Abstract
Successful resolution of infections by intracellular pathogens requires gamma interferon (IFN-γ). DNA vaccines promote T helper 1 (Th1) responses by triggering interleukin-12 (IL-12) release by dendritic cells (DC) through Toll-like receptor 9 (TLR9). In humans TLR9 is restricted to plasmacytoid DC. Here we show that DNA-Salmonella enterica serovar Typhimurium primer-booster vaccination, which provides alternative ligands to bind TLR4 on myeloid DC, strongly biases towards Th1 responses compared to vaccination with DNA alone. This results in higher immunoglobulin G2a (IgG2a) responses compared to IgG1 responses, higher IFN-γ responses compared to IL-10 CD4+-T-cell responses, and enhanced protection against Leishmania major infection in susceptible BALB/c mice.
Leishmaniasis and tuberculosis affect millions of people globally, but, aside from vaccination with Mycobacterium bovis bacillus Calmette-Guérin (BCG), which provides variable protection against pulmonary tuberculosis (8), there are no vaccines in routine use. Successful resolution of primary infection relies on the induction and maintenance of T cells that produce gamma interferon (IFN-γ) to activate macrophage antimicrobial activity (4, 31, 33). This implies that successful immunization will also rely on induction of IFN-γ-producing T helper 1 (Th1) cells. Recombinant protein-based vaccines can be effective at eliciting Th1 cells but on their own fail to elicit long-term memory (11). Novel adjuvants such as CpG motifs (29, 30) and monophosphoryl lipid A (6, 25), which trigger interleukin-12 (IL-12) release and promote a Th1 response by acting as ligands for Toll-like receptors (TLRs), provide one approach to enhancing memory T-cell responses. Naked DNA, which has endogenous adjuvant activity through the binding of CpG motifs in the plasmid backbone to TLR9, provides another. In mice, DNA vaccines elicit long-lived cellular (CD4+ and CD8+ T-cell) immunity against a variety of pathogens (10, 13), including that causing murine leishmaniasis (12, 21). Interestingly, myeloid rather than plasmacytoid dendritic cells (DC) are the most potent producers of interleukin IL-12 p70 in mice in response to CpGs (29). In humans, TLR9 expression is restricted to plasmacytoid DC and B cells (reviewed in reference 26). However, human myeloid DC produce high levels of IL-12 p70 in response to adjuvants that act as ligands for TLR3, TLR4, and TLR7 (19). Hence, alternative vaccine strategies that target TLRs on myeloid DC should enhance vaccine-induced Th1 responses. Live-attenuated Salmonella enterica serovar Typhimurium, which has multiple ligands for a range of TLRs, is a proven vaccine vehicle that stimulates both humoral and cellular immune responses (14). We postulated that a primer-booster strategy based on DNA vaccination followed by a boost with live recombinant Salmonella might provide an optimal strategy to focus and then enhance Th1 responses and hence protection against intracellular pathogens. Using the nontoxic C-terminal domain of Clostridium tetani tetanus toxin (fragment C or TetC) as a model antigen to optimize the vaccination regimen, we demonstrate that DNA-Salmonella primer-booster vaccination biases towards Th1 responses compared to vaccination with Salmonella or DNA alone. This bias translates into enhanced protection against the intracellular pathogen L. major with the use of DNA-Salmonella primer-booster vaccination in mice vaccinated with the Leishmania homologue of the receptor for activated C kinase (LACK) (24) antigen.
DNA and Salmonella vaccines.
