Abstract
Mucus overproduction is a significant component of the pathophysiology of obstructive lung diseases. Currently, there are only a few medications available that inhibit mucus production. Previous studies showed that glycyrrhizin, a triterpenoid in Glycyrrhiza uralensis (G. uralensis) inhibits mucin 5AC (MUC5AC) mRNA and protein expression. Other potential mucus production inhibitory compounds contained within in G. uralensis have not been fully investigated. The aim of the present study was to determine if the G. uralensis flavonoid 7,4'-dihydroxyflavone (7,4'-DHF), inhibits MUC5AC gene expression, mucus production and secretion and if so, to elucidate the mechanism of this inhibition. 7,4'-DHF significantly decreased phorbol 12-myristate 13-acetate (PMA) stimulated NCI-H292 human airway epithelial cell MUC5AC gene expression and mucus production, at a 28 fold lower concentration than glycyrrhizin (IC50 value of 1.4µM vs 38 µM, respectively), 7,4'-DHF also inhibited MUC5AC mucus secretion. Inhibition was associated with the suppression of NF-κB, STAT6 activation and enhanced HDAC2 expression. In a murine model of asthma, 7,4'-DHF treated mice exhibited a marked reduction in MUC5AC secretion in the bronchoalveolar lavage (BAL) fluid compared with control mice. These findings, together with previous findings linking NF-κB, STAT6 and HDAC2 modulation to the control of MUC5AC expression, demonstrate that 7,4'-DHF is a newly identified component of G. uralensis that regulates MUC5AC expression and secretion via regulation of NF-κB, STAT6 and HDAC2.
Keywords: Glycyrrhiza uralensis, NCI-H292, immunoblot, ovalbumin, bronchoalveolar lavage, MTT
Introduction
The mucin/mucus layers of the airways are part of the innate immune defense system, trapping pathogens and environmental toxins in inspired air, thereby preventing infection and damage to the distal airways. The central role of mucus overproduction in severe asthma was recognized in the 19th and early 20th century, and mucus plugging of airways is a common finding in fatal asthma attacks (Evans et al., 2009). Among the 20 mucus genes encoding human mucins, mucin 5AC (MUC5AC), which is mainly expressed in goblet cells, is the major mucin protein in the asthmatic airway, where expression was 60% higher in asthmatics as compared with normal subjects (Kraft et al., 2008). Conventional therapy for mucus hypersecretion is limited to indirectly inhibiting production by suppressing inflammation with corticosteroids, or by the use of anticholinergics that inhibit mucus production by blocking parasympathetic nerve activity. Novel compounds that directly inhibit epithelial goblet cell mucus production would be of great interest.
Using the human pulmonary mucoepidermoid carcinoma cell line NCI-H292 as a model, a number of environmental toxins, cytokines, and herbal derived compounds that either stimulate or inhibit MUC5AC production have been identified (Kim et al., 2012; Lee et al., 2011; Shao et al., 2004). PMA is a widely used as a model inflammatory stimulant, modulating a variety of cellular events, including NCI-H292 MUC5AC cell gene and protein expression (Hewson et al., 2004).
G. uralensis has been well documented as an effective treatment for productive cough and wheezing in Traditional Chinese Medicine (TCM) (Bensky D and Gamble A, 1993). Glycyrrhizin, a triterpenoid found in G. uralensis, inhibits MUC5AC mRNA and protein expression in PMA-stimulated NCI-H292 human epithelial cells (Lee et al., 2011; Nishimoto et al., 2010). Other compounds contained within G. uralensis that also inhibit mucus production have not been investigated.
The Anti-Asthma Herbal Medicine Intervention (ASHMI, or ASHMITM) an extract of three Chinese herbal herbs, one of which is G. uralensis, has immunomodulatory effects (Srivastava et al., 2014; Zhang et al., 2010) and decreases MUC5AC mRNA and the number of airway goblet cells in animal models of asthma (Busse et al., 2010). Using a systematic bioassay-guided purification protocol, our lab isolated and identified a number of flavonoid compounds with anti-inflammatory properties similar to G. uralensis. Three of these compounds, liquiritigenin (LQ), isoliquiritigenin (ILQ), and 7,4'-dihydroxyflavone (7,4'-DHF), inhibit human fetal lung fibroblast eotaxin-1 production more potently than glycyrrhizin (Jayaprakasam et al., 2009). They also suppressed the Th2 cytokines IL-4 and IL-5 production by cultured mouse T cells (7,4'-DHF being the most potent), as well as the number of airway goblet cells in OVA sensitized and challenged mice (Yang et al., 2013). In the current study, we investigated if 7,4'-DHF inhibited NCI-H292 cell MUC5AC gene expression, production, secretion and if so, which signaling pathways were involved. We also explored the in-vivo inhibitory effect of 7,4’-DHF on MUC5AC production in an OVA sensitized mouse model of asthma.
Materials and Methods
Compounds isolation and identification
G. uralensis aqueous extract was manufactured by the Sino-Lion Pharmaceutical Company (a GMP certified facility in Weifang, China) as described previously (Kelly-Pieper et al., 2009). Three flavonoids LQ, ILQ, 7,4'-DHF and glycyrrhizin were isolated from G. uralensis using published methods (Jayaprakasam et al., 2009) with modification. A dried aqueous extract of G. uralensis (200 g) dissolved in distilled-water was loaded onto a macroporous resin column (Amberlite XAD-7 HP, Acros Organics, Fair Lawn, NJ), and sequentially eluted with water and 20%, 70%, and 95% aqueous ethanol. 70% ethanol and 95% ethanol elutes were combined, concentrated and subjected to silica gel column chromatography, sequentially eluted with varying dilutions of dichloromethane-ethyl acetate (19:1, 9:1, 3:1), and dichloromethane-methanol (9:1, 3:1). Based on TLC and HPLC profiles, the eluates were combined to yield four major sub-fractions. These sub-fractions were further fractionated and purified using silica gel, Sephadex LH20 column chromatography and recrystallization to yield single compounds: LQ, ILQ, 7,4'-DHF, and glycyrrhizin. The structures of isolated compounds were identified by TLC, HPLC, liquid chromatographic mass spectrometry (LC-MS) and nuclear magnetic resonance (NMR) spectra comparison as shown in our previous publication (Jayaprakasam et al., 2009). The chemical structures of LQ, ILQ, 7,4'-DHF, and glycyrrhizin are shown in Figure 1.
