Abstract
Experiments outlined here investigate the role of nitric oxide (NO) in the pathogenesis of Plasmodium falciparum-induced malarial anemia (MA). The results show that ex vivo and in vitro NO synthase (NOS) activity in peripheral blood mononuclear cells (PBMCs) is significantly elevated in children with MA and inversely associated with hemoglobin levels. Additional experiments using PBMCs from non-malaria-exposed donors demonstrate that physiologic amounts of P. falciparum-derived hemozoin augment NOS type 2 (NOS2) transcripts and NO production. Results of these experiments illustrate that elevated NO production in children with MA is associated with decreased hemoglobin concentrations and that hemozoin can induce NOS2-derived NO formation in cultured blood mononuclear cells.
A lack of acquired immunity to Plasmodium falciparum malaria in young children appears to underlie the high rates of morbidity and mortality from malaria in areas of sub-Saharan Africa where malaria is endemic (7). In areas of high transmission, the predominate manifestations of severe malaria are hyperparasitemia and malarial anemia (MA) (7). Although the molecular mechanisms responsible for effective malarial immunity remain elusive, production of nitric oxide (NO) appears to be an important marker and potential mediator of disease severity. Previous studies show that elevated levels of NO metabolites (NO2− plus NO3− [NOx]) in plasma are associated with an enhanced parasite clearance time in Gabonese adults and children with malaria (14). In addition, previous results show that healthy, malaria-exposed Gabonese children with a history of mild malaria have significantly elevated levels of peripheral blood mononuclear cell (PBMC) NO production and nitric oxide synthase (NOS) enzyme activity compared to their age-matched cohorts with a history of severe malaria (19). Protection against severe malaria in this population of children appears to be, at least in part, related to a polymorphism in the NOS type 2 gene (NOS2, inducible NOS), which produces high levels of NO during an inflammatory event. For example, it was recently shown that a single-nucleotide polymorphism in the promoter region of the NOS2 gene (NOS2Lamberéné [NOS2 G954C]) is associated with increased in vivo and in vitro baseline NO production and protection against malaria (15, 16). Although increased NO production appears to be associated with protection against malaria in the Gabonese children that were previously investigated, elevated levels of NO can suppress erythropoiesis (26) and induce apoptosis in cultured CD34+ cells (23). Moreover, since the studies examining the functional significance of the NOS2Lamberéné polymorphism were conducted with healthy children, we extended our previous investigations by examining the association between NO production and anemia during acute P. falciparum malaria.
(Portions of this work were presented previously [C. C. Keller, P. G. Kremsner, J. B. Hittner, M. A. Misukonis, J. B. Weinberg, and D. J. Perkins, Abstr. 52nd Ann. Meet. Am. Soc. Trop. Med. Hyg., abstr. 300, 2003].)
NOS enzyme activity in ex vivo PBMCs.
To determine if NO production is altered in children with MA, NOS enzyme activity was measured in ex vivo PBMCs from healthy, malaria-exposed children (n = 26) and children with mild (n = 19) or severe (n = 14) malaria according to previously described methods (32). PBMCs were selected for investigation because monocytes are a primary source of NO during blood stage malaria. Furthermore, NOS enzyme activity was selected as the index for determining NO production, since this assay, unlike that for plasma NOx, is not influenced by dietary intake of nitrates. Participants were recruited from a longitudinal prospective study at the Albert Schweitzer Hospital in Lambaréné, Gabon, in the province of Moyen-Ogooue. Severe malaria cases were defined according to World Health Organization guidelines (>250,000 parasites/μl of blood and/or the presence of severe anemia, i.e., ≤5 g of hemoglobin [Hb] per dl of blood). Mild malaria cases were defined as those in which patients had <100,000 parasites/μl of blood and an absence of any signs or symptoms of severe malaria. Routine clinical evaluations and laboratory measures were used to evaluate the subjects; all blood samples were obtained prior to treatment with antimalarials and/or antipyretics. Children with acute malaria were given antimalarials and the appropriate supportive therapy as required. Healthy children were defined as those participants with a previous episode(s) of malaria and the absence of a positive thick blood film for malaria, or any other illnesses, within the previous 4 weeks. Informed consent was obtained from the parents of all participating children. The study was approved by the ethics committees of the International Foundation of the Albert Schweitzer Hospital in Lambaréné, the University of Tübingen, Duke University Medical Center Investigational Review Board, and the University of Pittsburgh Investigational Review Board.
