Abstract
Synapsin is a phosphoprotein reversibly associated with synaptic vesicles. We investigated synapsin function in mediating synaptic activity during intense stimulation at Drosophila motor boutons. Electron microscopy analysis of synapsin(−) boutons demonstrated that synapsin maintains vesicle clustering over the periphery of the bouton. Cyclosporin A pretreatment disrupted peripheral vesicle clustering, presumably due to increasing synapsin phosphorylated state. Labeling recycling vesicles with a fluorescent dye FM1-43 followed by photoconversion of the dye into electron dense product demonstrated that synapsin deficiency does not affect mixing of the reserve and recycling vesicle pools but selectively reduces the size of the reserve pool. Intense stimulation produced a significant increase in vesicle abundance and vesicle redistribution toward the central core of synapsin (+) boutons, while in synapsin (−) boutons the area occupied by vesicles did not change and the increase in vesicle numbers was not as prominent. However, intense stimulation produced an increase in basal release at synapsin(−) but not in synapsin(+) boutons, suggesting that synapsin may direct vesicles to the reserve pool. Finally, synapsin deficiency inhibited an increase in quantal size and formation of endosome-like cisternae, which was activated either by intense electrical stimulation or by high K+ application. Taken together, these results elucidate a novel synapsin function, specifically, promoting vesicle reuptake and reserve pool formation upon intense stimulation.
INTRODUCTION
The question of how neuronal terminals maintain synaptic transmission during intense neuronal stimulation remains fascinating and controversial. It is now understood that synaptic vesicles undergo a series of preparatory steps to be properly activated for neurotransmitter release, and, according to their functional state, vesicles can be subdivided into several functional pools (Neher, 1998 and Schneggenburger et al., 2002 for review). The recycling pool maintains exo/endocytosis at moderate stimulation paradigms, and it is refilled by newly recycled vesicles, while the reserve pool is a depot of synaptic vesicles which contribute to release only during intense stimulation (Rizzoli and Betz, 2005 for review). Multiple pathways have been proposed for recycling of synaptic vesicles upon exocytosis, but the relationship between these pathways and functional vesicle pools is not fully understood.
At the majority of synapses, the recycling and reserve pools are spatially intermixed (Rizzoli and Betz, 2005 for review). However, at Drosophila Ib type motor boutons, optical studies relying on vesicle staining with FM1-43 dye lead to a view that the recycling pool occupies the periphery of the bouton and the reserve pool is spread towards its central core (Kidokoro et al., 2004; Kuromi and Kidokoro, 2005 for review; Verstreken et al., 2005). This view has been recently revised by two ultrastructural studies (Akbergenova and Bykhovskaia, 2009a; Denker et al., 2009) demonstrating that at this synapse vesicles generally cluster over the periphery of the bouton, but the reserve and recycling pools are spatially intermixed. Furthermore, it was demonstrated that intense stimulation produces an increase in vesicle abundance, as well as vesicle redistribution towards the central core of the bouton (Akbergenova and Bykhovskaia, 2009a). This form of plasticity was associated with an increased synaptic enhancement during a subsequent stimulation.
Vesicle recycling may follow different routs or pathways, including active zone recycling, clathrin-mediated endocytosis, endosomal intermediates, or bulk membrane uptake (Wu et al, 2007; Smith et al., 2008; Cousin 2009 for review). An intense synaptic activity is often associated with the recycling pathway that involves a formation of endosome-like structures or cisternae (Heuser and Reese, 1973; Takei et al., 1996; Marxen et al., 1999; Richards et al., 2000; Teng and Wilkinson, 2000; Holt et al., 2003; de Lange et al., 2003). Recently, it was recognized that endosome-like structures may be capable of exocytosis (Coggins et al., 2007), and that this process may produce enlarged neurosecretory quanta (He et al., 2009; Akbergenova and Bykhovskaia, 2009b). Thus, formation of endosome-like cisternae and enlarged vesicles is likely to represent a pathway that enhances synaptic efficacy.
In summary, a neuronal terminal may sustain an intense stimulation by mobilizing reserve vesicles into the recycling pathway, by formation of extra vesicles, or by formation of enlarged vesicles and quanta. Our goal in this study was to understand how these mechanisms are regulated by a synaptic vesicle protein synapsin.
Synapsins, abundant and highly conserved family of phoshoproteins reversibly associated with vesicles (Greengard et al., 1993 for review), were implicated in maintaining the reserve pool (Bloom et al., 2003; Akbergenova and Bykhovskaia, 2007; Gitler et al., 2008) and regulating mobilization of vesicles into the recycling pool (Chi et al., 2003; Cousin et al., 2003; Menegon et al., 2006; Baldelli et al., 2007). In their dephosphorylated form, synapsins attach to synaptic vesicles and trigger actin polymerization (Bloom et al., 2003), while synapsin phosphorylation causes its dissociation from vesicles (Hosaka et al., 1999). Synapsin-dependent regulation of the reserve and recycling vesicle pools was found to be critical during sustained stimulation (Humeau et al., 2001). Consistently, synapsin deficiency produces enhanced synaptic depression (Rosahl et al., 1995; Gitler et al.2004, Samigullin et al., 2004).
To investigate the role of synapsin in activity dependent plasticity at the Drosophila motor boutons, we took advantage of the synapsin knockout (synapsin(−)) fly (Godeneschwege et al., 2004) that was shown to have defects in complex behavior, learning and memory (Michels et al., 2005). We have investigated how synaptic ultrastructure and activity is regulated upon intense stimulation in synapsin (−) boutons.
EXPERIMENTAL PROCEDURES
Preparations and chemicals
A WT strain of Drosophila melanogaster, Canton S, a single gene mutant of Drosophila (Godenschwege et al., 2004) Syn97 (synapsin(−)), and the rescue transgenic line (Lohr et al., 2002) were used in this study. Experiments were performed on Ib boutons (Lnenicka and Keshishian, 2000; Dasari and Cooper, 2004) of the muscles 6 and 7 of abdominal segments 2, 3 or 4 of the third instar larvae. Preparations were dissected in physiological solution containing (in mM) 130 NaCl, 36 sucrose, 5 KCl, 2 CaCl2, 2 MgCl2, and 5 HEPES, pH 7.3. (Jan and Jan 1976), pinned to sylgard, cut open along the dorsal midline, and internal organs were removed to expose the nerves and the muscles. In cyclosporin A pretreated preparations, cyclosporin A (20 μM, A.G.Scientific Laboratories) was applied for 20 minutes and washed.
Electron microscopy
Preparations were fixed in 1% glutaraldehyde/ 4% paraformaldehyde in 0.1 M cocodylate buffer for 2 hours at room temperature and then incubated at 4°C overnight. After washing in 0.1 M cocodylate buffer with 0.1 M sucrose added, samples were post-fixed for 1 hour in 1% osmium tetroxide, dehydrated through a graded series of ethanol and acetone, and embedded in Embed 812 epoxy resin (Electron Microscopy Sciences). Thin sections (70-90 nm) were collected on Formvar coated single slot grids and contrasted with lead citrate. Samples were examined on a Phillips 420 transmission electron microscope at 100 kV. To ascertain that only Ib type boutons were analyzed, we selected micrographs showing boutons of at least 2 μm diameter with small (approximately 30 nm diameter) clear vesicles (Atwood et al., 1993, Jia et al., 1993). In action potential stimulated preparations, we labeled the stimulated segment with a cactus needle, which was always clearly seen in the tissue fixed for electron microscopy (EM), and thus the stimulated segment was readily identified in the semi-thin slices and in the micrographs. Micrographs were analyzed employing ImageJ 1.36b software (NIH) and Photoshop 9.0 as described in (Akbergenova and Bykhovskaia, 2009a). Endosome-like structures were identified as described in (Akbergenova and Bykhovskaia, 2009).
