Abstract
Sexual reproduction in plants requires development of haploid gametophytes from somatic tissues. Pollen is the male gametophyte and develops within the stamen; defects in the somatic tissues of the stamen and in the male gametophyte itself can result in male sterility. The maize fuzzy tassel (fzt) mutant has a mutation in dicer-like1 (dcl1), which encodes a key enzyme required for microRNA (miRNA) biogenesis. Many miRNAs are reduced in fzt, and fzt mutants exhibit a broad range of developmental defects, including male sterility. To gain further insight into the roles of miRNAs in maize stamen development, we conducted a detailed analysis of the male sterility defects in fzt mutants. Early development was normal in fzt mutant anthers, however fzt anthers arrested in late stages of anther maturation and did not dehisce. A minority of locules in fzt anthers also exhibited anther wall defects. At maturity, very little pollen in fzt anthers was viable or able to germinate. Normal pollen is tricellular at maturity; pollen from fzt anthers included a mixture of unicellular, bicellular, and tricellular pollen. Pollen from normal anthers is loaded with starch before dehiscence, however pollen from fzt anthers failed to accumulate starch. Our results indicate an absolute requirement for miRNAs in the final stages of anther and pollen maturation in maize. Anther wall defects also suggest that miRNAs have key roles earlier in anther development. We discuss candidate miRNAs and pathways that might underlie fzt anther defects, and also note that male sterility in fzt resembles water deficit-induced male sterility, highlighting a possible link between development and stress responses in plants.
Introduction
Unlike animals that set aside germ cells early in embryogenesis, plants germ cells are specified from somatic cells within the reproductive organs in the adult plant. The male and female reproductive organs (stamens and carpels, respectively) that ultimately produce germ cells are found in flowers. Most flowers produce both stamens and carpels, however some plants segregate male and female flowers to separate plants (dioecy) or separate inflorescence (monoecy). Maize is monoecious and the tassel produces staminate (male) flowers, while the ear produces the pistillate (female) flowers. Floral meristems (FM) contain undifferentiated stem cells that will give rise to floral organs, including stamens and carpels. The molecular regulation of stamen development and male fertility is of particular interest in maize and other crop plants because male sterile lines with normal female fertility greatly facilitate the production of hybrid seed [1–4].
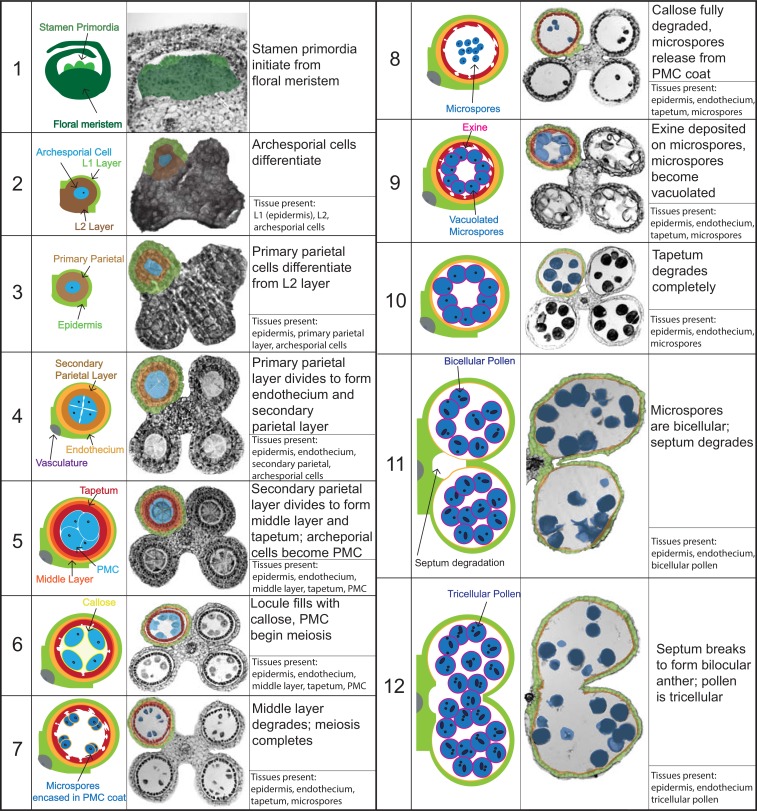
Stamens contain both the sporogenic cells that ultimately produce microspores (pollen) and the surrounding somatic tissue essential to support the developing pollen. Each stamen is composed of a filament, or stalk, that provides water and nutrients, and a four-lobed anther. Each anther lobe, or locule, houses the developing pollen in the interior, surrounded by somatic cells that support pollen development. Stamen development in maize and other plants follows a stereotypical developmental program that involves cell division, differentiation and cell death programs (Fig 1) [5–9]. The four anther locules are produced nearly simultaneously from the stamen primordia and consist of an L1-derived epithelium outer layer and an internal mass of undifferentiated L2-derived cells [10–12]. Pre-meiotic archesporial cells (AR) are specified from these L2-derived cells followed by two additional distinct cell layers, the secondary parietal layer and endothecium [8, 12]. The AR undergo a brief period of proliferation and maturation before differentiating into the pollen mother cells (PMC; also called microspore mother cells) [12, 13]. The secondary parietal layer divides anticlinally to form the tapetal and middle cell layers [8, 12]. The tapetum functions as a nurse tissue, providing nutrients and other materials for the developing pollen [13, 14], while the function of the middle layer is still unclear. The PMC undergo meiosis I and meiosis II, yielding haploid microspores [13]. Microspores undergo two rounds of mitosis (mitosis 1 and mitosis 2), which results in tricellular pollen at maturity [15]. Stamen and pollen development are tightly coordinated [16]; the middle layer degrades after meiosis and the tapetum degrades immediately preceding mitosis 1 [9]. Stamen development culminates with rupturing of the septum between adjacent locules, pollen dehiscence, and finally stamen abscission [16].
Fig 1. Summary of maize stamen development.
Major cellular events of stamen anther development are shown; left column, cartoon of locule; middle column, anther cross section with colors overlaid to indicate cell types (same colors used in cartoons and cross sections); right column, description of cell types present and defining characteristics for each stage. Stages based on Sanders et al., 1999.