Figure 1.
Chemical structures of liquiritigenin (LQ), isoliquiritigenin (ILQ), 7,4′-dihydroxyflavone (7,4′-DHF), and glycyrrhizin isolated from Glycyrrhizin uralensis. The flavonoids LQ, ILQ, 7,4′-DHF, and the triterpenoid glycyrrhizin were isolated from G. uralensis using column chromatographic separation methods. The structures of isolated compounds were identified by TLC, HPLC, liquid chromatography–mass spectrometry, and nuclear magnetic resonance spectra as we previously described.
Cell culture
The human pulmonary epithelial cell line NCI-H292 was purchased from American Type Collection Culture (ATCC) and cultured in 24-well tissue culture plates (1x104 cells/well seeding density) in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) and penicillin (100U/mL), streptomycin (100µg/mL) and HEPES buffer (25 mM) at 37°C in a humidified, 5% CO2/95% air, water-jacketed incubator. For serum deprivation the cells were washed twice with PBS then re-cultured in RPMI 1640 supplemented with 0.2% FCS for 24 hours.
Treatment of cells
After 24 hours of serum deprivation, the cells were washed with PBS twice and left untreated or pretreated for 30 minutes with buffer (serum free RPMI 1640) or compounds at various concentrations. 10ng/mL PMA (Fisher BioReagents, Pittsburgh, PA) was then added in serum free RPMI 1640 as previously described (Lee et al., 2011). After 24 hours, supernatants were collected to measure the secretion of MUC5AC protein. Cells were lysed with a buffer solution containing 25 mM Tris, 1% NP-40, 150 mM NaCl, 1 mM EDTA, 5% glycerol, and a protease inhibitor cocktail (Pierce Biotechnology, Rochford, IL, USA) and whole cell extracts were collected to measure the production of MUC5AC protein. Protein concentrations were determined using manufacturer's protocol (Bio-Rad laboratories, Hercules, CA), and stored at −80°C. In a parallel experiment, Total RNA was isolated from cells for MUC5AC mRNA measurement as details described below.
MUC5AC mucin analysis using ELISA
MUC5AC protein levels were measured using an indirect ELISA assay (Lee et al., 2011). Cell culture supernatants and lysates were prepared with a 1:2 dilution of carbonate/bicarbonate coating buffer, and 100µL of each sample was added to a 96-well ELISA plate and incubated at 37°C overnight until dry. Cell culture media without cell exposure served as a blank control. Plates were then washed three times with PBS-0.05% Tween 20 and blocked with 2% BSA for 1 hour at room temperature, then washed and incubated with 100µL of a mouse monoclonal MUC5AC antibody 1:300 (ab3649, Abcam, Cambridge, MA,) in PBS-0.05% Tween 20 for 60 minutes. Plates were washed and 100uL of a horseradish peroxidase conjugated goat anti-mouse IgG conjugate (12–349, Millipore, Temecula, CA) diluted 1:3000 in PBS-0.05% Tween 20 was added. After 15 minutes the plates were washed and developed using a 3,3’,5,5’-tetramethylbenzidine (TMB) Substrate Reagent Set (BD Biosciences, San Diego, CA) per manufacturer instructions. Absorbance was read at 450nm using a Vmax Kinetic ELISA microplate reader and the value of the blank control was subtracted from the sample values.
MTT cell viability assay
MTT cell viability assays were performed as previous described (Jayaprakasam et al., 2009). 100µL of NCI-H292 cells at a concentration of 2x105/mL were added to a 96-well tissue culture plate and allowed to adhere. After 24 hours the cells were washed with 200µL of PBS three times and then left untreated or treated with PMA 10ng/mL alone or PMA 10ng/mL plus various concentrations of 7,4'-DHF in serum free RPMI. After 24 hours, 50µL of 2.0 mg/mL (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) in RPMI was added and incubated for 3 hours at 37°C in a humidified, 5% CO2/95% air, water-jacketed incubator. The supernatants were then discarded and replaced with 200µL of DMSO and the plate was placed on an orbital shaker at room temperature. After 30 minutes absorbance was read at 595nm using a Vmax Kinetic ELISA microplate reader.
RNA isolation and real-time RT-PCR
Total RNA was isolated from cells using AllPrep DNA/RNA Mini Kit (Qiagen, Valencia, CA) and resuspended in 40µL. 5µL of this RNA preparation was reverse transcribed in a 20µL reaction volume using Improm-IITM Reverse Transcription system (Promega, Madison, WI) with random hexamer primers for cDNA synthesis step. 2µL of this resulting cDNA mixture were used in 50µL qPCR reaction volumes using the Maxima SYBR Green/ROX qPCR Master Mix (2X) (Fisher Scientific, Pittsburgh, PA). Primers for MUC5AC were (forward) 5’-TGCCAGGAGCTGCGGACCTC-3’ and (reverse) 5’-CCGAGCTCAGAGGACATATGGG-3’. The size of expected fragments amplified by PCR was 281bp. A constitutively expressed housekeeping gene Rig/S15 was also quantified. Primers for Rig/S15 were (forward) 5’-TTCCGCAAGTTCACCTACC-3’ and (reverse) 5’-CGGGCCGGCCATGCTTTACG-3’. The size of expected fragment amplified by PCR was 361bp. For qPCR, by using ABI PRISM® 7900HT Sequence Detection System (Life Technologies, Norwalk, CT), the mixture was denatured at 50 °C for 2mins, 94 °C for 10 mins, followed by 40 cycles at 95 °C for 15s, 60 °C for 30s and 72 °C for 30s. Normalized target gene expression was done using the 2(-ΔΔCt) method (Lee et al., 2011; Livak and Schmittgen, 2001).