FM1-43 staining
FM1-43 (2.5 μM, Molecular Probes) was loaded in the presynaptic boutons during the stimulation. After the dye loading, preparations were briefly washed in Ca2+ free solution containing 75 μM Advasep-7 (Biotium) and then rinsed in physiological saline without Advasep-7. Application of Advasep-7 (Kay et al., 1999) reduced background fluorescence and sharpened the borders of the boutons.
Synapsin immunostaining
Preparations were fixed for 20 minutes in 4% formaldehyde, washed for 15 minutes in PBS (pH 7.6) and incubated in blocking solution containing 0.1% Tween-20 for 30 minutes. Then preparations were incubated overnight at 4°C in primary mouse anti-Drosophila antibody (3C11 (anti SYNORF1) from The Developmental Studies Hybridoma Bank at the University of Iowa, 1:10 dilution), washed, and then incubated for two hours in Alexa Fluor 488 (Molecular Probes) secondary antibody (1:100 dilution).
Confocal imaging
All the stained boutons were imaged with identical settings using a Zeiss FS2 microscope with a x63 water immersion objective (0.95 NA) connected to a real-time confocal unit (Ultraview, PerkinElmer Life Sciences) equipped with a CCD camera (ORCA ER, Hamamatsu). Fluorescence was exited at 488 nm Kr/Ar laser wavelength, and a 500 nm long-pass emission filter was used for detection. Z series were taken at 0.3 Pm steps to image the entire bouton. To ascertain that we collect the data from only one type of the boutons (Ib), only large boutons (3 Pm diameter or larger) were analyzed. The quantitative analysis of confocal stacks was performed as described in our earlier study (Akbergenova and Bykhovskaia, 2007).
Photoconversion of the dye FM1-43
Photoconversion procedure was performed as described in (Akbergenova and Bykhovskaia, 2009b). Briefly, preparations were fixed for 15 minutes in a regular EM fixative, washed for 30 min in physiological saline, preincubated in DAB (1.5 mg/ml in physiological saline) for 10 min, and illuminated for 20 min under x63 water immersion objective using a mercury lamp with 485±10 bandpass excitation filter. We have found that a longer illumination does not produce any further increase in the photoconversion. At this point, fluorescence staining was completely bleached, and DAB reaction product was visible. After a brief superfusion with a physiological saline, the illuminated site was marked by a cactus needle to be identified in subsequent EM analysis. Then the preparations were left overnight in a regular EM fixative and processed as for conventional EM.
Recordings and quantal analysis of postsynaptic responses
The nerve was stimulated via suction electrode, and synaptic responses were recorded focally from the boutons visualized with DIC optics using macropatch electrodes of 5-10 μm tip diameter. The electrodes were manually bent to enable recordings under x63 magnification water immersion objective (Zeiss) with 1.8 mm working distance. Recordings were digitized with Digidata A/D board and Axoscope software (Axon Instruments) and analyzed off-line. Quantal content of synaptic responses was determined as described earlier (Akbergenova and Bykhovskaia, 2007) employing in-house software (Bykhovskaia, 2008). To determine quantal size, spontaneous activity was recorded for five minutes at each recording site.
Statistical analysis
Datasets were compared employing two-sided t-test and one-way ANOVA
RESULTS
Synapsin maintains peripheral vesicle distribution but does not affect mixing of the reserve and recycling pools
Earlier optical studies (Kuromi and Kidokoro, 1998, 1999) demonstrated that when the recycling vesicle pool was stained employing the uptake of the dye FM1-43 during a mild stimulation (1-3 Hz frequency), the staining occurred predominantly over the periphery of the bouton. Furthermore, it was demonstrated (Akbergenova and Bykhovskaia, 2007) that in synapsin null mutants a similar FM1-43 loading paradigm produced more even distribution of the dye, and FM1-43 staining spread towards the center of the bouton. These results were interpreted as a peripheral distribution of the recycling pool and more central distribution of the reserve pool (Kidokoro et al., 2004 for review), and it was suggested that this spatial segregation of the recycling and reserve pools is maintained by synapsin (Akbergenova and Bykhovskaia, 2007). However, two subsequent studies (Akbergenova and Bykhovskaia, 2009a; Denker et al., 2009) demonstrated that the entire pool of synaptic vesicles is distributed predominantly over the periphery of wild type (WT) Ib boutons, with the central core of the bouton being largely devoid of vesicles. Thus, to determine conclusively the role of synapsin in the distribution and mixing of vesicle pools, we performed EM analysis of synapsin (−) Ib type boutons coupled with a photoconversion of the FM1-43 dye.
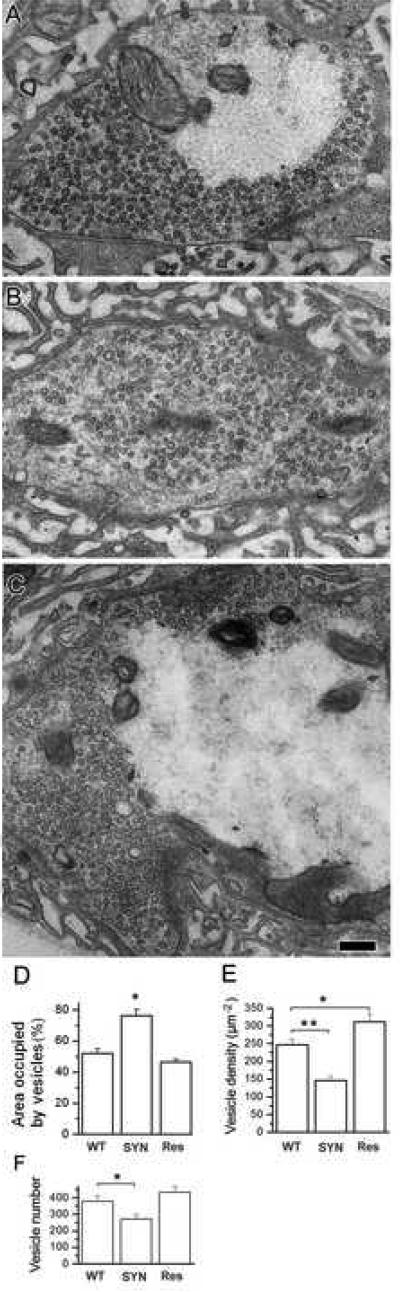
We found that in synapsin (−) boutons, unlike WT, vesicles were spread towards the center of the bouton, and void areas were significantly diminished (Fig. 1 A, B); the peripheral vesicle distribution was restored in the rescue line (Fig. 1 C). This observation was confirmed by morphometric analysis, which demonstrated that vesicles occupied 52±2.9% of the bouton area in WT, 76±4.2% in synapsin (−), and 47±1.9% in the rescue mutant (Fig. 1 D). In addition, vesicle density in synapsin (−) boutons was reduced (Fig. 1 E) and the total number of vesicles per bouton was significantly diminished (Fig. 1 F).
