Abstract
The CpxAR (Cpx) two-component regulator controls the expression of genes in response to a variety of environmental cues. The Cpx regulator has been implicated in the virulence of several gram-negative pathogens, although a role for Cpx in vivo has not been demonstrated directly. Here we investigate whether positive or negative control of gene expression by Cpx is important for the pathogenesis of Salmonella enterica serotype Typhimurium. The Cpx signal pathway in serotype Typhimurium was disrupted by insertional inactivation of the cpxA and cpxR genes. We also constitutively activated the Cpx pathway by making an internal in-frame deletion in cpxA (a cpxA* mutation). Activation of the Cpx pathway inhibited induction of the envelope stress response pathway controlled by the alternative sigma factor σE (encoded by rpoE). Conversely, the Cpx pathway was highly up-regulated (>40-fold) in a serotype Typhimurium rpoE mutant. The cpxA* mutation, but not the cpxA or the cpxR mutation, significantly reduced the capacity of serotype Typhimurium to adhere to and invade eucaryotic cells, although intracellular replication was not affected. The cpxA and cpxA* mutations significantly impaired the ability of serotype Typhimurium to grow in vivo in mice. To our knowledge, this is the first demonstration that the Cpx system is important for a bacterial pathogen in vivo.
Salmonellae are capable of surviving in a number of environmental niches. A variety of signal transduction systems allow salmonellae to perceive alterations in the external environment or damage to their cellular components and to make appropriate changes in their physiology in order to prolong survival. The response to alterations in the cell envelope is governed by at least three extracytoplasmic stress response (ESR) pathways in Salmonella spp. One pathway is controlled by the alternative sigma factor σE (RpoE) (12, 22), another by the two-component regulator CpxAR (32, 36), and a third, recently described, by the two-component regulator BaeSR (34).
The alternative sigma factor σE is activated in response to the accumulation of misfolded polypeptides, in particular outer membrane proteins, in the periplasm (23). Until recently only a few σE-regulated genes were known, but a number of new Escherichia coli σE-regulated genes have now been described (6, 37). The products of these genes include enzymes involved in the correct folding or degradation of proteins in the periplasm or in the biosynthesis of envelope components. σE is critically important for the virulence of Salmonella enterica serovar Typhimurium (13, 41) and is also involved in the pathogenesis of other bacteria (2, 19, 44).
The CpxAR two-component regulator system (referred to below as Cpx) consists of a histidine kinase sensor (HKS), CpxA, and a cognate response regulator (RR), CpxR (5, 36). A third component, CpxP, binds CpxA and inhibits its activity (4). The Cpx pathway is activated in E. coli by alterations in the cell envelope composition, particularly changes in the levels of the lipoprotein NlpE (40), changes in pH (4, 28), and overexpression of misfolded envelope proteins (5, 16). The first genes that were shown to be Cpx regulated (htrA, dsbA, and ppiA) are involved in protein folding or degradation in the periplasm (3, 5). More-recent work on the CpxAR regulon has identified a greater range of targets for phosphorylated CpxR in E. coli (7, 10), indicating a role for the Cpx regulon beyond the periplasm.
The Cpx regulon has been implicated in the virulence of a number of bacterial pathogens. CpxA has been shown to be involved in the pH-dependent regulation of Shigella flexneri VirF, a positive regulator of the invasion genes ipaBCD (29). More recently, in serovar Typhimurium, Cpx has been shown to be involved in the pH-dependent regulation of HilA, a positive regulator of invasion genes (27). Also, DsbA is required for the folding and secretion of the IpaBCD proteins by S. flexneri and of other virulence factors such as the enterotoxin produced by Vibrio cholerae (45, 46). A TnphoA insertion in cpxA reduces the ability of Salmonella enterica serovar Typhi to adhere to and invade epithelial cells in vitro (20).
All the above studies used in vitro assays, and there is no direct in vivo evidence that the Cpx regulon is important for the virulence of salmonellae or other pathogens. We have constructed strains of serovar Typhimurium with separate mutations in cpxA and cpxR that either inactivate or constitutively activate the Cpx pathway, and we have investigated the effects of these mutations on the ability of serovar Typhimurium to interact with eucaryotic cells in vitro and to infect mice.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Bacterial strains and plasmids used or constructed in this study are listed in Table 1. All strains were maintained on Luria-Bertani (LB) or M9 minimal medium, prepared as described elsewhere (38, 39). Where required, the medium was supplemented with 100 μg of ampicillin/ml, 50 μg of kanamycin/ml, 100 μg of streptomycin/ml, 50 μg of chloramphenicol/ml, 50 μg of gentamicin/ml, and 50 μg of 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal)/ml.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| Salmonella serovar Typhimurium | ||
| SL1344 | his mutant mouse virulent strain | 12a |
| GVB368 | SL1344 cpxR::Kmr | This study |
| GVB822 | SL1344 cpxA::Gmr | This study |
| GVB870 | SL1344 cpxA* | This study |
| GVB311 | SL1344 rpoE::Kmr | 13 |
| SMS438 | SL1344 rpoS::Apr | 30 |
| E. coli SM10 λpir | thi-1 thr1 leuB6 tonA21 lacY1 supE44 recA::RP4-2-Tc::Mu Kmr λpir | 17 |
| Plasmids | ||
| pCRII | PCR cloning vecor | Invitrogen |
| pWSK29 | Apr; low-copy-number vector | 43 |
| pRDH10 | λpir-based suicide vector; Tetr Cmr SacB | R. Haigh (Leicester University, Leicester, United Kingdom |
| pUC4K | Source of Kmr gene (aphI) cassette | 42 |
| pBBR1MCS-5 | Source of Gmr gene (accC1) | 18 |
| pSH105 | cpxR in pCRII | This study |
| pSH115 | pRDH10 cpxR::Kmr | This study |
| pSH130 | pRDH10 cpxA::Gmr | This study |
| pSH137 | pRDH10 ΔcpxA (in-frame deletion) | This study |
| pcpxRA | cpxRA in pWSK29 | This study |
| pTL61T | lacZ reporter plasmid | 21 |
| pcpxR::lacZ | cpxRA promoter in pTL61T | This study |
| prpoEP3::lacZ | rpoEP3 promoter in pTL61T | 26 |
Recombinant DNA methods.