Western blot analysis
Western blot analysis was performed as previously described (Wang et al., 2012) using the manufacturer’s protocol (Active Motif nuclear extract kit, Active Motif, Carlsbad, CA). Whole cell protein was extracted from serum deprived NCI H292 cells pretreated with 7, 4'-DHF for 24 hours followed by PMA stimulation for 30 minutes. Protein concentrations in whole cell extracts were determined according to the manufacturer’s protocol (Bio-Rad Protein Assay, Dye Reagent Concentrate, Bio-Rad laboratories, Hercules, CA), and proteins were stored at −80 °C until western blot analysis. Total cell lysates (30µg) were separated by SDS-PAGE and were transferred to nitrocellulose membranes. The blots were stained with 0.2% Ponceau S red to verify equal protein loading. The membranes were blocked with 5% bovine serum albumin in TBS-Tween 20 solution at 4°C for 1 hour and were incubated with anti phospho-IκBα (Ser32, rabbit, 1:1000), total IκBα (rabbit, 1:1000), phospho-NFκB (p-p65, rabbit, 1:1000), total NFκB (p65, rabbit, 1:1000), phospho-STAT6 (Tyr641, rabbit, 1:1000), total STAT6 (rabbit, 1:1000), total HDAC-2 (rabbit, 1:1000), phospho-ERK1/2 (Thr202/Tyr204, rabbit, 1:1000), total ERK1/2 (rabbit, 1:1000) and GADPH (D16H11, rabbit, 1:5000) at 4°C overnight. All antibodies were from Cell Signal Technology (Beverly, MA). Membranes were then incubated with anti-rabbit immunoglobulin (EMD Millipore, Billerica, MA) diluted 1:3000 in TBS-Tween 20 for 1 hour at room temperature with continuous shaking. Reactive bands were visualized with HRP-coupled anti-rabbit antibody using the Chemiluminescent HRP Antibody Detection Reagent (Denville Scientific, South Plainfield, NJ) according to the manufacturer’s recommended procedures. Densitometry of band intensity was expressed relative to that of non-stimulation, non-treatment control set at 100%. Phosphorylation status was present as the ratio between phosphorylated and total protein.
OVA sensitization/challenge and 7, 4'-DHF treatment in murine model
The OVA sensitization/challenge and 7, 4'-DHF treatment in a murine model were described previously (Yang et al., 2013). Six-week-old female BALB/c mice were purchased from the Jackson Laboratory (Bar Harbor, ME). All animal experiments were conducted according to ethical guidelines of Icahn School of Medicine at Mount Sinai, New York. To generate an allergic asthma model, female BALB/c mice were sensitized weekly, intraperitoneally with 200 mg OVA (Type V, Sigma-Aldrich) absorbed on 2mg alum (Pierce Biotechnology Inc., Rockford, IL) in 0.4 mL phosphate-buffered saline for 2 weeks. After the last sensitization, five intratracheal (i.t.) challenges were performed on days 14, 21, 28, 45 and 46 with 100 mg OVA in 0.05mL of phosphate-buffered saline. Three mice received 6µg of 7,4'-DHF in 1mL of 0.1% DMSO in water intragastrically, divided into two feedings daily for one month beginning 24 h after the first i.t. challenge. Three OVA sensitized/challenged mice receiving 0.5 ml of 0.1% DMSO in water twice daily for one month served as sham control mice for the experiment. Three mice not sensitized/challenged or treated served as the naïve control group. To obtain the BAL fluid, experimental mice lungs were lavaged three times with 1 mL of Hank's Balanced Salt Solution (HBSS) forty-eight hours after the final i.t. challenge. All recovered fluids were centrifuged at 700g for 15 minutes at 4°C to isolate cells. Supernatants were stored at −80°C for MUC5AC protein concentration measurements.
Statistical analysis
All statistical analyses were performed using GraphPad Prism4 software (GraphPad Software Inc, La Jolla, CA). Differences between multiple groups were analyzed by one-way ANOVA followed by the Dunnett test. A p value ≤0.05 was considered statistically significant.
Results
G. uralensis flavnoids inhibited PMA-induced MUC5AC production
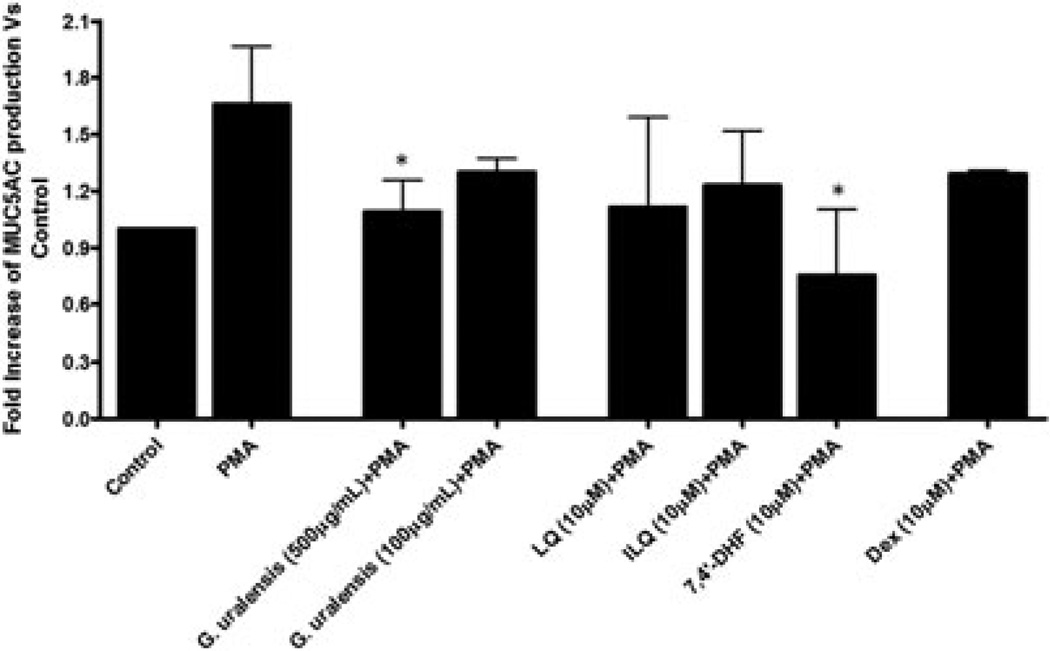
We investigated the regulatory effects of G. uralensis (500 and 100 µg/mL), three isolated flavonoids (7, 4'-DHF, LQ, and ILQ) (10µM), and the steroid dexamethasone (Dex, 10µM), on the PMA-induced MUC5AC production by H292 cells. Only G. uralensis at the concentration 500µg/mL and 7, 4'-DHF at 10µM, but not the other two flavonoids (LQ and ILQ) nor Dex, significantly inhibited PMA-induced MUC5AC production by H292 cells (Figure 2).