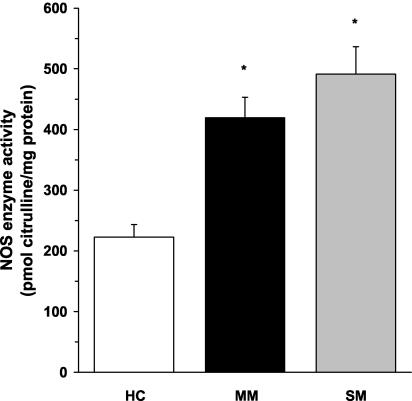
As shown in Fig. 1, NOS enzyme activity was significantly higher in ex vivo PBMCs from children with mild (P < 0.01) and severe (P < 0.01) malaria than in healthy children. Although the severe-malaria group had higher NOS enzyme activity than the mild-malaria group, the difference between the two groups was not significant (P = 0.14) (Fig. 1). As a control, PBMC lysates were incubated with specific (L-NIL) and nonspecific (L-NMMA) NOS2 inhibitors, which demonstrated that the NOS enzyme activity in the assays was NOS2 specific. Taken together, these experiments provide the first evidence illustrating that NOS enzyme activity is significantly elevated in circulating mononuclear cells from children with acute MA.
FIG. 1.
NOS enzyme activity in ex vivo PBMCs. Venous blood (3 ml) was obtained and PBMCs were collected from healthy Gabonese children (HC; n = 26) and children with mild malaria (MM; n = 19) or severe malaria (SM; n = 14). Cell lysates were prepared, and ex vivo NOS enzyme activity (in picomoles of citrulline per milligram of protein) was determined by measuring the conversion of l-[14C]arginine to l-[14C]citrulline. The graph shows the means ± the standard error of the means (SEM) of results from each of the groups. Statistical significance was determined by the Mann-Whitney U test. *, P value of <0.01 compared to results for HC.
Association of NOS enzyme activity with MA.
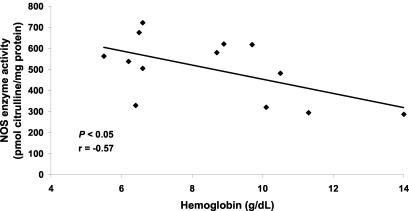
To further assess the relationship between elevated NO production and MA, we examined the association between ex vivo NOS enzyme activity and Hb levels in children with mild and severe MA. There was a significant inverse correlation between NOS enzyme activity and Hb concentration (r = −0.57, P < 0.05) (Fig. 2), illustrating that elevated PBMC NO production is associated with MA. Consistent with previous observations of asymptomatic, malaria-exposed children (4), the significant association between elevated NO production and decreased Hb levels in children with acute malaria shown here illustrates that increased NO production may be involved in the pathogenesis of MA. Since NO can inhibit erythropoiesis (26) and induce apoptosis (23) in hematopoietic precursors, we postulate that excessively high levels of NO during acute malaria may contribute to suppression of erythropoiesis. However, based on the present study design, a noncausal relationship between increased NO production and decreased Hb concentrations in children with MA cannot be ruled out.
FIG. 2.
Association of NOS enzyme activity with Hb. Venous blood (3 ml) was obtained and PBMCs were collected from children with malaria (n = 13), and cell lysates were prepared for ex vivo NOS enzyme activity determination (in picomoles of citrulline per milligram of protein). Hb was measured with a Hemocue. Regression analysis was used to examine the relationship between NOS enzyme activity and Hb levels.
Baseline and stimulated levels of NOS enzyme activity in cultured PBMCs.