Figure 1. Synapsin maintains the distribution of vesicles over the periphery of the bouton.
A-C Representative micrographs showing vesicle distribution in type Ib boutons of WT (A), synapsin (−) (B) and rescue (C) genotypes. Note a central core devoid of vesicles in WT and rescue, but not in syanpsin (−) boutons.
D-F. The results of a morphometric analysis show that the area occupied by vesicles in synapsin (−) boutons significantly exceeds that of WT or rescue (Res) boutons (D), while the vesicle density (E) and vesicle numbers (F) are reduced in synapsin (−) boutons. For each line, 3 larvae (22-27 boutons) were used to collect the data.
This result suggests reinterpretation of previous findings. Specifically, it suggests that synapsin maintains peripheral vesicle clustering rather than a segregation of the reserve and recycling pools. To address this issue conclusively, we performed photoconversion of the dye FM1-43 into electron dense product after dye loading at a mild stimulation paradigm. This approach enabled us to discern the vesicles that did uptake the dye (lumens dark due to the dye uptake, Fig. 2 A,B), and those that did not uptake the dye (translucent, Fig. 2 A,B). We have demonstrated earlier (Akbergenova and Bykhovskaia, 2009a) that the above stimulation paradigm produces approximately 30% of dark vesicles in WT boutons, suggesting that the recycling pool in our preparation may constitute approximately 30% of all the vesicles. It is important to note that the photoconversion rate in our experiments is likely to be close to 100%, since our study cited above showed that over 90% of all the vesicles in the terminal had dark lumens after the nerve was stimulated at a high frequency in the presence of FM1-43.
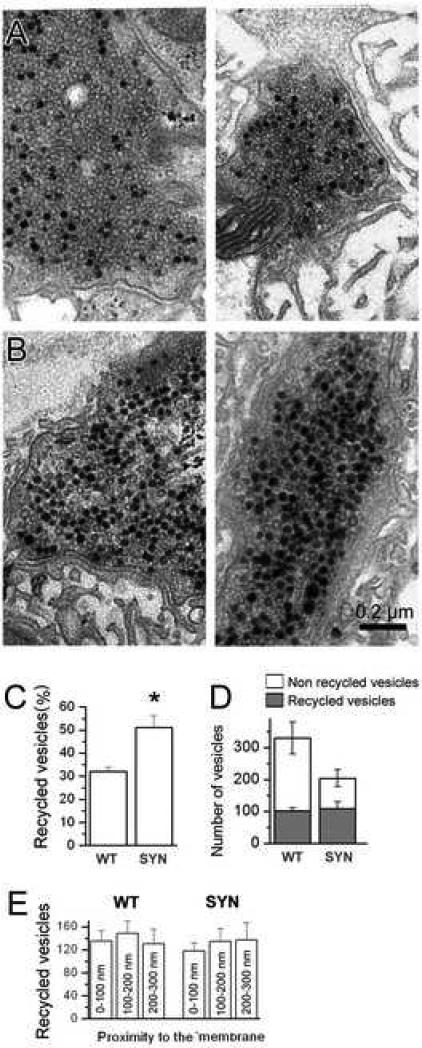
Figure 2. Synapsin does not affect the spatial mixing between the reserve and recycling pools but decreases the proportion of recycling vesicles.
A,B. Representative micrographs showing recycling (black due photoconverted FM1-43) and reserve (white lumens) vesicles in WT (A) and synapsin (−) (B) boutons.
C. The proportion of recycling vesicles is significantly increased in synapsin (−) (SYN) boutons.
D. The size of the recycling pool is not affected by synapsin gene deletion, while the size of the reserve pool and, consequentially, the total pool of vesicles, is diminished.
E. Spatial mixing between reserve and recycling vesicles is not affected by synapsin. The graph shows the number of recycling vesicles observed in the vicinity of synaptic membrane (within 100 nm), and inside the terminal (within 200 or 300 nm from the synaptic membrane). The vesicles are evenly distributed in both genotypes. For reader's convenience, this panel includes the vesicle distribution at WT synapses reported in (Akbergenova and Bykhovskaia, 2009a). For each line, 3 preparations (11-13 boutons) were used to collect the data.
We have loaded the dye FM1-43 during a stimulation at a 3 Hz frequency for 5 min and processed the preparations for photoconversion and a subsequent EM analysis. We found that in synapsin (−) boutons the proportion of dark vesicles was significantly higher than in WT (Fig. 2, A-C), suggesting a higher proportion of recycling vesicles. Notably, the total number of dark (recycling) vesicles in synapsin (+) and synapsin (−) did not significantly differ (Fig. 2 D), although the total number of vesicles in synapsin (−) boutons was diminished. Furthermore, synapsin did not affect spatial mixing of the reserve and recycling pools (Fig. 2). Indeed, in both WT and synapsin (−) boutons the reserve and recycling pools were uniformly distributed, and no preferential peripheral location of recycling vesicles was detected in either phenotype (Fig. 2 E). This result suggests that in our preparation synapsin deficiency selectively affects the size of the reserve pool of vesicles, without affecting either the size of the recycling pool or mixing the pools.
Thus, our results demonstrate that synapsin maintains the reserve pool of vesicles, as well as peripheral vesicle clustering. To elucidate the specific mechanism of the latter synapsin action, we employed synapsin immunolabeling to examine its distribution (Fig. 3A). Synapsin localization was compared to the vesicle distribution assessed by FM1-43 labeling at mild stimulation paradigms (Fig. 3 B). In both cases (Fig. 3 A, B), a peripheral distribution of fluorescence was observed. This result suggests that synapsin may co-localize with vesicles and tether them in a cluster over the periphery of the bouton.
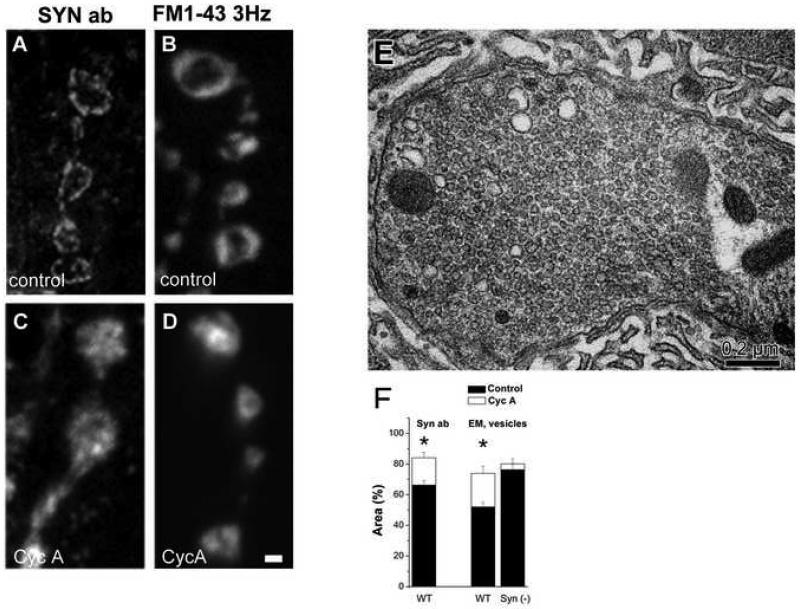
Figure 3. The peripheral distribution of both synapsin and vesicles is disrupted after CycA pretreatment.
