 ng/ml), serving as the positive control for MMP-9 production [13], substantially increased MMP-9 production, compared to its DMSO-control group (Figure 2).
Effect of TCZ concentration on MMP-9 production by C28/I2 chondrocytes
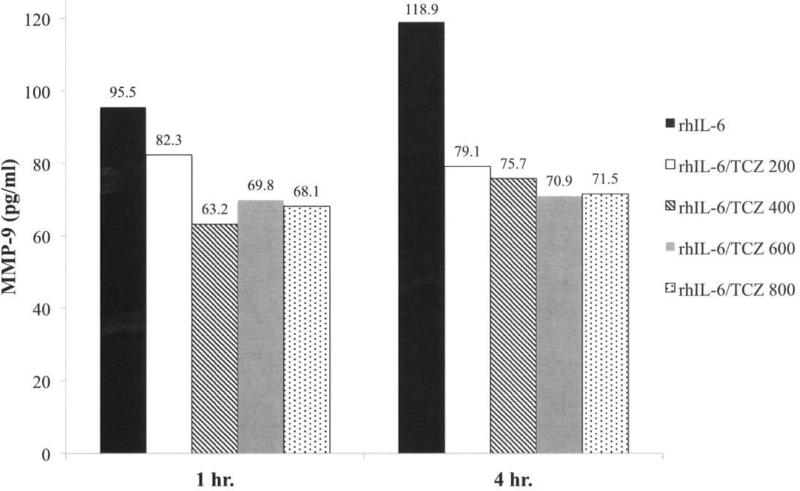
We next examined the effect of varying the concentration of TCZ between 200 ng/ml and 800 ng/ml on MMP-9 production by C28/I2 chondrocytes in response to rhIL-6 (50 ng/ml). Although the average of duplicate determinations from the MMP-9 ELISA showed that TCZ (200 ng/ml) reduced rhIL-6-induced MMP-9 after 1 hr by Δ=−16% (Figure 3) this difference did not reach statistical significance. However, TCZ at concentrations ranging from 400 ng/ml to 800 ng/ml significantly reduced MMP-9 after 1 hr (Figure 3). Furthermore, the reduction in MMP-9 in response to the combination of rhIL-6 plus TCZ was sustained after 4 hrs (Figure 3). Of note, concentrations of TCZ greater than 200 ng/ml did not increase its inhibitory effect on MMP-9 production at 1 hr (Figure 3) or at 4 hrs (Figure 3). The result of the chi-square analyses for all of the group comparisons is summarized in Table 1.
Figure 3.
Effect of TCZ on rhIL-6-Mediated MMP-9 Production by C28/I2 Chondrocytes. The concentration of rhIL-6 was 50 ng/ml. The concentration of TCZ was varied from 200 ng/ml to 800 ng/ml.
Table 1.
χ2 Analysis: Effect of TCZ on MMP-9 Production by C28/I2 Chondrocytes.
| Group | [TCZ] | 60 min | 4 hrs |
|---|---|---|---|
| 1 | 200 ng/ml1 | χ2=0.38; p = 0.54 | χ2=9.6; p = 0.002* |
| 2 | 400 ng/ml2 | χ2=10.1; p = 0.001* | χ2=18.7; p = 1.5 × 10−5* |
| 3 | 600 ng/ml3 | χ2=3.96; p = 0.046* | χ2=17.6; p = 2.6 × 10−5* |
| 4 | 800 ng/ml4 | χ2=5.16; p = 0.02* | χ2=17.6; p = 2.6 × 10−5* |
Ratio of [TCZ] to [rhIL-6]:
4:1
8:1
12:1
16:1