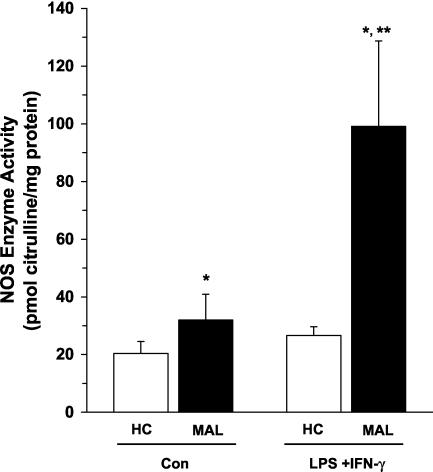
Since elevated ex vivo PBMC NOS enzyme activity could arise from stimulation by both host-derived inflammatory cytokines and parasite products, PBMCs isolated from children with and without malaria were cultured for 7 days according to previously described methods (33). Culturing for this length of time should remove the influence of the in vivo milieu on NO production. Briefly, PBMCs were plated in Dulbecco's modified Eagle's medium supplemented with 10 mM HEPES, 10 mM penicillin-streptomycin, and 10% pooled human serum (heat inactivated at 56°C for 30 min). PBMCs from healthy children (n = 26) and children with MA (n = 14; Hb levels ranging from 6.2 to 10.7 g/dl) were cultured under baseline conditions (medium alone [controls]) and following treatment with NO-inducing stimuli (lipopolysaccharide [LPS; 100 ng/ml; Alexis Corp., San Diego, Calif.] and gamma interferon [IFN-γ; 200 U/ml; BD Pharmingen, San Diego, Calif.]). PBMC cultures from children with severe MA (with an Hb level of <5.0 g/dl) were not prepared, since the anemia precluded drawing enough blood for our in vitro experimental design. It was previously shown that stimulation of cultured human PBMCs with LPS and IFN-γ increases NO production in culture supernatants through augmentation of NOS enzyme activity in immune-activated patients with rheumatoid arthritis (28). Consistent with findings for chronic inflammatory disease, children with malaria had significantly higher baseline (P < 0.05) and LPS- and IFN-γ-promoted (P < 0.01) NOS enzyme activity than malaria-exposed healthy control children (Fig. 3). However, LPS and IFN-γ stimulation failed to increase NOS enzyme activity in PBMCs from healthy children. This may be a consequence of the absence of in vivo immune activation and/or priming of PBMCs in healthy children. As noted above, specific (L-NIL) and nonspecific (L-NMMA) NOS2 inhibitors were used to demonstrate that the NOS enzyme activity was NOS2 specific. These results demonstrate that cultured PBMCs from children with MA have significantly elevated baseline and stimulated NO production during acute disease.
FIG. 3.
NOS enzyme activity in cultured PBMCs. Venous blood (3 ml) was obtained and PBMCs were collected from healthy Gabonese children (HC; n = 26) and children with malaria (MAL; n = 14). PBMCs (106 cells/ml) were cultured for 7 days with medium alone (control [Con]) or LPS (100 ng/ml) and IFN-γ (200 U/ml). Cell lysates were prepared from cultured PBMCs, and NOS enzyme activity (in picomoles of citrulline per milligram of protein) was determined. The graph shows means ± SEM of results for each of the groups. Statistical significance was determined by the Mann-Whitney U test. *, P value of <0.05 compared to results for HC; **, P value of <0.01 compared to results with unstimulated PBMCs from children with malaria.
Estimated hemozoin concentrations in children with malaria.
Since PBMCs from children with MA were cultured for 7 days and therefore no longer influenced by positive and negative in vivo NOS2 regulatory factors (e.g., cytokines), we postulated that a malarial product(s) encountered in vivo may be responsible for elevated levels of NOS enzyme activity in children with acute malaria. Based on previous studies illustrating that the in vivo acquisition of hemozoin (malarial pigment) alters the production of cytokines and effector molecules (17, 20), we investigated hemozoin as a potential mediator of augmented NO production in children with MA. Since there are presently no reports defining the concentrations of hemozoin in phagocytic cells in children with various degrees of malaria disease severity, we estimated the hemozoin content in children with mild and severe malaria to ensure that the amount of hemozoin used for the in vitro experiments was physiologically relevant. Table 1 illustrates our method for calculating the concentration of hemozoin in circulating blood in children with malaria. The geometric mean parasitemia level used for our calculations was based on those of previous studies of Gabonese children with mild and severe malaria (21). The concentration of hemozoin per parasitized red blood cell (pRBC) was derived from previous studies using in vitro cultures of P. falciparum (10). Estimated concentrations of hemozoin were determined by multiplying the geometric mean levels of parasitemia in children with mild and severe malaria (41,369 and 275,005 parasites/μl of blood, respectively) by the concentration of isolated hemozoin per pRBC (47 fg) (Table 1). Based on these calculations, children with mild malaria would have 1.9 μg of hemozoin per ml of circulating blood, while children with severe malaria would have 12.9 μg of hemozoin per ml of circulating blood. Based on these calculations, the estimated physiological concentrations of hemozoin selected for the in vitro experiments were 10, 1.0, and 0.1 μg/ml.