MicroRNAs (miRNAs) are 20–22 nucleotide RNAs that post-transcriptionally regulate gene expression in plants and animals by repressing translation or triggering cleavage and degradation of target mRNAs [17, 18]. In plants, miRNAs are key regulators of development and physiology, including phase change, leaf polarity, inflorescence development and responses to biotic and abiotic stresses [19–22]. In addition, miRNAs direct the production of trans-acting siRNAs (tasi-RNAs) and phased RNAs (phasi-RNAs) that also play key roles in development [23–25]. We recently published a report describing characterization and cloning of the maize fzt mutant, which is caused by a hypomorphic mutation in dicer-like1 (dcl1) [26]. fzt has reduced levels of some, but not all miRNAs, resulting in a broad range of vegetative and reproductive defects. fzt mutant inflorescences have multiple defects including loss of stem cell homeostasis in meristems, which leads to fasciation in the inflorescence meristem and indeterminacy in other determinate meristems (spikelet pair, spikelet, and floral meristems). In addition, fzt florets make abnormal floral organs, including abnormal stamens that never shed pollen. Both dcl1-null and dcl1-fzt alleles are transmitted normally through pollen, indicating that fzt is not required in pollen, but rather in the somatic tissues of the stamen. Here, we conduct a detailed analysis of stamen development in fzt mutants. We find that fzt is required at multiple stages of stamen development and is essential for the final stages of pollen maturation and release. This work implicates miRNA-regulated pathways in multiple stages of stamen development and lays the foundation to identify novel regulators of male fertility in maize.
Methods
Histology and morphological analyses
Plants for analyses were grown in the Howell Biology Science Complex Biological Greenhouses under long day growth conditions (16 hours light) between March 2014 and March 2015. Normal sibling control plants were grown in parallel with fzt mutant plants (backcrossed a minimum of four times to the A619 inbred) for all experiments. Developing stamens were collected every 3–4 days starting approximately 6 weeks after planting. Time of dehiscence of normal plants was noted and samples were named relative to this time point (D 0.0).
Tassel florets were dissected and fixed overnight in either FAA (50% ethanol, 5% acetic acid, 3.7% formaldehyde, 1% DMSO and 0.5% triton; for young florets prior to tassel emergence) or Carnoy’s fixative (for older florets after tassel emergence) as previously described [27]. After fixation, stamens for sectioning were dehydrated in a graded ethanol series, embedded in paraffin, and sectioned (8 microns) using a Reichert-Jung Biocut 2030 Paraffin Microtome. Sectioned tissue was dewaxed with xylene and stained using Toluidine Blue (0.05% in water). To visualize pollen nuclei, pollen was manually collected from stamens (fixed in Carnoy’s fixative) on a glass cover slip and stained with 0.5 μg/mL DAPI (Life Technologies; cat.# D1306) in 1x phosphate buffered saline (PBS) at 4°C overnight.
Pollen viability was assessed using a modified Alexander stain as previously described [28]. Stamens were collected from at least two plants for each time point. Stamens from greenhouse-grown plants (pollen collected between 8-9AM) were cut on a glass slide to liberate pollen and pollen was incubated on pollen germination media [29] for 3 hours then fixed using pollen germination media fixative [30] to kill and preserve the pollen. Plates were stored at 4°C until all samples were collected and ready for analysis. All pollen within a 5cm2 section of each plate was scored. Starch accumulation in stamens was determined by fixing stamens with Carnoy’s fixative and incubating with Gram’s iodine solution (Sigma-Aldrich, cat. #90107) at room temperature overnight.
Stamens/pollen were visualized on a LSM Zeiss 700 confocal (toluidine blue and DAPI staining) or a Zeiss Axio Observer Z1 (Alexander and starch staining) microscope. All images were processed using Adobe Photoshop and/or Zeiss ZEN imaging software.
Results
fzt is male sterile and makes abnormal stamens
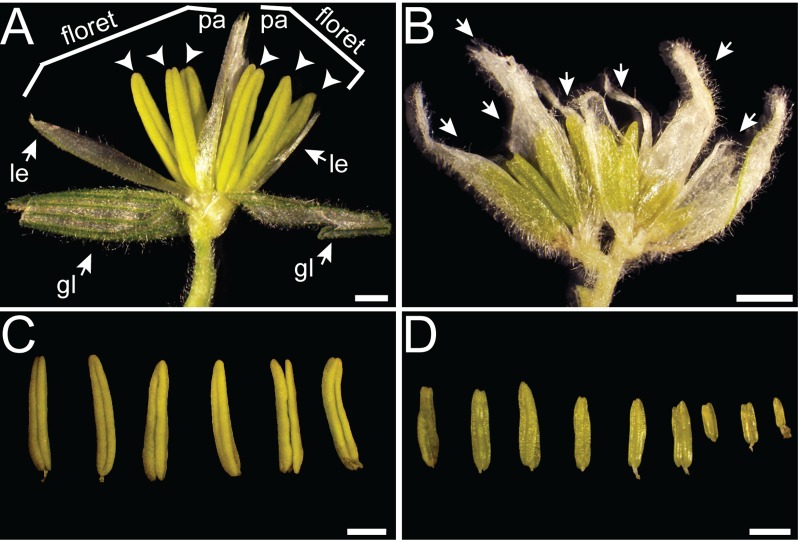
Grass florets are borne in spikelets, which contain one or more florets. Maize spikelets initiate two florets, which are the product of the upper and lower floral meristem. Both tassel and ear floral meristems initiate stamen and carpel primordia and sex determination occurs via stamen arrest in the ear and carpel abortion in the tassel [31]. At maturity, maize tassel florets are composed of two bract-like organs (lemma and palea), two lodicules (analogous to petals) and three stamens (Fig 2A). In normal spikelets, the upper floret (UF) and lower floret (LF) each produce three stamens, resulting in six stamens per spikelet. Stamens in the UF develop approximately one day ahead of the LF [32], although there are no morphological differences between stamen development in the upper and lower floret at maturity. In normal plants, the three stamens from a single floret initiate and develop synchronously. Normal anthers are a uniform yellow color and are composed of four locules, which support pollen development and contain pollen at maturity (Fig 2C). In contrast, fzt florets contained too many or too few stamens with abnormal morphology (Fig 2B and 2D). Whereas normal anthers were a uniform shape, color, and size (5–6.5mm at maturity), fzt anthers varied in size from less than 1mm to 4mm (Table 1), were often flat or twisted, and ranged in color from yellow to brown (Fig 2D).
Fig 2. Male reproductive defects in fzt.