For DNA vaccines LACK (amino acids 143 to 312) was amplified from clone lmk5 (accession number W88311), obtained from an L. major substrain LV39 (MRHO/SU/59/P) cDNA library (17), and inserted downstream of the cytomegalovirus promoter into a modified version (minus the neomycin resistance gene) of the expression vector pcDNA3 (Invitrogen, Paisley, United Kingdom). A synthetic TetC gene optimized for high-level expression (1) was subcloned from ptech2 (kindly provided by A. Khan, Newcastle University, Newcastle, United Kingdom) into unmodified pcDNA3.1 (Invitrogen). Empty modified pcDNA3 was used as a vector control. Plasmid DNA was purified using Endofree plasmid maxikits (Qiagen Ltd., Crawley, United Kingdom) and resuspended in pyrogen-free phosphate-buffered saline. For Salmonella vaccines, the plasmid pkk/ppagC/Cfrag, which contains TetC under the control of the in vivo-inducible pagC promoter, was kindly provided by S. Dunstan, Imperial College, London, United Kingdom. A novel plasmid, pkk/ppagC/LACK, was engineered by replacing TetC in pkk/ppagC/Cfrag with LACK (amino acids 143 to 312). pkk/ppagC/Cfrag or pkk/ppagC/LACK was transferred into the r−m+ S. enterica serovar Typhimurium LB5010 strain by electroporation and was then transduced into an attenuated aroD mutant strain of S. enterica serovar Typhimurium, C5 (C5aroD; kindly provided by C. Hormaeche, University of Cambridge, Cambridge, United Kingdom), with the use of bacteriophage P22 (int−) (22), and glycerol stocks were prepared. Bacteria were streaked onto Luria-Bertani (LB) agar, and a colony was picked and cultured in LB broth overnight at 37°C with 50 μg of ampicillin/ml. To test for in vitro expression of TetC and LACK by recombinant salmonellae, experiments were performed using N medium [5 mM KCl, 7.5 mM (NH4)2SO4, 0.5 mM K2SO4, 1 mM KH2PO4, and 100 mM Tris-HCl (pH 7.4)] supplemented with 0.1% Casamino Acids; 38 mM glycerol; 40 μg each of tryptophan, tyrosine, and phenylalanine/ml; and 10 μg each of para-aminobenzoic acid, para-hydroxybenzoic acid, and 2,3 dihydrobenzoate/ml. MgCl2 was added across the range 1 μM to 100 mM, and cultures were induced for 2.5 h at 37°C. Expression of TetC protein by recombinant C5aroD was demonstrated by Western blotting with polyclonal rabbit anti-TetC antibody (kindly provided by S. Clare, Imperial College). The expected protein band at 50 kDa was observed (data not shown). Expression of LACK could not be detected by Western blotting with sera from DNA-vaccinated mice.
Immunization and infection.
Female BALB/c mice (5 to 6 weeks old; Charles River Laboratories, Margate, United Kingdom) were injected subcutaneously in the shaven rump with two doses of 100 μg of TetC, LACK, or vector DNA, 2 weeks apart. At 4 weeks, mice were boosted intravenously with 5 × 105 recombinant C5aroD bacteria expressing TetC (TetC-Sal) or LACK (LACK-Sal) or containing the empty pkk/ppagC plasmid (Vec-Sal). Mice were maintained in the Central Biomedical Services (University of Cambridge) facility under pathogen-free conditions. All procedures were carried out under United Kingdom Government Home Office guidelines. For TetC immunogenicity studies, in vivo recall via injection with 4 μg of recombinant TetC (kindly provided by A. Stevenson, University of Glasgow, Glasgow, United Kingdom) in the rump was performed 8 weeks post-TetC-Sal or Vec-Sal boost, i.e., allowing time for viable Salmonella to clear in immunized mice. Forty-eight hours later, mice were sacrificed and bled for serum, and the draining inguinal lymph nodes and spleens were removed. For infectious challenge, LACK-vaccinated mice were injected with 2 × 106 stationary-phase (days 5 to 6) L. major substrain LV39 promastigotes into the hind footpad 8 weeks postboost. Footpad depth was determined by weekly measurement with Vernier calipers. Statistical differences between vaccine groups over the course of infection were determined using Hotelling's T-squared generalized means test, which provides a conservative estimate for the difference in means profiles.
Antibody and T-cell responses.