Figure 2.
Effect of Glycyrrhizin uralensis flavonoids on phorbol 12-myristate 13-acetate (PMA)-induced mucin 5AC (MUC5AC) protein production. Serum-deprived NCI-H292 cells were pretreated with G. uralensis (500 or 100 µg/mL) or the G. uralensis flavonoids liquiritigenin (LQ), isoliquiritigenin (ILQ), and 7,4′-dihydroxyflavone (7,4′-DHF; 10 µM), or dexamethasone (Dex; 10 µM) for 30 min and then stimulated with PMA (10 ng/mL) for 24 h. MUC5AC protein production in cell lysis was measured by ELISA. Each represents a mean ± SD of three independent experiments in comparison with that of control set at 1. * p < 0.01, versus PMA stimulation, non-treatment group.
G. uralensis flavnoids inhibited PMA-induced MUC5AC secretion
We also evaluated the inhibitory effects of G. uralensis, three isolated flavonoids (7, 4'-DHF, LQ, and ILQ), and the steroid dexamethasone (Dex), on the PMA-induced MUC5AC secretion by H292 cells. PMA induced a 12 fold increase of MUC5AC protein secretion. G. uralensis at the concentration 500µg/mL, all three G. uralensis flavanoids at 10µM, and Dex at 10µM, significantly inhibited PMA-induced MUC5AC secretion by H292 cells, while 7, 4'-DHF was the most potent inhibitor (compared with LQ, and ILQ) of MUC5AC secretion at the concentration (10µM) tested (Figure 3).
Figure 3.
Effect of Glycyrrhizin uralensis flavonoids on phorbol 12-myristate 13-acetate (PMA)-induced mucin 5AC (MUC5AC) protein secretion. Serum-deprived NCI-H292 cells were pretreated with G. uralensis (500 or 100 µg/mL) or the G. uralensis flavonoids liquiritigenin (LQ), isoliquiritigenin (ILQ), and 7,4′-dihydroxyflavone (7,4′-DHF; 10 µM), or dexamethasone (Dex; 10 µM) for 30 min and then stimulated with PMA (10 ng/mL) for 24 h. MUC5AC protein secretion in supernatants was measured by ELISA. Each represents a mean ± SD of three independent experiments in comparison with that of control set at 1. ** p < 0.01, versus PMA stimulation, nontreatment group; # p < 0.05, versus PMA stimulation, 7,4′-DHF treatment group.
7,4'-DHF inhibited PMA-induced MUC5AC secretion
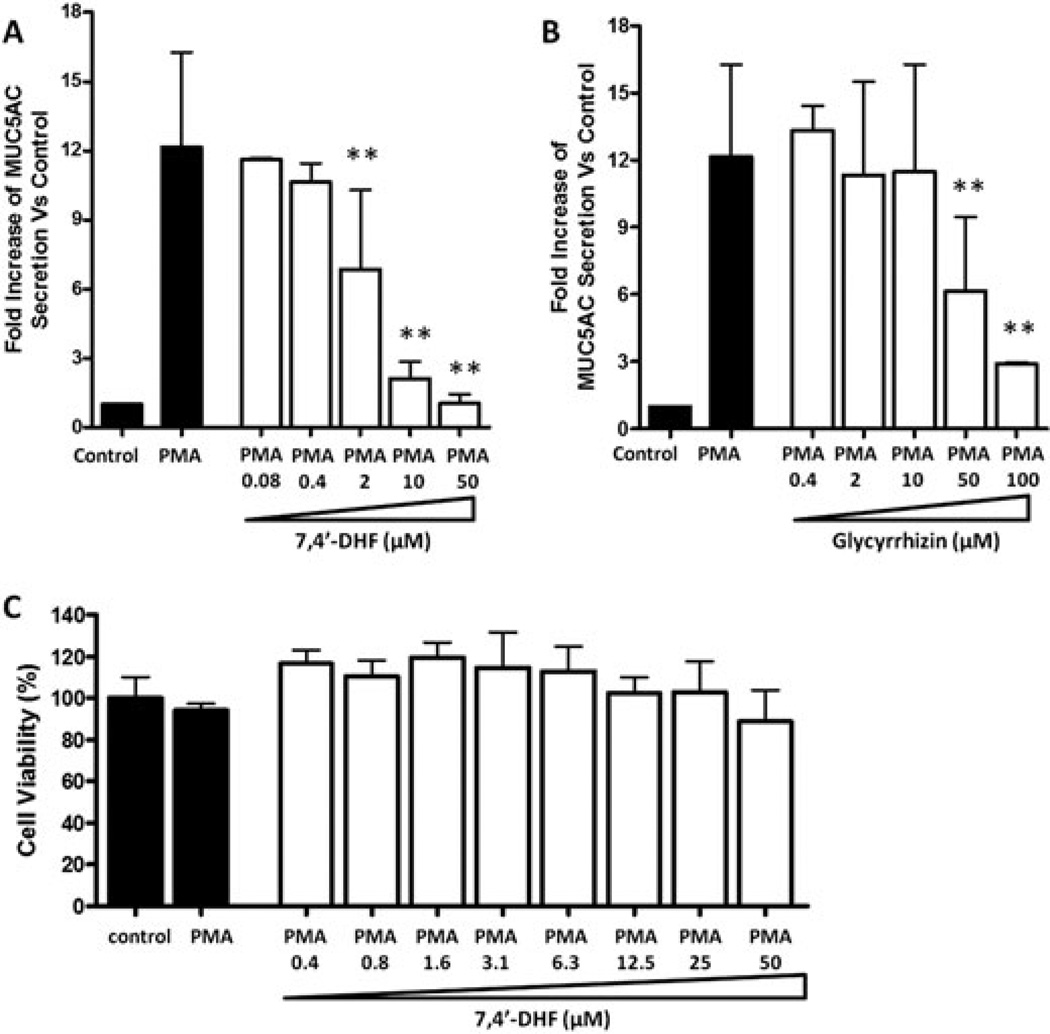
We focused on 7,4'-DHF and compared its inhibition of MUC5AC protein secretion to that of glycyrrhizin. Both glycyrrhizin (at 50 and 100µM) and 7,4'-DHF (at 2, 10, and 50µM) dose dependently inhibited MUC5AC secretion by PMA stimulated NCI-H292 cells (Figure 4A, B, p<0.01) Interestingly, 7,4'-DHF suppressed MUC5AC secretion at a 28 fold lower concentration than glycyrrhizin: IC50 values of 1.4 (0.95–2.0) and 38 (33–44) µM (95% CI), respectively. MTT cell viability assays revealed no cytotoxic effects of 7,4'-DHF at concentrations up to 50µM, the highest dose tested (Figure 4C).
