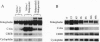Abstract
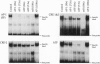
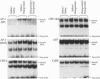
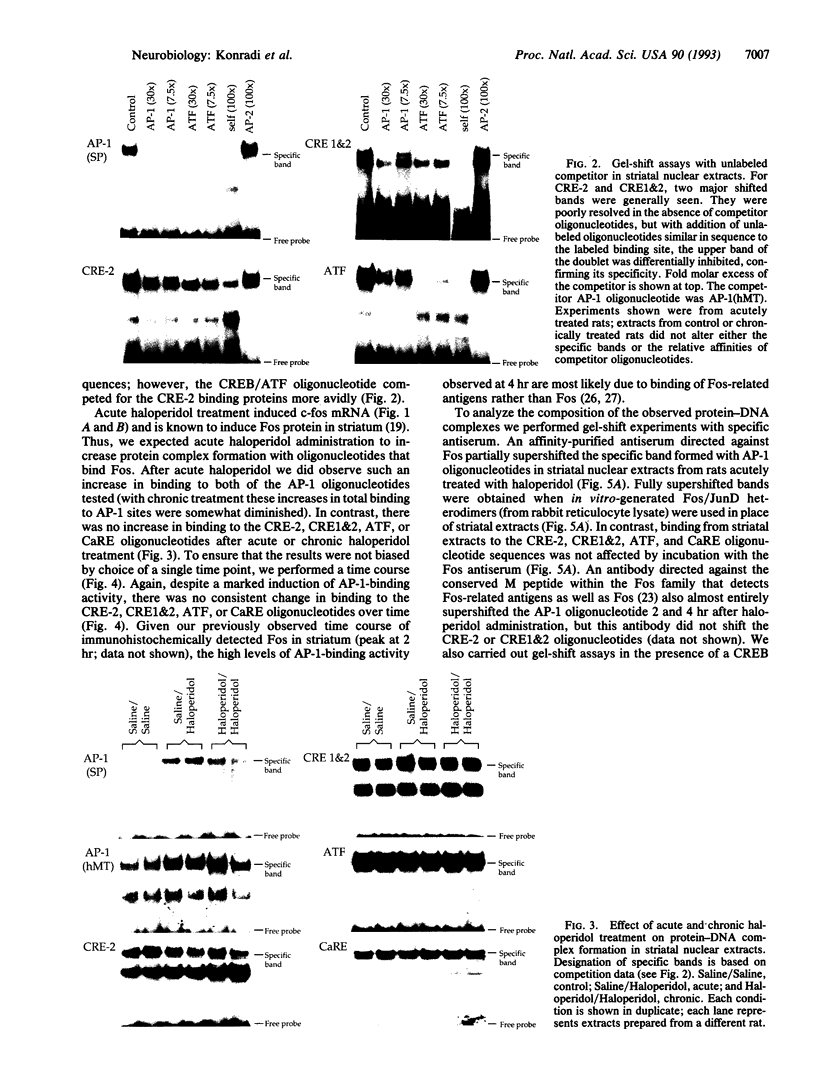
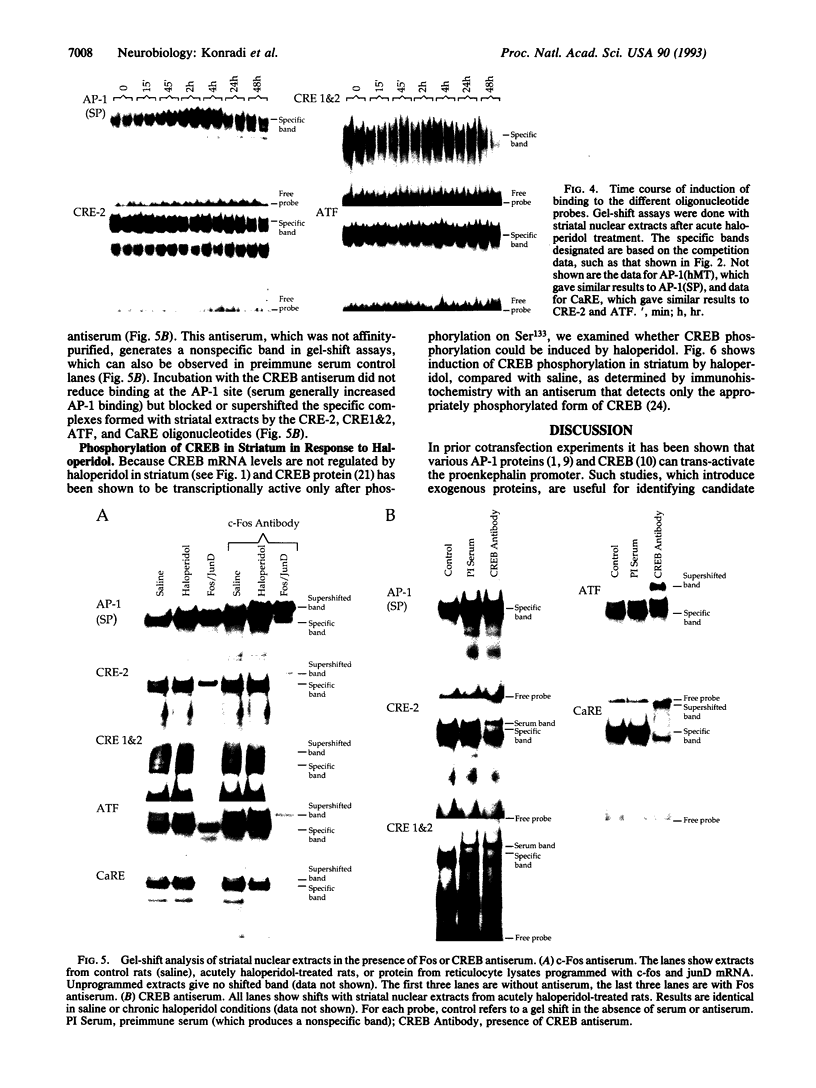
The proenkephalin gene is a well-studied model of transcription factor-target gene interaction in the nervous system and has been proposed as a regulatory target of the protein product of the immediate-early gene c-fos. This regulatory mechanism has been proposed, in part, because the cAMP response element 2 (CRE-2) site, the key DNA regulatory element within the proenkephalin second-messenger-inducible enhancer, avidly binds AP-1 proteins, including Fos, in vitro. However, we observe a dissociation in the time course of activation of c-fos and proenkephalin mRNA in rat striatum after administration of the dopamine D2 receptor antagonist haloperidol. This result prompted us to investigate the composition of protein complexes in striatal nuclear extracts that bind to the CRE-2 site. Even though our striatal nuclear extracts had substantial basal and haloperidol-inducible AP-1-binding activities that contained Fos, we could not detect Fos in complexes bound to the CRE-2 element. Instead, as determined by antibody supershift analysis, we detect CRE-binding protein (CREB)-like proteins binding to CRE-2 in both basal and haloperidol-stimulated conditions. Finally, we show that haloperidol induces CREB protein phosphorylation in striatum.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cohen D. R., Ferreira P. C., Gentz R., Franza B. R., Jr, Curran T. The product of a fos-related gene, fra-1, binds cooperatively to the AP-1 site with Jun: transcription factor AP-1 is comprised of multiple protein complexes. Genes Dev. 1989 Feb;3(2):173–184. doi: 10.1101/gad.3.2.173. [DOI] [PubMed] [Google Scholar]
- Comb M., Birnberg N. C., Seasholtz A., Herbert E., Goodman H. M. A cyclic AMP- and phorbol ester-inducible DNA element. 1986 Sep 25-Oct 1Nature. 323(6086):353–356. doi: 10.1038/323353a0. [DOI] [PubMed] [Google Scholar]
- Comb M., Mermod N., Hyman S. E., Pearlberg J., Ross M. E., Goodman H. M. Proteins bound at adjacent DNA elements act synergistically to regulate human proenkephalin cAMP inducible transcription. EMBO J. 1988 Dec 1;7(12):3793–3805. doi: 10.1002/j.1460-2075.1988.tb03264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragunow M., Robertson G. S., Faull R. L., Robertson H. A., Jansen K. D2 dopamine receptor antagonists induce fos and related proteins in rat striatal neurons. Neuroscience. 1990;37(2):287–294. doi: 10.1016/0306-4522(90)90399-o. [DOI] [PubMed] [Google Scholar]
- Draisci G., Iadarola M. J. Temporal analysis of increases in c-fos, preprodynorphin and preproenkephalin mRNAs in rat spinal cord. Brain Res Mol Brain Res. 1989 Jul;6(1):31–37. doi: 10.1016/0169-328x(89)90025-9. [DOI] [PubMed] [Google Scholar]