Antigen-specific antibody subclasses were measured by enzyme-linked immunosorbent assay (ELISA) with recombinant TetC-coated plates and biotinylated rabbit anti-mouse immunoglobulin G1 (IgG1) or IgG2a (Zymed Laboratories Inc., South San Francisco, Calif.) detecting antibodies. Detection was with streptavidin-horseradish peroxidase (Dako) and o-phenylenediamine substrate. Plates were read at 492 nm (Opti-Max; Molecular Devices, Wokingham, United Kingdom). For cytokine assays, pooled cells from draining lymph nodes or spleens were cultured in RPMI 1640 (Invitrogen) at 6 × 105 cells/well in U-bottomed 96-well plates and stimulated for 72 h at 37°C in 5% CO2 with or without 10 μg of TetC recombinant protein/ml. For blocking experiments, cells were incubated with 10 μg of inhibitory CD4 (H129.19, noazide/low endotoxin [NA/LE]; PharMingen) or CD8 (53-6.7, NA/LE; PharMingen) monoclonal antibody (MAb) or isotype control (R35-95, NA/LE; PharMingen) per ml. For LACK-vaccinated mice, pooled splenocytes were plated onto syngeneic bone marrow-derived DC (15) that had been infected 24 h previously with L. major LV39 stationary-phase promastigotes at a 10:1 ratio to act as antigen-presenting cells. Noninfected DC acted as a control. Supernatants were removed at 72 h, and IFN-γ, IL-4, and IL-10 levels were measured by sandwich ELISA with antibodies from PharMingen. The number of IFN-γ-secreting cells was determined by ELISPOT assay with 96-well multiscreen Immobilon P filtration plates (Millipore Corp., Bedford, Mass.) and cytokine ELISPOT pair MAb reagents (PharMingen) according to the manufacturer's instructions. Splenocytes were washed in phosphate-buffered saline containing 0.5% bovine serum albumin, 2 mM EDTA, and 0.09% sodium azide and resuspended in BD IMag anti-mouse CD4 (clone GK1.5) or CD8 (clone 53-6.7) particles-direct magnets, and cells were positively selected. CD4+ or CD8+ T cells resuspended in RPMI 1640 (Invitrogen) plus 10% fetal calf serum at 2.5 × 105 or 1 × 104 cells per well, DC at 105 or 104 cells per well, and TetC recombinant protein at 10 μg/ml were added to coated plates for 24 h at 37°C. Plates were washed and further processed according to the manufacturer's instructions, and developed plates were visualized under a dissecting microscope.
DNA-Salmonella primer-booster vaccination biases towards Th1 responses.
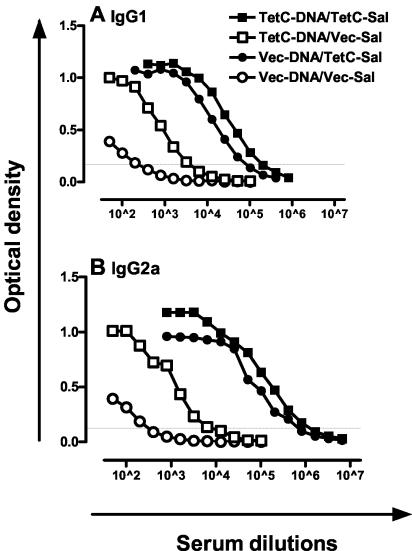
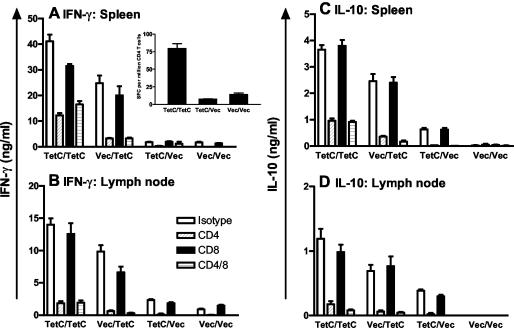
IgG2a levels are dependent on IFN-γ, whereas IgG1 levels correlate with IL-4 (3). IgG2a and IgG1 were therefore used as surrogate markers for Th1 and Th2 responses in TetC-immunized mice. As in previous studies (5) immunization with recombinant Salmonella expressing TetC (Vec-DNA/TetC-Sal) resulted in strong IgG1 and IgG2a responses (Fig. 1). Mice boosted with TetC-Sal after TetC-DNA priming showed the highest titers of TetC-specific IgG1 and IgG2a, with significant boosting of the response compared to that of mice immunized with TetC-DNA alone (Fig. 1). IgG2a/IgG1 ratios based on endpoint titers were 5.4 for TetC-Sal-boosted mice compared to 1.9 for mice immunized with TetC-DNA alone. Immunization with recombinant Salmonella alone (Vec-DNA/TetC-Sal) also resulted in strong IFN-γ and IL-10 responses in draining lymph node and spleen cells isolated