Standard methods were used for preparation of plasmid or chromosomal DNA, restriction analysis, and ligation. DNA hybridization was carried out by using the Gene Images labeling and detection kit (Amersham Life Sciences). DNA for sequencing was isolated by using QIAGEN plasmid preparation columns. Sequencing reactions were carried out by using the Thermo Sequenase kit (Amersham) and were run on a LI-COR 4000L automated DNA sequencer. All PCRs were carried out using Taq polymerase (Reddymix; ABgene). The PCR template was generated from a single bacterial colony. Standard methods were used for transformation and conjugation of plasmid DNA from E. coli to serovar Typhimurium.
Cloning and mutation of the cpxR and cpxA genes.
The serovar Typhimurium cpxR gene was amplified by using primers cpxR1 (5′-GAGCTGACTTCCCTATATTAAA-3′) and cpxR2 (5′-GTCGAAAGGCGTCAGGCGTT-3′). The serovar Typhimurium cpxA gene was amplified by using primers cpxAfw (5′-CCGGAACGCAAAGACGGTCA-3′) and cpxArv (5′-TCGACGGCGAGATAAAAAAT-3′). PCR products were cloned directly into pCR2.I (Invitrogen) according to the manufacturer's instructions. The clones were checked by DNA sequencing.
The cpxR gene was subcloned from pCR2.I into pBluescript by digestion with HindIII and XbaI to form pSH105. The cpxR gene was mutated by the introduction of a BglII site by inverse PCR at positions 324 to 329 to produce pSH110. The kanamycin resistance gene of pUC4K (aphI encoding kanamycin kinase; Kmr) was isolated by digestion with BamHI and ligated with BglII-digested pSH110. The disrupted cpxR gene (cpxR::Kmr) was subcloned into the suicide vector pRDH10 to generate pSH115. pRDH10 requires the pir product for replication and possesses sacB from Bacillus subtilis, allowing for positive selection for allelic exchange (13). The use of this suicide vector for introducing mutations into serovar Typhimurium has been described previously (13). The wild-type (WT) SL1344 cpxR gene was replaced with cpxR::Kmr by allelic exchange following introduction of pSH115 by conjugation.
The cpxA gene was inactivated by inserting a gentamicin resistance (Gmr) gene into a StuI site that had been engineered at bp 1483 of cpxA by inverse PCR. The Gmr gene (acc1) was amplified by PCR from plasmid pBBR1MCS-5 and cloned into pCR2.1. This construct was then digested with EcoRV and EclI, generating acc1 with blunt ends, which was cloned into the StuI site of the engineered cpxA gene. The cpxA::Gmr construct was then subcloned into pRDH10 and used to produce an SL1344 cpxA mutant by allelic exchange.
An in-frame deletion mutation was created in the cpxA gene to generate a mutation that rendered the Cpx system constitutive (cpxA* mutation). The mutation was engineered by overlapping PCR, which created a deletion removing amino acids 92 to 104 of CpxA. The deletion also created a new EcoRI restriction site that was used to screen for the mutation. The PCR product containing the ΔcpxA gene was then subcloned into pRDH10 and used to generate a serovar Typhimurium mutant as described above. The genotype of the mutants was analyzed and confirmed to be correct by PCR and Southern hybridization (data not shown).
The complete cpxRA operon, including the upstream promoter region, was amplified by PCR using primers cpxR3 (5′-GCTTGTAACAACTTCAGCTGCGTGG-3′) and cpxR4 (5′-GCTTGGGCAACATCAGGACCAGCATT-3′) and was cloned into the low-copy-number vector pWSK29 (pcpxRA). This plasmid was used to complement the cpx mutations.
Construction of the pcpxR::lacZ reporter plasmid and β-galactosidase assay.
The promoter region of cpxR was amplified by using primers cpxP1Hind (5′-CCCAAGCTTCAAACATGCGTCAGGGGGTG-3′) and cpxP1Xba (5′-GCTCTAGAGTTTACGTACCTCCGAGGCAC-3′), which possessed HindIII and XbaI restriction endonuclease sites, respectively. The PCR product was digested with the appropriate enzymes and then ligated with the lacZ reporter vector pTL61T (21), which had been similarly treated. The correct products were selected by overnight growth on plates containing ampicillin and X-Gal. A single blue colony was selected and sequenced to confirm that the promoter had been cloned correctly. The plasmid was then transformed by electroporation into WT and mutant serovar Typhimurium strains. The same vector was used previously to generate a reporter for monitoring expression from the σE-dependent promoter (rpoEP3) of the rpoE operon (26).
Overnight cultures of strains containing the appropriate plasmid(s) were diluted 1:1,000 into fresh LB broth with appropriate antibiotics, and the cultures were incubated at 37°C with aeration. Samples were taken every hour and assayed for β-galactosidase activity (24), adapted to a 96-well plate format. A mean activity was calculated from seven samples.
Amikacin sensitivity test.
Overnight bacterial cultures were diluted in fresh LB to an optical density of 0.25 at 600 nm and were grown for 2 h at 30 or 37°C. After growth, 100-μl aliquots were used to inoculate 3 ml of top agar, which was used to form a lawn on LB agar plates. Disks containing 30 U of amikacin (Oxoid) were placed on the lawns, and the plates were incubated aerobically overnight at 37°C. The diameter of the zone of inhibition was determined.
Invasion and persistence of serovar Typhimurium in phagocytic and nonphagocytic cells.