A normal spikelet (A) contains two florets; each floret is composed of a palea (pa), two lodicules (not visible) and three stamens (arrowheads). Two glumes (gl) subtend the two florets. A fzt spikelet (B) contains extra florets (arrows) and lacks glumes; fzt florets often contain abnormal floral organs, including abnormal stamens, and do not make the normal complement of floral organs. (C) Stamens from normal spikelet shown in (A). All stamens (three stamens per floret) in a normal spikelet are ~6mm at maturity, yellow in color, and have four plump locular lobes. (D) Stamens from fzt spikelet shown in (B). fzt spikelets produce more that six stamens; number of stamens per floret is variable. At maturity, fzt stamens are smaller than normal and variable in size; fzt stamens are often twisted or shrivelled and appear yellow to brown in color. Scale bars = 2mm.
Table 1. Length of mature anthers from normal and fzt mutant plants.
| Anther size (mm) | <1 | 1–2 | 2–3 | 3–4 | 4–5 | 5–5.5 | 5.5–6.5 |
|---|---|---|---|---|---|---|---|
| Normal (n = 28) | 0 | 0 | 0 | 0 | 0 | 36% | 64% |
| fzt (n = 150) | 11% | 49% | 25% | 15% | 0 | 0 | 0 |
fzt is required for late stages of stamen development
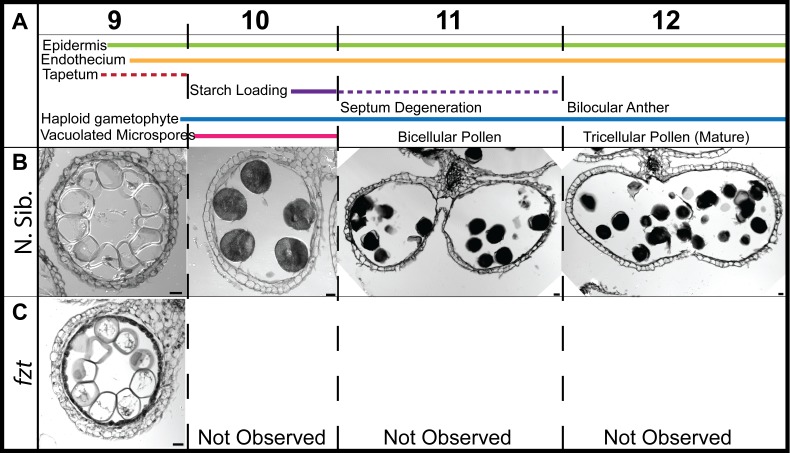
Male sterility can result from multiple defects, including defects in microsporogenesis itself, as well as defects in somatic cell developmental programs in the developing stamen [1, 33]. Stamen development follows a stereotypical program of cell proliferation, differentiation, and degradation and has been well-characterized in maize [7, 12]. The development and degradation of the tapetum, which provides nutrients to the developing pollen, plays a particularly important role in supporting development of viable pollen, and mutants that affect tapetum function are often male sterile [34–36]. To gain a better understanding of the cause of male sterility in fzt, we examined normal and fzt anthers in transverse sections during development. Anther length and developmental stage are usually tightly correlated in maize and anther length is often used as a proxy for developmental stage. fzt mutants, however, are much smaller than normal and we did not observe this close correlation between anther length and developmental stage (for example, some fzt stamens contain tricellular pollen, but never reach the size of normal anthers with tricellular pollen). Therefore, we used defining hallmarks of stamen development (as outlined by [16]) to determine developmental stage of fzt anthers (Fig 1). fzt anthers often included both normal and defective locules in the same anther. Therefore, we scored the number of locules with developmental defects, rather than the number of defective anthers. In approximately 70% of fzt anther locules, development was normal until late stages of anther/pollen development (stage 9) (S1 Fig), at which point microspores have become vacuolated (Table 2). We never observed fzt locules that progressed past stage 9, the stage immediately preceding complete degradation of the tapetum (stage 10), breakdown of the septum (stage 11 and 12), and dehiscence (stage 13) (Fig 3). In arrested locules, the tapetum either fails to degrade at all, or fails to degrade completely since we always observed at least some tapetal remnants in arrested locules. In the remaining ~30% of locules (not arrested at stage 9), we observed phenotypes consistent with slightly earlier defects in development, including collapsed, shrunken locules, and locules with enlarged and vacuolated cell layers (S2 Fig). Collapsed locules often had one to three somatic cell layers and contained crushed pollen (based on auto-fluorescence of the native exine) (S3 Fig). In addition, we often observed locules in developing anthers with three cell layers, a degrading inner cell layer and degenerating microspores (S2 Fig). These phenotypes are consistent with defects in the anther wall, including aberrant (premature or late) degradation of cell layers, vacuolated cells or cells that fail to differentiate normally. We did not observe phenotypes that indicated defects in pre-meiotic anthers (S1 Fig).
Table 2. Frequency of locule phenotypes in mature fzt anthers.
| Morphologically normal stage 9 locules | Collapsed locules | Locules with vacuolated inner layers(s) | |
|---|---|---|---|
| fzt (n = 100) | 71% | 21% | 8% |
Fig 3. fzt anthers arrest at late stages of stamen maturation and do not dehisce.
(A) Stage of anther development is indicated, as well as the cell types and physiological events that occur at each stage. (B) Normal anthers fully mature and produce mature pollen; the septum degrades immediately prior to dehiscence. (C) fzt anthers often progress to stage 9, however never progress past stage 9 and do not dehisce. Scale bars = 20μm.