following in vivo recall and restimulation with recombinant protein in vitro (Fig. 2). While recall responses to DNA alone (TetC-DNA/Vec-Sal) were low at this time point, they were significantly enhanced by being boosted with Salmonella (TetC-DNA/TetC-Sal) to levels much higher than those seen with Salmonella alone. Indeed, the combined DNA-Salmonella primer-booster regimen provided a significant boost to both IFN-γ and IL-10 responses compared to regimens with either Salmonella alone or DNA alone (Fig. 2). Release of IL-4 was not measurable in lymph node cells and was equivalently low (<0.2 ng/ml) in spleen cells following either vaccination regimen (data not shown). For lymph node and spleen cells, anti-CD4 but not anti-CD8 treatment blocked IFN-γ and IL-10 release (Fig. 2). Addition of anti-CD4 and anti-CD8 antibody together did not block either IFN-γ or IL-10 release more than did anti-CD4 added alone. The ratio of IFN-γ to IL-10 was 11.4 ± 0.35 for spleen cells and 12.8 ± 0.70 for lymph node cells from DNA-Salmonella primer-booster-immunized mice, compared to 3.0 ± 0.31 and 6.2 ± 0.20, respectively, for mice immunized with TetC-DNA alone. ELISPOT assays confirmed a higher frequency of IFN-γ-secreting CD4+ T cells in the spleens of DNA-Salmonella primer-booster-immunized mice than in the spleens of TetC-DNA-immunized mice (Fig. 2A, inset). An increase in IFN-γ-secreting CD8+ T cells was not observed in ELISPOT assays for either vaccination regimen (data not shown). Taken together, these data confirm a strong bias towards a Th1 response in mice receiving a Salmonella boost compared to those vaccinated with TetC-DNA alone.
FIG. 1.
TetC-specific IgG2a and IgG1 antibody levels presented as the average of duplicate absorbance (optical density) units for pooled sera from three mice/group with 12 serial twofold dilutions. The faint horizontal line represents the line at which endpoint titers were determined. Data shown are representative of two experiments comparing DNA-Salmonella primer-booster (TetC-DNA/TetC-Sal), Salmonella-alone (Vec-DNA/TetC-Sal), DNA-alone (TetC-DNA/Vec-Sal), and vector control (Vec-DNA/Vec-Sal) regimens. Note that numbers following superscript carets are exponents.
FIG. 2.
Cytokine responses in BALB/c mice vaccinated with a DNA-Salmonella primer-booster (TetC-DNA/TetC-Sal [TetC/TetC]), Salmonella-alone (Vec-DNA/TetC-Sal [Vec/TetC]), DNA-alone (TetC-DNA/Vec-Sal [TetC/Vec]), or vector control (Vec-DNA/Vec-Sal [Vec/Vec]) regimen. Pooled spleen (A and C) or lymph node (B and D) cells (n = 3 mice/group) were restimulated in vitro with recombinant protein with or without anti-CD4, anti-CD8, or isotype control MAb, and IFN-γ (A and B), IL-10 (C and D), or IL-4 (data not shown) levels were determined by ELISA. Data are representative of two experiments: P < 0.0001 for all TetC/TetC-versus-TetC/Vec comparisons; 0.002 < P < 0.02 for all TetC/TetC-versus-Vec/TetC comparisons; and 0.0001 < P < 0.0006 for TetC/Vec-versus-Vec/Vec comparisons, except for IFN-γ in spleen cells, where this comparison was not significant. The inset in panel A shows ELISPOT-determined frequencies of CD4+ T cells producing IFN-γ in the spleens of mice vaccinated with a DNA-Salmonella primer-booster (TetC/TetC), DNA-alone (TetC/Vec), or vector control (Vec/Vec) regimen.