TABLE 1.
Estimated hemozoin concentrations in blood from children with malariaa
| Severity of malaria | Geometric mean no. of parasites/μla | Hemozoin concn (fg/pRBC)b | Estimated hemozoin concn in blood from children with malaria (μg/ml)c |
|---|---|---|---|
| Mild | 41,369 | 47 | 1.9 |
| Severe | 275,005 | 47 | 12.9 |
Calculated from blood of children with mild (n = 17) and severe (n = 12) malaria (21).
Calculated from cultured parasites (10).
Determined by multiplying the concentration of hemozoin (in femtograms per pRBC) obtained from cultured parasites by the geometric mean number of parasites per microliter in children with mild and severe malaria.
Effect of hemozoin on PBMC NOx production.
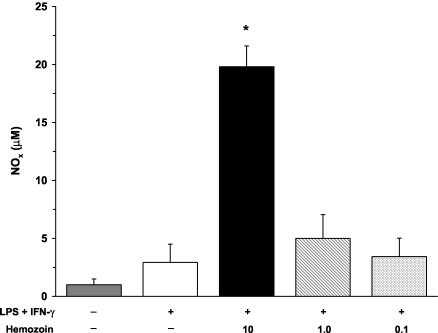
Hemozoin is an insoluble coordinated polymer of heme subunits formed during the detoxification of heme by plasmodia (27). Upon rupture of pRBCs, hemozoin is released into circulation and is rapidly phagocytosed by mononuclear cells (25). Hemozoin exists as a coordinated heme polymer containing a conglomeration of host- and parasite-derived lipids and proteins (6, 11). Therefore, we used a crude isolate of hemozoin that was not subjected to proteinase K treatment and/or acetone washing, since it more closely mimics the moiety acquired during a natural infection. Crude hemozoin was isolated from in vitro cultures of P. falciparum-infected RBCs (strain Pf-D6) when the level of parasitemia was 3 to 5% and late trophozoites and early schizonts were the predominate forms. RBCs were spun at 2,000 rpm for 10 min, and the resulting pellet was resuspended in 40 ml of 0.01 M phosphate-buffered saline (pH 7.2) with 2 ml of saponin for 10 min. The solution was then spun at 14,000 rpm for 15 min, and the pelleted material was washed in phosphate-buffered saline until the resulting pellet was dark red and free from the white RBC cellular components (four to seven times). The final pellet was dried, weighed, and resuspended in filter-sterilized H2O at a final concentration of 1.0 mg/ml, and the final solution was extensively sonicated to disperse the hemozoin. Since larger volumes of blood were required for the in vitro assays, experiments with hemozoin were conducted with cultured PBMCs from healthy, non-malaria-exposed adults from the United States. Cultured PBMCs were stimulated with medium alone, LPS and IFN-γ, or LPS and IFN-γ in the presence of hemozoin (10, 1.0, or 0.1 μg/ml) for 48 h. The stimulation of cells with hemozoin was performed in the presence of LPS and IFN-γ since cultured human blood mononuclear cells typically require priming and activation to produce NO (31). Production of NO was determined by measuring NOx in culture supernatants according to previously described methods (19). It was also confirmed that hemozoin does not interfere with the Griess reaction. LPS and IFN-γ stimulation nonsignificantly increased levels of NOx (Fig. 4). The addition of a high dose (10 μg/ml) of hemozoin to LPS- and IFN-γ-stimulated PBMCs significantly augmented NOx levels (P < 0.01), while the intermediate and low doses of hemozoin (1.0 and 0.1 μg/ml, respectively) failed to significantly elevate NOx levels (Fig. 4). Moreover, additional experiments revealed that hemozoin alone, in the absence of stimulation, failed to significantly increase NOx levels (data not shown). The results presented here illustrate that a crude preparation of hemozoin increases NO production in human PBMCs.
FIG. 4.
Effect of hemozoin on NOx production in cultured PBMCs. Venous blood (40 ml) was obtained from healthy, malaria-naïve U.S. donors, and PBMCs were isolated. Cultured PBMCs (106 cells/ml) were stimulated with medium alone, LPS (100 ng/ml) and IFN-γ (200 U/ml), or LPS and IFN-γ in the presence of hemozoin (10, 1.0, or 0.1 μg/ml). The NOx concentration in culture supernatants was determined at 48 h via the Griess reaction by previously defined methods (19). Values shown are the means ± SEM of results from 10 subjects. Statistical significance was determined by the Mann-Whitney U test. *, P value of <0.05 compared to results for LPS+IFN-γ stimulation conditions.