fzt anthers produce non-viable pollen that arrests at multiple developmental time points
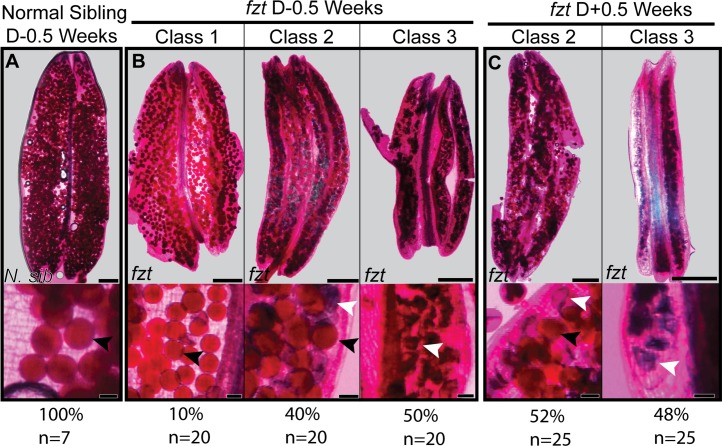
Our developmental analysis indicated that approximately 70% of fzt locules developed normally until the final stages of anther development, however pollen did not appear to fully mature and dehisce. To ask if this immature pollen was viable, we stained fzt anthers with a modified Alexander stain to assess pollen viability [28]. Pollen that contains a cytoplasm (viable) stains light fuchsia, whereas pollen that lacks a cytoplasm (nonviable) stains blue. We assayed viability of pollen from fzt anthers at multiple time points relative to dehiscence in normal siblings, including immediately before dehiscence (dehiscence minus 0.5 weeks; D-0.5), during dehiscence (D 0.0) and several time points after dehiscence (D+0.5, D+1.0, D+2.0). Normal anthers immediately before (D-0.5) and during dehiscence (D 0.0) contained pollen that stained light fuchsia and was uniformly plump, indicating that pollen was viable (Fig 4A) (S1 Table). In contrast, many fzt anthers produced shrivelled pollen that stained dark blue, indicating the pollen was not viable (Fig 4B and 4C). fzt anthers differed in the amount of viable pollen they contained and we classified fzt anthers based on the ratio of viable/nonviable pollen. Class I anthers contained >90% viable pollen, class II anthers contained a mixture of viable and nonviable pollen and class III anthers contained >90% nonviable pollen (Fig 4B and 4C). At early time points (D -0.5, D+0.0), ~25% of fzt anthers were class I (mostly viable pollen), ~25% class II (mixture of viable/nonviable pollen) and ~50% class III (mostly nonviable pollen; Fig 4B and 4C) (S1 Table). At later time points (D+0.5, D+1.0, and D+2.0), <5% of fzt anthers were class I, ~47% were class II and ~47% were class III. Class I anthers were usually the largest anthers in the florets, however even fzt class I anthers contained more nonviable pollen than normal anthers. Class III anthers were typically the smallest anthers in florets and were thin, with shrivelled or flat locule lobes.
Fig 4. Pollen in fzt anthers has reduced viability.
Modified Alexander stain to assess pollen viability; viable pollen stains pink (black arrowheads), nonviable pollen stains blue (white arrowheads). Top box depicts whole anther, bottom box depicts close-up of pollen grains. (A) Almost all pollen from normal anthers is viable; pollen are round and plump in shape and stain pink. (B) fzt anthers (D-0.5) are heterogeneous. Class 1 anthers contain almost entirely viable pollen, similar to normal anthers. Class 2 anthers contain a mixture of viable and non-viable pollen grains. Nonviable pollen stains blue and is shrivelled. Class 3 anthers contain almost entirely nonviable pollen. C) fzt anthers (D+0.5) are all Class 2 or Class 3. Scale bars: top row = 500 μm; bottom row = 50 μm.
Although Alexander staining indicated that fzt anthers contain some viable pollen, fzt homozygotes are male sterile. Therefore, we performed in vitro germination assays to determine if pollen from fzt anthers could germinate. Nearly all pollen (~92%) collected from normal anthers at dehiscence (D 0.0) germinated (Table 3); pollen grains from normal anthers were uniformly large, pale yellow spheres. Since fzt anthers never dehisce, we manually collected pollen by breaking open anthers to release the pollen. We assayed pollen germination at four time points to ensure any apparent germination defect reflected a real defect and not simply a developmental delay in fzt. At all time points, <3% of pollen from fzt anthers germinated (Table 3). Pollen from fzt anthers was heterogenous in appearance and included small, shrivelled, clear pollen grains, as well as larger, opaque, pale yellow pollen grains (data not shown). Thus, even though fzt stamens make some viable pollen, very little pollen can germinate.
Table 3. Germination frequencies of pollen from normal and fzt anthers.
| Genotype (time point) | % pollen germinated (n) |
|---|---|
| Normal (D 0.0) | 92.7% (466) |
| fzt (D+0.0) | 2.9% (136) |
| fzt (D+1.0) | 0.9% (656) |
| fzt (D+1.5) | 0.2% (720) |
| fzt (D+2.5) | 0% (73) |
Based on our developmental analysis, fzt anthers fail to develop past stage 9, at which point unicellular microspores are vacuolated. To ask if pollen maturation also prematurely arrest in fzt anthers, we stained pollen with DAPI to visualize the number of nuclei. Normal mature pollen have undergone two rounds of mitosis, yielding tricellular pollen with two compact germ nuclei and one large vegetative nucleus. Nearly all pollen from normal anthers at dehiscence (D 0.0) were tricellular (94%; Table 4). In contrast, pollen from fzt anthers of the same age contained a large proportion of unicellular and bicellular pollen, indicating that pollen from fzt anthers fails to develop to maturity (Table 4). We examined pollen from fzt anthers at multiple time points to account for any variability in developmental timing. At the first two developmental time points (D -0.5, D+0.0), approximately 75% of pollen from fzt anthers was unicellular, and the remaining pollen was bicellular (24%), suggesting that pollen development in fzt anthers is delayed or arrested. At the final time point assayed (D+1.0), a third of the pollen from fzt anthers was tricellular, while the remaining pollen was unicellular or bicellular (Table 4). We could not examine later time points because pollen would not separate from the anther.
Table 4. fzt pollen arrests at the unicellular and bicellular stage.