- Gerfen C. R., Engber T. M., Mahan L. C., Susel Z., Chase T. N., Monsma F. J., Jr, Sibley D. R. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990 Dec 7;250(4986):1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Ginty D. D., Kornhauser J. M., Thompson M. A., Bading H., Mayo K. E., Takahashi J. S., Greenberg M. E. Regulation of CREB phosphorylation in the suprachiasmatic nucleus by light and a circadian clock. Science. 1993 Apr 9;260(5105):238–241. doi: 10.1126/science.8097062. [DOI] [PubMed] [Google Scholar]
- Hope B., Kosofsky B., Hyman S. E., Nestler E. J. Regulation of immediate early gene expression and AP-1 binding in the rat nucleus accumbens by chronic cocaine. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5764–5768. doi: 10.1073/pnas.89.13.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggenvik J. I., Collard M. W., Stofko R. E., Seasholtz A. F., Uhler M. D. Regulation of the human enkephalin promoter by two isoforms of the catalytic subunit of cyclic adenosine 3',5'-monophosphate-dependent protein kinase. Mol Endocrinol. 1991 Jul;5(7):921–930. doi: 10.1210/mend-5-7-921. [DOI] [PubMed] [Google Scholar]
- Hyman S. E., Comb M., Lin Y. S., Pearlberg J., Green M. R., Goodman H. M. A common trans-acting factor is involved in transcriptional regulation of neurotransmitter genes by cyclic AMP. Mol Cell Biol. 1988 Oct;8(10):4225–4233. doi: 10.1128/mcb.8.10.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman S. E., Comb M., Pearlberg J., Goodman H. M. An AP-2 element acts synergistically with the cyclic AMP- and phorbol ester-inducible enhancer of the human proenkephalin gene. Mol Cell Biol. 1989 Jan;9(1):321–324. doi: 10.1128/mcb.9.1.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobierski L. A., Chu H. M., Tan Y., Comb M. J. cAMP-dependent regulation of proenkephalin by JunD and JunB: positive and negative effects of AP-1 proteins. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10222–10226. doi: 10.1073/pnas.88.22.10222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. C. Induction of c-fos mRNA expression in rat striatum by neuroleptic drugs. J Neurochem. 1990 Apr;54(4):1453–1455. doi: 10.1111/j.1471-4159.1990.tb01983.x. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Bilezikjian L. M. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature. 1987 Jul 9;328(6126):175–178. doi: 10.1038/328175a0. [DOI] [PubMed] [Google Scholar]
- Nguyen T. V., Kosofsky B. E., Birnbaum R., Cohen B. M., Hyman S. E. Differential expression of c-fos and zif268 in rat striatum after haloperidol, clozapine, and amphetamine. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4270–4274. doi: 10.1073/pnas.89.10.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishina H., Sato H., Suzuki T., Sato M., Iba H. Isolation and characterization of fra-2, an additional member of the fos gene family. Proc Natl Acad Sci U S A. 1990 May;87(9):3619–3623. doi: 10.1073/pnas.87.9.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normand E., Popovici T., Onteniente B., Fellmann D., Piatier-Tonneau D., Auffray C., Bloch B. Dopaminergic neurons of the substantia nigra modulate preproenkephalin A gene expression in rat striatal neurons. Brain Res. 1988 Jan 26;439(1-2):39–46. doi: 10.1016/0006-8993(88)91459-x. [DOI] [PubMed] [Google Scholar]