p-value for significance was <0.05
ICC analysis of MMP-9 production by C28/I2 chondrocytes and PANC-1
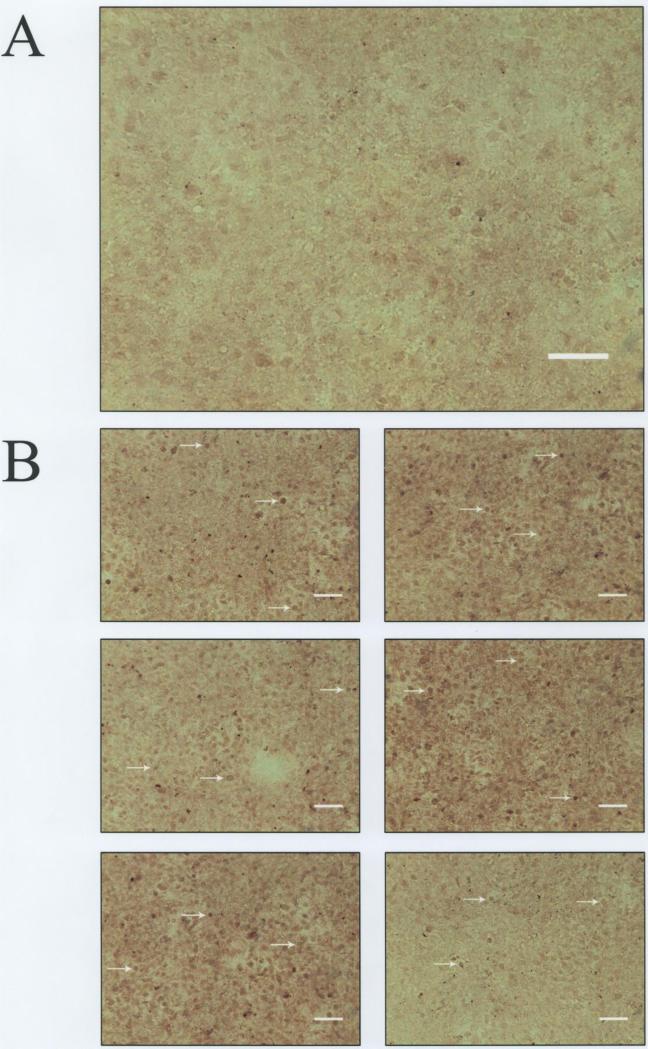
MMP-9 production was also analyzed by ICC using an anti-MMP-9 antibody that is reactive with both pro-MMP-9 and activated MMP-9. The photomicrograph in Figure 4A shows the appearance of the negative C28/I2 chondrocyte control for MMP-9. This image was arrived at by processing the cells for ICC but without applying primary anti-MMP-9 antibody. The other photomicrographs (Figure 4B) show C28/I2 chondrocytes from the “no additions” control group which were identified by Metamorph® as MMP-9-positive (arrows) in 6 regions of the cover slip encompassing the microscopic fields enumerated for the ICC analysis.
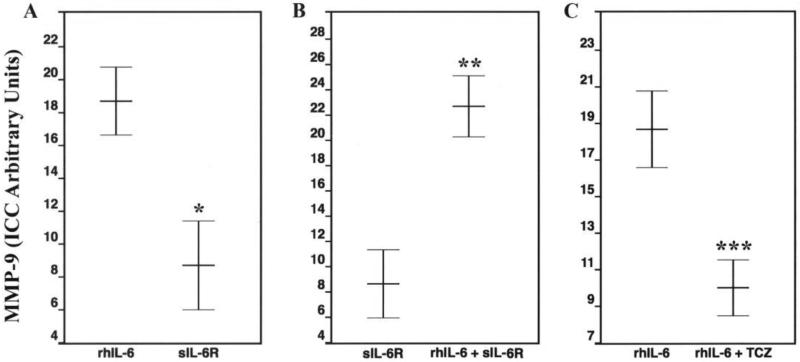
Figure 4.
Anti-MMP-9 Antibody-Mediated Immunocytochemistry (ICC).
Panel A: C28/I2 chondrocytes-negative control (– = 100 μm).
Panel B: Arrows indicate MMP-9-positive chondrocytes (– = 100 μm)
The ICC of anti-MMP-9 antibody-positive C28/I2 chondrocytes treated with rhIL-6 (50 ng/ml) for 24 hrs is shown in Figure 5 and compared to C28/I2 chondrocytes in the “no additions” control or 10% FBS control group. The quantitative analysis showed that rhIL-6 significantly increased the number of anti-MMP-9 antibody positive chondrocytes compared to the “no additions” control group [i.e. DMEM/F12 (1:1) containing 0.5% FBS; (p=1.23 × 10−7)]. As a further determination regarding the specificity of the rhIL-6 effect on C28/I2 chondrocyte MMP-9 production, PANC-1 cells were also incubated with rhIL-6 (50 ng/ml) for 24 hrs. The number of MMP-9-positive PANC-1 cells was not significantly altered by rhIL-6 (“no additions” control, Arbitrary Units, 17.3 ± 0.28; rhIL-6, 15.5 ± 0.78; mean ± SD, n=5; p=0.67).
Figure 5.