| Genotype (time point) | One nuclei | Two nuclei | Three nuclei |
|---|---|---|---|
| Normal (D 0.0) (n = 51) | 0.0% | 5.8% | 94.1% |
| fzt (D-0.5) (n = 154) | 75.3% | 24.0% | 0.6% |
| fzt (D 0.0) (n = 66) | 68.1% | 30.3% | 1.5% |
| fzt (D+1.0) (n = 107) | 34.6% | 36.4% | 28.9% |
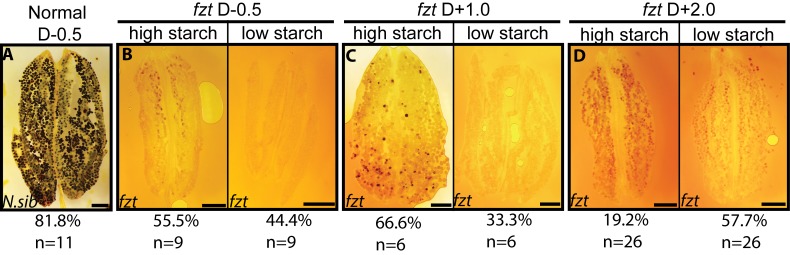
During the final stages of anther maturation, pollen accumulates starch, which functions both as an energy source and as a terminal regulator of pollen maturation [37]. Pollen that fails to accumulate normal starch levels often arrests prematurely [37, 38]. The failure of most of the pollen from fzt anthers to mature into tricellular viable pollen could be due to insufficient starch accumulation [39, 40]. To test this hypothesis, we assayed starch accumulation in fzt and normal sibling anthers by staining pollen with iodine [37]. Pollen grains fully loaded with starch stain dark brown or black, while pollen without starch do not stain and appear clear or light brown. Pollen from normal and fzt mutant anthers had dramatic differences in starch accumulation (Fig 5) (S2 Table). Half a week before dehiscence (D -0.5), most anthers from normal plants contained pollen grains that stained black (81%), indicating these pollen grains were fully loaded with starch (Fig 5A). The remaining anthers contained a mixture of pollen that stained brown and black, consistent with partial starch loading. Similar to previous assays, we assayed starch accumulation in fzt anthers at multiple time points to account for any developmental delay in fzt. At all developmental time points, pollen in fzt anthers had dramatically reduced starch accumulation compared to normal anthers. Approximately 50% of fzt anthers produced pollen with no detectable starch (low starch), while pollen from the other 50% of fzt anthers had minimal starch accumulation (high starch, Fig 5B–5D). Thus, fzt anthers fail to accumulate starch and arrest prior to maturation.
Fig 5. Pollen in fzt anthers does not accumulate starch.
(A) Pollen from normal anthers (D-0.5) stain black with iodine indicating high starch accumulation. (B) Pollen from fzt anthers (D-0.5) do not stain (low starch) or stain light brown (high starch) indicating minimal starch accumulation. (C, D) Pollen from fzt anthers one week (C) and two weeks (D) after normal siblings dehisced still had minimal starch accumulation. “Low starch” and “high starch” anthers show the range of phenotypes observed. Scale bars = 500μm.
Discussion
fzt functions in multiple stages of development in the somatic tissue of the anther
The maize fzt mutant has a broad range of developmental defects that affect both vegetative and reproductive tissues. Some, but not all, miRNAs are reduced in the fzt mutant; reduced levels of a subset of miRNAs and misregulation of target mRNAs are likely responsible for the developmental defects in fzt. We recently described the cloning and characterization of the fzt mutant [26]; however, we did not investigate the cause of male sterility in fzt plants in detail. MiRNAs have been implicated in stamen development in Arabidopsis and rice and the roles of a handful miRNAs/miRNA-targets in stamen development have been investigated in greater detail. To gain a better understanding of the role of microRNAs in maize stamen development, we conducted a detailed characterization of stamen and pollen development in fzt plants.
Male sterility in fzt is caused by defects in development of the somatic sporophytic tissue and not defects in the gametophyte. Both dcl1-fzt and dcl1-null alleles are transmitted normally through pollen suggesting that male sterility in fzt mutants is caused by defects in sporophytic development [26]. Our analysis indicates that fzt is absolutely required to progress past stage 9 of stamen development, at which point microspores have become vacuolated and the tapetum is completely degraded. While some pollen from fzt anthers does reach the tricellular stage, most pollen arrests at the unicellular or bicellular stage consistent with a late stage arrest in stamen/pollen development. Furthermore, pollen from fzt plants fails to accumulate significant levels of starch, which might underlie this developmental arrest. We also observed several other defects in fzt anthers that result from developmental defects in the anther wall, such as vacuolated cells and improper degradation of cell layers. The anther wall is critical to support the developing pollen and defects in the cell wall often leads to male sterility. In particular, the tapetum functions as a nurse tissue providing macromolecules and other nutrients to the developing pollen and defects in tapetum function and degradation (premature or late) result in male sterility [34–36]. Together these results suggest that miRNAs and their targets have multiple roles in stamen development, including the differentiation and degradation of the somatic cell layers, starch accumulation, stamen maturation, and dehiscence.
The phenotypes observed in fzt anthers indicate that miRNAs play key roles in development of the anther wall and the final stages of anther maturation and dehiscence. We did not observe any defects in premeiotic anthers, suggesting either that miRNAs do not play key roles in these early stages of stamen development or miRNAs that function at these early stages are present at sufficient levels in fzt mutants (notably, not all miRNAs are decreased in fzt). Although miRNAs are clearly required for late stages of stamen development, no roles for miRNAs in premeiotic anthers have been defined. Much less is known about premeiotic anther development in general, however. Many miRNAs are present in premeiotic anthers in maize [41] and two miRNAs, miR2118 and miR2275, are required to produce multiple phasi-RNAs in premeiotic anthers [42], suggesting that miRNAs might also play a role in premeiotic anthers.
Candidate miRNAs that contribute to fzt male sterility
MiRNAs have key roles in stamen development in multiple plant species. In Arabidopsis, the hypomorphic dcl1 allele, caf1, and a null allele of HYPONASTIC LEAVES1 (HYL1, encodes a dsRNA-binding protein required for accurate miRNA processing) make abnormal anthers [43, 44]. Both caf1 and hyl1 mutant anthers contain only two locules rather than the four locules present in normal anthers. Despite these defects, both caf1 and hyl1 mutant anthers produce functional pollen capable of producing progeny. In contrast, maize fzt mutant stamens contain four locules similar to normal stamens but exhibit other defects in development in the somatic tissues within the anther. Importantly, fzt does not produce functional pollen; most fzt anthers arrest at stage 9 and pollen from fzt anthers often arrests prematurely at the unicellular and bicellular stages. In addition, pollen from fzt anthers fails to accumulate normal levels of starch and does not germinate. Similar defects have not been reported in miRNA biogenesis mutants from Arabidopsis, suggesting that miRNAs have novel roles in stamen development in maize compared to Arabidopsis.
Anther maturation requires a complex interplay of hormones, which often act through miRNA-regulated transcription factors (TFs). Three miRNAs that likely play key roles in maize stamen development include miR159, which targets gibberellin (GA)-induced MYB (GAMYB) TFs, miR167, which targets auxin response factor (ARF) TFs, and miR319, which targets TCP TFs. TCP TFs have been linked to jasmonic acid (JA) biosynthesis. MiR159, miR167, and miR319 are reduced ~5, 30 and 7-fold in fzt tassel primordia, respectively, and thus reduction of these miRNAs and misregulation of their target mRNAs are excellent candidates to underlie male sterility in fzt.
