Key Clinical Message
Insulin detemir is commonly used in obese patients with diabetes mellitus as it is considered hepatoselective and causes less weight gain. We describe a relative lack of effectiveness of detemir in patients with significant NAFLD and hypertriglyceridemia, compared to isophane insulin. This may affect how such patients are managed with insulin.
Keywords: detemir, hepatoselective, NAFLD, hypertriglyceridaemia
Background
Insulin detemir has now become established as an effective basal insulin preparation and is widely used in the management of both type 1 and type 2 diabetes. Although it is similar in efficacy to isophane insulin, we have observed a relative lack of efficacy in two patients with significant hypertriglyceridemia and established nonalcoholic fatty liver disease, suggesting that there are circumstances where the efficacy of detemir may be adversely affected by specific metabolic parameters.
Case Report
Patient 1 was a 56‐year‐old Caucasian man with a 4‐year history of poorly controlled type 2 diabetes and marked hypertriglyceridemia (random triglycerides were 28.7 mmol/L and total cholesterol was 7.9 mmol/L at diagnosis). His mother had type 2 diabetes, but there was no other family history of note. He denied alcohol consumption for the previous 14 years and had been only a light drinker previously. At the time of starting insulin he was being treated with metformin, gliclazide, fenofibrate, and omacor.
On examination, he was obese with a body weight of 109 kg and BMI of 32 kg/m2. He had eruptive xanthomata and evidence of splenomegaly but no hepatomegaly. Glycated hemoglobin (HbA1c) was 69 mmol/mol (8.5%), fasting triglycerides 8.4 mmol/L, and total cholesterol 4.5 mmol/L. Serum creatinine was 110 μmol/L (eGFR 102 mL/min/1.73 m2) and liver enzymes were slightly elevated: AST 71 iU/L (ref 10–50) and γGT 60 iU/L (1–55). Serum albumin was normal 47 g/dL (35–50), INR 1.04, and hepatitis serology was negative. Ultrasound confirmed a 17.7‐cm spleen and CT demonstrated nonalcoholic fatty liver disease (NAFLD). In view of splenomegaly, he underwent a bone marrow aspirate and trephine biopsy, both of which were normal. A liver biopsy confirmed hepatosteatosis with marked steatosis, mild inflammatory activity, and hepatocellular ballooning (Fig. 1A). In part of the biopsy, bridging fibrosis with parenchymal nodule formation was in keeping with cirrhotic transformation (Fig. 1B).
Figure 1.
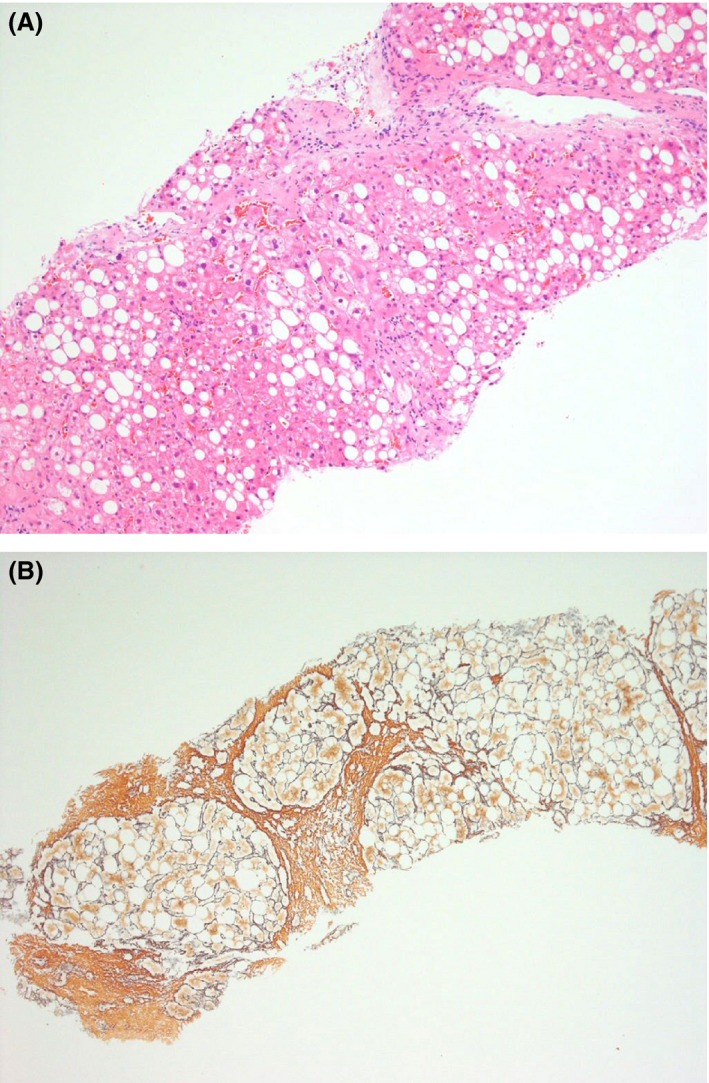
(A) Liver with marked steatosis and hepatocellular ballooning (H&E). (B) Reticulin stain (Gordon and Sweet) reveals, in part of the biopsy core, bridging fibrosis with parenchymal nodule formation in keeping with cirrhotic transformation.