Effect of 0.5% FBS, 10% FBS or rhIL-6 (50 ng/ml)-containing DMEM/F12 (1:1) on MMP-9 Production by C28/I2 Chondrocytes (– = 100 μm).
Values are mean ± SD (n=6 microscopic fields)
C28/I2 chondrocytes maintained in DMEM/F12 (1:1) containing 10% FBS for 24 hrs also increased the number of MMP-9-positive chondrocytes compared to the “no additions” control containing 0.5% FBS (p<2 × 10−3). This result provided substantive justification for maintaining C28/I2 chondrocytes in 0.5% FBS for determining MMP-9 production in the various treatment groups.
Although C28/I2 chondrocytes incubated with rhIL-6 alone had a significantly increased number of MMP-9-positive chondrocytes compared to the “no additions” control group, the combination of rhIL-6 plus sIL-6R also significantly increased the number of MMP-9-positive chondrocytes compared to sIL-6R (p=3.1 × 10−5) (Figure 6), whereas sIL-6R alone significantly reduced the number of MMP-9-positive chondrocytes compared to rhIL-6 (p=2.2 × 10−4). This was also the case for the rhIL-6 plus TCZ group when compared to rhIL-6 (p=9.7 × 10−4) (Figure 6). Importantly, TCZ alone had no significant effect (p=0.07) on chondrocyte MMP-9-positivity compared to rhIL-6.
Figure 6.
Effect of Various Incubation Conditions on MMP-9 Production by C28/I2 Chondrocytes: Anti-MMP-9 Antibody-Mediated ICC. Values are mean ± SD (n=5) * p = 2.2 × 10−4; ** p = 3.1 × 10−5; *** p = 9.7 × 10−4
ICC analysis of NGAL production by C28/I2 chondrocytes
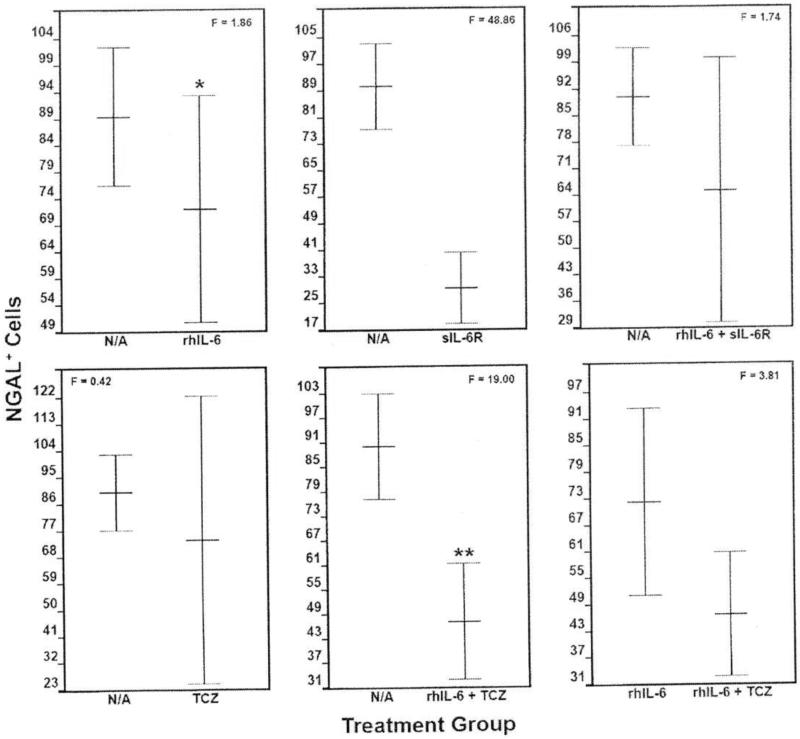
The number of NGAL-positive cells was significantly reduced (F=48.86; p=4.3 × 10−4) by sIL-6R compared to the “no additions” control group as well as by the combination of rhIL-6 plus TCZ (F=19.00; p=4.7 × 10−3) (Figure 7). By contrast, none of the other incubation conditions, altered NGAL production (Figure 7). Notably, rhIL-6 plus TCZ failed to significantly reduce NGAL compared to rhIL-6. In general, the ICC analysis of the various treatment groups for NGAL-positive chondrocytes mirrored results obtained with the NGAL ELISA (data not shown).
Figure 7.
