Key Clinical Message
Sporadic Medullary Carcinoma of the Thyroid is a relatively uncommon entity and at the time of diagnosis, most already present loco‐regional metastasis. Therapy should be aggressive to reduce recurrence and mortality. Follow‐up period should continue lifelong and should also include calcium/pentagastrin infusion test, as well as 6‐month interval diagnostic imaging.
Keywords: Neoadjuvant Radiation Therapy, Recurrent Spinal Metastasis, Spinal Metastasis, Sporadic Medullary Carcinoma of the Thyroid, Thyroid Metastasis
Introduction
Medullary thyroid carcinoma (MTC) is an undifferentiated, neuroendocrine tumor which arises from the parafollicular cells or C‐cells of the thyroid gland. The particular characteristic of this tumor is the production of calcitonin 1 as well as ACTH, histaminase, corticotrophin, vasoactive intestinal peptide, serotonin, and carcinoembriogenic antigen (CEA) 2, 3. MTC can be either sporadic (SMTC) (65–70%) or familial (FMTC) as part of the multiple endocrine neoplasia type 2 (MEN2) syndrome 4. In most patients with a newly diagnosed MTC, a loco‐regional metastasis has already taken place, and a distant metastasis is present in 15–20% of patients 4, 5, 6. The most common sites of metastasis presentation are the lungs, liver, and bones, and metastasis rarely occurs in the brain, skin, and breast 7, 8.
We present the case and outcome of a male patient with a recurrent spinal metastasis of a SMTC after radiation therapy with normal levels of calcitonin, CEA, and calcium, and we also present a review of the literature. To our knowledge, there is no standardized treatment, follow‐up, or even a case for recurrent spinal metastasis from a SMTC reported in the literature.
Case Report
A 49‐year‐old male patient was referred to our institution with a two‐month history of progressive paraparesis of the lower extremities and a paresthesia below T7. On presentation, the patient had an insecure and slightly forward flexion gait, associated with a 4+/5 paresis of the left L2‐3‐4‐5 dermatome muscles, as well as paresthesia below T7 and hypoesthesia of the L3‐4 dermatome. No urinary or defecatory problems were evident upon examination.
The patient's history was significant for a medullary carcinoma of the thyroid (T3,N1,M1), which already had metastasized to the spinal column. The initial diagnosis was made 2 years before, after the patient had developed a paraparesis of the lower extremities. An MRI reported an epidural tumor infiltration with compression of the thecal sac and the thoracic myelon at the T7 level. (Fig. 1) A hemilaminectomy and tumor resection at this level was performed under the assumption of an epidural metastasis from a cancer of unknown origin. The histopathological diagnosis reported a metastasis of an MTC with high expression of calcitonin. At this time, laboratory findings were within normal levels, including calcium and CEA. Shortly after that, the patient underwent a total thyroidectomy, along with bilateral neck dissection surgery. After the complete work‐up, the specimen was classified as a SMTC. Following the procedure, the patient was treated with radiation therapy from T7‐L5, where he also had lytic lesions within the bodies and laminae of several vertebrae, with a dose prescription of 2 Gy five times a week until 40 Gy was achieved. The initial follow‐up showed a stable spine disease at the operated level. (Fig. 2) The patient recovered well, except for a slightly left‐sided paresis of the L2‐3 dermatome muscles. Other than that, a well‐controlled hypertension was also significant in his past medical history. After 2 years of stable and asymptomatic disease, the patient re‐developed a paraparesis.
Figure 1.

Preoperative MRI before the first operation. Sagittal T1‐sequence without (A) and with contrast (B) of the thoracic and lumbar spine showing the number of the vertebrae marked, as well as the initial thecal sac compression at the level of T7 and the other bone metastasis at T12 and L4. (C) Axial T2‐sequence at the level T7. A nearly complete compression of the thecal sac can be seen.
Figure 2.
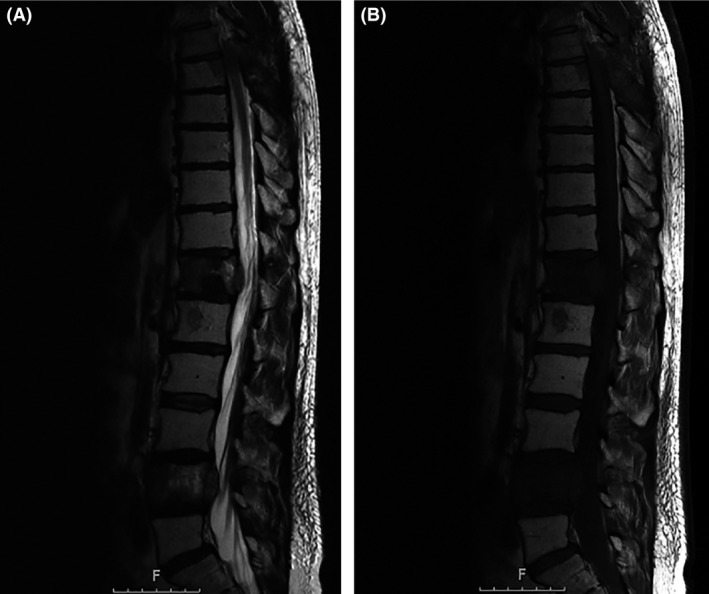
MRI after the first radiation cycle. Sagittal T2‐sequence (A) and T1‐sequence without contrast (B) showing a stable disease at the operated level T7 and the suspicion of progressive disease at T12 and L4 levels.