A-D. Immunolabeling for synapsin (A) shows that it is distributed over the periphery of the bouton, similar to FM1-43 staining (B) at a mild stimulation paradigm (5 min at 3 Hz). After CycA pretreatment, both synapsin (C) and FM1-43 (D) fluorescence becomes distributed over the entire bouton, spreading towards its central core.
E. A representative electron micrograph showing a bouton after CycA pretreatment and mild stimulation (5 min at 3 Hz). Note that vesicles are distributed over the entire bouton, including its central core.
F. Quantitative analysis shows that after a CycA treatment followed by a mild stimulation, synapsin (left column) or vesicles disperse and occupy larger areas than at rest. This does not occure in synapsin (−) boutons (right column). Synapsin immunostaining data are collected from 8 CycA untreated preparations (31 bouton) for and 5 CycA treated preparations (18 boutons). EM data collected from 3 preparations (15-20 boutons) for each line at each condition.
To test this hypothesis further, we assessed the effect of calcineurin inhibitor cyclosporin A (CycA) on the distribution of synapsin and vesicles. Earlier optical studies have demonstrated that CycA pretreatment produces FM1-43 staining of entire boutons, including their central cores, even during mild stimulation paradigms (Kuromi and Kidokoro, 1999; Akbergenova and Bykhovskaia, 2007); this CycA action was ascribed to a mobilization of the reserve pool into the recycling pathway. However, CycA treatment was also shown to increase synapsin phosphorylated state (Jovanovic et al., 2001). Thus, CycA effect could be also ascribed to an increase in synapsin phosphorylated forms and, consequentially, its dispersion from vesicles (Hosaka at al. 1999). To test this suggestion, we employed synapsin immunolabeling of CycA pretreated preparations.
We found that CycA pretreatment disrupted the peripheral distribution of synapsin (Fig. 3 C, F). The distribution of synapsin and the distribution of vesicles stained by FM1-43 at a mild stimulation paradigm (5 min at a 3 Hz frequency) were similar in CycA treated preparations (Fig. 3 C,D). This result suggests that CycA treatment disrupts the peripheral distribution of both synapsin and vesicles. To confirm this at the ultrastructural level, we performed EM analysis of CycA treated and mildly stimulated preparations. We found that, indeed, in these preparations peripheral vesicle distribution was disrupted (Fig. 3 E), and vesicles occupied a significantly larger area than in control preparations (Fig. 3 F). Interestingly, the effect of CycA described above was only observed when CycA pretreatment was followed by nerve stimulation. The CycA treatment alone had no effect on vesicle distribution (data not shown), suggesting that the CycA produced vesicle dispersion is activity-dependent. Importantly, this CycA-produced dispersion was not observed in synapsin (−) boutons (Fig. 3 F), where vesicles occupy over 80% of the bouton at rest, and the area occupied by vesicles does not further increase upon the treatment.
Taken together, our results suggest that a calcineurin-dependent synapsin phosphorylation/dephosphorylation cycle may regulate activity dependent dispersion/clustering of synaptic vesicles.
Synaptic potentiation during high-frequency stimulation is synapsin-dependent
Next, we tested whether synapsin would affect the ability of the nerve terminal to potentiate in response to intense activity. Our recent study (Akbergenova and Bykhovskaia, 2009a) demonstrated that an intense stimulation (10 Hz for 15 min followed by a 10 min rest) produces a structural potentiation, i.e. formation of extra vesicles and their distribution towards the central core of Ib boutons. It could be suggested that areas devoid of vesicles may serve as a reserve space for a potential increase in vesicle numbers. In this case, we would expect that the structural potentiation would be disrupted in synapsin (−) mutants.
To test this suggestion, we employed the potentiation paradigm (15 min at 10 Hz + 10 min rest) and processed preparations for EM (Fig. 4, A,B). We found that the increase in vesicle numbers upon intense stimulation was substantially diminished in the absence of synapsin (from 59 to 27%, Fig. 4 C), although it remained statistically significant. Furthermore, in synapsin (−) boutons, the area occupied by vesicles did not increase upon stimulation (Fig. 4 D). These results contrast to the observations made for WT boutons, where the area occupied by vesicles significantly increased upon potentiation. These results may indicate that the lack of peripheral vesicle organization in synapsin (−) boutons may compromise their ability to increase vesicle numbers in response to an intense stimulation.
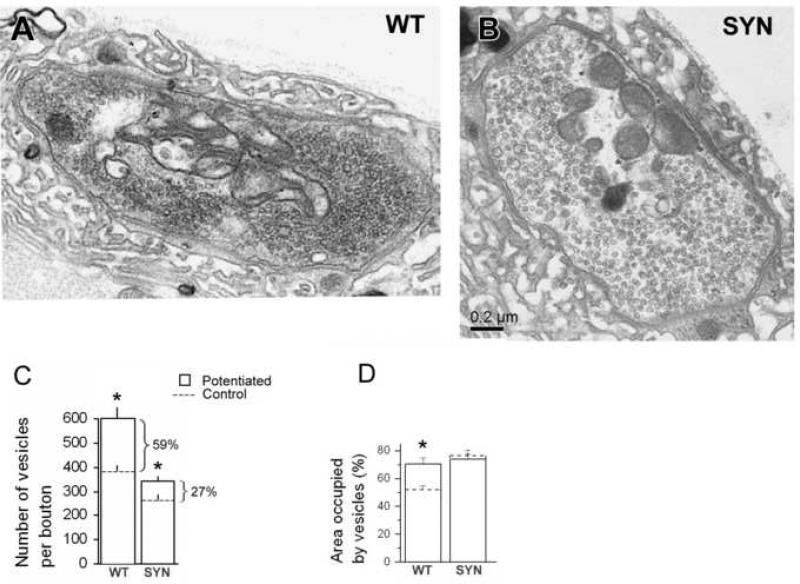
Figure 4. Synapsin promotes activity-dependent vesicle formation.
A,B. Representative micrographs showing WT (A) and synapsin (−) boutons (B) fixed after employing the potentiation paradigm (15 min at 10 Hz + 10 min rest). Note that in both genotypes vesicles are dispersed largely over entire boutons.
C. Vesicle numbers significantly increased upon potentiation in both genotypes, but in WT this increase is more prominent. Asterisks indicate significant (p<0.05) difference between resting (control) and potentiated states.
D. Area occupied by vesicles increased upon potentiation in WT but not in synapsin (−) (SYN) boutons. Asterisk indicates significant (p<0.05) difference between resting (control) and potentiated states. Data collected from 3 preparations (17-20 boutons) for each line at each condition.