The abilities of the different serovar Typhimurium strains to invade and survive in phagocytic and nonphagocytic cells were assessed by using the macrophage-like cell line RAW 264.7 and the epithelial cell lines HEp2 and Caco-2. Cells were routinely cultured in Dulbecco's modified Eagle medium (DMEM; Gibco BRL) supplemented with 4 mM l-glutamine and 10% (vol/vol) fetal bovine serum. For invasion assays, cells were seeded into 24-well tissue culture plates (Costar) at 105 per well and were incubated at 37°C with 5% CO2 overnight or until confluent (5 to 6 days for Caco-2). Where appropriate, cells were incubated with cytochalasin D (CD) (0.5 μg/ml) for 1 h prior to infection, and CD was maintained in the medium throughout the assay. Prior to infection, the monolayers were washed twice with phosphate-buffered saline (PBS). Bacteria from overnight cultures in LB medium were diluted in 1 ml of DMEM to give a multiplicity of infection of approximately 1:1. The inocula were added to monolayers and then incubated for 1 to 2 h as above. The monolayers were washed twice with sterile PBS, overlaid with 1 ml of DMEM containing 100 μg of polymyxin B/ml, and incubated for 1 h as above. Then monolayers were washed twice with PBS, and the cells were either lysed (with sterile water plus 1% Triton X-100) or overlaid with 1 ml of DMEM containing 10 μg of polymyxin B/ml and incubated for a further 3 or 21 h before being washed and lysed as above. Following lysis of the cells, the number of viable bacteria released from the cells was determined by plating serial dilutions on Luria agar. To investigate adhesion to cells in the absence of invasion, cells were incubated with CD (0.5 μg/ml) for 1 h prior to infection, and CD was maintained in the medium throughout the assay to prevent bacterial internalization. The assay was then performed as described above except that the monolayer was washed four times instead of two and cells were directly lysed and not incubated in the presence of polymyxin B. Statistical differences in the number of bacteria recovered were analyzed by analysis of variance.
Determination of in vivo CI.
Salmonellae grown statically overnight in LB medium were recovered by centrifugation and resuspended in sterile PBS (pH 7.2). Groups of three or four female BALB/c mice (6 to 8 weeks old; Charles River, Margate, United Kingdom) were challenged with an inoculum containing equal numbers (∼103 CFU) of two strains of bacteria by either the intraperitoneal or the intravenous route. The actual number of bacteria present was determined by viable counts. Three or four days later, mice were killed, and the numbers of each strain present in the liver and spleen were determined. The competitive index (CI) is calculated as (output CFU of strain A/output CFU of strain B)/(input CFU of strain A/input CFU of strain B). Each assay was performed at least twice. A CI of ∼1.0 indicates that the strains compete equally well. To determine whether the difference in CFU recovered versus CFU inoculated for the pair of strains was statistically significant, the ratio of CFU recovered to CFU administered for each strain was calculated for individual mice [i.e., for mouse 1, (output CFU of strain A)/(input CFU of strain A) and (output CFU of strain B)/(input CFU of strain B) were calculated], and results were compared by the Mann-Whitney U test.
RESULTS
Construction of serovarTyphimurium cpx mutants.
A number of in vitro studies have indicated that the Cpx regulon may be involved in the virulence of several bacterial pathogens, including Salmonella spp. We and others have shown that the arm of the ESR controlled by σE is critically important for the growth and survival of serovar Typhimurium in mice (13). We therefore wished to see if the Cpx regulon is also involved in serovar Typhimurium pathogenesis. We constructed several serovar Typhimurium strains with mutations in cpxA or cpxR that inactivate or constitutively activate the Cpx regulon (see Materials and Methods). The most profound cpx phenotypes described in E. coli are attributed to constitutive activation of the Cpx regulon due to particular mutations in cpxA designated cpxA* (9). These mutations were generally found to map to the region of cpxA that codes for the periplasmic domain of CpxA and were thought to make the protein signal blind (36). We created an in-frame deletion in cpxA which removed amino acids 92 to 104 from CpxA to create a serovar Typhimurium cpxA* mutant as described above.
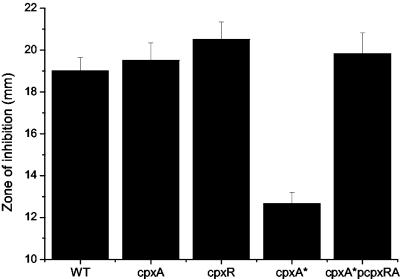
To confirm that the mutants had the expected phenotypes, we examined the activity of the cpxRA promoter (see below) in the different mutants as well as the mutants' sensitivity to the antibiotic amikacin. E. coli cpxA* strains are much less sensitive to amikacin than WT strains (9); we therefore checked whether our putative serovar Typhimurium cpxA* strain had altered amikacin sensitivity. Like E. coli cpxA* strains, the serovar Typhimurium cpxA* strain was found to be significantly (P < 0.001) more resistant to amikacin than its WT parent (Fig. 1). This phenotype was complemented by a plasmid (pcpxRA) carrying a WT copy of the cpxRA genes. Both the cpxR and the cpxA mutant were more sensitive to amikacin than the WT strain, but only the cpxR mutant was significantly so (P < 0.05).
FIG. 1.
The cpxA* mutation renders serovar Typhimurium more resistant to the antibiotic amikacin. Serovar Typhimurium strains were tested for sensitivity to amikacin by a disk diffusion assay. Bars, mean diameters of the zone of inhibition (in millimeters); error bars, standard deviations for six replicates.
Effect of cpx mutations on cpxRA expression in serovar Typhimurium.
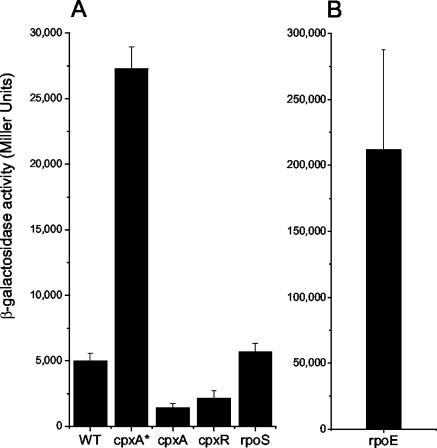
The activity of the serovar Typhimurium cpxRA promoter in the WT and mutant strains was analyzed by using a lacZ reporter plasmid, pcpxR::lacZ (Fig. 2). The activity of pcpxR::lacZ in the cpxA* mutant was much higher than those of the WT (∼5-fold), cpxA, and cpxR (∼20-fold for both mutants) strains, indicating that the Cpx pathway is constitutively active in this strain. In contrast, the β-galactosidase activities of the cpxA- and cpxR-null mutants were ∼2- to ∼3.5-fold lower than that of the WT strain. In E. coli the cpxRA promoter is reported to be dependent on the alternative sigma factor RpoS for activity (8). There was no difference between the β-galactosidase activity of a serovar Typhimurium rpoS mutant and that of the WT strain after 5 h of growth (Fig. 2) or at other stages in the growth curve (data not shown). Therefore, it would appear that in serovar Typhimurium, cpxRA expression is not dependent on RpoS.