MiR159-regulation of GAMYB TFs is a particularly intriguing with regard to fzt stamen defects. GAMYB TFs have key roles in stamen development in Arabidopsis, rice and barley [45–49]. Notably, anthers in barley HvGAMYB overexpression lines arrest at stage 9 and fail to dehisce; these defects are strongly reminiscent of the maize fzt stamen phenotype [46]. In contrast to fzt, however, pollen from HvGAMYB overexpression lines accumulate starch, suggesting slightly different roles for GAMYBs in maize and barley stamen development. The role of GAMYBs genes in maize anther development has not been investigated, although the late stage arrest we observe in fzt is consistent with GAMYB overexpression
fzt stamen defects resemble water deficit-induced male sterility
Male gametophyte development is very sensitive to heat stress and water deficit, which can induce male sterility in both rice and wheat, and negatively impact grain production [50–53]. Plants that experience water deficit often produce sterile anthers and exhibit a range of defects including vacuolated cell layers, tapetum degradation, tapetum and microspore collapse, and arrested pollen that fails to accumulate starch [50–52]. We noticed that fzt anthers often exhibited defects reminiscent of those reported in drought-stressed plants from rice and wheat. In particular, fzt anthers exhibited vacuolated cell layers; pollen often arrests before completion of the two rounds of mitosis and does not accumulate starch. Heat and drought stress responses have been linked to both miRNA and GA hormone-regulated responses [54–57]. Particularly relevant to this discussion, the miR159-GAMYB regulatory module appears to function in both heat and drought responses in wheat, rice, and Arabidopsis and miR159 and GAMYB levels are modulated in response to heat and drought stress, however the exact response (up- or downregulation) depends on both the tissue assayed and the species [52, 58–61]. An intriguing possibility is that down-regulation of miR159 (or another miRNA) in fzt anthers may elicit a drought response even under well-watered conditions, and contribute to male sterility fzt anthers.
Conclusions
Our results indicate an absolute requirement for miRNAs in the final stages of anther and pollen maturation in maize. Anther wall defects also suggest that miRNAs have key roles earlier in anther development. The male sterility in fzt resembles water deficit-induced male sterility, highlighting a possible link between development and stress responses in plants.
Supporting Information
(A) Stage of anther development is indicated, as well as the cell types and physiological events that occur at each stage. (B) Normal anther development. (C) In ~70% of fzt locules, development is indistinguishable from normal through stage 8. Scale bars = 20μm.
(TIF)
(A) Normal anther immediately before dehiscence contains mature pollen and is bilocular. (B-D) Examples of abnormal locules in fzt anthers from mature plants. (B) fzt anther contains two "normal" locules arrested at stage 9 (arrowheads), and two collapsed locules. (C) Close-up of collapsed locule in (B); collapsed locule contains three tissue layers, indicating defects in cell layer degradation. (D) Locule from fzt anther has three cell layers; cells are vacuolated. (E) Locule from developing fzt anther contains three cell layers, a degrading inner cell layer and degenerating microspores. Scale bars = 100μm.
(TIF)
(A) fzt stamen with three collapsed locules. Exine in crushed pollen auto-fluoresces. B) Close-up of collapsed locule in (A, red box). (C-D) Normal stage 7 locule prior to exine deposition. (C) Normal stage 7 locule, bright field with fluorescence overlaid. Pollen does not auto-fluorescence because it lacks a pollen coat with exine. (D) Normal stage 7 locule in (C), showing only fluorescence. (E) Normal stage 12 locule, bright field with fluorescence overlaid. Mature pollen auto-fluoresces because pollen coats contains exine. (F) Normal stage 12 locule shown in (E), showing only fluorescence. All images were taken using the same confocal conditions normalized to exine fluorescence. Scale bars = 20μm.
(TIF)
(DOCX)
(DOCX)
Acknowledgments
We thank Julie Marik for care of greenhouse plants, Cathy Herring and Jim Holland for field support, and Elizabeth Ables for help with microscopy and reagents. We thank Thompson lab members for critical reading and comments on the manuscript.
Data Availability
All relevant data are within the paper and its supporting information files.
Funding Statement
This work was supported by National Science Foundation grant IOS-1148971 to BT. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1.Skibbe D, Schnable P. Male sterility in maize. Maydica. 2005;50:367–76. [Google Scholar]
- 2.Wilson ZA, Zhang D-B. From Arabidopsis to rice: pathways in pollen development. J. Exp. Bot. 2009;60(5):1479–92. 10.1093/jxb/erp095 [DOI] [PubMed] [Google Scholar]
- 3.Duvick DN. Cytoplasmic pollen sterility in corn. Adv. Genet. 1965;13(1):51–6. [Google Scholar]
- 4.Laughnan JR, Gabay-Laughnan S. Cytoplasmic male sterility in maize. Annu. Rev. Genet. 1983;17(1):27–48. [DOI] [PubMed] [Google Scholar]
- 5.Kelliher T, Egger RL, Zhang H, Walbot V. Unresolved issues in pre-meiotic anther development. Front. Plant Sci. 2014;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bedinger P. The remarkable biology of pollen. Plant Cell. 1992;4(8):879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang MT, Neuffer MG. Maize microsporogenesis. Genome. 1989;32(2):232–44. [Google Scholar]
- 8.Timofejeva L, Skibbe DS, Lee S, Golubovskaya I, Wang R, Harper L, et al. Cytological characterization and allelism testing of anther developmental mutants identified in a screen of maize male sterile lines. G3: Genes| Genomes| Genetics. 2013;3(2):231–49. 10.1534/g3.112.004465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warmke H, Lee S-LJ. Mitochondrial degeneration in Texas cytoplasmic male-sterile corn anthers. J. Hered. 1977;68(4):213–22. [Google Scholar]
- 10.Dawe RK, Freeling M. The role of initial cells in maize anther morphogenesis. Development. 1992;116(4):1077–85. [DOI] [PubMed] [Google Scholar]