Insulin detemir (14 units nocte) was commenced as his random glucose values were 17–22 mmol/L. The dose was rapidly titrated upward. Within five months he was using 158 units per day of detemir, in divided doses, plus insulin aspart 24 units with each meal. Despite these doses, his capillary glucose values were consistently more than 10 mmol/L and HbA1c remained unsatisfactory at 69 mmol/mol (8.5%). Basal insulin was then changed to insulatard 70 units per day in divided doses (insulin aspart dose unchanged) and within four months his HbA1c had fallen to 51 mmol/mol (6.8%) and by 12 months he had lost 6 kg of weight.
Patient 2 was a 68‐year‐old man with a 20‐year history of type 2 diabetes and type V hyperlipidemia. For two years, after the diagnosis of diabetes, he was managed with oral hypoglycemic agents alone. He then developed necrotizing pancreatitis (considered secondary to hypertriglyceridemia) and was treated with human isophane insulin and human soluble insulin for ten years before being switched to detemir and novorapid. He was referred due to the patient's concern of escalating insulin dose and associated weight gain of 20 kg over the previous five years. At the time of referral the detemir dose was 120 units twice daily and novorapid 120 units with each meal (total of 600 units/day). HbA1c was 55 mmol/mol (7.2%). He did not drink alcohol.
On examination he was obese with body weight of 126 kg and BMI of 44.6 kg/m2. Random triglycerides were markedly elevated (31.9 mmol/L), total cholesterol was 6.3 mmol/L, and HDL was 0.3 mmol/L. There was no clinical or biochemical evidence of any endocrinopathy to explain insulin resistance and no evidence of immune‐mediated insulin resistance. Liver enzymes and synthetic function were normal but there was mild renal impairment (serum creatinine 145 μmol/L; eGFR 77mL/min/1.73 m2). Liver ultrasound showed extensive fat infiltration consistent with NAFLD. Detemir was changed to isophane insulin 100 units BD and novorapid reduced to 100 units/meal. After six months, the dose of insulatard had stabilized at 140 units BD and Novorapid 50 units TDS (430 units/day – 30% reduction in total daily dose), his weight remained unchanged and despite the reduction in insulin dose, HbA1c improved to 50 mmol/mol (6.7%).
Discussion
The two cases presented in this report showed that in patients with a history of severe hypertriglyceridemia and significant fat infiltration of the liver, a hepatoselective insulin (detemir) appeared less efficacious in achieving glycemic control. Very high insulin doses were required and weight gain was problematic.
Insulin detemir differs from human insulin by the deletion of the amino acid threonine in position 30 of the B chain, plus the addition of a C14 fatty acid chain at position 29 of the B chain. This allows detemir to reversibly bind to serum albumin 1 and is a characteristic that has led to it being considered a hepatoselective insulin: the albumin–insulin molecule is unable to pass through the capillary endothelial cell barrier to reach peripheral adipocytes, whereas in the liver, the albumin–detemir molecule is able to pass freely through the sinusoids. This allows it to exert a greater effect on hepatocytes than in peripheral tissues 2.
Hypothetically, the efficacy of hepatoselective insulin may be reduced in NAFLD by less hepatic exposure to insulin (due to increased insulin clearance or to portosystemic shunting), or from direct hepatic parenchymal cell damage. Given that the usual action of insulin is to stimulate the liver to store glucose in the form of glycogen and to switch off gluconeogenesis, underexposure of hepatocytes to insulin would lead to hyperglycemia.
A portosystemic shunt was thought to be unlikely in these patients as there were no radiological features of collateral (variceal) circulation. Increased clearance of detemir might have occurred; premarketing studies suggested that hepatic impairment affected the bioavailability of insulin. In these studies there was a 35–47% faster clearance of detemir in patients with severe/moderate liver insufficiency as compared to healthy subjects 3. By contrast, clearance of human insulin is usually reduced in liver failure 4 and insulin clamp studies with human insulin have shown decreased clearance in patients with fat infiltration of the liver 5.
In a mouse model of NAFLD (induced by high‐fat feeding), there was decreased insulin activation of glycogen synthase and increased gluconeogenesis 6 during a euglycemic–hyperinsulinemic clamp, suggesting a direct, adverse consequence of hepatic fat accumulation. There are no equivalent data for human NAFLD. The closest model available is of detemir's action in obesity where the presence of NAFLD may be inferred 7. A pharmacodynamic study of 18 subjects showed that in the presence of Grade 1 obesity (BMI 30–35 kg/m2) a lower glucose infusion rate was required to maintain normoglycemia after administration of detemir, compared to Neutral Protamine Hagedorn (NPH) insulin or glargine 8. This suggests reduced efficacy in obesity. However, it was not reported whether the participants in this study had NAFLD. Advanced hepatocellular fibrosis may be associated with a change in the configuration of the sinusoidal endothelium, including loss of fenestrations, which would reduce the exchanges between hepatocytes and sinusoidal blood. Therefore, this would suggest that in severe NAFLD/cirrhosis, higher doses of detemir insulin would be required.