Since it is likely that a major supply source of extra vesicles upon intense stimulation is excess endocytosis (Akbergenova and Bykhovskaia, 2009a), we explored the balance between endo- and exocytosis in synapsin (−) boutons by examining the rundown of synaptic activity during prolonged continuous stimulation (Fig. 5). We found that during prolonged stimulation synaptic activity decays significantly faster in the absence of synapsin, and this effect is reversed in the rescue transgenic line (Fig. 5 B, C). To further explore the balance between exo- and endocytosis, we compared the activity rundown in untreated and bafilomycin-treated (10 μM applied for 20 min prior to stimulation, Akbergenova and Bykhovskaia, 2007) preparations (Fig. 6 A). Bafilomycin (Baf) is a selective blocker of activity-dependent vesicle refilling (Amara and Kuhar, 1993; Cousin and Nichols, 1997; Kuromi and Kidokoro, 2000; Cavelier and Attwell, 2007), therefore the difference between the activity in treated and untreated preparations (Fig. 6 B) can serve as a measure of endocytic vesicle replenishment during continuous activity. Figure 6 A shows the magnitude of the synaptic response, as the nerve is continuously stimulated in the presence (triangles) or absence of Baf (squares). The difference between the responses in Baf-untreated and Baf-treated preparations is presented in Fig. 6 B, and it reflects the replenishment of the recycling pool, since the replenishment affects the synaptic response in Baf-untreated but not in Baf-treated preparations. In both lines, the replenishment initially overshoots the rundown, and that produces an initial increase in activity beyond the basal level (Fig. 6, synaptic responses (A, squares) and replenishment (B) exceed 100%). However, at synapsin (−) terminals this initial overshoot is relatively brief, and after 4-6 minute stimulation it is followed by a steady decay in the vesicle replenishment (Fig. 6 B, open circles). In contrast, the replenishment in WT terminals remains steady (Fig. 6B, solid circles), and this enables the terminals to maintain a steady activity that consistently exceeds the baseline. Thus, it appears that the process of vesicle replenishment occurs in two phases, with the initial phase (within initial 5 min) being synapsin-independent, and with a slower continuous phase requiring the presence of synapsin.
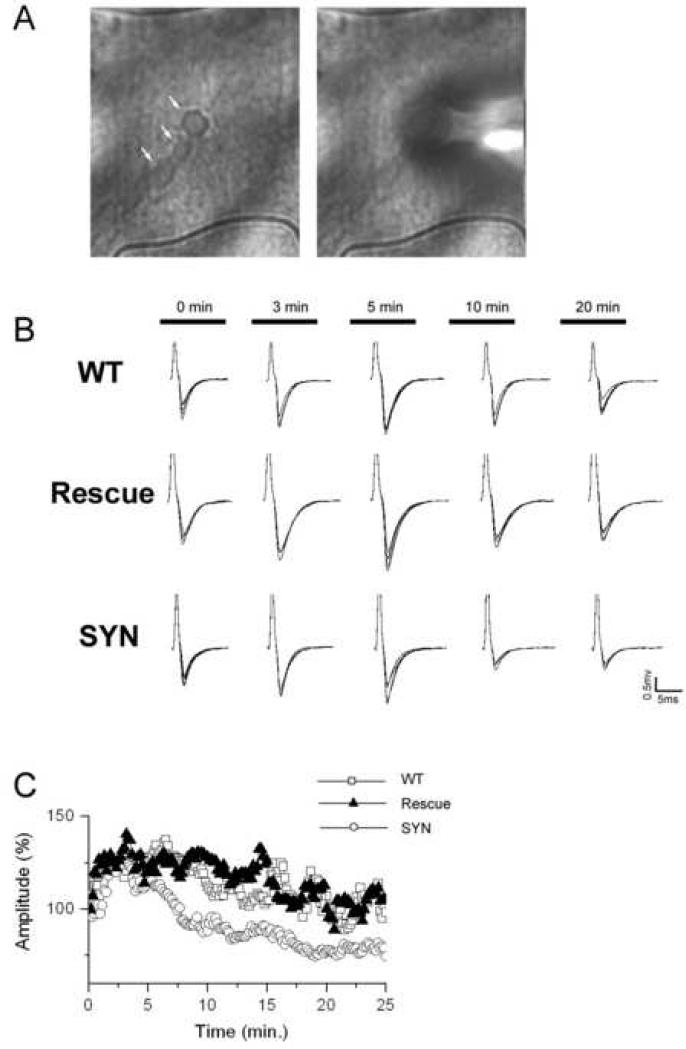
Figure 5. Synapsin is required for maintaining synaptic activity during sustained stimulation.
A. Recordings of synaptic activity. A string of boutons (left, arrows) and the macropatch electrode positioned over a bouton (right).
B. EPSPs recorded in the begining of stimulation (10 Hz) and after 3, 5, 10, and 20 min of sustained stimulation. Note a decrease in the EPSP size in the synapsin (−) mutant.
C. Synaptic depression is significantly enhanced in synapsin (−) boutons (SYN).
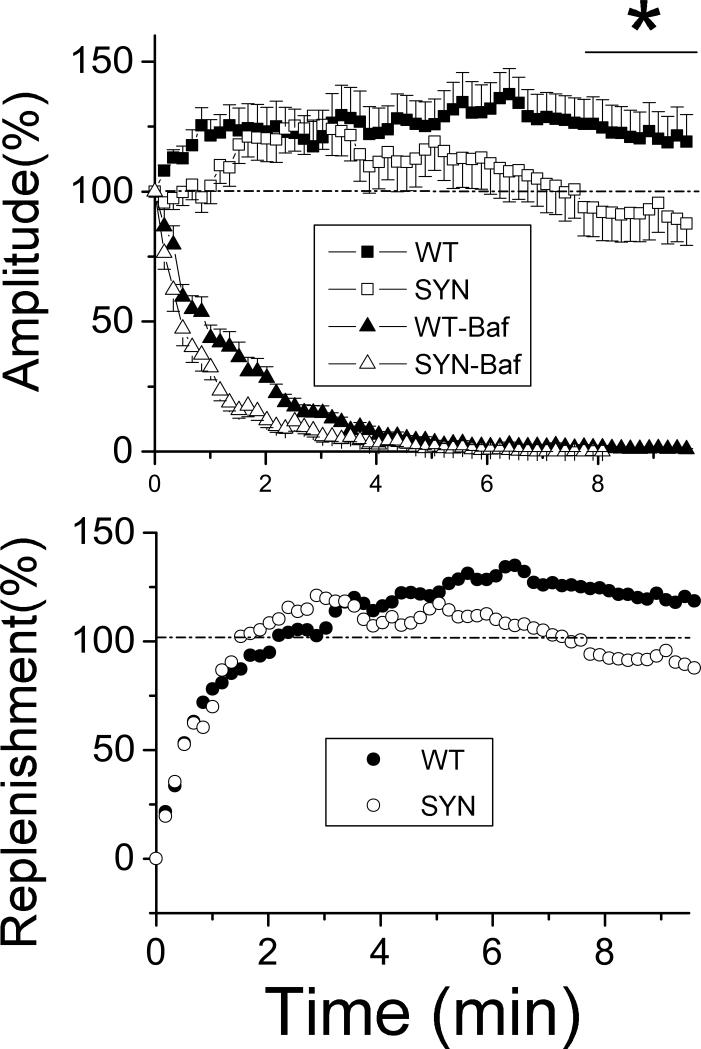
Figure 6. Vesicle replenishment during sustained activity is compromised in synapsin (−) boutons.