- 11.Dawe RK, Freeling M. Clonal analysis of the cell lineages in the male flower of maize. Dev. Biol. 1990;142(1):233–45. [DOI] [PubMed] [Google Scholar]
- 12.Kelliher T, Walbot V. Emergence and patterning of the five cell types of the Zea mays anther locule. Dev. Biol. 2011;350(1):32–49. 10.1016/j.ydbio.2010.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C-JR, Nan G-L, Kelliher T, Timofejeva L, Vernoud V, Golubovskaya IN, et al. Maize multiple archesporial cells 1 (mac1), an ortholog of rice TDL1A, modulates cell proliferation and identity in early anther development. Development. 2012;139(14):2594–603. 10.1242/dev.077891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu L, Fan X-d. Tapetum: regulation and role in sporopollenin biosynthesis in Arabidopsis. Plant Mol. Biol. 2013;83(3):165–75. 10.1007/s11103-013-0085-5 [DOI] [PubMed] [Google Scholar]
- 15.Hsu S, Peterson P. Relative stage duration of microsporogenesis in maize [Meiosis through pollen maturation]. Iowa State Journal of Research. 1981. [Google Scholar]
- 16.Sanders PM, Bui AQ, Weterings K, McIntire K, Hsu Y-C, Lee PY, et al. Anther developmental defects in Arabidopsis thaliana male-sterile mutants. Sex. Plant Reprod. 1999;11(6):297–322. [Google Scholar]
- 17.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–33. Epub 2009/01/27. S0092-8674(09)00008-7 [pii] 10.1016/j.cell.2009.01.002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogers K, Chen X. Biogenesis, turnover, and mode of action of plant microRNAs. Plant Cell. 2013;25(7):2383–99. 10.1105/tpc.113.113159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun G. MicroRNAs and their diverse functions in plants. Plant Mol. Biol. 2012;80(1):17–36. 10.1007/s11103-011-9817-6 [DOI] [PubMed] [Google Scholar]
- 20.Nag A, Jack T. Chapter twelve-sculpting the flower; the role of microRNAs in flower development. Curr. Top. Dev. Biol. 2010;91:349–78. 10.1016/S0070-2153(10)91012-0 [DOI] [PubMed] [Google Scholar]
- 21.Chen X. Small RNAs and their roles in plant development. Annu. Rev. Cell Dev. Biol. 2009;25:21–44. Epub 2009/07/07. 10.1146/annurev.cellbio.042308.113417 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Voinnet O. Origin, biogenesis, and activity of plant microRNAs. Cell. 2009;136(4):669–87. Epub 2009/02/26. S0092-8674(09)00128-7 [pii] 10.1016/j.cell.2009.01.046 . [DOI] [PubMed] [Google Scholar]
- 23.Allen E, Xie Z, Gustafson AM, Carrington JC. microRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell. 2005;121(2):207–21. [DOI] [PubMed] [Google Scholar]
- 24.Nogueira FT, Madi S, Chitwood DH, Juarez MT, Timmermans MC. Two small regulatory RNAs establish opposing fates of a developmental axis. Genes Dev. 2007;21(7):750–5. Epub 2007/04/04. 21/7/750 [pii] 10.1101/gad.1528607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arikit S, Zhai J, Meyers BC. Biogenesis and function of rice small RNAs from non-coding RNA precursors. Curr. Opin. Plant Biol. 2013;16(2):170–9. 10.1016/j.pbi.2013.01.006 [DOI] [PubMed] [Google Scholar]
- 26.Thompson BE, Basham C, Hammond R, Ding Q, Kakrana A, Lee TF, et al. The dicer-like1 homolog fuzzy tassel is required for the regulation of meristem determinacy in the inflorescence and vegetative growth in maize. Plant Cell. 2014;26(12):4702–17. 10.1105/tpc.114.132670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruzin SE. Plant microtechnique and microscopy: Oxford University Press New York; 1999. [Google Scholar]
- 28.Peterson R, Slovin JP, Chen C. A simplified method for differential staining of aborted and non-aborted pollen grains. International Journal of Plant Biology. 2010;1(2):13. [Google Scholar]
- 29.Freeling M, Walbot V. The maize handbook: Springer Science & Business Media; 2013. [Google Scholar]
- 30.Pfahler PL. In vitro germination characteristics of maize pollen to detect biological activity of environmental pollutants. Environ. Health Perspect. 1981;37:125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng PC, Greyson RI, Walden DB. Organ initiation and the development of unisexual flowers in the tassel and ear of Zea mays. Am. J. Bot. 1983;70(3):450–62. [Google Scholar]
- 32.Hsu S, Huang Y-C, Peterson P. Development pattern of microspores in Zea mays L. Maydica. 1988;33(2):77–98. [Google Scholar]
- 33.Horner HT, Palmer RG. Mechanisms of genic male sterility. Crop Sci. 1995;35(6):1527–35. [Google Scholar]
- 34.Kawanabe T, Ariizumi T, Kawai-Yamada M, Uchimiya H, Toriyama K. Abolition of the tapetum suicide program ruins microsporogenesis. Plant Cell Physiol. 2006;47(6):784–7. [DOI] [PubMed] [Google Scholar]
- 35.Vizcay-Barrena G, Wilson ZA. Altered tapetal PCD and pollen wall development in the Arabidopsis ms1 mutant. J. Exp. Bot. 2006;57(11):2709–17. [DOI] [PubMed] [Google Scholar]
- 36.Shi Y, Zhao S, Yao J. Premature tapetum degeneration: a major cause of abortive pollen development in photoperiod sensitive genic male sterility in rice. J. Integr. Plant Biol. 2009;51(8):774–81. 10.1111/j.1744-7909.2009.00849.x [DOI] [PubMed] [Google Scholar]
- 37.Datta R, Chamusco KC, Chourey PS. Starch biosynthesis during pollen maturation is associated with altered patterns of gene expression in maize. Plant Physiol. 2002;130(4):1645–56. 10.1104/pp.006908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wen L-Y, Chase CD. Mitochondrial gene expression in developing male gametophytes of male-fertile and S male-sterile maize. Sex. Plant Reprod. 1999;11(6):323–30. [Google Scholar]
- 39.Regan SM, Moffatt BA. Cytochemical analysis of pollen development in wild-type Arabidopsis and a male-sterile mutant. The Plant Cell Online. 1990;2(9):877–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Datta R, Chourey PS, Pring DR, Tang HV. Gene-expression analysis of sucrose-starch metabolism during pollen maturation in cytoplasmic male-sterile and fertile lines of sorghum. Sex. Plant Reprod. 2001;14(3):127–34. [Google Scholar]