Effect of hemozoin on NOS2 transcripts.
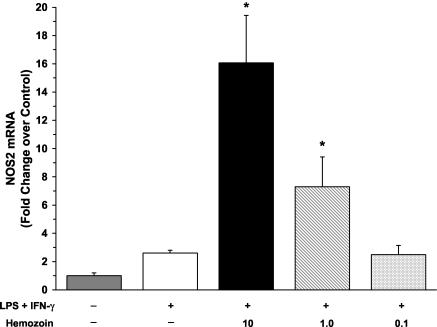
To determine if hemozoin increased levels of NOx by induction of NOS2 mRNA, we cultured PBMCs from healthy, malaria-naïve U.S. adults with medium alone, LPS and IFN-γ, or LPS and IFN-γ in the presence of hemozoin (10, 1.0, or 0.1 μg/ml) for 48 h and measured NOS2 mRNA by real-time reverse transcription (RT)-PCR. To accomplish this, total RNA was isolated from cultured PBMCs by the GITC method (8) and reverse transcribed into cDNA. NOS2 gene expression was analyzed by quantitative real-time RT-PCR on an ABI Prism 7700 sequence detection system (Applied Biosystems, Foster City, Calif.) with NOS2-specific primers and a probe (assay identification no. Hs00167248_m1; Applied Biosystems) according to the parameters specified by the manufacturer. To control for nonspecific background fluorescence, no-template controls were included in triplicate. An endogenous control gene, β-actin (assay identification no. 4326315E; Applied Biosystems), was used as a reference gene to normalize cDNA loadings among samples. Stimulation of PBMCs with LPS and IFN-γ nonsignificantly elevated levels of NOS2 transcripts at 48 h (Fig. 5). The addition of both large (10 μg/ml) and intermediate (1.0 μg/ml) amounts of hemozoin significantly augmented levels of LPS- and IFN-γ-induced NOS2 transcripts (P < 0.05) (Fig. 5). Previous studies have reported difficulties in detecting NOS2 transcripts in human mononuclear cells (for a review, see reference 31); however, using sensitive quantitative methods such as real-time RT-PCR, we were able to detect the NOS2 message. Although NOS2 transcripts were not highly abundant in cultured PBMCs, the addition of hemozoin significantly increased the de novo NOS2 message, demonstrating that hemozoin-induced NO production can occur through increased NOS2 transcription.
FIG. 5.
Effect of hemozoin on NOS2 mRNA expression. NOS2 mRNA was quantified for three individuals by real-time RT-PCR. PBMCs (106 cells/ml) were obtained from healthy, malaria-naïve U.S. donors and cultured with medium alone, LPS (100 ng/ml) and IFN-γ (200 U/ml), or LPS and IFN-γ in the presence of hemozoin (10, 1.0, or 0.1 μg/ml). Cells were collected at 48 h for NOS2 mRNA determination by real-time RT-PCR. Data were compared using the ΔΔCT method, where the endogenous control gene (β-actin) cycle threshold (CT) value was subtracted from the experimental gene (NOS2) CT for each sample. The change in CT (ΔCT) for each experimental sample was then subtracted from the ΔCT of the unstimulated (medium alone) control sample. Change (n-fold) was expressed as 2−ΔΔCT relative to results from unstimulated conditions. Values are the means ± SEMs of results from three samples. Statistical significance was determined by the Mann-Whitney U test. *, P value of <0.05 compared to results for LPS+IFN-γ stimulation conditions.
The results presented here show that NOS enzyme activity is elevated in children with mild and severe MA and that high levels of NOS enzyme activity are associated with anemia. The role of NO in malaria pathogenesis remains controversial, since some studies of subjects with cerebral malaria have shown that NO is associated with protection (5, 14, 19, 24), while others have found either no effect or adverse consequences of elevated NO production (1-3, 18, 30). However, this is the first report that has directly examined NO production during acute MA. Although polymorphisms in the NOS2 promoter (NOS2Lamberéné [NOS2 G954C] and C1173T) have been associated with protection against malaria (12, 15, 16), there were not enough children in our sample population with these particular polymorphisms to provide meaningful statistically valid comparisons (data not shown).