Figure 4.
Effect of 7,4′-dihydroxyflavone (7,4′-DHF) and glycyrrhizin on phorbol 12-myristate 13-acetate (PMA)-induced mucin 5AC (MUC5AC) protein secretion. Serum-deprived NCI-H292 cells were pretreated with compounds for 30 min and then stimulated with PMA (10 ng/mL) for 24 h. MUC5AC protein secretion in supernatants was measured by ELISA. (A) Concentration-dependent effect of 7, 4′-DHF on PMA-induced MUC5AC protein secretion. (B) Concentration-dependent effect of glycyrrhizin on PMA-induced MUC5AC protein secretion. Each represents a mean ± SD of three to eight independent experiments in comparison with that of control set at 1. ** p < 0.01, versus PMA stimulation, nontreatment group. (C) Cell viability of 7,4′-DHF-pretreated PMA-stimulated NCI-H292 cells. The cell viability percentage was calculated by comparison with the medium alone group, which was set at 100%.
7,4'-DHF inhibited PMA-induced MUC5AC gene expression
To evaluate 7,4'-DHF inhibition of MUC5AC gene expression, MUC5AC mRNA levels were determined by real-time PCR. Ct values obtained for the MUC5AC gene and the housekeeping gene Rig/S15 were 30.0 ±0.5, and 21.5±1.0, respectively, under control conditions. PMA stimulation caused a 4-fold increase in MUC5AC mRNA levels when compared with untreated controls. 7,4'-DHF (10µM) decreased PMA-stimulated MUC5AC mRNA expression levels compared to PMA unstimulated control cells (Figure 5).
Figure 5.
Effect of 7,4′-dihydroxyflavone (7,4′-DHF) on phorbol 12-myristate 13-acetate (PMA)-induced mucin 5AC (MUC5AC) mucin gene expression. Serum-deprived NCI-H292 cells were pretreated with 7,4′-DHF (0, 2, and 10 µM) for 30 min followed by stimulation with PMA (10 ng/ml) for 24 h. Mucin 5AC mucin gene expression was measured by quantitative real-time RT-PCR. As a quantitative control for mRNA input, Rig/S15 rRNA, a constitutively expressed housekeeping gene, was measured. Each represents a mean ± SD of three independent experiments compared with that of control value set at 1. ** p < 0.01, versus PMA stimulation alone group.
7,4'-DHF Effects on NF-κB, STAT6, ERK1/2 activation and HDAC2 expression
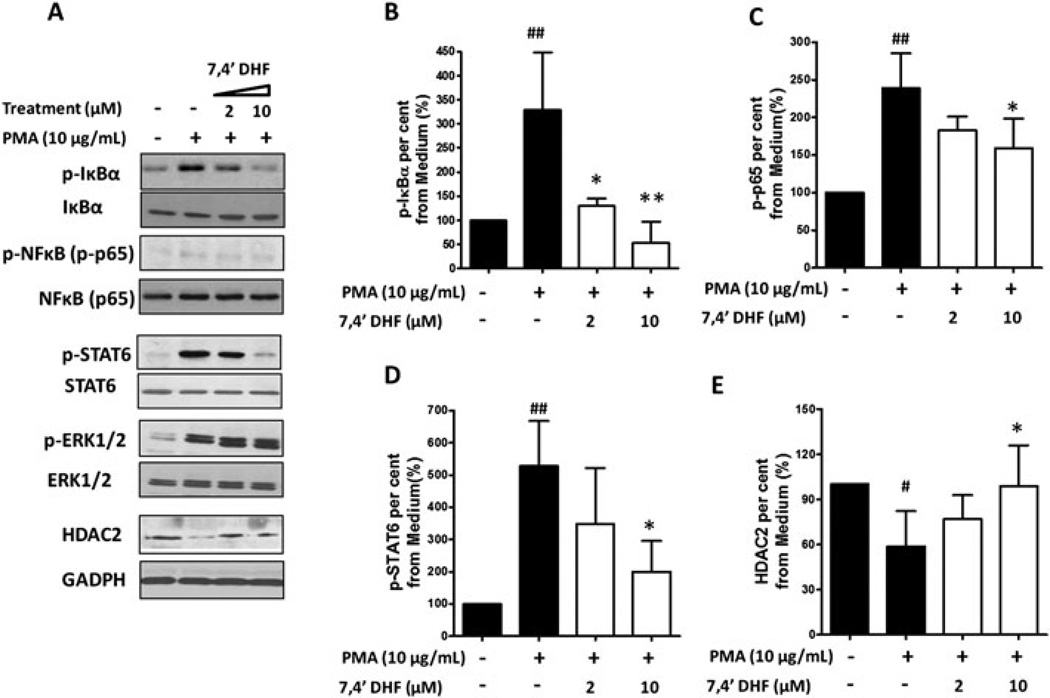
We next determined 7,4'-DHF effects on IκB, NF-κB, STAT6, ERK1/2 phosphorylation and HDAC-2 expression, which are signaling molecules previously shown to modulate MUC5AC expression. NCI-H292 cells were pretreated with 7, 4'-DHF (0, 2, 10 µM) for 24 hours, then stimulated with 10ng/mL PMA for 30 minutes. As shown in Figure 6, PMA stimulation increased IκBα phosphorylation, which was reduced by 7, 4'-DHF pretreatment (Figure 6A, B). 7, 4'-DHF inhibited PMA stimulated NF-κB (p65) activation (Figure 6A, C).7, 4'-DHF also reduced PMA induced STAT6 phosphorylation. (Figure 6A, D). PMA reduced HDAC-2 expression, which was prevented by 10µM 7, 4'-DHF (Figure 6A, E). 7, 4'-DHF did not affect PMA-induced ERK1/2 phosphorylation levels (Figure 6A). 7, 4'-DHF didn’t show significant regulatory effects on IκB, NF-κB(p65), STAT6, and ERK1/2 expression.