FIG. 2.
Effect of mutations in stress response regulators on expression of the cpx genes. β-Galactosidase activities were determined for serovar Typhimurium strains harboring the pcpxR::lacZ reporter plasmid. Strains were grown for 5 h (late-log phase) in LB broth at 37°C with aeration. Bars represent means for seven replicates; error bars, standard deviations.
The cpxRA promoter is highly active in a serovar Typhimurium cpxA* mutant; however, it is even more active (∼43-fold more than in the WT strain) in a serovar Typhimurium rpoE mutant (Fig. 2). This indicates that the absence of rpoE causes a massive activation of the Cpx pathway. Overexpression of σE does not reduce expression of cpxR or cpxA (data not shown), so it seems unlikely that a member of the σE regulon is a negative regulator of cpxRA. The Cpx pathway is probably highly activated in the rpoE mutant as a response to the envelope stress experienced by this strain.
In E. coli the Cpx pathway negatively regulates expression of rpoE directly (10). Recently it also was shown that the rpoE transcript was present in a higher concentration in a serovar Typhimurium cpxR mutant (26). The serovar Typhimurium rpoErseABC operon possesses three promoters, the first two of which are σE independent, while the third, rpoEP3, is σE dependent (26).
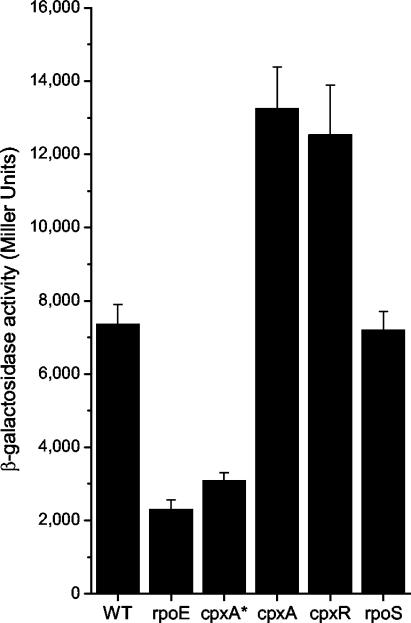
In order to examine the effect of the Cpx pathway on induction of the σE regulon in serovar Typhimurium, we examined the activity of rpoEP3 in the different mutants by using a lacZ reporter construct (prpoEP3::lacZ). Compared to that of the WT strain, the expression of prpoEP3::lacZ is reduced approximately two- to threefold in the rpoE and cpxA* strains, respectively (Fig. 3). Conversely, the activity of prpoEP3::lacZ is approximately doubled in the cpx-null mutants relative to that in the WT strain. Thus, in serovar Typhimurium, as in E. coli, activation of the Cpx pathway has a negative impact on induction of the σE regulon. The β-galactosidase activity of the rpoS mutant is similar to that of the WT strain, indicating that rpoS does not influence expression of the σE-dependent rpoEP3 promoter.
FIG. 3.
CpxAR suppresses activation of the σE regulon. β-Galactosidase activities were determined for serovar Typhimurium strains harboring the prpoEP3::lacZ reporter plasmid. Strains were grown for 5 h (late log phase) in LB broth at 37°C with aeration. Bars represent means for seven replicates; error bars, standard deviations.
Effect of the cpx mutations on the ability of serovar Typhimurium to enter and replicate within eucaryotic cells.
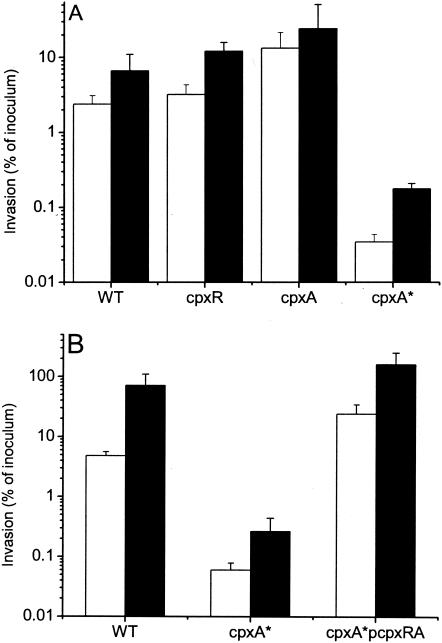
A serovar Typhimurium rpoE mutant invades macrophages normally but survives poorly intracellularly (13). We analyzed the abilities of the cpx mutants to invade and persist in macrophages and epithelial cells. Both cpx-null mutants invaded the macrophage-like cell line RAW 264.7 as well as the WT strain did; in fact the cpxA strain consistently exhibited a slight but significant (P < 0.05) increase in invasiveness over that of its WT parent (Fig. 4A). The serovar Typhimurium cpxA* strain was significantly less invasive than the WT, cpxA, and cpxR strains (P < 0.001). The reduced invasiveness of the cpxA* mutant could be complemented with plasmid pcpxRA (Fig. 4B). At 24 h postinfection, the intracellular numbers of all bacteria had increased, indicating that none of the cpx mutations affect the intracellular survival or replication of serovar Typhimurium. Similar results were obtained when the assay was performed with HEp2 or Caco-2 epithelial cells (data not shown). In order to ascertain whether the cpxA* strain was defective in initial adhesion or in entry into cells, the invasion assay was repeated in the presence of CD to prevent bacterial internalization. The presence of CD did not affect the results of the invasion assay; significantly fewer CFU of the cpxA* strain than of the WT strain were recovered (data not shown). Similarly, centrifugation of the cpxA* mutant onto cells did not overcome the internalization defect of the strain. Collectively these results suggest that adhesion to eucaryotic cells rather than cell entry is affected in the serovar Typhimurium cpxA* mutant.