A. Synaptic activity during continuous stimulation at bafilomycin-treated (Baf, triangles) versus untreated (squares) preparations demonstrates that synapsin deficiency produces a rundown of activity after 4-6 minutes of stimulation, at almost complete decay of activity at Baf-treated preparations, thus suggesting that synapsin affects vesicle replenishment. Data collected from 21-29 preparations for each line at each condition. The asterisk indicates significant difference between the WT and Syn lines over the outlined time interval.
B. The rate of vesicle replenishment derived as a difference of activity at Baf-untreated versus Baf-treated preparations. The rate of replenishment initially overshoots the basal activity both lines, and subsequently decays in synapsin (−) but not in synapsin (+) preparations.
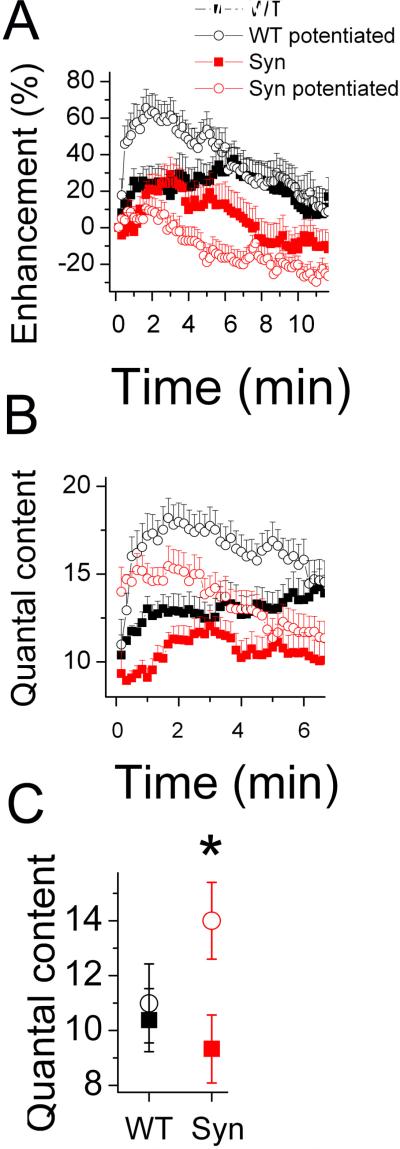
Since the stimulation-dependent vesicle supply produces a functional potentiation, that is manifested as an increased synaptic enhancement in potentiated boutons (Akbergenova and Bykhovskaia, 2009a), we tested whether this potentiation would occur in synapsin(−) boutons. We potentiated the terminals employing the (15 min at 10 Hz + 10 min rest) stimulation paradigm, and then recorded synaptic activity from potentiated and non-potentiated boutons during a continuous stimulation at a 10 Hz or 3 Hz frequency (Fig. 7). In WT boutons, a strong synaptic enhancement was observed in potentiated boutons during the stimulation at a 10 Hz frequency, compared to naive boutons (Fig. 7 A, B, back open circles versus black solid squares). However, the potentiation paradigm did not modify basal quantal release recorded at a 3 Hz frequency (Fig. 7, C). In contrast, synapsin (−) mutants showed a significant increase in basal quantal release but no synaptic enhancement after the potentiation paradigm (Fig. 6, red open circles versus red solid squares).
Figure 7. Potentiation affects basal release in synapsin (−) but not in synapsin(+) boutons, and it increases synaptic enhancement during a subsequent stimulation in synapsin(+) but not in synapsin (−) boutons.
A,B Synaptic activity recorded during a continuous stimulation at a 10 Hz frequency in potentiated (open circles) and naïve (solid squares) synapsin (+) (black) and synapsin (−) (red) boutons. Each data point represent the average of 100 subsequent responses.
C. Basal quantal release (recorded at a 3 Hz stimulation frequency) in potentiated and nonpotentiated boutons. Data collected from 22-30 preparations for each line at each condition.
These results suggest that functional properties of extra vesicles formed upon intense stimulation depend on synapsin. Normally, these extra vesicles may not be available for release during a mild stimulation but become involved into the recycling pathway upon a subsequent intense stimulation. This functional property classifies these extra vesicles as those belonging to the reserve pool. In contrast, the increase in quantal content observed at synapsin (−) boutons upon the potentiation paradigm indicates that the newly formed vesicles are not directed to the reserve pool and are available for release. These results suggest that synapsin is responsible for directing the newly formed vesicles into the reserve pool, and this synapsin function enables a synapse to sustain a continuous stimulation.
Synapsin mediates the process of formation of enlarged vesicles and quanta during maintained depolarization
It was demonstrated recently (He at al., 2009, Akbergenova and Bykhovskaia, 2009b) that maintained depolarization may activate the recycling pathway that produces enlarged vesicles and endosome-like cisternae, and this pathway may enhance synaptic transmission by exocytosis of some of the cisternae / enlarged vesicles and, respectively, producing enlarged neurosecretory quanta. In this study, we tested whether this process would depend on the presence of synapsin.
First, we quantified all the enlarged vesicles and endosome-like structures (Fig. 8 A, B, arrowheads) in potentiated (15 min at 10 Hz + 10 min rest) boutons. A size cutoff of 45 nm was used to discriminate between normal and enlarged vesicles; this cutoff was determined by fitting the vesicle size at rest by a Gaussian distribution (Akbergenova and Bykhovskaia, 2009b). To test whether some of these structures may represent invaginations of the synaptic membrane or parts of other organelles, we performed serial sectioning at several boutons and indentified the endosome-like structures on subsequent sections. A number of enlarged vesicles/endosome-like structures could be traced on two-three serial sections (Fig. 8 B), and neither of them were linked to any other membranous structure. We found that the number of enlarged vesicles/endosome-like cistrenae was significantly increased upon potentiation in synapsin (+) terminals, but remained unchanged in synapsin (−) terminals (Fig. 8 C).
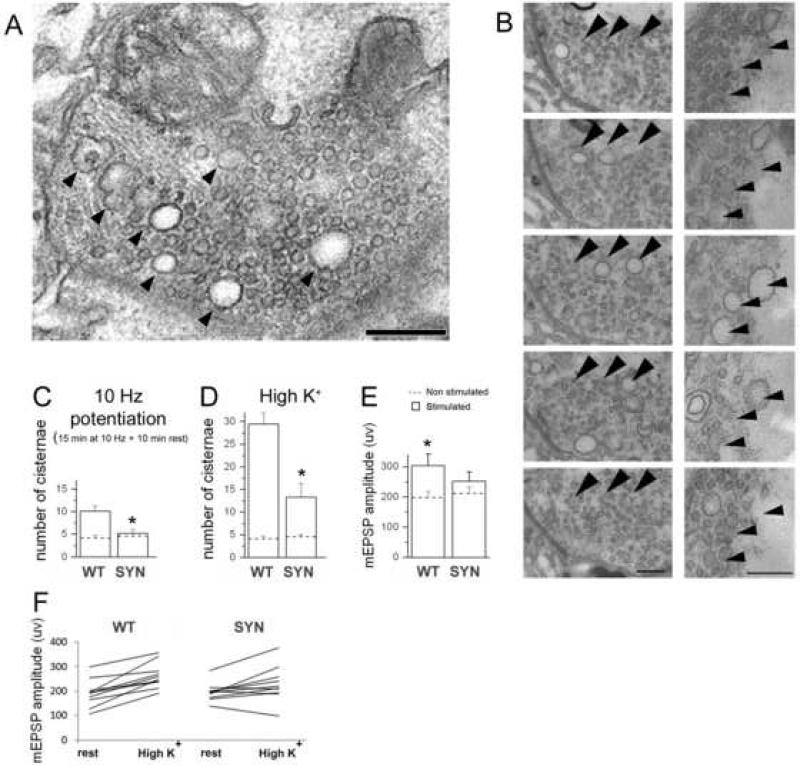
Figure 8. Synapsin promotes a formation of enlarged vesicles and quanta.