Figure 6.
Regulatory effects of 7,4′-dihydroxyflavone (7,4′-DHF) on phorbol 12-myristate 13-acetate (PMA)-stimulated phosphorylated IκBα (p-IκBα), phosphorylated NFκB (p-NF-κB), phosphorylated signal transducer and activator of transcription 6 (p-STAT6), phosphorylated extracellular signal-regulated kinase 1/2 (p-ERK1/2), and histone deacetylase 2 (HDAC2) expression. Serum-deprived NCI-H292 cells were pretreated with 7,4′-DHF (0, 2, and 10 µM) for 24 h followed by PMA (10 ng/mL) stimulation for 30 min. (A) The expression of p-IκBα, IκBα, p-NF-κB (p-p65), NF-κB(p65), p-STAT6, STAT6, p-ERK1/2, ERK1/2, HDAC2, and GAPDH (loading control) in whole cell extracts was evaluated by western blot analysis. Quantitative western blot analysis of p-IκBα (B), p-NF-κB (p-p65, C), p-STAT6 (D), and HDAC2 (E) expression was measured in three independent experiments. Densitometry of band intensity was expressed relative to that of non-stimulation, nontreatment control set at 100%. Phosphorylation status was present as the ratio between phosphorylated and total protein.* p < 0.05 ** p < 0.01, versus PMA stimulation alone group; # p < 0.05 ## p < 0.01, versus medium alone group.
7,4'-DHF inhibited MUC5AC secretion in vivo
As expected, BAL levels of MUC5AC proteins were significantly increased in OVA sensitized/challenged mice compared with naïve group as shown in Figure 7. 7, 4'-DHF 6µg/mL exhibited a significant decrease approximately 38% in BAL levels of MUC5AC after 4 week treatment as compared with sham group.
Discussion
Current treatment options for mucus hypersecretion, a component the chronic lung diseases asthma, chronic obstructive pulmonary disease (COPD), and cystic fibrosis, are limited. Corticosteroids remain the cornerstone of mucus treatment, however, systemic side effects limit long-term applicability. Of the non-steroidal mucoregulators, macrolides appear effective but can lead to life-threatening cardiovascular complications and antibiotic resistance with long-term use, new therapeutic agents are needed to reduce the associated morbidity and mortality (Cerveri and Brusasco, 2010; Ehre et al., 2014; Rogers, 2007). This has led to efforts in the identification of new compounds that inhibit mucus production and gene expression. It was previously found that glycyrrhizin, derived from G. uralensis, inhibits MUC5AC gene expression (Lee et al., 2011). However, the present study found that 7,4'-DHF was 28 times more potent in inhibiting MUC5AC secretion than glycyrrhizin with IC50 values of 1.4 and 38 µM, respectively without detectable cytotoxicity. 10µM 7,4'-DHF also significantly inhibited PMA stimulated MUC5AC gene expression whereas a ten-fold higher concentration of glycyrrhizin (100 µM) was required (Lee et al., 2011). Flavonoids are widely distributed secondary metabolites and currently consumed in large amounts in the daily diet with anti-inflammatory, antilipidemic, antihyperglycemic, antiviral, hepatoprotective, gastric antiulcer, cardioprotective, neuroprotective, antioxidant and anticancer actions (Romano et al., 2013). The flavonoids luteolin (Lee et al., 2014), apigenin and wogonin (Kim et al., 2012; Sikder et al., 2014) were reported to significantly inhibit stimulated MUC5AC secretion, production and gene expression at concentrations ranges of 5–20µM via regulation of the NF-κB signaling pathway. In the present study, 7,4'-DHF at 10µM, showed similar MUC5AC inhibitory effects via regulation of not only NF-κB, but also HDAC2 and STAT6 activation.
Among a variety of transcriptional regulators, the NF-κB pathway, which plays a central role in normal innate and adaptive immunes response, also plays an important role in mucin production (Fujisawa et al., 2009). Over expression of NF-κB is associated with a number of chronic inflammatory conditions including asthma (Li and Verma, 2002) . Nuclear translocation of NF-κB depends on the phosphorylation of inhibitory proteins associated with cytoplasmic NF-κB, known as IκBs. Decreased levels of phosphorylated IκB-α (p-IκBα) is an indicator of NF-κB inhibition (Li and Verma, 2002). Another signaling pathway involved in MUC5AC regulation is the signal transducer and activator of transcription 6 (STAT6), which plays a role in both the normal immune response and Th-2 mediated inflammatory diseases such as asthma. Mice deficient in STAT6 are completely protected from ovalbumin-induced goblet cell formation and mucus production (Kuperman et al., 1998). Other stimulus including nitrogen dioxide (Ji et al., 2015), secondhand cigarette smoke (Singh et al., 2011), and mycoplasma pneumonia (Hao et al., 2014) exposure also induce overexpression of airway mucins via the activation of STAT6 pathway in vivo. STAT6 is phosphorylated by Janus kinases (JAKs) after stimulation by the cytokines interleukin-4 (IL-4) and interleukin 13 (IL-13) or PMA (Arinobu et al., 2000). In contrast, histone deacetylase 2 (HDAC-2) suppresses NF-κB activation and loss of HDAC-2 can lead to activation of the NF-κB signaling pathway (Adcock et al., 2005). Knockdown of HDAC2 by HDAC2-specific siRNA prevented the Dex-induced repression of MUC5AC in NHBE and A549 cells (Chen et al., 2012). PMA induced MUC5AC mucus secretion results in part by activation of the mitogen-activated protein kinase superfamily, extracellular-signal regulated kinase 1/2 (ERK1/2) signaling pathway. ERK1/2 has been implicated in MUC5AC up-regulation in asthma (Stewart, 2001; Zassadowski et al., 2012). Our work indicated, PMA up-regulated H292 cells phosphorylation of NF-κB, IκB, STAT6, and ERK1/2, leading to MUC5AC gene expression and mucin protein production/secretion. On the other hand, HDAC-2, which represses MUC5AC gene expression, is inactivated. Our characterization of 7,4'-DHF MUC5AC inhibition shows that it acts, at least partially, through inhibition of both NF-κB and STAT6, as well as activation of HDAC-2 with no effect on ERK1/2.