FIG. 4.
Effects of cpx mutations on serovar Typhimurium invasion of and survival within macrophages. Bacteria at a multiplicity of infection of ∼1:1 were incubated with the murine macrophage cell line RAW 264.7. The assay was performed as described in the text. Graphs show the number of viable bacteria (as a percentage of the initial inoculum) inside the macrophages at 3 h (open bars) and 24 h (solid bars) after infection. Each bar represents the mean from triplicate experiments. Error bars, standard deviations. (A) Effects of cpx mutations; (B) complementation of the cpxA* mutation.
Effects of cpx mutations on Salmonella virulence.
The capacities of the cpxR- and cpxA-null mutants to compete for growth in murine tissues with the WT strain were compared by a competition assay. The CI is determined by dividing the ratio of the two strains isolated from murine tissues by the ratio of the two strains in the inoculum. The CI for the cpxR mutant versus the WT was 0.53; the difference in the recovery of the two strains was not significant. The cpxA-null strain was significantly (P < 0.05) less able than the WT strain to compete in vivo (CI, 0.198). Because the cpxA* strain does not possess a selectable marker, it is not possible to directly distinguish it from the WT strain in a competition assay. Instead, the cpxA* strain was made to compete with the cpxR strain, whose virulence is very similar to that of the WT strain. The CI was 0.0358 (P < 0.05).
We investigated whether the cpx mutations affected the ability of serovar Typhimurium to infect mice by the natural route of infection by infecting mice orally and determining the number of CFU in different tissues postinfection. At a dose of ∼5 × 106 CFU, the cpxR mutant infected the Peyer's patches (PP), mesenteric lymph nodes, liver, and spleen as well as the WT strain did (data not shown). In contrast, most mice challenged with either the cpxA or the cpxA* strain did not become infected, and for those that did, very few organisms could be recovered.
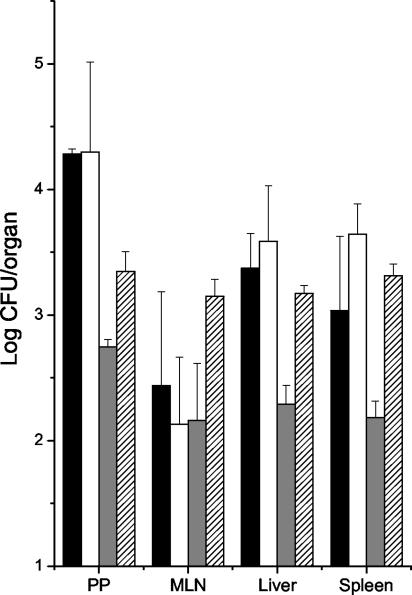
At a higher dose (∼2 ×108 CFU to 3 ×108 CFU), both the cpxA and cpxA* strains colonized the PP and translocated to deeper tissues. Mice were culled on days 4 and 7, the organs were removed, and CFU were determined (Fig. 5). The cpxA* strain was found at lower levels than the cpxA strain in all of the organs at day 4; although this difference was not statistically significant, it may possibly indicate a role for cpxA* in the early stages of serovar Typhimurium infection. By day 7 the difference between the two mutants was less apparent. Also, 2 days after oral challenge, much higher numbers of the cpxA strain than of the cpxA* strain were shed in the feces of mice (data not shown).
FIG. 5.
Effects of the cpxA mutations on the ability of serovar Typhimurium to colonize murine organs following oral infection. Mice were infected with either the cpxA (∼2 × 108 CFU) or the cpxA* (∼3 ×108 CFU) strain. Numbers of bacteria present in different organs of the mice were determined on days 4 and 7 postinfection. Bard represent means for four mice; error bars, standard deviations. MLN, mesenteric lymph node. Solid bars, cpxA strain on day 4; open bars, cpxA strain on day 7; shaded bars, cpxA* strain on day 4; hatched bars, cpxA* strain on day 7.
DISCUSSION
Our results indicate that the Cpx signal transduction system plays a role in the pathogenesis of salmonellae. Null mutations in the genes that encode either the sensor (cpxA) or the activator (cpxR) do not adversely affect the attachment and internalization of serovar Typhimurium into eucaryotic cells under the conditions tested in our assay. However, constitutive activation of the Cpx pathway significantly reduced the ability of serovar Typhimurium to enter host cells. This appeared to be due to a reduced ability of the serovar Typhimurium cpxA* mutant to adhere to eucaryotic cells rather than to a defect in making contact with eucaryotic cells or in internalization. This is because the same difference in cell association between the cpxA* mutant and the WT strain was evident if internalization was inhibited or if contact with eucaryotic cells was increased by centrifugation.
In E. coli, all of the phenotypes of cpxA* mutants can be accounted for by elevated levels of phosphorylated CpxR (CpxR-P) (9). Mutations in the periplasmic loop region of CpxA, such as that in our serovar Typhimurium cpxA* strain, are signal blind and are not subject to negative regulation by the cytoplasmic protein CpxP, which down-regulates the Cpx pathway in the absence of stress (35). The molecular mechanism of the effect of CpxP on CpxA activity is not known but is thought to affect the balance of kinase and phosphatase activities (9, 35). The defect in the serovar Typhimurium cpxA* mutant was complemented by a WT copy of the cpxA gene (cpxRA) provided in trans on a plasmid. The WT CpxA protein is responsive to CpxP, which presumably represses its kinase activity and enhances phosphatase activity. Presumably this is sufficient to dephosphorylate the CpxR-P present in the cell and accounts for the complementation observed. However, it is possible that the WT CpxA gene affects the activity of the mutant CpxA* protein directly or indirectly to reduce its ability to phosphorylate CpxR. It would appear that the WT cpxA allele is dominant over the cpxA* allele. However, more studies would be needed to determine if this is the case, since the WT gene is present in multiple copies and there is only a single copy of the cpxA* gene.