Laboratory findings were within normal levels, including calcitonin, CEA and calcium levels. An MRI showed a tumor with a dorsally oriented mass‐effect at the levels T6‐7, T12, and L4, as well an intravertebral tumor infiltration at L1 level. (Fig. 3)
Figure 3.
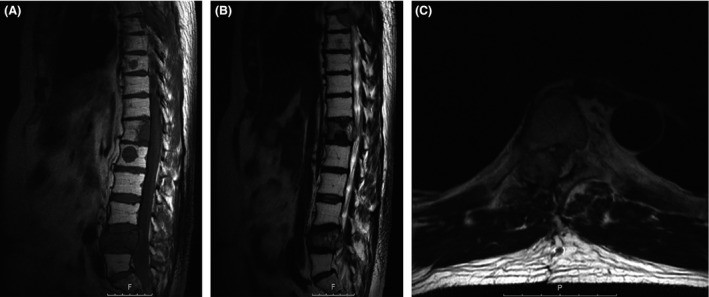
Preoperative MRI before the second operation. Sagittal T1‐sequence (A) without contrast and T2‐sequence (B) of the thoracic and lumbar spine showing the recurrent growth of the metastasis with the corresponding thecal sac compression at the level of T7 and the other bone metastasis at T12, L1, and L4. (C) Axial T2‐sequence at the level T7. An almost identical imaging to the initial MRI, where a nearly complete compression of the thecal sac can be observed.
We performed a decompressive laminectomy at T7 and tumor resection, which was confined to the T7 level, to relieve the thoracic myelon, followed by a dorsal instrumentation at T5 to T9 (DePuy Expedium, Raynham, MA, USA). Postoperatively, almost all the symptoms were relieved, except for the known paresis of the left L2‐3 dermatome muscles.
The histopathological examination of the recurrent lesion again revealed an intra‐ and paravertebral metastasis from a neuroendocrine carcinoma. The immunohistochemical work‐up of the specimen showed a strong positive reaction for calcitonin, cytokeratin 8 and 18, and thyroid transcription factor‐1, so that the findings corresponded again with a metastasis of the MTC. (Fig. 4) The patient recovered well from the operation and was then referred to radiation therapy applied at the same location as 2 years before and with the same prescription dose.
Figure 4.
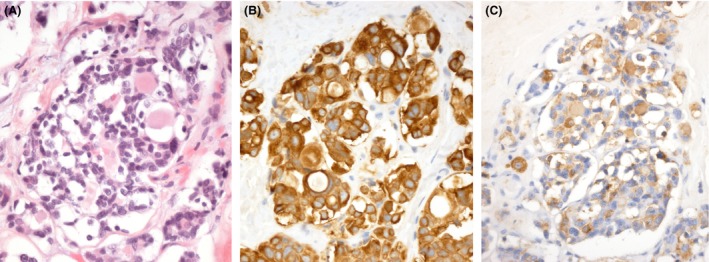
Histology – second surgery. (A) Hematoxylin‐ and eosin‐stained tissue sections demonstrated a highly cellular, epithelial tumor consisting of cells with medium‐sized, round nuclei and pale, eosinophilic cytoplasm. The tumor grew in small to large nests with an occasional follicular histological pattern. Not only was the tumor strongly immunopositive for cytokeratin 18 (B), but it also exhibited positive immunostaining for calcitonin in the majority of the tumor cells (C).
The 3‐, 6‐, and 12‐month follow‐ups showed a normal and secure gait with the known nonprogressive 4 + /5 paresis of the left L2‐3 dermatome muscles. The spinal MRI at 6 months following radiation therapy again showed a stable spine disease. (Fig. 5) Calcitonin, CEA, and calcium levels also remained unaltered. After 18 months following the second operation, no other metastasis was documented.
Figure 5.
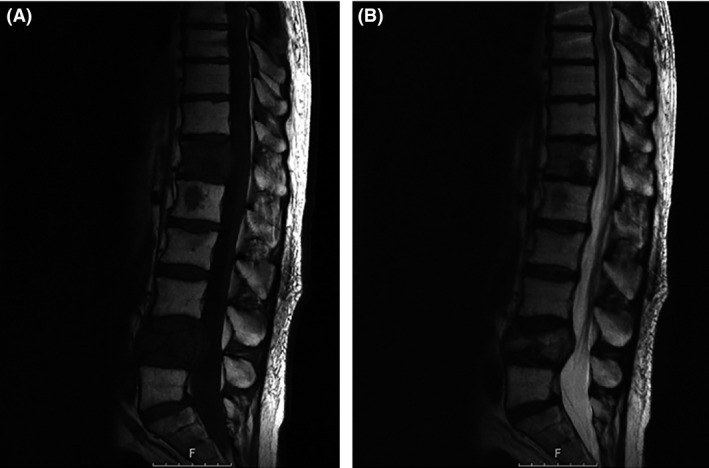
MRI after the second radiation cycle. Sagittal T1‐sequence (A) without contrast and T2‐sequence (B) showing a stable disease at the reoperated level T7 and a stable disease at T12 and L4 levels.
Discussion
Description and epidemiology
The most common cancer of the endocrine system is thyroid carcinoma (TC). Of all cancers diagnosed, it is estimated that each year less than 3% will be TC, and less than 4% of these will result in death 9. In 2012, approximately 56,460 new cases of TC were diagnosed in the United States alone, with a female predisposition of 4:1 and a median age at diagnosis of 50 years 10. TC is classified into major subtypes: differentiated (papillary [>85%], follicular [5–15%]), poorly differentiated (insular, solid, or trabecular), undifferentiated or anaplastic (1–2%), medullary (5%), and primary malignant lymphoma (2%) 11, 12. Each histologic type of TC has its different and varied biologic behavior 2. TC incidence has increased almost 2.6‐fold from 1973 to 2006 13. This increment in the diagnosis is attributed to the optimization of small cancer detection; however, mortality remains unchanged at 0.5 per 100,000 of the population 14.