A. Endosome-like structures (arrowheads) formed upon sustained depolarization.
B. Tracing several endosome-like structures (arrowheads) through subsequent serial sections demonstrates that they are not connected to the plasma membrane or mitochondria.
B. Potentiation activates formation of endosome-like structures/enlarged vesicles in WT but not in synapsin (−) boutons. Asterisk indicate significant difference (p<0.05) between WT and synapsin (−) genotypes upon potentiation. Dotted lines – before stimulation; bars – after stimulation.
C. High K+ treatment drastically increases the number of endosome-like structures/enlarged vesicles in WT boutons, and this effect is not as prominent in synapsin (−) boutons. EM data collected from 3 preparations (16-19 boutons) for each line at each condition.
D, F. After high K+ treatment, quantal size significantly increases in synapsin (+) but not in synapsin (−) boutons, as could be seen from mean mEPSP amplitudes (D) or those in individual experiments (F). Asterisks indicate significant difference (p<0.05) between WT and synapsin (−) high K+ treated preparations. Data collected from 22 WT and 26 synapsin (−) preparations.
Next, we employed high K+ application (90 mM for 5 min), a treatment that was demonstrated to be the most efficient activator of the recycling pathway producing enlarged vesicles/quanta in our preparation (Akbergenova and Bykhovskaia, 2009b). WT and synapsin (−) preparations were fixed immediately following the high K+ treatment and processed for the EM analysis. WT preparations responded to the treatment by formation of numerous endosome-like structures, and this effect was substantially diminished in synapsin (−) boutons (Fig. 8 D).
To test physiological consequences of this synapsin function, we recorded spontaneous activity from synapsin (+) or synapsin (−) boutons before and after high K+ application. Spontaneous activity was recorded for 5 min, then high K+ solution (90 mM for 5 min) was applied, and finally the preparations were washed and original recordings repeated. We found that whereas WT boutons show consistent and significant increase in quatal size upon treatment in every experiment, synapsin (−) boutons do not show a consistent increase in quantal size, and although an overall positive trend is observed, it does not reach significance (Fig. 8 E, F).
Thus, a synapse may respond to maintained depolarization by enhancing a recycling pathway that involves a formation of enlarged vesicles/quanta, and this pathway critically depends on synapsin functioning.
DISCUSSION
We took advantage of the Drosophila synapsin null mutant to demonstrate a synapsin function in enabling a nerve terminal to maintain and enhance its activity during sustained stimulation. This synapsin function is likely to be mediated by several mechanisms. First, synapsin maintains vesicle organization that leaves void and, possibly, reserve spaces; these reserve spaces may be filled with extra vesicles upon intense stimulation. Second, synapsin is likely to participate in the process of vesicle recycling more directly, by directing the extra vesicles formed upon intense stimulation into the reserve pool. Finally, synapsin is required for an activation of the recycling pathway that produces enlarged vesicles/quanta upon maintained depolarization.
Synapsin maintains a peripheral vesicle organization
We demonstrated that synapsin functioning is responsible for peripheral vesicle organization in Drosophila Ib motor boutons. Normally, vesicles in these boutons cluster over the periphery of the bouton leaving void spaces (Akbergenova and Bykhovskaia, 2009a, Denker et al., 2009). In this study, we have demonstrated that this vesicle organization is disrupted in synapsin null mutants. Furthermore, we have demonstrated that synapsin, similar to vesicles, has a peripheral distribution. This result suggests that synapsin may perform its function by binding to vesicles and tethering them at a peripheral cluster. In support of this idea, we have also demonstrated that a treatment by a calcineurin inhibitor cycA disrupts both peripheral vesicle clustering and the peripheral distributiuon of synapsin.
The latter findings compel revisiting an earlier hypothesis that CycA treatment mobilizes reserve vesicles into the recycling pathway (Kuromi and Kidokoro, 1999). The above interpretation was largely based upon the observation that FM1-43 fluorescence spread towards the center of the bouton when a mild stimulation paradigm was employed in the presence of CycA. Our data suggests a different interpretation for this CycA action. It is known that CycA application completely blocks synapsin dephosphorylation at sites 4,5,6 (Jovanovic et al., 2001), which are controlled by MAP kinase/calcineurine activity. Interestingly, one of these sites (S62) is conserved between mammals and Drosophila (Genbank, COBALT alignment). Thus, it is likely that one of the consequences of CycA treatment is an increase in synapsin phosphorylated forms, and, consequentially, synapsin dispersion from vesicles followed by a dispersion of vesicles over the entire bouton.
The synapsin function in maintaining vesicle clustering appears to be rather general among different types of organisms and synapses. Indeed, the studies performed at the giant lamprey synapse showed that synapsin neutralization disrupts vesicle clustering (Bloom et al., 1993; Hilfiker et al., 1998). Vesicle clustering is widely observed at mammalian CNS synapses (Peters et al., 1991; Harris and Sultan, 1995; Schikorski and Stevens, 2001) even though regularities in vesicle distribution are not immediately evident. In synapsin null mutants vesicles are generally depleted (Rosahl et al., 1995; Li et al., 1995; Gitler et al., 2004; the present study), and the degree of depletion may depend on the terminal region in relation to the plasma membrane (Li et al., 1995; Samigullin et al., 2004; Coleman et al., 2008). A tomography study (Siksou et al., 2007) demonstrated that vesicles are linked into clusters by a filamentous matrix, and that synapsin belongs to these clusters. Thus, the general synapsin function in vesicle clustering is evident. Our study suggests an important physiological function of this clustering. Specifically, it suggests that synapsin may enable nerve terminals to maintain reserve spaces that can be filled by newly formed vesicles upon intense stimulation.
Synapsin positively regulates vesicle supply upon intense stimulation and directs newly supplied vesicles into the reserve pool
We have demonstrated that synapsin is a critical player in the process of structural potentiation, a form of plasticity which produces formation of extra vesicles upon intense stimulation (Akbergenova and Bykhovskia, 2009a). We believe that the major underlying mechanism of this structural potentiation is excess endocytosis (Engisch and Nowycky, 1998), that may be accelerated by intense synaptic activity (Wu et al., 2005). We cannot, however, completely exclude the possibility that a part of the newly formed vesicle pool is produced by anterograde axonal vesicle trafficking (Levitan et al., 2007; Goldstein et al., 2008).