An alternative hypothesis is that hypertriglyceridemia may have a direct, adverse effect on detemir action. The binding of detemir to albumin could be affected by the lipid content of plasma. Competition experiments with fatty acids have shown that levemir can be displaced from human albumin by long‐chain fatty acids (containing at least 12 carbon atoms) but with much weaker competition by a medium chain fatty acid 9. The significance of this is uncertain, but potentially it could lead to loss of biological activity, or unpredictable release or clearance of levemir.
Detemir‐specific insulin antibodies were not measured in the two cases. Antibody development has been observed in phase 3 clinical trials of detemir, but did not impact on metabolic control 10.
Hypertriglyceridemia and NAFLD in these cases were attributed to insulin resistance secondary to obesity. There were no specific features of alternative cause(s) for a relative lack of efficacy of insulin, such as lysosomal storage disease or monogenic insulin resistance syndromes ‐ and these were not tested for. It is possible that the cases described relate to less common etiologies of insulin resistance.
In managing patients with diabetes, interindividual differences in response to insulin may be encountered; a relative lack of efficacy with one type of insulin may not be seen with an alternative insulin. Such a response may be unrelated to the degree of hepatoselectivity of the insulin chosen.
In conclusion, these two cases suggest that detemir may be less efficacious in patients with significant hypertriglyceridemia complicated by NAFLD. It is not possible to determine a precise mechanism to explain this observation but this could be due primarily to hypertriglyceridemia or due to the effects of infiltration of hepatic parenchyma with lipid.
Conflict of Interest
None declared.
Clinical Case Reports 2016; 4(1): 83–86
References
- 1. Olsen, H. B. , and Kaarsholm N. C.. 2000. Structural effects of protein lipidation as revealed by LysB29‐myristoyl, des(B30) insulin. Biochemistry 39:11893–11900. [DOI] [PubMed] [Google Scholar]
- 2. Hordern, S. V. , Wright J. E., Umpleby A. M., Shojaee‐Moradie F., Amiss J., and Russell‐Jones D. L.. 2005. Comparison of the effects on glucose and lipid metabolism of equipotent doses of insulin detemir and NPH insulin with a 16‐h euglycaemic clamp. Diabetologia 48:420–426. [DOI] [PubMed] [Google Scholar]
- 3. Health Canada . Summary basis of decision. 2005. http://www.hc-sc.gc.ca/dhp-mps/prodpharma/sbd-smd/drug-med/sbd_smd_2006_levemir_081683-eng.php (accessed 10 Dec 2014).
- 4. Letiexhe, M. R. , Scheen A. J., Gerard P. L., Bastens B. H., Pirotte J., Belaiche J., et al. 1993. Insulin secretion, clearance, and action on glucose metabolism in cirrhotic patients. J. Clin. Endocrinol. Metab. 77:1263–1268. [DOI] [PubMed] [Google Scholar]
- 5. Kotronen, A. , Vehkavaara S., Seppala‐Lindroos A., Bergholm R., and Yki‐Jarvinen H.. 2007. Effect of liver fat on insulin clearance. Am. J. Physiol. Endocrinol. Metab. 293:E1709–E1715. [DOI] [PubMed] [Google Scholar]
- 6. Samuel, V. T. , Liu Z. X., Qu X., Elder B. D., Bilz S., Befroy D., et al. 2004. Mechanism of hepatic insulin resistance in non‐alcoholic fatty liver disease. J. Biol. Chem. 279:32345–32353. [DOI] [PubMed] [Google Scholar]
- 7. Ruhl, C. E. , and Everhart J. E.. 2003. Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology 124:71–79. [DOI] [PubMed] [Google Scholar]
- 8. Porcellati, F. , Lucidi P., Rossetti P., Candeloro P., Andreoli A. M., Marzotti S., et al. 2011. Differential effects of adiposity on pharmacodynamics of basal insulins NPH, glargine, and detemir in type 2 diabetes mellitus. Diabetes Care 34:2521–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kurtzhals, P. , Havelund S., Jonassen I., and Markussen J.. 1997. Effect of fatty acids and selected drugs on the albumin binding of a long‐acting, acylated insulin analogue. J. Pharm. Sci. 86:1365–1368. [DOI] [PubMed] [Google Scholar]
- 10. Bartley, P. C. , Bogoev M., Larsen J., and Philotheou A.. 2008. Long‐term efficacy and safety of insulin detemir compared to Neutral Protamine Hagedorn insulin in patients with Type 1 diabetes using a treat‐to‐target basla‐bolus regimen with insulin aspart at meals: a 2‐year, randomised, controlled trial. Diabet. Med. 25:442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]