It is known that a differentiated TC requires a different therapy approach and has an overall better prognosis. The other subtypes are usually treated more aggressively and the prognosis is significantly poorer. Therefore, the treatment and follow‐up necessities for these patients are contrasting.
The most common sites of metastasis presentation are the lungs, liver, and bones, and metastasis rarely occurs in the brain, skin, and breast 7, 8. Because the description of other metastatic sites goes beyond the scope of this review, we will only focus on bone metastasis, specifically to the spine. It is important to mention that a metastasis to the bone from a TC most often arises from a papillary TC (28%), followed by follicular (22%), insular (20%), anaplastic (13%), hurthle‐cell TC (11%), and MTC (6%) 15. When a metastasis to the bone has occurred, the most typical places are the vertebrae (54%), pelvis (50%), ribs (31%), femur (21%), skull (13%), humerus (11%), and other locations (17%) 16.
The spine, specifically the thoracic spine (60–80%), is the most affected region, followed by the lumbar spine (15–30%) and the cervical spine (<10%). The vertebral body is the preferred site (85%), especially the posterior part (66%), followed by the paravertebral spaces (10–15%) and the epidural spaces (<5%) 17.
Of all patients with thyroid nodules, 0.2–0.4% have MTC, representing 5–10% of all TC [18,] and are responsible for up to 13.4% of all deaths caused by TC 3. MTC can be either sporadic (SMTC) (65–70%) or familial (FMTC) as part of the MEN2 syndrome 4. MTC can affect patients of all ages, but has a peak incidence between the fourth and sixth decades for SMTC, and the second and third decades for FMTC 19. MTC has a slightly female preponderance in a ratio of 1:1.05 19. Although the role of the patient's age still remains controversial [20, 21, 22, 23, 24, 25, 26, 27,] the prognosis for MTC depends on the grade of cancer, extrathyroidal spread and size, as well as the quality of the initial surgical treatment 2, 18, 20, 28, 29, 30. The overall prognosis rate is good, with a 5‐year survival of 78–91% and a 10‐year survival of 61–88% 24, 30, 31. Whereas a biochemical cure (i.e., undetectable calcitonin levels, normal CEA, and calcium levels) could predict a survival rate as high as 97% at 10 years, a recurrence was found in 4.9% of the patients. Interestingly, noncured patients have a good survival as well, with a rate of 80% and 70% at 5 and 10 years, respectively 22.
Diagnosis
As in any TC, patients most commonly present with a palpable, painless, nontender, growing, and generally asymptomatic thyroid nodule. Most of these nodules are found incidentally. Occasionally, the patient presents associated symptoms such as hoarseness, stridor, dysphagia, or even dyspnea. In some rare cases, the patient might present diarrhea either as a chief complaint or associated symptom, which has a higher incidence in patients with metastatic disease. Because the parafollicular cells are predominantly located in the upper portion of the thyroid lobe, most of the MTC are found in this area 1, 2, 32, 33, 34, 35.
In the present case, the SMTC was incidentally diagnosed, and none of the common signs or symptoms were present. It is important to mention that in less than 10% of the patients with a bone metastasis from a TC, the presenting symptom is spinal cord compression 16, 36, 37.
There are many guidelines one could follow once a nodule is found. In summary, laboratory tests should initiate the diagnosis. Depending on the results, an ultrasound and a fine‐needle aspiration biopsy are indicated. Specifically for MTC, calcitonin, CEA, and calcium levels should be determined, and when a FMTC is suspected, RET mutation analysis, that is, genetic testing, as well as a MEN2‐syndrome work‐up should be attained 8, 35, 38, 39, 40, 41, 42. In patients with MTC, the calcitonin level will be above normal, and after a calcium or pentagastrin infusion, the level will increase even more. The few cases seen with a normal calcitonin level would also respond to an infusion of calcium or pentagastrin 2. In most patients with a newly diagnosed MTC, a metastasis has already taken place 4. A distant metastasis is present in 15–20% of the patients 5, 6. Therefore, a complete neck ultrasound as well as a complete whole‐body screening are indicated 4. The presence of histaminase in the serum and a calcitonin level above 400 pg/mL suggest a metastasis 2, 39.
Treatment and follow‐up
The treatment for FMTC requires a multidisciplinary approach and goes beyond the scope of this review, so we will only focus on the treatment for SMTC.
The only cure for MTC is a complete surgical treatment of the gland (i.e., total thyroidectomy) and any loco‐regional metastasis. An aggressive surgical treatment should be performed. The high incidence of multifocality, the lack of effective adjuvant therapies, and the better outcome and prognosis in patients with a completely resected pathology justify an aggressive surgical treatment. The treatment for those patients with a residual or recurrent disease, as well as those with distant metastasis, is not well established 43, 44.
It is fundamental to the postoperative management to have preoperative serum baseline levels of calcitonin, CEA, and calcium, and in specific cases, ACTH, histaminase, corticotrophin, vasoactive intestinal peptide, and serotonin. As previously stated, one should also evaluate the need for a calcium/pentagastrin infusion test in order to have the baseline level as well. All these markers will form the cornerstone of follow‐up treatment and will be indicative of a possible recurrence or progression of the disease.