Previously we found that treatment with 7, 4'-DHF in a murine model of allergic asthma not only significantly reduced eosinophilic pulmonary inflammation, serum IgE levels, IL-4 and IL-13 levels, and increased IFN-γ production in lung cell cultures, but also decreased airways mucus-positive goblet cells in response to antigen stimulation (Yang et al., 2013). In the current study, we treated OVA sensitized/challenged mice with 7, 4'-DHF at 6µg/mL (1.5µM), which was based on our in vitro IC50 concentration that inhibits MUC5AC secretion. We found that 7, 4'-DHF exhibited a significant decrease of approximately 38% of MUC5AC protein in BAL fluids of OVA sensitized mice as compared with a vehicle treated group. These findings provide an important in vivo correlate to our studies performed in vitro and suggest that chronic oral ingestion of 7,4'-DHF can reduce mucus production during allergic lung inflammation.
In conclusion, this study extends our knowledge of G. uralensis flavonoid regulation of MUC5AC protein secretion, production and gene expression. For the first time, we demonstrated that the G. uralensis flavonoid 7,4'-DHF suppressed PMA stimulated epithelial cell MUC5AC secretion 28 times more potently than the G. uralensis triterpenoid, glycyrrhizin without evidence of cellular toxicity. 7,4'-DHF also strongly suppressed MUC5AC production and gene expression, which was at least partially linked to suppression of activation of NF-κB and STAT6 and upregulation of HDAC-2 expression. In a asthma murine model, 7,4'-DHF treated mice exhibited reduced BAL MUC5AC secretion compared with the OVA-induced mice. 7,4'-DHF is a novel component of G. uralensis that regulates MUC5AC gene expression, protein production and secretion and is a potential treatment option for alleviating excess mucus hypersecretion in asthma and other lung diseases.
Acknowledgement
We thank Mr. Henry Ehrlich for reading the manuscript and suggesting changes, and Dr. Hugh A. Sampson for his continued support.
Funding
This study was supported by NIH/NCCAM center grant # 1P01 AT002644725-01 “Center for Chinese Herbal Therapy (CHT) for Asthma”, the Sean Parker Foundation “ASHMI active compounds for asthma therapy”, and the Winston Wolkoff Fund for Integrative Medicine for Allergies and Wellness.
Footnotes
Authors’ disclosure
Dr. Xiu-Min Li shares the patent for the use of ASHMI (PCT/US05/08600 for ASHMI) with Herbal Spring LLC. The other authors have no financial interests to disclose.
Reference List
- Adcock IM, Ito K, Barnes PJ. Histone deacetylation: an important mechanism in inflammatory lung diseases. COPD. 2005;2:445–455. doi: 10.1080/15412550500346683. [DOI] [PubMed] [Google Scholar]
- Arinobu Y, Sugimoto R, Akaiwa M, Arima K, Otsuka T, Hamasaki N, Izuhara K. Augmentation of signal transducer and activation of transcription (STAT)6 and STAT3 expression in stimulated B and T cells. Biochem Biophys Res Commun. 2000;277:317–324. doi: 10.1006/bbrc.2000.3674. [DOI] [PubMed] [Google Scholar]
- Bensky D, Gamble A. Chinese Herbal Medicine: Materia Medica. Seattle: Eastland Press; 1993. [Google Scholar]
- Busse PJ, Schofield B, Birmingham N, Yang N, Wen MC, Zhang T, Srivastava K, Li XM. The traditional Chinese herbal formula ASHMI inhibits allergic lung inflammation in antigen-sensitized and antigen-challenged aged mice. Ann Allergy Asthma Immunol. 2010;104:236–246. doi: 10.1016/j.anai.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerveri I, Brusasco V. Revisited role for mucus hypersecretion in the pathogenesis of COPD. Eur Respir Rev. 2010;19:109–112. doi: 10.1183/09059180.00002710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Watson AM, Williamson CD, Rahimi M, Liang C, Colberg-Poley AM, Rose MC. Glucocorticoid receptor and histone deacetylase-2 mediate dexamethasone-induced repression of MUC5AC gene expression. Am J Respir Cell Mol Biol. 2012;47:637–644. doi: 10.1165/rcmb.2012-0009OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehre C, Ridley C, Thornton DJ. Cystic fibrosis: an inherited disease affecting mucin-producing organs. Int J Biochem Cell Biol. 2014;52:136–145. doi: 10.1016/j.biocel.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans CM, Kim K, Tuvim MJ, Dickey BF. Mucus hypersecretion in asthma: causes and effects. Curr Opin Pulm Med. 2009;15:4–11. doi: 10.1097/MCP.0b013e32831da8d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa T, Velichko S, Thai P, Hung LY, Huang F, Wu R. Regulation of airway MUC5AC expression by IL-1beta and IL-17A; the NF-kappaB paradigm. J Immunol. 2009;183:6236–6243. doi: 10.4049/jimmunol.0900614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Kuang Z, Jing J, Miao J, Mei LY, Lee RJ, Kim S, Choe S, Krause DC, Lau GW. Mycoplasma pneumoniae modulates STAT3-STAT6/EGFR-FOXA2 signaling to induce overexpression of airway mucins. Infect.Immun. 2014;82:5246–5255. doi: 10.1128/IAI.01989-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewson CA, Edbrooke MR, Johnston SL. PMA induces the MUC5AC respiratory mucin in human bronchial epithelial cells, via PKC, EGF/TGF-alpha, Ras/Raf, MEK, ERK and Sp1-dependent mechanisms. J Mol Biol. 2004;344:683–695. doi: 10.1016/j.jmb.2004.09.059. [DOI] [PubMed] [Google Scholar]
- Jayaprakasam B, Doddaga S, Wang R, Holmes D, Goldfarb J, Li XM. Licorice flavonoids inhibit eotaxin-1 secretion by human fetal lung fibroblasts in vitro. J Agric Food Chem. 2009;57:820–825. doi: 10.1021/jf802601j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X, Han M, Yun Y, Li G, Sang N. Acute nitrogen dioxide (NO2) exposure enhances airway inflammation via modulating Th1/Th2 differentiation and activating JAK-STAT pathway. Chemosphere. 2015;120:722–728. doi: 10.1016/j.chemosphere.2014.10.039. [DOI] [PubMed] [Google Scholar]