- Robertson G. S., Vincent S. R., Fibiger H. C. D1 and D2 dopamine receptors differentially regulate c-fos expression in striatonigral and striatopallidal neurons. Neuroscience. 1992 Jul;49(2):285–296. doi: 10.1016/0306-4522(92)90096-k. [DOI] [PubMed] [Google Scholar]
- Romano G. J., Shivers B. D., Harlan R. E., Howells R. D., Pfaff D. W. Haloperidol increases proenkephalin mRNA levels in the caudate-putamen of the rat: a quantitative study at the cellular level using in situ hybridization. Brain Res. 1987 Apr;388(1):33–41. doi: 10.1016/0169-328x(87)90018-0. [DOI] [PubMed] [Google Scholar]
- Sabol S. L., Yoshikawa K., Hong J. S. Regulation of methionine-enkephalin precursor messenger RNA in rat striatum by haloperidol and lithium. Biochem Biophys Res Commun. 1983 Jun 15;113(2):391–399. doi: 10.1016/0006-291x(83)91739-4. [DOI] [PubMed] [Google Scholar]
- Sheng M., McFadden G., Greenberg M. E. Membrane depolarization and calcium induce c-fos transcription via phosphorylation of transcription factor CREB. Neuron. 1990 Apr;4(4):571–582. doi: 10.1016/0896-6273(90)90115-v. [DOI] [PubMed] [Google Scholar]
- Sonnenberg J. L., Rauscher F. J., 3rd, Morgan J. I., Curran T. Regulation of proenkephalin by Fos and Jun. Science. 1989 Dec 22;246(4937):1622–1625. doi: 10.1126/science.2512642. [DOI] [PubMed] [Google Scholar]
- Tang F., Costa E., Schwartz J. P. Increase of proenkephalin mRNA and enkephalin content of rat striatum after daily injection of haloperidol for 2 to 3 weeks. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3841–3844. doi: 10.1073/pnas.80.12.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nguyen T., Kobierski L., Comb M., Hyman S. E. The effect of depolarization on expression of the human proenkephalin gene is synergistic with cAMP and dependent upon a cAMP-inducible enhancer. J Neurosci. 1990 Aug;10(8):2825–2833. doi: 10.1523/JNEUROSCI.10-08-02825.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorn P., Roest G., Groenewegen H. J. Increase of enkephalin and decrease of substance P immunoreactivity in the dorsal and ventral striatum of the rat after midbrain 6-hydroxydopamine lesions. Brain Res. 1987 Jun 2;412(2):391–396. doi: 10.1016/0006-8993(87)91149-8. [DOI] [PubMed] [Google Scholar]
- Young W. S., 3rd, Bonner T. I., Brann M. R. Mesencephalic dopamine neurons regulate the expression of neuropeptide mRNAs in the rat forebrain. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9827–9831. doi: 10.1073/pnas.83.24.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]