Immediately after surgical treatment, the patient should receive a thyroxin substitution therapy and should be monitored clinically and biochemically within a month. It is important to emphasize that the parafollicular cells or C‐cells of the thyroid gland do not respond to TSH, so neither a serum suppression of TSH concentrations nor adjuvant therapy with radioiodine is indicated 18, 44, 45, 46, 47, 48, 49, 50
At 3 and 6 months after initial diagnosis and treatment, serum concentrations of calcitonin, CEA, and calcium should be controlled. The patient is considered biochemically cured if the serum CEA and calcium levels are normal and the calcitonin serum level is undetectable 24, 44. Further appropriate follow‐up intervals are 6–12 months 35. In the present case, all the laboratory levels were normal, that is, biochemically cured.
It is also important to mention that the serum calcitonin levels fall slowly in some patients; therefore, a measurement at 3 and 6 months is mandatory. Normally, a fall in the first 72 hours is expected 34, 51. After this time, if still altered in comparison to the reference levels, a residual or recurrent disease could be assumed. Each case should be treated individually, and a calcium/pentagastrin infusion testing should also be carried out at some point during the follow‐up period.
If a residual or recurrent MTC is suspected, one should perform a completely new diagnostic evaluation, that is, neck ultrasound and whole‐body screening. Other imaging studies for the detection of loco‐regional or distant metastasis are described in the literature, but their sensitivity and specificity are still controversial 7, 39, 50, 52, 53, 54, 55, 56, 57, 58.
Once a recurrence or residual disease has been diagnosed, the treatment options will vary depending on the site of metastasis. In most cases, a surgical resection is indicated. In some cases, laparoscopy or even arteriography for abdominal metastasis should be evaluated 35, 59.
The use of radiotherapy is still controversial. Most of the studies showed no benefit with respect to the overall survival, but radiotherapy did play an important role in disease control and a longer progression‐free disease for MTC 60, 61, 62, 63, 64, 65, 66, 67, 68, 69.
Radiotherapy is indicated when a patient did not receive a curative resection, when the patient was diagnosed with extrathyroidal disease, or when nonsurgical bone metastasis is present 2, 39, 44. The amount of residual disease will determine the radiation dosage, which normally requires 60–70 Gy, unless the distant metastasis is around the spinal cord, where the tissue tolerance for radiation is up to 45 Gy 2, 44.
Normally, systemic therapy is only considered for patients with a progressive metastatic disease in which a contraindication for surgery or radiotherapy exists 44. Small clinical trials have shown promising results with certain monoclonal antibodies, but none of them have demonstrated to improve survival. There are only two monoclonal antibodies, Vandetanib and Cabozantinib, that have completed a phase III clinical trial, and they are recommended only for patients with tumor progression 70.
Although treatment with bisphosphonates in MTC has not been studied, in the setting of osteolysis‐induced hypercalcemia, its use has been shown to control symptoms and regulate calcium levels 49, 71.
Our perspective
To our knowledge, there is no standardized treatment for MTC with bone metastasis, particularly in cases involving the spine. The treatment for bone metastasis, surgical or medical, has been suggested through small case series, varied surgical experience and preference, and has provided some degree of consensus as to the optimal management in differentiated TC. The extrapolation of this management has not yet been proven for MTC 49, 70. There is no evidence regarding bone metastasis in MTC, but it is known that of all cancers metastasizing to the spine, thyroid cancer has the best prognosis 36.
As previously stated, radiation has been proposed as a neoadjuvant therapy for the treatment of loco‐regional or distant metastasis in MTC, for it has shown to lead to a considerable reduction in pain in up to 80% of patients with symptomatic bone lesions, as well as to play an important part in disease control and a longer progression‐free disease 60, 61, 62, 63, 64, 65, 66, 67, 68, 69.
It is known that patients with a bone metastasis may have painful lesions, fractures, structural instability of the spine, neurological dysfunction, and, when there is spinal involvement, spinal cord compression with or without paraplegia. There are different managing options or recommendations depending on the situation. Either way, the main role of surgical therapy is to promote pain reduction, restore the stability of the spine, and prevent or correct the neurological deficit 36, 49.
Our review focuses on spinal bone metastasis, which was the cause of the chief complaint in the present case, after a previously treated and radiologically stable disease.
According to the literature, patients with a fracture, an impending fracture, or painful bone metastasis have diverse treatment options. A minimally invasive percutaneous procedure should be considered when a patient has a contraindication for surgery or radiation therapy. This type of procedure could also be used in combination with radiation therapy when no contraindication is present 49.
Radiation therapy is also indicated in patients with a disease progression and/or possible invasion to adjacent structures, as well as postoperative bone metastasis patients with incomplete resection 49.
According to the American Thyroid Association, for patients with an isolated, asymptomatic bone metastasis surgery will not be curative, but it can be performed. It is in these cases and in patients with small bone metastasis, which do not pose an immediate threat or cause neurological symptoms, where a conservative non‐surgical therapy could be implemented 49. We strongly disagree on this point, for a radical surgery in TC metastasis could represent a better local and systemic control, prolong the progression‐free disease period, and lower the recurrence rate.
In some instances, the patient presents a gross tumor burden or even spinal cord compression. In both cases, surgery is indicated and, as noted before, postoperative radiation should be initiated 49.