- Kelly-Pieper K, Patil SP, Busse P, Yang N, Sampson H, Li XM, Wisnivesky JP, Kattan M. Safety and tolerability of an antiasthma herbal Formula (ASHMI) in adult subjects with asthma: a randomized, double-blinded, placebo-controlled, dose-escalation phase I study. J Altern Complement Med. 2009;15:735–743. doi: 10.1089/acm.2008.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JO, Sikder MA, Lee HJ, Rahman M, Kim JH, Chang GT, Lee CJ. Phorbol ester or epidermal growth-factor-induced MUC5AC mucin gene expression and production from airway epithelial cells are inhibited by apigenin and wogonin. Phytother Res. 2012;26:1784–1788. doi: 10.1002/ptr.4650. [DOI] [PubMed] [Google Scholar]
- Kraft M, Adler KB, Ingram JL, Crews AL, Atkinson TP, Cairns CB, Krause DC, Chu HW. Mycoplasma pneumoniae induces airway epithelial cell expression of MUC5AC in asthma. Eur Respir J. 2008;31:43–46. doi: 10.1183/09031936.00103307. [DOI] [PubMed] [Google Scholar]
- Kuperman D, Schofield B, Wills-Karp M, Grusby MJ. Signal transducer and activator of transcription factor 6 (Stat6)-deficient mice are protected from antigen-induced airway hyperresponsiveness and mucus production. J.Exp.Med. 1998;187:939–948. doi: 10.1084/jem.187.6.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Lee SY, Bae HS, Kim JH, Chang GT, Seok JH, Lee CJ. Inhibition of airway MUC5AC mucin production and gene expression induced by epidermal growth factor or phorbol ester by glycyrrhizin and carbenoxolone. Phytomedicine. 2011;18:743–747. doi: 10.1016/j.phymed.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Seo HS, Ryu J, Yoon YP, Park SH, Lee CJ. Luteolin inhibited the gene expression, production and secretion of MUC5AC mucin via regulation of nuclear factor kappa B signaling pathway in human airway epithelial cells. Pulm Pharmacol Ther. 2014 doi: 10.1016/j.pupt.2014.09.008. (in press) [DOI] [PubMed] [Google Scholar]
- Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Nishimoto Y, Hisatsune A, Katsuki H, Miyata T, Yokomizo K, Isohama Y. Glycyrrhizin attenuates mucus production by inhibition of MUC5AC mRNA expression in vivo and in vitro. J Pharmacol Sci. 2010;113:76–83. doi: 10.1254/jphs.09344fp. [DOI] [PubMed] [Google Scholar]
- Rogers DF. Physiology of airway mucus secretion and pathophysiology of hypersecretion. Respir Care. 2007;52:1134–1146. [PubMed] [Google Scholar]
- Romano B, Pagano E, Montanaro V, Fortunato AL, Milic N, Borrelli F. Novel insights into the pharmacology of flavonoids. Phytother Res. 2013;27:1588–1596. doi: 10.1002/ptr.5023. [DOI] [PubMed] [Google Scholar]
- Shao MX, Nakanaga T, Nadel JA. Cigarette smoke induces MUC5AC mucin overproduction via tumor necrosis factor-alpha-converting enzyme in human airway epithelial (NCI-H292) cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L420–L427. doi: 10.1152/ajplung.00019.2004. [DOI] [PubMed] [Google Scholar]
- Sikder MA, Lee HJ, Ryu J, Park SH, Kim JO, Hong JH, Seok JH, Lee CJ. Apigenin and Wogonin Regulate Epidermal Growth Factor Receptor Signaling Pathway Involved in MUC5AC Mucin Gene Expression and Production from Cultured Airway Epithelial Cells. Tuberc Respir Dis (Seoul) 2014;76:120–126. doi: 10.4046/trd.2014.76.3.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SP, Gundavarapu S, Pena-Philippides JC, Rir-Sima-ah J, Mishra NC, Wilder JA, Langley RJ, Smith KR, Sopori ML. Prenatal secondhand cigarette smoke promotes Th2 polarization and impairs goblet cell differentiation and airway mucus formation. J.Immunol. 2011;187:4542–4552. doi: 10.4049/jimmunol.1101567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava KD, Dunkin D, Liu C, Yang N, Miller RL, Sampson HA, Li XM. Effect of Antiasthma Simplified Herbal Medicine Intervention on neutrophil predominant airway inflammation in a ragweed sensitized murine asthma model. Ann Allergy Asthma Immunol. 2014;112:339–347. doi: 10.1016/j.anai.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AG. Airway wall remodelling and hyperresponsiveness: modelling remodelling in vitro and in vivo. Pulm Pharmacol Ther. 2001;14:255–265. doi: 10.1006/pupt.2001.0290. [DOI] [PubMed] [Google Scholar]
- Wang R, Liu C, Xia L, Zhao G, Gabrilove J, Waxman S, Jing Y. Ethacrynic acid and a derivative enhance apoptosis in arsenic trioxide-treated myeloid leukemia and lymphoma cells: the role of glutathione S-transferase p1-1. Clin Cancer Res. 2012;18:6690–6701. doi: 10.1158/1078-0432.CCR-12-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Patil S, Zhuge J, Wen MC, Bolleddula J, Doddaga S, Goldfarb J, Sampson HA, Li XM. Glycyrrhiza uralensis flavonoids present in anti-asthma formula, ASHMI, inhibit memory Th2 responses in vitro and in vivo. Phytother Res. 2013;27:1381–1391. doi: 10.1002/ptr.4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zassadowski F, Rochette-Egly C, Chomienne C, Cassinat B. Regulation of the transcriptional activity of nuclear receptors by the MEK/ERK1/2 pathway. Cell Signal. 2012;24:2369–2377. doi: 10.1016/j.cellsig.2012.08.003. [DOI] [PubMed] [Google Scholar]
- Zhang T, Srivastava K, Wen MC, Yang N, Cao J, Busse P, Birmingham N, Goldfarb J, Li XM. Pharmacology and immunological actions of a herbal medicine ASHMI on allergic asthma. Phytother Res. 2010;24:1047–1055. doi: 10.1002/ptr.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]