Th1 bias following DNA-Salmonella primer-booster vaccination translates into enhanced protection against an intracellular pathogen.
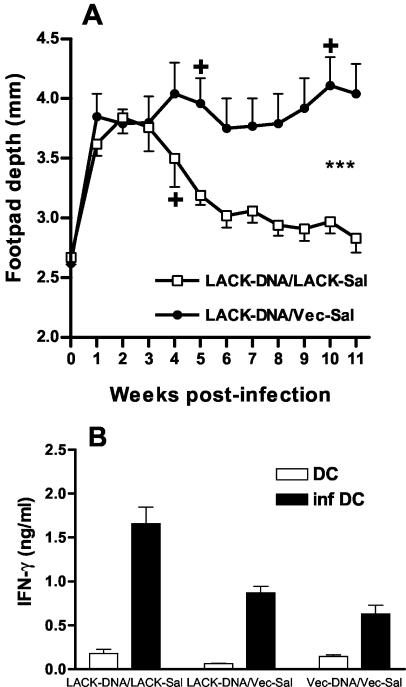
Results obtained using TetC as a model antigen suggested that DNA-Salmonella primer-booster vaccination should provide enhanced protection against intracellular pathogens compared to that for DNA alone. Using LACK-vaccinated mice, we observed significant (P < 0.001) protection in LACK-DNA/LACK-Sal-vaccinated mice compared to those vaccinated with LACK-DNA alone (Fig. 3A). This correlated with enhanced prechallenge IFN-γ release by spleen cells isolated from mice 2 days after live parasite challenge and restimulated in vitro with infected DC as antigen-presenting cells (Fig. 3B). IL-4 and IL-10 were not detectable in these supernatants. These results are again consistent with a bias towards a Th1 response elicited by DNA-Salmonella primer-booster vaccination compared to that with DNA alone that translates into enhanced protection against challenge infection. In the experiment shown (Fig. 3A) we did not compare DNA alone with Salmonella alone since in previous experiments (data not shown) we had not seen any evidence for protection with LACK-Sal vaccination alone.
FIG. 3.
Clinical outcome (A) and IFN-γ (B) responses following L. major challenge in mice vaccinated with DNA-Salmonella primer-booster (LACK-DNA/LACK-Sal) regimen compared to those for mice vaccinated with DNA alone (LACK-DNA/Vec-Sal) (n = 10 mice/group). Responses for mice vaccinated with LACK-DNA/Vec-Sal did not differ significantly from those for mice vaccinated with Vec-DNA/Vec-Sal (data not shown). Clinical outcome was measured as footpad depth following injection of L. major into the hind footpad. Asterisks indicate significance at P = 0.001 over the course of infection determined by using Hotelling's T-squared generalized means test. IFN-γ levels were measured in supernatants from pooled spleen cells (n = 3 mice/group) restimulated in vitro with the use of infected DC. Student's t tests demonstrated differences at P < 0.001 for LACK-DNA/LACK-Sal versus LACK-DNA/Vec-Sal and P < 0.05 for LACK-DNA/Vec-Sal versus Vec-DNA/Vec-Sal.
Conclusions.
Resolution of primary infection with intracellular pathogens is dependent upon induction of Th1 cells making IFN-γ (4, 31, 33). Vaccination strategies that bias towards Th1 responses might therefore offer enhanced protection. Here we report that DNA-Salmonella primer-booster vaccination with the use of TetC as a model antigen induces a strong bias towards Th1 responses compared to those for mice immunized with either Salmonella or DNA alone. As a further test of this vaccine strategy we showed that DNA-Salmonella primer-booster vaccination with the use of the LACK antigen resulted in enhanced IFN-γ production and protection against L. major challenge in susceptible BALB/c mice compared to vaccination with DNA alone. These results suggest that heterologous primer-booster vaccination employing DNA-Salmonella might provide an optimal strategy for vaccines against intracellular pathogens, at least in the context of vaccines against leishmaniasis.