In the present case, the patient was incidentally diagnosed with a metastasized SMTC after spinal surgery. The serum levels after the initial treatment, as well as the follow‐up levels, revealed no alterations, which could have made a residual or recurrent disease or even the metastatic disease suspicious. The initial surgical treatment, that is, hemilaminectomy, caused a significant, almost complete, reduction of the symptoms. Although the patient received adjuvant radiotherapy, the tumor grew in the exact same location where the first hemilaminectomy and resection of the epidural tumor mass were performed, causing the same symptoms once again. The importance of this radiological finding (Fig. 3) and the fact that this type of metastasis has a good prognosis raises the question of whether a complete removal, that is, total vertebrectomy, could achieve an improvement in disease control, prevent a recurrence, and, possibly, prolong the overall survival rate. The aggressiveness of the surgery could provide a better local control, prolong the progression‐free disease period, and lower the recurrence rate 72, 73.
It is important to mention that a calcium/pentagastrin infusion test was never performed on this patient, and furthermore, only a single follow‐up MRI of the spine was performed a year after the initial diagnosis and treatment. Therefore, we include in our recommendations the use of the calcium/pentagastrin infusion test in the follow‐up work‐up at least two times after the first surgery or when the suspicion of a metastasis arises. An MRI of the spine should be performed at an interval of 3–6 months once a metastasis has been diagnosed. It is also our recommendation to perform an aggressive treatment, that is, total en bloc spondylectomy, in a patient with a single metastatic lesion from a MTC to the spine. The use of monoclonal therapies should also be considered in patients showing distant metastases.
All these suggestions will allow the patients with a progressive disease to be diagnosed and treated earlier, and most importantly, contribute to a longer progression‐free disease interval. The follow‐up period should continue for the entire life of patients with distant metastatic disease in SMTC. Although most patients have a stable disease, marked disease progression has been reported after years, and in some series, calcitonin‐negative MTC have also been reported 36, 45, 59.
Conclusion
The reported patient recovered well from surgery and radiation therapy of his spinal SMTC metastasis, and the 1‐year follow‐up showed a clinically and radiologically stable disease. There are no evidence‐based guidelines to treat recurrent spinal metastasis from this type of tumor. After a thorough review of the literature, we strongly suggest treating spinal metastasis from this rare type of tumor aggressively in order to significantly reduce local recurrence, which directly correlates to its mortality. The follow‐up period should continue for the entire life of patients with distant metastatic disease in SMTC and should include the calcium/pentagastrin infusion test in the follow‐up work‐up, as well as 6‐month interval diagnostic imaging.
Clinical Case Reports 2016; 4(1): 9–18
References
- 1. Tuttle, R. M. 2012. Clinical manifestations and staging of medullary thyroid cancer In UpToDate, Ross D. S., ed. UpToDate, Waltham, MA, USA. [Google Scholar]
- 2. Lal, G. , O'Dorisio T., McDougall R., and Weigel R. J.. 2008. Cancer of the endocrine system Pp. 1271–1306 in Abeloff M. D., ed. Abeloff's clinical oncology. Elsevier, USA. [Google Scholar]
- 3. Favia, G. , and Iacobone M.. 2005. Medullary thyroid carcinoma: state of the art. G. Chir. 26:405. [PubMed] [Google Scholar]
- 4. Saad, M. F. , Ordonez N. G., Rashid R. K., Guido J. J., Hill C. S. Jr, Hickey R. C., et al. 1984. Medullary carcinoma of the thyroid: a study of the clinical features and prognostic factors of 161 patients. Medicine (Baltimore) 63:319. [PubMed] [Google Scholar]
- 5. Bergholm, U. , Adami H. O., Bergstrom R., Johansson H., Lundell G., Telenius‐Berg M., et al. 1989. Clinical characteristics in sporadic and familial medullary thyroid carcinoma: a nationwide study of 249 patients in Sweden from 1959 through 1981. Cancer 63:1196. [DOI] [PubMed] [Google Scholar]
- 6. Sippel, R. S. , Kunnimalaiyaan M., and Chen H.. 2008. Current management of medullary thyroid cancer. Oncologist 13:539. [DOI] [PubMed] [Google Scholar]
- 7. Giraudet AL, D. V. , Leboulleux S., Aupérin A., Dromain C., Chami L., Ny Tovo N., et al. 2007. Imaging medullary thyroid carcinoma with persistent elevated calcitonin levels. J. Clin. Endocrinol. Metab. 92:4185. [DOI] [PubMed] [Google Scholar]
- 8. Pacini, F. , Castagna M. G., Brilli L., and Pentheroudakis G.. 2010. Thyroid cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann. Oncol. 21:214. [DOI] [PubMed] [Google Scholar]