The Effect of Various Incubation Conditions on NGAL Production by C28/I2
Chondrocytes: Anti-NGAL Antibody-Mediated ICC. Among the various incubation conditions, only sIL-6R (p=4.3 × 10−4) and the combination of rhIL-6 + TCZ (p=4.7 x 10−3) significantly reduced the number of NGAL-positive cells compared to the “no additions” control group. However, the combination of rhIL-6 plus TCZ did not decrease the number of NGAL-positive cells compared to rhIL-6 alone. Values are mean ± SD (n=5).
Discussion
The principal objectives of this study were 1) to determine the extent to which rhIL-6 stimulated MMP-9 and NGAL production by immortalized human juvenile chondrocyte lines; and 2) to determine whether sIL-6R or TCZ, the latter a fully humanized IgG1(κ) monoclonal antibody which neutralizes the interaction between IL-6 and IL-6Rα, augmented or inhibited MMP-9 and/or NGAL.
The principal result of MMP-9 ELISA was that rhIL-6 alone stimulated MMP-9 production by C28/I2, but not T/C28a2 immortalized human chondrocytes. In the MMP-9 ELISA, the combination of rhIL-6 and sIL-6R significantly decreased MMP-9 production in C28/I2 chondrocytes and most importantly, the combination of rhIL-6 plus TCZ also reduced MMP-9 compared to rhIL-6. In analyzing both the MMP-9 ELISA and anti-MMP-9 ICC results, we found that the combination of rhIL-6 plus TCZ reduced MMP-9, but TCZ by itself had no effect on chondrocyte-derived MMP-9 compared to rhIL-6. These data strongly suggested that neutralizing the interaction between rhIL-6 and IL-6R with TCZ in vitro reduced C28/I2 chondrocyte MMP-9 production. However, it remains to be determined the extent to which TCZ would be an effective therapeutic strategy to inhibit MMP-9 production by articular chondrocytes in vivo. This should first be tested in well-validated animal models of RA and osteoarthritis. Although TCZ, a potent inhibitor of IL-6-mediated JAK/STAT activation [5] reduced MMP-9 production, TCZ will not be helpful in sorting out which of three potential IL-6 pathways (i.e. IL-6Rα/gp130, mIL-6R, sIL-6R) is altered by TCZ, since TCZ does not selectively inhibit the IL-6Rα/gp130 pathway [14].
Even though we employed an MMP-9 ELISA and an anti-MMP-9 antibody-based ICC quantitative analysis, we could not determine the extent to which any of the various treatment groups altered either or both pro-MMP-9 and/or activated MMP-9 because the anti-MMP-9 antibody used in the ICC analysis does not distinguish between the two enzyme forms. Thus, we have come to recognize this as an important limitation of this study.
It is well known that IL-6 activates the JAK/STAT pathway in many cell types [5], including chondrocytes [4,11]. In that regard, only two previously published in vitro studies were designed to correlate MMP-9 production per se with STAT protein phosphorylation. In one of these studies, Dechow et al. [15] indicated that there appeared to be a “strong” relationship between MMP-9 gene expression and the level of tyrosine phosphorylated STAT3 (termed STAT3c) in primary breast cancer specimens. In the other study, Kothari et al. [16] showed that IL-6-induced macrophage MMP-9 gene expression was mainly dependent on p-ERK1/2 although MMP-9 gene expression was also inhibited by a JAK-dependent product, identified as IL-10. In view of these results, future in vitro experiments should determine the precise relationship between activation of STAT proteins by rhIL-6 and activation of pro-MMP-9 gene activity as well as identifying any cofactors that may also be involved in pro-MMP-9 gene expression [17]. In that regard, it is generally agreed that these studies will have to employ quantitative PCR for assessing MMP-9 gene expression as well as gelatin-impregnated zymography to determine the extent to which pro-MMP-9 is activated under the various culture conditions. In fact, “gelatin-zymography” will be critical to this assessment in the analysis of pro-MMP-9 activation [18]. This is due to the fact that intracellular processing of latent pro-MMP-9 can produce different forms of active gelatinase, with molecular sizes ranging from 64 kDa to 82 kDa.
Finally, rhIL-6 failed to stimulate NGAL production in C28/I2 chondrocytes. This result contrasted with previous results obtained for NGAL production by human osteoarthritis chondrocytes in response to rhIL-1β [9]. This difference may merely represent a differential response of human chondrocytes to IL-1β versus IL-6 or differences between human OA chondrocytes and the C28/I2 human chondrocyte line.