As shown here, cultured PBMCs from children with MA have higher baseline and stimulated NOS enzyme activity than those from healthy, malaria-exposed children. Furthermore, a crude isolate of P. falciparum-derived hemozoin enhances NOS2 transcripts and NO production in cultured human PBMCs, suggesting that ingestion of hemozoin may account for increased NOS activity in children with acute malaria. These results are in agreement with those of a recent report showing that a purified preparation of hemozoin and a synthetic preparation of hemozoin (β-hematin) increased IFN-γ-induced NOS2 transcripts and NO production in a murine macrophage cell line (13). Although concentrations of hemozoin used in those studies were 2.5 to 5 times higher than the estimated concentrations for children with mild and severe malaria presented here (Table 1), the present studies demonstrate that the ingestion of physiologically relevant concentrations of hemozoin (10 and 1.0 μg/ml) significantly enhances LPS- and IFN-γ-promoted NOS2 transcripts and NO production in human PBMCs. These results are in contrast to those of several studies of cultured murine peritoneal macrophages in which P. vinckei-derived hemozoin reduced NO production (22) and β-hematin decreased LPS-induced NO and tumor necrosis factor alpha production (29). The apparent discrepancy between those results and results presented here may be a consequence of the murine origin of the macrophages and/or the concentration of β-hematin, which was 10-fold higher in that study than concentrations used in the present study. Moreover, since regulation of the human NOS2 gene is substantially different than that of the murine NOS2 gene (9), hemozoin-induced activation and regulation of NOS2 and subsequent NO production may be different in human and murine systems.
Based on previous results (15, 19) and results presented here, we propose that elevated baseline levels of NO in healthy children, and increased levels of NO during the early phases of the immune response to acute malaria, protect against the development of severe disease. However, if parasite growth is not effectively limited during the early phases of the immune response, sustained overproduction of NO may lead to the development of severe MA. We are currently testing this hypothesis in a hospital-based study of Kenyan children with severe MA.
Acknowledgments
We thank the following staff members of the Albert Schweitzer Hospital in Lambaréné, Gabon, for their cooperation and technical assistance: Anita van den Biggelaar, Judith Jans, Hanna Knoop, Doris Luckner, Barbara Moritz, Anselme Ndzengue, Marcel Nkeyi, Daniela Schmid, and Milena Sovric.
This work was conducted at the Albert Schweitzer Hospital, Duke University, and the University of Pittsburgh and was supported in part by the National Institutes of Health grants AI-51305-01 (D.J.P.) and AI-41764 (J.B.W.), the VA Research Service (J.B.W.), and the University of Pittsburgh Competitive Research Development Fund (D.J.P.).
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Agbenyega, T., B. Angus, G. Beduaddo, B. Baffoebonnie, G. Griffin, P. Vallance, and S. Krishna. 1997. Plasma nitrogen oxides and blood lactate concentrations in Ghanaian children with malaria. Trans. R. Soc. Trop. Med. Hyg. 91:298-302. [DOI] [PubMed] [Google Scholar]
- 2.Al-Yaman, F., M. M. Awburn, and I. A. Clark. 1997. Serum creatinine levels and reactive nitrogen intermediates in children with cerebral malaria in Papua New Guinea. Trans. R. Soc. Trop. Med. Hyg. 91:303-305. [DOI] [PubMed] [Google Scholar]
- 3.Al-Yaman, F. M., D. Mokela, B. Genton, K. A. Rockett, M. P. Alpers, and I. A. Clark. 1996. Association between serum levels of reactive nitrogen intermediates and coma in children with cerebral malaria in Papua New Guinea. Trans. R. Soc. Trop. Med. Hyg. 90:270-273. [DOI] [PubMed] [Google Scholar]