The only previous study (23) employing a similar strategy against intracellular pathogens reported that DNA-Salmonella primer-booster vaccination with the ESAT-6 antigen provided partial organ-specific protection against Mycobacterium tuberculosis challenge in the spleen but not in the liver or lung. The strategy employed was different from ours in that the attenuated S. enterica serovar Typhimurium strain was engineered to secrete ESAT-6 via the hemolysin secretion system of Escherichia coli, and the DNA was administered via gene gun. Gene gun delivery has frequently been reported to bias towards IgG1 production and mixed Th1-Th2 responses compared to intramuscular injection (e.g., see reference 32). Prior DNA vaccination experiments using ESAT-6 had similarly resulted in minor protection (16, 18, 20), and the authors (23) conclude along with others (2) that ESAT-6 is not very immunogenic and difficult to use for immunization. In contrast the LACK antigen used here is immunodominant, and susceptibility of BALB/c mice to L. major depends on the early production of IL-4 by CD4+ T cells which react to the LACK antigen (28). Although previous studies reported successful vaccination against L. major Friedlin (12) or L. major WHOM/IR/-173 (9) with the use of LACK DNA vaccination, DNA alone is not protective against L. major LV39 in our hands. This may reflect differing Th1-Th2 profiles observed in BALB/c mice with the use of these different strains of L. major (reviewed in reference 27). The important observation made here is that manipulation of the vaccination regimen to bias towards a Th1 response has elicited protection against the strain of the parasite that most biases towards a Th2 response during primary infection. In the previous studies that reported protection with the use of LACK DNA alone, in vivo depletion studies demonstrated a role for both CD4+ and CD8+ cells (12). Although we did not observe a contribution for CD8+ T cells in IFN-γ production in vitro, we did not look for cytotoxic T-lymphocyte activity in vitro and our results do not preclude a role for CD8+ T cells in contributing to effector or memory T-cell responses in vivo.
Although the use of live recombinant Salmonella vaccines in humans has always been an attractive option because of the possibility for oral delivery (7), high prior exposure to Salmonella infections in the Third World may compromise the use of Salmonella as a vaccine vehicle in human populations. The proof of principle demonstrated here is that boosting DNA vaccination with a delivery system that targets innate immunity via alternative TLRs has facilitated vaccination against leishmanial infection with use of a normally strong Th2 response-promoting antigen and a strong Th2 response-promoting parasite strain. This fuels further research into vaccination strategies that promote Th1 immunity by combining the power of multiple adjuvants that trigger IL-12 production by both myeloid and plasmacytoid DC via a range of different TLRs.
Acknowledgments
This work was supported by a studentship to U.G.L. from the Elmore Trust and the James Baird Fund and a program grant to J.M.B. from the British Medical Research Council.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Anderson, R., X. M. Gao, A. Papakonstantinopoulou, N. Fairweather, M. Roberts, and G. Dougan. 1997. Immunization of mice with DNA encoding fragment C of tetanus toxin. Vaccine 15:827-829. [DOI] [PubMed] [Google Scholar]
- 2.Brandt, L., M. Elhay, I. Rosenkrands, E. B. Lindblad, and P. Andersen. 2000. ESAT-6 subunit vaccination against Mycobacterium tuberculosis. Infect Immun. 68:791-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coffman, R. L., D. A. Lebman, and P. Rothman. 1993. Mechanism and regulation of immunoglobulin isotype switching. Adv. Immunol. 54:229-270. [DOI] [PubMed] [Google Scholar]