It noteworthy that Zerenga et al. [19] did not find any evidence for NGAL protein in either normal human adult articular cartilage or tracheal cartilage or for that matter, any NGAL produced by cultured rat embryo chondrocytes. However, NGAL protein synthesis could be induced in the rat chondrocyte cultures by lipopolysaccharide (LPS) [19], indicating that the NGAL gene was up-regulated by LPS and potentially by other pro-inflammatory mediators.
Changes in NGAL production were also evaluated following treatment of C28/I2 chondrocytes with rhIL-6 plus sIL-6 or rhIL-6 plus TCZ. Thus, whereas the combination of rhIL-6 and TCZ inhibited MMP-9 production compared to rhIL-6 alone, the results of the anti-NGAL antibody ICC showed that rhIL-6 plus TCZ did not significantly affect NGAL production compared to rhIL-6, although rhIL-6 plus TCZ significantly reduced NGAL compared to the “no additions” control group.
Finally, the results of this in vitro study could have substantive implications for potentially employing TCZ to regulate rhIL-6-induced MMP-9 and/or NGAL production by articular cartilage in various arthritic conditions. Since MMP-9 forms a complex with NGAL with the MMP-9/NGAL complex maintaining MMP-9 in its activated state [10], the putative inability of TCZ to effectively regulate C28/I2 chondrocyte NGAL production may have significance for future translational research. Although sIL-6R as well as the combination of rhIL-6 plus TCZ significantly dampened NGAL production compared to the “no additions” control group, the overall results suggested that employing therapeutic levels of TCZ in arthritic disorders might be most effective for regulating elevated levels of chondrocyte NGAL when NGAL is over-produced in response to the combined effects of other pro-inflammatory cytokines implicated in arthritis which include, IL-1β [4], TNF-α [4], IL-17A [20] and OSM [21].
Acknowledgements
This study was supported by a contract between Genentech/Roche Group, Charles J. Malemud, Ph.D., Principal Investigator, and Case Western Reserve University (CWRU), and by NICHD R01-069819-01A1, Principal Investigator; Sam Mesiano, PhD and NEI P30-EY11373, The CWRU Visual Sciences Core Center Grant; Co-Principal Investigators, Eric Pearlman and Irina Pikuleva,. We thank Scott Howell, Ph.D. for his support in performing the ICC analyses.
Footnotes
Conflicts of interest statement
The authors declare no conflicts of interest.
References
- 1.Malemud CJ. Matrix metalloproteinases (MMPs) in health and disease: an overview. Front Biosci. 2006;11:1696–1701. doi: 10.2741/1915. Crossref. [DOI] [PubMed] [Google Scholar]
- 2.Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:529–543. doi: 10.2741/1817. Crossref. [DOI] [PubMed] [Google Scholar]
- 3.Martel-Pelletier J, Welsch DJ, Pelletier JP. Metalloproteases and inhibitors in arthritic diseases. Best Pract Res Clin Rheumatol. 2001;15:805–829. doi: 10.1053/berh.2001.0195. Crossref. [DOI] [PubMed] [Google Scholar]
- 4.Malemud CJ. Immunotherapies and rheumatoid arthritis-Introduction. J Clin Cell Immunol. 2013 doi: 10.4172/2155-9899.1000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shetty A, Hanson R, Korsten P, Shawagfeh M, Arami S, et al. Tocilizumab in the treatment of rheumatoid arthritis and beyond. Drug Des Devel Ther. 2014;8:349–364. doi: 10.2147/DDDT.S41437. Crossref. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kokenyesi R, Tan L, Robbins JR, Goldring MB. Proteoglycan production by immortalized human chondrocyte cell lines cultured under conditions that promote expression of the differentiated phenotype. Arch Biochem Biophys. 2000;383:79–90. doi: 10.1006/abbi.2000.2044. Crossref. [DOI] [PubMed] [Google Scholar]
- 7.Goldring MB. Culture of immortalized chondrocytes and their use as models of chondrocyte function. Methods Mol Med. 2004;100:37–52. doi: 10.1385/1-59259-810-2:037. Crossref. [DOI] [PubMed] [Google Scholar]