- 9. Jemal, A. , Siegel R., Xu J., and Ward E.. 2010. Cancer statistics, 2010. Cancer J. Clin. 60:277. [DOI] [PubMed] [Google Scholar]
- 10. Howlader, N. , Noone A. M., Krapcho M., Neyman N., Aminou R., Altekruse S. F., et al. 2011. SEER cancer statistics review, 1975–2009 (vintage 2009 populations). National Cancer Institute, Philadelphia, PA, USA. [Google Scholar]
- 11. Kumar, V. 2009. Robbins and Cotran pathologic basis of disease. Saunders‐Elsevier, Philadelphia, PA, USA. [Google Scholar]
- 12. Melmed, S. 2011. Williams textbook of endocrinology. Saunders‐Elsevier, Philadelphia, PA, USA: [Google Scholar]
- 13. Cramer, J. D. , Fu P., Harth K. C., Margevicius S., and Wilhelm S. M.. 2010. Analysis of the rising incidence of thyroid cancer using the surveillance, epidemiology and end results National Cancer Data Registry. Surgery 148:1147. [DOI] [PubMed] [Google Scholar]
- 14. Davies, L. , and Welch H. G.. 2006. Increasing incidence of thyroid cancer in the United States, 1973‐2002. JAMA 295:2164. [DOI] [PubMed] [Google Scholar]
- 15. Tickoo, S. K. , Pittas A. G., Adler M., Fazzari M., Larson S. M., Robbins R. J., et al. 2000. Bone metastases from thyroid carcinoma: a histopathologic study with clinical correlates. Arch. Pathol. Lab. Med. 124:1440. [DOI] [PubMed] [Google Scholar]
- 16. Pittas, A. G. , Adler M., Fazzari M., Tickoo S. K., Rosai J., Larson S. M., et al. 2000. Bone metastases from thyroid carcinoma: clinical characteristics and prognostic variables in one hundred forty‐six patients. Thyroid 10:261. [DOI] [PubMed] [Google Scholar]
- 17. Ramadan, S. , Ugas M. A., Berwick R. J., Notay M., Cho H., Jerjes W., et al. 2012. Spinal metastasis in thyroid cancer. Head Neck Oncol. 4:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Noullet, S. , Tresallet C., Godiris‐Petit G., Hoang C., Leenhardt L., and Menegaux F.. 2011. Surgical management of sporadic medullary thyroid cancer. J. Visc. Surg. 148:e244. [DOI] [PubMed] [Google Scholar]
- 19. Raue, F. 1998. German medullary thyroid carcinoma/multiple endocrine neoplasia registry. German MTC/MEN study group. Medullary thyroid carcimoma/multiple endocrine neoplasia type 2. Langenbecks Arch Surg. 383:334. [DOI] [PubMed] [Google Scholar]
- 20. de Groot, J. W. , Plukker J. T., Wolffenbuttel B. H., Wiggers T., Sluiter W. J., and Links T. P.. 2006. Determinants of life expectancy in medullary thyroid cancer: age does not matter. Clin. Endocrinol. 65:729. [DOI] [PubMed] [Google Scholar]
- 21. Dottorini, M. E. , Assi A., Sironi M., Sangalli G., Spreafico G., and Colombo L.. 1996. Multivariate analysis of patients with medullary thyroid carcinoma: prognostic significance and impact on treatment of clinical and pathologic variables. Cancer 77:1556. [DOI] [PubMed] [Google Scholar]
- 22. Kebebew, E. , Ituarte P. H., Siperstein A. E., Duh Q. Y., and Clark O. H.. 2000. Medullary thyroid carcinoma: clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer 88:1139. [DOI] [PubMed] [Google Scholar]
- 23. Mirallie, E. , Vuillez J. P., Bardet S., Frampas E., Dupas B., Ferrer L., et al. 2005. High frequency of bone/bone marrow involvement in advanced medullary thyroid cancer. J. Clin. Endocrinol. Metab. 90:779. [DOI] [PubMed] [Google Scholar]
- 24. Modigliani, E. , Cohen R., Campos J. M., Conte‐Devoix B., Maes B., Boneu A., et al. 1998. Prognostic factors for survival and for biochemical cure in medullary thyroid carcinoma: results in 899 patients. The GETC Study Group. Groupe d'étude des tumeurs à calcitonine. Clin. Endocrinol. (Oxf) 48:265–273. [DOI] [PubMed] [Google Scholar]
- 25. Roman, S. , Lin R., and Sosa J. A.. 2006. Prognosis of medullary thyroid carcinoma: demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer 107:2134. [DOI] [PubMed] [Google Scholar]
- 26. Scopsi, L. , Sampietro G., Boracchi P., Del Bo R., Gullo M., Placucci M., et al. 1996. Multivariate analysis of prognostic factors in sporadic medullary carcinoma of the thyroid: a retrospective study of 109 consecutive patients. Cancer 78:2173. [PubMed] [Google Scholar]
- 27. Van Heerden, J. A. , Grant C. S., Gharib H., Hay I. D., and Ilstrup D. M.. 1990. Long‐term course of patients with persistent hypercalcitonemia after apparent curative primary surgery for medullary thyroid carcinoma. Ann. Surg. 212:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cupisti, K. , Wolf A., Raffel A., Schott M., Miersch D., Yang Q., et al. 2007. Long‐term clinical and biochemical follow‐up in medullary thyroid carcinoma: a single institution's experience over 20 years. Ann. Surg. 246:815. [DOI] [PubMed] [Google Scholar]
- 29. Grozinsky‐Glasberg, S. , Benbassat C. A., Tsvetov G., Feinmesser R., Peretz H., Shimon I., et al. 2007. Medullary thyroid cancer: a retrospective analysis of a cohort treated at a single tertiary care center between 1970 and 2005. Thyroid 17:549. [DOI] [PubMed] [Google Scholar]