- 4.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunstan, S. J., C. P. Simmons, and R. A. Strugnell. 1998. Comparison of the abilities of different attenuated Salmonella typhimurium strains to elicit humoral immune responses against a heterologous antigen. Infect. Immun. 66:732-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans, J. T., C. W. Cluff, D. A. Johnson, M. J. Lacy, D. H. Persing, and J. R. Baldridge. 2003. Enhancement of antigen-specific immunity via the TLR4 ligands MPL adjuvant and Ribi 529. Expert Rev. Vaccines 2:219-229. [DOI] [PubMed] [Google Scholar]
- 7.Everest, P., P. Griffiths, and G. Dougan. 1995. Live Salmonella vaccines as a route towards oral immunisation. Biologicals 23:119-124. [DOI] [PubMed] [Google Scholar]
- 8.Fine, P. E. 1995. Variation in protection by BCG: implications of and for heterologous immunity. Lancet 346:1339-1345. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalo, R. M., G. del Real, J. R. Rodriguez, D. Rodriguez, R. Heljasvaara, P. Lucas, V. Larraga, and M. Esteban. 2002. A heterologous prime-boost regime using DNA and recombinant vaccinia virus expressing the Leishmania infantum P36/LACK antigen protects BALB/c mice from cutaneous leishmaniasis. Vaccine 20:1226-1231. [DOI] [PubMed] [Google Scholar]
- 10.Gurunathan, S., D. M. Klinman, and R. A. Seder. 2000. DNA vaccines: immunology, application, and optimization. Annu. Rev. Immunol. 18:927-974. [DOI] [PubMed] [Google Scholar]
- 11.Gurunathan, S., C. Prussin, D. L. Sacks, and R. A. Seder. 1998. Vaccine requirements for sustained cellular immunity to an intracellular parasitic infection. Nat. Med. 4:1409-1415. [DOI] [PubMed] [Google Scholar]
- 12.Gurunathan, S., D. L. Sacks, D. R. Brown, S. L. Reiner, H. Charest, N. Glaichenhaus, and R. A. Seder. 1997. Vaccination with DNA encoding the immunodominant LACK parasite antigen confers protective immunity to mice infected with Leishmania major. J. Exp. Med. 186:1137-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurunathan, S., C. Y. Wu, B. L. Freidag, and R. A. Seder. 2000. DNA vaccines: a key for inducing long-term cellular immunity. Curr. Opin. Immunol. 12:442-447. [DOI] [PubMed] [Google Scholar]
- 14.Hormaeche, C. E. 1991. Live attenuated Salmonella vaccines and their potential as oral combined vaccines carrying heterologous antigens. J. Immunol. Methods 142:113-120. [DOI] [PubMed] [Google Scholar]
- 15.Inaba, K., M. Inaba, N. Romani, H. Aya, M. Deguchi, S. Ikehara, S. Muramatsu, and R. M. Steinman. 1992. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 176:1693-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamath, A. T., C. G. Feng, M. Macdonald, H. Briscoe, and W. J. Britton. 1999. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect. Immun. 67:1702-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levick, M. P., J. M. Blackwell, V. Connor, R. M. Coulson, A. Miles, H. E. Smith, K. L. Wan, and J. W. Ajioka. 1996. An expressed sequence tag analysis of a full-length, spliced-leader cDNA library from Leishmania major promastigotes. Mol. Biochem. Parasitol. 76:345-348. [DOI] [PubMed] [Google Scholar]
- 18.Li, Z., A. Howard, C. Kelley, G. Delogu, F. Collins, and S. Morris. 1999. Immunogenicity of DNA vaccines expressing tuberculosis proteins fused to tissue plasminogen activator signal sequences. Infect. Immun. 67:4780-4786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lore, K., M. R. Betts, J. M. Brenchley, J. Kuruppu, S. Khojasteh, S. Perfetto, M. Roederer, R. A. Seder, and R. A. Koup. 2003. Toll-like receptor ligands modulate dendritic cells to augment cytomegalovirus- and HIV-1-specific T cell responses. J. Immunol. 171:4320-4328. [DOI] [PubMed] [Google Scholar]