- 4.Anstey, N. M., D. L. Granger, M. Y. Hassanali, E. D. Mwaikambo, P. E. Duffy, and J. B. Weinberg. 1999. Nitric oxide, malaria, and anemia: inverse relationship between nitric oxide production and hemoglobin concentration in asymptomatic, malaria-exposed children. Am. J. Trop. Med. Hyg. 61:249-252. [DOI] [PubMed] [Google Scholar]
- 5.Anstey, N. M., J. B. Weinberg, M. Hassanali, E. D. Mwaikambo, D. Manyenga, M. A. Misukonis, D. R. Arnelle, D. Hollis, M. I. McDonald, and D. L. Granger. 1996. Nitric oxide in Tanzanian children with malaria. Inverse relationship between malaria severity and nitric oxide production/nitric oxide synthase type 2 expression. J. Exp. Med. 184:557-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashong, J. O., I. P. Blench, and D. C. Warhurst. 1989. The composition of haemozoin from Plasmodium falciparum. Trans. R. Soc. Trop. Med. Hyg. 83:167-172. [DOI] [PubMed] [Google Scholar]
- 7.Breman, J. G., A. Egan, and G. T. Keusch. 2001. The intolerable burden of malaria: a new look at the numbers. Am. J. Trop. Med. Hyg. 64:iv-vii. [DOI] [PubMed] [Google Scholar]
- 8.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 9.de Vera, M. E., R. A. Shapiro, A. K. Nussler, J. S. Mudgett, R. L. Simmons, S. M. Morris, Jr., T. R. Billiar, and D. A. Geller. 1996. Transcriptional regulation of human inducible nitric oxide synthase (NOS2) gene by cytokines: initial analysis of the human NOS2 promoter. Proc. Natl. Acad. Sci. USA 93:1054-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egan, T. J. 2002. Physico-chemical aspects of hemozoin (malaria pigment) structure and formation. J. Inorg. Biochem. 91:19-26. [DOI] [PubMed] [Google Scholar]
- 11.Goldie, P., E. Roth, Jr., J. Oppenheim, and J. Vanderberg. 1990. Biochemical characterization of Plasmodium falciparum hemozoin. Am. J. Trop. Med. Hyg. 43:584-596. [DOI] [PubMed] [Google Scholar]
- 12.Hobbs, M. R., V. Udhayakumar, M. C. Levesque, J. Booth, J. M. Roberts, A. N. Tkachuk, A. Pole, H. Coon, S. Kariuki, B. L. Nahlen, E. D. Mwaikambo, A. L. Lal, D. L. Granger, N. M. Anstey, and J. B. Weinberg. 2002. A new NOS2 promoter polymorphism associated with increased nitric oxide production and protection from severe malaria in Tanzanian and Kenyan children. Lancet 360:1468-1475. [DOI] [PubMed] [Google Scholar]
- 13.Jaramillo, M., D. C. Gowda, D. Radzioch, and M. Olivier. 2003. Hemozoin increases IFN-gamma-inducible macrophage nitric oxide generation through extracellular signal-regulated kinase- and NF-kappaB-dependent pathways. J. Immunol. 171:4243-4253. [DOI] [PubMed] [Google Scholar]
- 14.Kremsner, P. G., S. Winkler, E. Wildling, J. Prada, U. Bienzle, W. Graninger, and A. K. Nussler. 1996. High plasma levels of nitrogen oxides are associated with severe disease and correlate with rapid parasitological and clinical cure in Plasmodium falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 90:44-47. [DOI] [PubMed] [Google Scholar]
- 15.Kun, J. F., B. Mordmuller, D. J. Perkins, J. May, O. Mercereau-Puijalon, M. Alpers, J. B. Weinberg, and P. G. Kremsner. 2001. Nitric oxide synthase 2(Lambaréné) (G-954C), increased nitric oxide production, and protection against malaria. J. Infect. Dis. 184:330-336. [DOI] [PubMed] [Google Scholar]
- 16.Kun, J. F. J., B. Mordmuller, B. Lell, L. G. Lehman, D. Luckner, and P. G. Kremsner. 1998. Polymorphism in promoter region of inducible nitric oxide synthase gene and protection against malaria. Lancet 351:265-266. [DOI] [PubMed] [Google Scholar]
- 17.Luty, A. J. F., D. J. Perkins, B. Lell, R. Schmidt-Ott, L. G. Lehman, D. Luckner, B. Greve, P. Matousek, K. Herbich, D. Schmid, J. B. Weinberg, and P. G. Kremsner. 2000. Low interleukin-12 activity in severe Plasmodium falciparum malaria. Infect. Immun. 68:3909-3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maneerat, Y., P. Viriyavejakul, B. Punpoowong, M. Jones, P. Wilairantana, E. Pongponratn, G. Turner, and R. Udomsangpetch. 2000. Inducible nitric oxide synthase expression is increased in the brain in fatal cerebral malaria. Histopathology 37:269-277. [DOI] [PubMed] [Google Scholar]