A serovar Typhi strain with a TnphoA insertion in cpxA exhibited reduced adhesion to and invasion of Int407 cells (20). The TnphoA insertion in cpxA mapped to nucleotide 267, which is within the region we deleted to construct the serovar Typhimurium cpxA* mutant. Therefore, it is possible that the serovar Typhi mutant is behaving as a cpxA* strain. Recently, it was reported that cpxA (but not cpxR) was required by serovar Typhimurium for expression of hilA at pH 6.0 (27). Mutation of cpxA (or cpxR) did not affect hilA expression at pH 8.0. HilA is a positive regulator of the serovar Typhimurium invasion genes (1). When cultured in a medium at pH 6.0 (but not pH 8.0), the serovar Typhimurium cpxA mutant exhibited reduced internalization of eucaryotic cells. Interestingly, at pH 8.0, both the cpxA and cpxR mutants were internalized slightly more efficiently than the WT strain (27). This is in agreement with our results, where we observed a small but consistent increase in the invasiveness of the cpxA and cpxR mutants over that of the WT strain. In our experiments, invasion assays were performed with bacteria cultured overnight in LB medium; the pH of these cultures was ∼8.0.
We postulate that the cpxA* mutation affects the adhesiveness of serovar Typhimurium. There are a number of studies demonstrating the involvement of the Cpx system in various adhesive processes in E. coli. A functional CpxAR pathway has been shown to be important for surface sensing and adhesion to inanimate surfaces by E. coli (31). It was postulated by the authors that the role of the CpxAR pathway was to regulate genes required to maintain stable contact with the inanimate surface. CpxAR also directly and indirectly affects production of the P pili in E. coli. In the absence of glucose in the culture medium, CpxR-P binds upstream of the pap genes to activate their expression (15). CpxAR also regulates the expression of proteins such as DsbA and PpiA, which assist in pilin assembly in the periplasm. The P pili produced by a cpxR mutant are shorter than those of WT E. coli (15). Conversely, the Cpx system negatively regulates expression of curli in E. coli (33). Thus, in E. coli, the Cpx system can positively or negatively regulate the expression of fimbriae.
Salmonellae, including serovar Typhimurium, have the potential to express a diverse number of fimbriae, most of which have only been shown to be expressed in vivo (14). During infection the appropriate expression of fimbriae is important for the interaction of bacteria with host tissues and other bacteria. If, as in E. coli, CpxAR regulates the expression of several fimbriae in serovar Typhimurium, this could account for the reduced adhesion to eucaryotic cells in vitro and the reduced colonization of murine tissues in vivo exhibited by the serovar Typhimurium cpxA* mutant. CpxAR could be involved in negatively regulating the expression of curli in serovar Typhimurium, as is the case with E. coli. Preliminary studies have indicated that this may be the case (unpublished observation).
Signals that activate the Cpx pathway have been studied extensively in E. coli and include overexpression of the lipoprotein NlpE (40). Adhesion of E. coli to inanimate surfaces induces the Cpx pathway in an NlpE-dependent manner (31). Interestingly, in the serovar Typhimurium LT2 genome sequence, the nlpE gene is a pseudogene containing a frameshift mutation 450 bp from the start codon, although the nlpE gene is intact in the sequenced serovar Typhi strain CT18. We have cloned and sequenced the nlpE gene from SL1344 and found 100% identity with serovar Typhi nlpE (data not shown). Therefore, NlpE should be functional in the strains used in this study. Also, we have found that overexpression of the NlpE protein activates the Cpx pathway in serovar Typhimurium, as it does in E. coli (data not shown).
Among serovar Typhimurium cpx mutants, cpxR was more virulent than cpxA, which in turn was more virulent than cpxA*. The cpxA* mutant would be expected to have excess CpxR-P; therefore; virulence would appear to correlate inversely with CpxR-P levels. If this is the case, then CpxR-P may either negatively regulate genes that are important for the infection of mice or, alternatively, positively regulate genes that interfere with the infection process. Appropriate activation of another two-component regulator has been shown to be critically important for serovar Typhimurium virulence. The PhoPQ two-component regulator controls the expression of genes that are needed for different stages in the pathogenesis of serovar Typhimurium infection. PhoP-activated genes (pag) are involved in survival within macrophages, and PhoP-repressed genes (prg) are involved in the invasion of epithelial cells. Consequently, serovar Typhimurium strains with null mutations in the RR gene phoP (which prevent expression of pag and allow constitutive expression of prg) and strains with deletions in the periplasmic domain of the sensor kinase PhoQ, which constitutively activate the PhoPQ pathway (constitutive activation of pag and complete repression of prg), are both attenuated (25). Therefore, the context of PhoPQ activation is very important for Salmonella infection. The same may also apply to CpxAR.
Because of the organization of the cpxRA operon, insertions into cpxA should not have any effect on cpxR expression, whereas mutations in the cpxR gene may also affect the expression of cpxA. Therefore, CpxR is likely to be expressed in the cpxA-null mutant. Although CpxR could not be phosphorylated by CpxA, other phosphate donors, such as acetyl phosphate, or an alternative HKS protein may activate CpxR. It is also possible that CpxA may interact with alternative RRs. Indeed, several studies have indicated that the CpxA and CpxR proteins can act independently; for example, mutation of cpxA but not cpxR affects the expression of hilA at pH 6.0 in serovar Typhimurium (27). Also, in E. coli, growth-dependent activation of CpxR is independent of CpxA (11).