- 20.Lowrie, D. B., C. L. Silva, M. J. Colston, S. Ragno, and R. E. Tascon. 1997. Protection against tuberculosis by a plasmid DNA vaccine. Vaccine 15:834-838. [DOI] [PubMed] [Google Scholar]
- 21.Mendez, S., S. Gurunathan, S. Kamhawi, Y. Belkaid, M. A. Moga, Y. A. Skeiky, A. Campos-Neto, S. Reed, R. A. Seder, and D. Sacks. 2001. The potency and durability of DNA- and protein-based vaccines against Leishmania major evaluated using low-dose, intradermal challenge. J. Immunol. 166:5122-5128. [DOI] [PubMed] [Google Scholar]
- 22.Miller, I. A., S. Chatfield, G. Dougan, L. Desilva, H. S. Joysey, and C. Hormaeche. 1989. Bacteriophage P22 as a vehicle for transducing cosmid gene banks between smooth strains of Salmonella typhimurium: use in identifying a role for aroD in attenuating virulent Salmonella strains. Mol. Gen. Genet. 215:312-316. [DOI] [PubMed] [Google Scholar]
- 23.Mollenkopf, H. J., D. Groine-Triebkorn, P. Andersen, J. Hess, and S. H. Kaufmann. 2001. Protective efficacy against tuberculosis of ESAT-6 secreted by a live Salmonella typhimurium vaccine carrier strain and expressed by naked DNA. Vaccine 19:4028-4035. [DOI] [PubMed] [Google Scholar]
- 24.Mougneau, E., F. Altare, A. E. Wakil, S. Zheng, T. Coppola, Z.-E. Wang, R. Waldmann, R. M. Locksley, and N. Glaichenhaus. 1995. Expression cloning of a protective Leishmania antigen. Science 268:563-566. [DOI] [PubMed] [Google Scholar]
- 25.Reed, S. G., R. N. Coler, and A. Campos-Neto. 2003. Development of a leishmaniasis vaccine: the importance of MPL. Expert Rev. Vaccines 2:239-252. [DOI] [PubMed] [Google Scholar]
- 26.Rothenfusser, S., E. Tuma, S. Endres, and G. Hartmann. 2002. Plasmacytoid dendritic cells: the key to CpG. Hum. Immunol. 63:1111-1119. [DOI] [PubMed] [Google Scholar]
- 27.Sacks, D., and N. Noben-Trauth. 2002. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2:845-858. [DOI] [PubMed] [Google Scholar]
- 28.Schilling, S., and N. Glaichenhaus. 2001. T cells that react to the immunodominant Leishmania major LACK antigen prevent early dissemination of the parasite in susceptible BALB/c mice. Infect. Immun. 69:1212-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah, J. A., P. A. Darrah, D. R. Ambrozak, T. N. Turon, S. Mendez, J. Kirman, C. Y. Wu, N. Glaichenhaus, and R. A. Seder. 2003. Dendritic cells are responsible for the capacity of CpG oligodeoxynucleotides to act as an adjuvant for protective vaccine immunity against Leishmania major in mice. J. Exp. Med. 198:281-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stacey, K. J., and J. M. Blackwell. 1999. Immunostimulatory DNA as an adjuvant in vaccination against Leishmania major. Infect. Immun. 67:3719-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swihart, K., U. Fruth, N. Messmer, K. Hug, R. Behin, S. Huang, G. Del Giudice, M. Aguet, and J. A. Louis. 1995. Mice from a genetically resistant background lacking the interferon gamma receptor are susceptible to infection with Leishmania major but mount a polarized T helper cell 1-type CD4+ T cell response. J. Exp. Med. 181:961-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toda, M., H. Sato, Y. Takebe, Y. Taniguchi, S. Saito, S. Inouye, T. Takemori, and M. Sakaguchi. 2000. Inhibition of immunoglobulin E response to Japanese cedar pollen allergen (Cry j 1) in mice by DNA immunization: different outcomes dependent on the plasmid DNA inoculation method. Immunology 99:179-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, Z. E., S. L. Reiner, S. Zheng, D. K. Dalton, and R. M. Locksley. 1994. CD4+ effector cells default to the Th2 pathway in interferon gamma-deficient mice infected with Leishmania major. J. Exp. Med. 179:1367-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]