- 30. Pelizzo, M. R. , Boschin I. M., Bernante P., Toniato A., Piotto A., Pagetta C., et al. 2007. Natural history, diagnosis, treatment and outcome of medullary thyroid cancer: 37 years experience on 157 patients. Eur. J. Surg. Oncol. 33:493. [DOI] [PubMed] [Google Scholar]
- 31. Alevizaki, M. , Saltiki K., Rentziou G., Papathoma A., Sarika L., Vasileiou V., et al. 2012. Medullary thyroid carcinoma: the influence of policy changing in clinical characteristics and disease progression. Eur. J. Endocrinol. 167:799. [DOI] [PubMed] [Google Scholar]
- 32. Chong, G. C. , Beahrs O. H., Sizemore G. W., and Woolner L. H.. 1975. Medullary carcinoma of the thyroid gland. Cancer 35:695. [DOI] [PubMed] [Google Scholar]
- 33. LiVolsi, V. A. 1990. Surgical pathology of the thyroid Pp. 22:164–166 in Benington J. L., ed. Major problems in pathology. WB Saunders, Philadelphia, PA, USA. [Google Scholar]
- 34. Moo‐Young, T. A. , Traugott A. L., and Moley J. F.. 2009. Sporadic and familial medullary thyroid carcinoma: state of the art. Surg. Clin. N. Am. 89:1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Perros, P. , British Thyroid Association , Royal College of Physicians 2007. Guidelines for the management of thyroid cancer. 2nd ed Royal College of Physicians, London, United Kingdom. [Google Scholar]
- 36. Quan, G. M. Y. , Pontillart V., Palussiere J., and Bonichon F.. 2012. Multidisciplinary treatment and survival of patients with vertebral metastases from thyroid carcinoma. Thyroid 22:125. [DOI] [PubMed] [Google Scholar]
- 37. Stojanodinovic, A. , Shoup M., Ghossein R. A., Nissan M., Brenan M. F., Shah J. P., et al. 2002. The role of operations for distantly metastatic well‐differentiated thyroid carcinoma. Surgery 131:636. [DOI] [PubMed] [Google Scholar]
- 38. Baloch, Z. , Carayon P., Conte‐Devoix B., Rasmussen U. F., Henry J. F., LiVolsi V., et al. 2003. Laboratory medicine practice guidelines: laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid 13:3. [DOI] [PubMed] [Google Scholar]
- 39. Cooper, D. S. , Doherty G. M., Hauge B. R., Kloos R. T., Lee S. L., Mandel S. J., et al. 2009. The American Thyroid Association (ATA) guidelines taskforce on thyroid nodules and differentiated thyroid cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19:1167. [DOI] [PubMed] [Google Scholar]
- 40. Frates, M. C. , Benson C. B., Charboneau J. W., Cibas E. S., Clark O. H., Coleman B. G., et al. 2005. Management of thyroid nodules detected at US: Society of Radiologists in ultrasound consensus conference statement. Radiology 237:794. [DOI] [PubMed] [Google Scholar]
- 41. Gharib, H. , Papini E., Paschke R., Duick D. S., Valcavi R., Hegedus L., et al. 2010. AACE/AME/ETA thyroid nodule guidelines. Endocr. Pract. 16:1.20497938 [Google Scholar]
- 42. Welker, M. J. , and Orlov D.. 2003. Thyroid nodules. Am. Fam. Physician 67:559. [PubMed] [Google Scholar]
- 43. Clayman, G. L. , and El‐Baradie T. S.. 2003. Medullary thyroid cancer. Otolaryngol. Clin. N. Am. 36:91. [DOI] [PubMed] [Google Scholar]
- 44. Tuttle, R. M . 2012. Treatment of medullary thyroid cancer In UpToDate, Ross D. S., ed. UpToDate, Waltham, MA, USA. [Google Scholar]
- 45. Conway, A. , Wiernik A., Rawal A., Lam C., and Mesa H.. 2012. Occult primary medullary thyroid carcinoma presenting with pituitary and parotid metastases: case report and review of the literature. Endocri. Pathol. 23:115. [DOI] [PubMed] [Google Scholar]
- 46. Erovic, B. M. , Kim D., Cassol C., Goldstein D. P., Irish J. C., Asa S. L., et al. 2012. Prognostic and predictive markers in medullary thyroid carcinoma. Endocr. Pathol. 23:232. [DOI] [PubMed] [Google Scholar]
- 47. Faik Erdogan M, A. G. , Erdogan G., and Kamel N.. 2006. Radioactive iodine treatment in medullary thyroid carcinoma. Nucl. Med. Commun. 27:359. [DOI] [PubMed] [Google Scholar]
- 48. Fialkowski, E. A. , and Moley J. F.. 2006. Current approaches to medullary thyroid carcinoma, sporadic and familial. J. Surg. Oncol. 94:737. [DOI] [PubMed] [Google Scholar]
- 49. Kloos, R. T. , Eng C., Evans D. B., Francis G. L., Gagel R. F., Gharib H., et al. 2009. The American Thyroid Association (ATA) guidelines taskforce on medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid 19:565. [DOI] [PubMed] [Google Scholar]
- 50. Treglia, G. , Rufini V., Salvatori M., Giordano A., and Giovanella L.. 2012. PET in recurrent medullary thyroid carcinoma. Int. J. Mol. Imaging 2012:324686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stepanas, A. V. , Samaan N. A., Hill C. S. Jr, and Hickey R. C.. 1979. Medullary thyroid carcinoma: importance of serial serum calcitonin measurement. Cancer 43:825. [DOI] [PubMed] [Google Scholar]
- 52. Berna, L. , Cabezas R., Mora J., Torres G., Estorch M., and Carrio I.. 1995. 111In‐Octreotide and 99mTc(V)‐dimercaptosuccinic acid studies in the imaging of recurrent medullary thyroid carcinoma. J. Endocrinol. 144:339. [DOI] [PubMed] [Google Scholar]