The rpoE gene is negatively regulated by CpxR-P. The induction of the σE-dependent rpoEP3 promoter in the serovar Typhimurium cpxA* mutant is similar to that in the serovar Typhimurium rpoE mutant. The rpoEP3 promoter is essential for expression of rpoE under conditions that activate the σE pathway; therefore, constitutive activation of the Cpx system may render σE levels suboptimal in vivo. Since σE is critical to serovar Typhimurium pathogenesis, the reduced virulence of the serovar Typhimurium cpxA* strain may be partly due to rpoE repression. However, the rpoE and cpxA* mutations affected the ability of serovar Typhimurium to adhere to, enter, and survive and replicate within eucaryotic cells in vitro in different ways. A serovar Typhimurium rpoE mutant could bind to and enter eucaryotic cells almost as well as the isogenic WT strain but could not survive intracellularly (13). In contrast, the serovar Typhimurium cpxA* mutant appears to be able to grow and survive normally within eucaryotic cells but was defective in the initial interaction with eucaryotic cells. We are currently identifying genes which are positively or negatively regulated by the Cpx system in serovar Typhimurium and investigating their role in the infectious process.
Acknowledgments
This work was supported by a grant (17/PRS12222) from the BBSRC. J.K was partially supported by grant 2/3010/23 from the Slovak Academy of Sciences and by grant 065027/Z/01/Z from the Wellcome Trust.
Editor: J. T. Barbieri
REFERENCES
- 1.Bajaj, V., R. L. Lucas, C. Hwang, and C. A. Lee. 1996. Co-ordinate regulation of Salmonella typhimurium invasion genes by environmental and regulatory factors is mediated by control of hilA expression. Mol. Microbiol. 22:703-714. [DOI] [PubMed] [Google Scholar]
- 2.Craig, J. E., A. Nobbs, and N. J. High. 2002. The extracytoplasmic sigma factor, final σE, is required for intracellular survival of nontypeable Haemophilus influenzae in J774 macrophages. Infect. Immun. 70:708-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danese, P. N., and T. J. Silhavy. 1997. The σE and the Cpx signal transduction systems control the synthesis of periplasmic protein-folding enzymes in Escherichia coli. Genes Dev. 11:1183-1193. [DOI] [PubMed] [Google Scholar]
- 4.Danese, P. N., and T. J. Silhavy. 1998. CpxP, a stress-combative member of the Cpx regulon. J. Bacteriol. 180:831-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danese, P. N., W. B. Snyder, C. L. Cosma, L. J. Davis, and T. J. Silhavy. 1995. The Cpx two-component signal transduction pathway of Escherichia coli regulates transcription of the gene specifying the stress-inducible periplasmic protease, DegP. Genes Dev. 9:387-398. [DOI] [PubMed] [Google Scholar]
- 6.Dartigalongue, C., D. Missiakas, and S. Raina. 2001. Characterization of the Escherichia coli sigma E regulon. J. Biol. Chem. 276:20866-20875. [DOI] [PubMed] [Google Scholar]
- 7.Deretic, V., M. J. Schurr, and H. Yu. 1995. Pseudomonas aeruginosa, mucoidy and the chronic infection phenotype in cystic fibrosis. Trends Microbiol. 3:351-356. [DOI] [PubMed] [Google Scholar]
- 8.De Wulf, P., O. Kwon, and E. C. Lin. 1999. The CpxRA signal transduction system of Escherichia coli: growth-related autoactivation and control of unanticipated target operons. J. Bacteriol. 181:6772-6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Wulf, P., and E. C. Lin. 2000. Cpx two-component signal transduction in Escherichia coli: excessive CpxR-P levels underlie CpxA* phenotypes. J. Bacteriol. 182:1423-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Wulf, P., A. M. McGuire, X. Liu, and E. C. Lin. 2002. Genome-wide profiling of promoter recognition by the two-component response regulator CpxR-P in Escherichia coli. J. Biol. Chem. 277:26652-26661. [DOI] [PubMed] [Google Scholar]
- 11.DiGiuseppe, P. A., and T. J. Silhavy. 2003. Signal detection and target gene induction by the CpxRA two-component system. J. Bacteriol. 185:2432-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erickson, J. W., and C. A. Gross. 1989. Identification of the sigma E subunit of Escherichia coli RNA polymerase: a second alternate sigma factor involved in high-temperature gene expression. Genes Dev. 3:1462-1471. [DOI] [PubMed] [Google Scholar]
- 12a.Hoiseth, S. K., and B. A. D. Stocker. 1981. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 291:238-239. [DOI] [PubMed] [Google Scholar]
- 13.Humphreys, S., A. Stevenson, A. Bacon, A. B. Weinhardt, and M. Roberts. 1999. The alternative sigma factor, σE, is critically important for the virulence of Salmonella typhimurium. Infect. Immun. 67:1560-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humphries, A. D., M. Raffatellu, S. Winter, E. H. Weening, R. A. Kingsley, R. Droleskey, S. Zhang, J. Figueiredo, S. Khare, J. Nunes, L. G. Adams, R. M. Tsolis, and A. J. Baumler. 2003. The use of flow cytometry to detect expression of subunits encoded by 11 Salmonella enterica serotype Typhimurium fimbrial operons. Mol. Microbiol. 48:1357-1376. [DOI] [PubMed] [Google Scholar]
- 15.Hung, D. L., T. L. Raivio, C. H. Jones, T. J. Silhavy, and S. J. Hultgren. 2001. Cpx signaling pathway monitors biogenesis and affects assembly and expression of P pili. EMBO J. 20:1508-1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones, C. H., P. N. Danese, J. S. Pinkner, T. J. Silhavy, and S. J. Hultgren. 1997. The chaperone-assisted membrane release and folding pathway is sensed by two signal transduction systems. EMBO J. 16:6394-6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolter, R., M. Inuzuka, and D. R. Helinski. 1978. Trans-complementation-dependent replication of a low molecular weight origin fragment from plasmid R6K. Cell 15:1199-1208. [DOI] [PubMed] [Google Scholar]
- 18.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop, and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175-176. [DOI] [PubMed] [Google Scholar]