- 41.Nakano M, Nobuta K, Vemaraju K, Tej SS, Skogen JW, Meyers BC. Plant MPSS databases: signature-based transcriptional resources for analyses of mRNA and small RNA. Nucleic Acids Res. 2006;34(Database issue):D731–5. 10.1093/nar/gkj077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhai J, Zhang H, Arikit S, Huang K, Nan G-L, Walbot V, et al. Spatiotemporally dynamic, cell-type–dependent premeiotic and meiotic phasiRNAs in maize anthers. Proc. Natl. Acad. Sci. U. S. A. 2015;112(10):3146–51. 10.1073/pnas.1418918112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacobsen SE, Running MP, Meyerowitz EM. Disruption of an RNA helicase/RNAse III gene in Arabidopsis causes unregulated cell division in floral meristems. Development. 1999;126(23):5231–43. Epub 1999/11/11. . [DOI] [PubMed] [Google Scholar]
- 44.Lian H, Li X, Liu Z, He Y. HYL1 is required for establishment of stamen architecture with four microsporangia in Arabidopsis. J. Exp. Bot. 2013;64(11):3397–410. 10.1093/jxb/ert178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alonso-Peral MM, Li J, Li Y, Allen RS, Schnippenkoetter W, Ohms S, et al. The microRNA159-regulated GAMYB-like genes inhibit growth and promote programmed cell death in Arabidopsis. Plant Physiol. 2010;154(2):757–71. 10.1104/pp.110.160630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murray F, Kalla R, Jacobsen J, Gubler F. A role for HvGAMYB in anther development. Plant J. 2003;33(3):481–91. . [DOI] [PubMed] [Google Scholar]
- 47.Millar AA, Gubler F. The Arabidopsis GAMYB-like genes, MYB33 and MYB65, are microRNA-regulated genes that redundantly facilitate anther development. Plant Cell. 2005;17(3):705–21. 10.1105/tpc.104.027920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaneko M, Inukai Y, Ueguchi-Tanaka M, Itoh H, Izawa T, Kobayashi Y, et al. Loss-of-function mutations of the rice GAMYB gene impair alpha-amylase expression in aleurone and flower development. Plant Cell. 2004;16(1):33–44. 10.1105/tpc.017327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aya K, Ueguchi-Tanaka M, Kondo M, Hamada K, Yano K, Nishimura M, et al. Gibberellin modulates anther development in rice via the transcriptional regulation of GAMYB. Plant Cell. 2009;21(5):1453–72. 10.1105/tpc.108.062935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saini H, Aspinall D. Effect of water deficit on sporogenesis in wheat (Triticum aestivum L.). Ann. Bot. 1981;48(5):623–33. [Google Scholar]
- 51.Saini H, Sedgley M, Aspinall D. Development anatomy in wheat of male sterility induced by heat stress, water deficit or abscisic acid. Funct. Plant Biol. 1984;11(4):243–53. [Google Scholar]
- 52.Jin Y, Yang H, Wei Z, Ma H, Ge X. Rice male development under drought stress: phenotypic changes and stage-dependent transcriptomic reprogramming. Mol Plant. 2013;6(5):1630–45. 10.1093/mp/sst067 . [DOI] [PubMed] [Google Scholar]
- 53.Dolferus R, Ji X, Richards RA. Abiotic stress and control of grain number in cereals. Plant Sci. 2011;181(4):331–41. 10.1016/j.plantsci.2011.05.015 [DOI] [PubMed] [Google Scholar]
- 54.Peleg Z, Blumwald E. Hormone balance and abiotic stress tolerance in crop plants. Curr. Opin. Plant Biol. 2011;14(3):290–5. 10.1016/j.pbi.2011.02.001 [DOI] [PubMed] [Google Scholar]
- 55.Wang YH, Irving HR. Developing a model of plant hormone interactions. Plant Signal. Behav. 2011;6(4):494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferdous J, Hussain SS, Shi BJ. Role of microRNAs in plant drought tolerance. Plant biotechnology journal. 2015;13(3):293–305. 10.1111/pbi.12318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Colebrook EH, Thomas SG, Phillips AL, Hedden P. The role of gibberellin signalling in plant responses to abiotic stress. J. Exp. Biol. 2014;217(1):67–75. [DOI] [PubMed] [Google Scholar]
- 58.Wang Y, Sun F, Cao H, Peng H, Ni Z, Sun Q, et al. TamiR159 directed wheat TaGAMYB cleavage and its involvement in anther development and heat response. PLoS One. 2012;7(11):e48445 10.1371/journal.pone.0048445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu H-H, Tian X, Li Y-J, Wu C-A, Zheng C-C. Microarray-based analysis of stress-regulated microRNAs in Arabidopsis thaliana. RNA. 2008;14(5):836–43. 10.1261/rna.895308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou L, Liu Y, Liu Z, Kong D, Duan M, Luo L. Genome-wide identification and analysis of drought-responsive microRNAs in Oryza sativa. J. Exp. Bot. 2010;61(15):4157–68. 10.1093/jxb/erq237 [DOI] [PubMed] [Google Scholar]
- 61.Reyes JL, Chua NH. ABA induction of miR159 controls transcript levels of two MYB factors during Arabidopsis seed germination. Plant J. 2007;49(4):592–606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Stage of anther development is indicated, as well as the cell types and physiological events that occur at each stage. (B) Normal anther development. (C) In ~70% of fzt locules, development is indistinguishable from normal through stage 8. Scale bars = 20μm.
(TIF)
(A) Normal anther immediately before dehiscence contains mature pollen and is bilocular. (B-D) Examples of abnormal locules in fzt anthers from mature plants. (B) fzt anther contains two "normal" locules arrested at stage 9 (arrowheads), and two collapsed locules. (C) Close-up of collapsed locule in (B); collapsed locule contains three tissue layers, indicating defects in cell layer degradation. (D) Locule from fzt anther has three cell layers; cells are vacuolated. (E) Locule from developing fzt anther contains three cell layers, a degrading inner cell layer and degenerating microspores. Scale bars = 100μm.
(TIF)
(A) fzt stamen with three collapsed locules. Exine in crushed pollen auto-fluoresces. B) Close-up of collapsed locule in (A, red box). (C-D) Normal stage 7 locule prior to exine deposition. (C) Normal stage 7 locule, bright field with fluorescence overlaid. Pollen does not auto-fluorescence because it lacks a pollen coat with exine. (D) Normal stage 7 locule in (C), showing only fluorescence. (E) Normal stage 12 locule, bright field with fluorescence overlaid. Mature pollen auto-fluoresces because pollen coats contains exine. (F) Normal stage 12 locule shown in (E), showing only fluorescence. All images were taken using the same confocal conditions normalized to exine fluorescence. Scale bars = 20μm.
(TIF)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its supporting information files.