- 53. Boi, F. , Maurelli I., Pinna G., Atzeni F., Piga M., Lai M. L., et al. 2007. Calcitonin measurement in wash‐out fluid from fine needle aspiration of neck masses in patients with primary and metastatic medullary thyroid carcinoma. J. Clin. Endocrinol. Metab. 92:2115. [DOI] [PubMed] [Google Scholar]
- 54. Gotthardt, M. , Behe M. P., and Beuter D.. 2006. Improved tumour detection by gastrin receptor scintigraphy in patients with metastasised medullary thyroid carcinoma. Eur. J. Nucl. Med. Mol. Imaging 33:1273. [DOI] [PubMed] [Google Scholar]
- 55. Krausz, Y. , Rosler A., Guttmann H., Ish‐Shalom S., Shibley N., Chisin R., et al. 1999. Somatostatin receptor scintigraphy for early detection of regional and distant metastases of medullary carcinoma of the thyroid. Clin. Nucl. Med. 24:256. [DOI] [PubMed] [Google Scholar]
- 56. Ong, S. C. , Schoder H., Patel S. G., Tabangay‐Lim I. M., Doddamane I., Gonen M., et al. 2007. Diagnostic accuracy of 18F‐FDG PET in restaging patients with medullary thyroid carcinoma and elevated calcitonin levels. J. Nucl. Med. 48:501. [DOI] [PubMed] [Google Scholar]
- 57. Oudoux, A. , Salaun P. Y., Bournaud C., Campion L., Ansguer C., Rousseau C., et al. 2007. Sensitivity and prognostic value of positron emission tomography with F‐18‐fluorodeoxyglucose and sensitivity of immunoscintigraphy in patients with medullary thyroid carcinoma treated with anticarcinoembryogenic antigen‐targeted radioimmunotherapy. J. Clin. Endocrinol. Metab. 92:4590. [DOI] [PubMed] [Google Scholar]
- 58. Udelsman, R. , Ball D., Baylin S. B., Wong C. Y., Osterman F. A. Jr, and Sostre S.. 1993. Preoperative localization of occult medullary carcinoma of the thyroid gland with single‐photon emission tomography dimercaptosuccinic acid. Surgery 114:1083. [PubMed] [Google Scholar]
- 59. Lupone, G. , Antonino A., Rosato A., Zenone P., Iervolino E. M., Grillo M., et al. 2012. Surgical strategy for the treatment of sporadic medullary thyroid carcinoma: our experience. G. Chir. 33:395. [PubMed] [Google Scholar]
- 60. Brierley, J. , Tsang R., Simpson W. J., Gospodarowicz M., Sutcliffe S., and Panzarella T.. 1996. Medullary thyroid cancer: analyses of survival and prognostic factors and the role of radiation therapy in local control. Thyroid 6:305. [DOI] [PubMed] [Google Scholar]
- 61. Fersht, N. , Vini L., A'Hern R., and Harmer C.. 2001. The role of radiotherapy in the management of elevated calcitonin after surgery for medullary thyroid cancer. Thyroid 11:1161. [DOI] [PubMed] [Google Scholar]
- 62. Fife, K. M. , Bower M., and Harmer C.. 1996. Medullary thyroid cancer: the role of radiotherapy in local control. Eur. J. Surg. Oncol. 22:588. [DOI] [PubMed] [Google Scholar]
- 63. Hyer, S. L. , Vini L., A'Hern R., and Harmer C.. 2000. Medullary thyroid cancer: multivariate analysis of prognostic factors influencing survival. Eur. J. Surg. Oncol. 26:686. [DOI] [PubMed] [Google Scholar]
- 64. Martinez, S. R. , Beal S. H., Chen A., Chen S. L., and Schneider P. D.. 2010. Adjuvant external beam radiation for medullary thyroid carcinoma. J. Surg. Oncol. 102:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Nguyen, T. D. , Chassard J. L., Lagarde P., Cutuli B., Le Fur R., Reme‐Saumon M., et al. 1992. Results of postoperative radiation therapy in medullary carcinoma of the thyroid: a retrospective study by the French Federation of Cancer Institutes–the Radiotherapy Cooperative Group. Radiother. Oncol. 23:1. [DOI] [PubMed] [Google Scholar]
- 66. Samaan, N. A. , Schultz P. N., and Hickey R. C.. 1988. Medullary thyroid carcinoma: prognosis of familial versus sporadic disease and the role of radiotherapy. J. Clin. Endocrinol. Metab. 67:801. [DOI] [PubMed] [Google Scholar]
- 67. Schwartz, D. L. , Rana V., Shaw S., Yazbeck C., Ang K. K., Morrison W. H., et al. 2008. Postoperative radiotherapy for advanced medullary thyroid cancer: local disease control in the modern era. Head Neck 30:883. [DOI] [PubMed] [Google Scholar]
- 68. Terezakis, S. A. , and Lee N. Y.. 2010. The role of radiation therapy in the treatment of medullary thyroid cancer. J. Natl. Compr. Canc. Netw. 8:532. [DOI] [PubMed] [Google Scholar]
- 69. Wilson, P. C. , Millar B. M., and Brierley J. D.. 2004. The management of advanced thyroid cancer. Clin. Oncol. 16:561. [DOI] [PubMed] [Google Scholar]
- 70. Smit, J. 2013. Treatment of advanced medullary thyroid cancer. Thyroid Res. 6:S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jimenez, C. , Hu I. M., and Gagel R. F.. 2008. Management of medullary thyroid carcinoma. Endocrinol. Metab. Clin. N. Am. 37:481. [DOI] [PubMed] [Google Scholar]
- 72. Abdel‐Wanis, M. E. , Kawahara N., Murata A., Murakami H., Nambu K., Ueda Y., et al. 2002. Thyroid cancer spinal metastases: report on 25 operations in 14 patients. Anticancer Res. 22:2509. [PubMed] [Google Scholar]
- 73. Demura, S. , Kawahara N., Murakami H., Abdel‐Wanis M. E., Kato S., Yoshioka K., et al. 2011. Total en bloc spondylectomy for spinal metastases in thyroid carcinoma. J. Neurosurg. Spine. 14:172. [DOI] [PubMed] [Google Scholar]