- 19.Kovacikova, G., and K. Skorupski. 2002. The alternative sigma factor σE plays an important role in intestinal survival and virulence in Vibrio cholerae. Infect. Immun. 70:5355-5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leclerc, G. J., C. Tartera, and E. S. Metcalf. 1998. Environmental regulation of Salmonella typhi invasion-defective mutants. Infect. Immun. 66:682-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Linn, T., and R. St Pierre. 1990. Improved vector system for constructing transcriptional fusions that ensures independent translation of lacZ. J. Bacteriol. 172:1077-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipinska, B., S. Sharma, and C. Georgopoulos. 1988. Sequence analysis and regulation of the htrA gene of Escherichia coli: a sigma 32-independent mechanism of heat-inducible transcription. Nucleic Acids Res. 16:10053-10067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mecsas, J., P. E. Rouviere, J. W. Erickson, T. J. Donohue, and C. A. Gross. 1993. The activity of sigma E, an Escherichia coli heat-inducible sigma-factor, is modulated by expression of outer membrane proteins. Genes Dev. 7:2618-2628. [DOI] [PubMed] [Google Scholar]
- 24.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 25.Miller, S. I. 1991. PhoP/PhoQ: macrophage-specific modulators of Salmonella virulence? Mol. Microbiol. 5:2073-2078. [DOI] [PubMed] [Google Scholar]
- 26.Miticka, H., G. Rowley, B. Rezuchova, D. Homerova, S. Humphreys, J. Farn, M. Roberts, and J. Kormanec. 2003. Transcriptional analysis of the rpoE gene encoding extracytoplasmic stress response sigma factor σE in Salmonella enterica serovar Typhimurium. FEMS Microbiol. Lett. 226:307-314. [DOI] [PubMed] [Google Scholar]
- 27.Nakayama, S., A. Kushiro, T. Asahara, R. Tanaka, L. Hu, D. J. Kopecko, and H. Watanabe. 2003. Activation of hilA expression at low pH requires the signal sensor CpxA, but not the cognate response regulator CpxR, in Salmonella enterica serovar Typhimurium. Microbiology 149:2809-2817. [DOI] [PubMed] [Google Scholar]
- 28.Nakayama, S., and H. Watanabe. 1995. Involvement of cpxA, a sensor of a two-component regulatory system, in the pH-dependent regulation of expression of Shigella sonnei virF gene. J. Bacteriol. 177:5062-5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakayama, S., and H. Watanabe. 1998. Identification of cpxR as a positive regulator essential for expression of the Shigella sonnei virF gene. J. Bacteriol. 180:3522-3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Neal, C. R., W. M. Gabriel, A. K. Turk, S. J. Libby, F. C. Fang, and M. P. Spector. 1994. RpoS is necessary for both the positive and negative regulation of starvation survival genes during phosphate, carbon, and nitrogen starvation in Salmonella typhimurium. J. Bacteriol. 176:4610-4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otto, K., and T. J. Silhavy. 2002. Surface sensing and adhesion of Escherichia coli controlled by the Cpx-signaling pathway. Proc. Natl. Acad. Sci. USA 99:2287-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pogliano, J., A. S. Lynch, D. Belin, E. C. Lin, and J. Beckwith. 1997. Regulation of Escherichia coli cell envelope proteins involved in protein folding and degradation by the Cpx two-component system. Genes Dev. 11:1169-1182. [DOI] [PubMed] [Google Scholar]
- 33.Prigent-Combaret, C., E. Brombacher, O. Vidal, A. Ambert, P. Lejeune, P. Landini, and C. Dorel. 2001. Complex regulatory network controls initial adhesion and biofilm formation in Escherichia coli via regulation of the csgD gene. J. Bacteriol. 183:7213-7223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raffa, R. G., and T. L. Raivio. 2002. A third envelope stress signal transduction pathway in Escherichia coli. Mol. Microbiol. 45:1599-1611. [DOI] [PubMed] [Google Scholar]
- 35.Raivio, T. L., D. L. Popkin, and T. J. Silhavy. 1999. The Cpx envelope stress response is controlled by amplification and feedback inhibition. J. Bacteriol. 181:5263-5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raivio, T. L., and T. J. Silhavy. 1997. Transduction of envelope stress in Escherichia coli by the Cpx two-component system. J. Bacteriol. 179:7724-7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rezuchova, B., H. Miticka, D. Homerova, M. Roberts, and J. Kormanec. 2003. New members of the Escherichia coli σE regulon identified by a two-plasmid system. FEMS Microbiol. Lett. 225:1-7. [DOI] [PubMed] [Google Scholar]
- 38.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 39.Schurr, M. J., H. Yu, J. M. Martinez-Salazar, N. S. Hibler, and V. Deretic. 1995. Biochemical characterization and posttranslational modification of AlgU, a regulator of stress response in Pseudomonas aeruginosa. Biochem. Biophys. Res. Commun. 216:874-880. [DOI] [PubMed] [Google Scholar]
- 40.Snyder, W. B., L. J. Davis, P. N. Danese, C. L. Cosma, and T. J. Silhavy. 1995. Overproduction of NlpE, a new outer membrane lipoprotein, suppresses the toxicity of periplasmic LacZ by activation of the Cpx signal transduction pathway. J. Bacteriol. 177:4216-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Testerman, T. L., A. Vazquez-Torres, Y. Xu, J. Jones-Carson, S. J. Libby, and F. C. Fang. 2002. The alternative sigma factor σE controls antioxidant defences required for Salmonella virulence and stationary-phase survival. Mol. Microbiol. 43:771-782. [DOI] [PubMed] [Google Scholar]
- 42.Vieira, J., and J. Messing. 1982. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene 19:259-268. [DOI] [PubMed] [Google Scholar]
- 43.Wang, R. F., and S. R. Kushner. 1991. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene 100:195-199. [PubMed] [Google Scholar]
- 44.Yu, H., J. C. Boucher, N. S. Hibler, and V. Deretic. 1996. Virulence properties of Pseudomonas aeruginosa lacking the extreme-stress sigma factor AlgU (σE). Infect. Immun. 64:2774-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu, J. 1998. Inactivation of DsbA, but not DsbC and DsbD, affects the intracellular survival and virulence of Shigella flexneri. Infect. Immun. 66:3909-3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu, J., H. Webb, and T. R. Hirst. 1992. A homologue of the Escherichia coli DsbA protein involved in disulphide bond formation is required for enterotoxin biogenesis in Vibrio cholerae. Mol. Microbiol. 6:1949-1958. [DOI] [PubMed] [Google Scholar]