- 8.Finger F, Schörle C, Zien A, Gebhard P, Goldring MB, et al. Molecular phenotyping of human chondrocyte cell lines T/C-28a2, T/C-28a4, and C-28/I2. Arthritis Rheum. 2003;48:3395–3403. doi: 10.1002/art.11341. Crossref. [DOI] [PubMed] [Google Scholar]
- 9.Gupta K, Shukla M, Malemud CJ, Cowland J, Haqqi TM. Neutrophil gelatinase-associated lipocalin (NGAL) is produced by IL-1ß-stimulated human osteoarthritis chondrocytes and protects MMP-9 from degradation. Osteoarthritis Cartilage 14 Suppl B. 2006:S87–S88. [Google Scholar]
- 10.Gupta K, Shukla M, Cowland JB, Malemud CJ, Haqqi TM. Neutrophil gelatinase-associated lipocalin is expressed in osteoarthritis and forms a complex with matrix metalloproteinase 9. Arthritis Rheum. 2007;56:3326–3335. doi: 10.1002/art.22879. Crossref. [DOI] [PubMed] [Google Scholar]
- 11.Aida Y, Honda K, Tanigawa S, Nakayama G, Matsumura H, et al. IL-6 and soluble IL-6 receptor stimulate the production of MMPs and their inhibitors via JAKSTAT and ERK-MAPK signalling in human chondrocytes. Cell Biol Int. 2012;36:367–376. doi: 10.1042/CBI20110150. Crossref. [DOI] [PubMed] [Google Scholar]
- 12.Kotake S, Sato K, Kim KJ, Takahashi N, Udagawa N, et al. Interleukin-6 and soluble interleukin-6 receptors in the synovial fluids from rheumatoid arthritis patients are responsible for osteoclast-like cell formation. J Bone Miner Res. 1996;11:88–95. doi: 10.1002/jbmr.5650110113. Crossref. [DOI] [PubMed] [Google Scholar]
- 13.Haq M, Shaeii AE, Zervos EE, Rosemurgy AS. In vitro and in vivo matrix metalloproteinase production by pancreatic cancer cells and by distant organs. Int J Surg Investig. 2000;1:459–465. Crossref. [PubMed] [Google Scholar]
- 14.Rose-John S, Waetzig GH, Scheller J, Grötzinger J, Seegert D. The IL-6/sIL-6R complex as a novel target for therapeutic approaches. Expert Opin Ther Targets. 2007;11:613–624. doi: 10.1517/14728222.11.5.613. Crossref. [DOI] [PubMed] [Google Scholar]
- 15.Dechow TN, Pedranzini L, Leitch A, Leslie K, Gerald WL, et al. Requirement of matrix metalloproteinase-9 for the transformation of human mammary epithelial cells by Stat3-C. Proc Natl Acad Sci U S A. 2004;101:10602–10607. doi: 10.1073/pnas.0404100101. Crossref. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kothari P, Pestana R, Mesraoua R, Elchaki R, Khan KM, et al. IL-6-mediated induction of matrix metalloproteinase-9 is modulated by JAK-dependent IL-10 expression in macrophages. J Immunol. 2014;192:349–357. doi: 10.4049/jimmunol.1301906. Crossref. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malemud CJ, Pearlman E. Targeting JAK/STAT signaling pathway in inflammatory diseases. Curr Signal Transduct Ther. 2009;4:201–221. [Google Scholar]
- 18.Toth M1, Sohail A, Fridman R. Assessment of gelatinases (MMP-2 and MMP-9) by gelatin zymography. Methods Mol Biol. 2012;878:121–135. doi: 10.1007/978-1-61779-854-2_8. Crossref. [DOI] [PubMed] [Google Scholar]
- 19.Zerenga B, Cermelli S, Michelis B, Cancedda R, Cancedda FD, et al. Expression of NRL/NGAL (neu-related lipocalin/neutrophil gelatinase-associated lipocalin) during mammalian embryonic development and in inflammation. Eur J Cell Biol. 2000;79(3):165–172. doi: 10.1078/s0171-9335(04)70019-9. Crossref. [DOI] [PubMed] [Google Scholar]
- 20.Moran EM, Mullan R, McCormick J, Connolly M, Sullivan O, et al. Human rheumatoid arthritis tissue production of IL-17A drives matrix and cartilage degradation: synergy with tumour necrosis factor-alpha, oncostatin M and response to biologic therapies. Arthritis Res Ther. 2009;11(4):R113. doi: 10.1186/ar2772. Crossref. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li WQ, Dehnade F, Zafarullah M. Oncostatin M-induced matrix metalloproteinase and tissue inhibitor of metalloproteinase-3 genes expression in chondrocytes requires Janus kinase/STAT signaling pathway. J Immunol. 2001;166(5):3491–3498. doi: 10.4049/jimmunol.166.5.3491. Crossref. [DOI] [PubMed] [Google Scholar]