- 19.Perkins, D. J., P. G. Kremsner, D. Schmid, M. A. Misukonis, M. A. Kelly, and J. B. Weinberg. 1999. Blood mononuclear cell nitric oxide production and plasma cytokine levels in healthy Gabonese children with prior mild or severe malaria. Infect. Immun. 67:4977-4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perkins, D. J., J. Moore, J. Otieno, Y. Shi, B. Nahlen, V. Udhayakumar, and A. A. Lal. 2003. In vivo acquisition of hemozoin by placental blood mononuclear cells suppresses PGE2, TNF-α, and IL-10. Biochem. Biophys. Res. Commun. 311:839-846. [DOI] [PubMed] [Google Scholar]
- 21.Perkins, D. J., J. B. Weinberg, and P. G. Kremsner. 2000. Reduced interleukin-12 and transforming growth factor-β1 in severe childhood malaria: relationship of cytokine balance with disease severity. J. Infect. Dis. 182:988-992. [DOI] [PubMed] [Google Scholar]
- 22.Prada, J., J. Malinowski, S. Muller, U. Bienzle, and P. G. Kremsner. 1996. Effects of Plasmodium vinckei hemozoin on the production of oxygen radicals and nitrogen oxides in murine macrophages. Am. J. Trop. Med. Hyg. 54:620-624. [DOI] [PubMed] [Google Scholar]
- 23.Reykdal, S., C. Abboud, and J. Liesveld. 1999. Effect of nitric oxide production and oxygen tension on progenitor preservation in ex vivo culture. Exp. Hematol. 27:441-450. [DOI] [PubMed] [Google Scholar]
- 24.Rockett, K. A., M. M. Awburn, W. B. Cowden, and I. A. Clark. 1991. Killing of Plasmodium falciparum in vitro by nitric oxide derivatives. Infect. Immun. 59:3280-3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwarzer, E., M. Alessio, D. Ulliers, and P. Arese. 1998. Phagocytosis of the malarial pigment, hemozoin, impairs expression of major histocompatibility complex class II antigen, CD54, and CD11c in human monocytes. Infect. Immun. 66:1601-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shami, P. J., and J. B. Weinberg. 1996. Differential effects of nitric oxide on erythroid and myeloid colony growth from CD34+ human bone marrow cells. Blood 87:977-982. [PubMed] [Google Scholar]
- 27.Slater, A. 1992. Malaria pigment. Exp. Parasitol. 74:362-365. [DOI] [PubMed] [Google Scholar]
- 28.St. Clair, E. W., W. E. Wilkinson, T. Lang, L. Sanders, M. A. Misukonis, G. S. Gilkeson, D. S. Pisetsky, D. L. Granger, and J. B. Weinberg. 1996. Increased expression of blood mononuclear cell nitric oxide synthase type 2 in rheumatoid arthritis patients. J. Exp. Med. 184:1173-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taramelli, D., S. Recalcati, N. Basilico, P. Olliaro, and G. Cairo. 2000. Macrophage preconditioning with synthetic malaria pigment reduces cytokine production via heme iron-dependent oxidative stress. Lab. Investig. 80:1781-1788. [DOI] [PubMed] [Google Scholar]
- 30.Taylor, A. M., N. P. J. Day, D. X. T. Sinh, P. P. Loc, T. T. H. Mai, T. T. Chau, N. H. Phu, T. T. Hien, and N. J. White. 1998. Reactive nitrogen intermediates and outcome in severe adult malaria. Trans. R. Soc. Trop. Med. Hyg. 92:170-175. [DOI] [PubMed] [Google Scholar]
- 31.Weinberg, J. B. 1998. Nitric oxide production and nitric oxide synthase type 2 expression by human mononuclear phagocytes: a review. Mol. Med. 4:557-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weinberg, J. B., D. L. Granger, D. S. Pisetsky, M. F. Seldin, M. A. Misukonis, S. N. Mason, A. M. Pippen, P. Ruiz, E. R. Wood, and G. S. Gilkeson. 1994. The role of nitric oxide in the pathogenesis of spontaneous murine autoimmune disease: increased nitric oxide production and nitric oxide synthase expression in MRL-lpr/lpr mice, and reduction of spontaneous glomerulonephritis and arthritis by orally administered NG-monomethyl-l-arginine. J. Exp. Med. 179:651-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weinberg, J. B., J. J. Muscato, and J. E. Niedel. 1981. Monocyte chemotactic peptide receptor. Functional characteristics and ligand-induced regulation. J. Clin. Investig. 68:621-630. [DOI] [PMC free article] [PubMed] [Google Scholar]