Hypothetically, actin/synapsin interactions could contribute to the latter mechanism by reducing vesicle mobility and promoting vesicle capturing in the terminals (McGuinness et al., 1989; Nunes et al., 2006), thus enhancing vesicle supply via axonal transport. However, recent studies (Gaffield and Betz, 2007; Wong et al., 2008; Levitan, 2008) indicate that this possibility is unlikely. There is, in contrast emerging evidence that synapsin may contribute to endocytosis upon intense stimulation. In particular, nerve stimulation has been shown to produce synapsin immunoreactivity in the region of endocytic zones (Bloom et al., 2003). Furthermore, sustained stimulation of synapsin null terminals produced enhanced synaptic depression, as was observed in several preparations (Rosahl et al., 1995; Gitler et al., 2004; Samigullin et al., 2004), including Drosophila NMJ (this study). We suggest, therefore, that a synapsin-dependent mechanism may enhance endocytosis upon sustained intense activity and direct the newly formed vesicles into the reserve pool.
Our study clearly demonstrated a critical role of synapsin in the latter mechanism, namely directing newly formed vesicles into the reserve pool, as well as maintaining the reserve pool. First, FM1-43 photoconversion experiments demonstrated that the reserve pool is diminished in synapsin (−) terminals, while the recycling pool is unaffected. Second, recordings of synaptic activity from potentiated boutons demonstrated that the vesicles newly formed upon potentiation are directed into the reserve pool in WT but not in synapsin (−) terminals. These results directly demonstrate synapsin functioning in forming and maintaining the reserve pool and suggest that this function is carried out via mediating compensatory endocytosis during sustained activity.
Synapsin mediates formation of enlarged vesicles/quanta upon maintained depolarization
Synapses may respond to maintained depolarization by activation of a recycling pathway that produces numerous endosome-like cisternae (Heuser and Reese, 1973; Marxen et al., 1999; Teng and Wilkinson, 2000; Holt et al., 2003; de Lange et al., 2003). This endosome-like structures are likely to represent a mixture of intermediates from three pathways, including bulk membrane uptake (Takei et al., 1996; de Lange et al., 2003), endosomal recycling (Wucherpfennig, 2003), and compound vesicles fusion (He et al., 2009; Akbergenova and Bykhovskaia, 2009b). Some of the endosome-like structures may fuse with the presynaptic membrane (Coggins et al., 2007).
A recent rigorous study at the calyx synapse (He et al., 2009) demonstrated that intense activity initiates compound vesicle fusion, which is followed by formation of enlarged vesicles, subsequent release of enlarged neurosecretory quanta, and a compensatory bulk membrane reuptake. Our study at the Drosophila nmj (Akbergenova and Bykhovskaia, 2009b) produced similar conclusions, showing no FM1-43 staining at some of the enlarged vesicles (presumably formed by compound vesicle fusion), a strong and uniform FM1-43 uptake at other endosome-like structures (presumably produced by bulk endocytosis), and a non-uniform staining pattern (presumably, a fusion of stained and non-stained vesicles). The formation of enlarged vesicles produces an increase in quantal size that is likely to represent a mechanism for enhancing synaptic activity in response to a sustained stimulation. Indeed, enlarged vesicles/quanta appear as a result of enhanced motor activity in vivo (Steinert et al., 2006), implying a physiological significance of this mechanism.
In this study, we demonstrated that both formation of enlarged vesicles/cisternae and release of enlarged quanta critically depends on the presence of synapsin. Synapsin (−) terminals lack the ability to increase the quantal size in response to sustained depolarization, since the formation of enlarged vesicles/cisternae is inhibited in synapsin (−) terminals. It is important to note that synapsin deficiency inhibits activity-dependent formation of both endosome-like structures and vesicles. This finding rules out the possibility that extra vesicles produced upon intense stimulation could be formed from endosome-like cisternae.
Thus, our study reveals a novel synapsin function, specifically, enhancing synaptic transmission via an increase in the size of a neurosecretory quantum. Together with earlier studies demonstrating that the formation of enlarged vesicles/cisternae depends on actin polymerization (Holt et al., 2003; Richards et al., 2004; Akbergenova and Bykhovskaia, 2009b), our results may elucidate a specific molecular pathway of the compound vesicle fusion, suggesting that some of the observed endosome-like structures may be formed via an actin/synapsin-dependent association of synaptic vesicles.
In summary, we have demonstrated that the ability of a synapse to respond to a sustained stimulation is compromised in synapsin (−) terminals in several ways. First, synapsin (−) terminals have a diminished reserve pool and a limited ability to sustain synaptic activity in response to continuous stimulation. Second, synapsin (−) terminals have a reduced ability to increase vesicle abundance in response to intense stimulation. Third, the vesicles newly formed in response to an intense stimulation are not directed to the reserve pool in the absence of synapsin. Finally, synapsin is required for an activation of a recycling pathway that produces enlarged vesicles/quanta in response to a maintained depolarization.
Acknowledgements
We thank D. Erich Buchner for kindly providing us with synapsin null and rescue mutants, as well as for constructive discussions and critical reading of our manuscript. The study was supported by the NIH grants R01 MH061059 and U54 NS039408.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Akbergenova Y, Bykhovskaia M. Synapsin maintains the reserve vesicle pool and spatial segregation of the recycling pool in Drosophila presynaptic boutons. Brain Res. 2007;1178:52–64. doi: 10.1016/j.brainres.2007.08.042. [DOI] [PubMed] [Google Scholar]
- Akbergenova Y, Bykhovskaia M. Stimulation-induced formation of the reserve pool of vesicles in Drosophila motor boutons. J.Neurophysiol. 2009;101:2423–2433. doi: 10.1152/jn.91122.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbergenova Y, Bykhovskaia M. Enhancement of the endosomal endocytic pathway increases quantal size. Mol.Cell Neurosci. 2009;40:199–206. doi: 10.1016/j.mcn.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara SG, Kuhar MJ. Neurotransmitter transporters: recent progress. Annu.Rev.Neurosci. 1993;16:73–93. doi: 10.1146/annurev.ne.16.030193.000445. [DOI] [PubMed] [Google Scholar]
- Atwood HL, Govind CK, Wu CF. Differential ultrastructure of synaptic terminals on ventral longitudinal abdominal muscles in Drosophila larvae. J.Neurobiol. 1993;24:1008–1024. doi: 10.1002/neu.480240803. [DOI] [PubMed] [Google Scholar]
- Baldelli P, Fassio A, Valtorta F, Benfenati F. Lack of synapsin I reduces the readily releasable pool of synaptic vesicles at central inhibitory synapses. J.Neurosci. 2007;27:13520–13531. doi: 10.1523/JNEUROSCI.3151-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom O, Evergren E, Tomilin N, Kjaerulff O, Low P, Brodin L, Pieribone VA, Greengard P, Shupliakov O. Colocalization of synapsin and actin during synaptic vesicle recycling. J.Cell Biol. 2003;161:737–747. doi: 10.1083/jcb.200212140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bykhovskaia M. Making quantal analysis more convenient, fast, and accurate: User-friendly software QUANTAN. J.Neurosci.Methods. 2008;168:500–513. doi: 10.1016/j.jneumeth.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavelier P, Attwell D. Neurotransmitter depletion by bafilomycin is promoted by vesicle turnover. Neurosci.Lett. 2007;412:95–100. doi: 10.1016/j.neulet.2006.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi P, Greengard P, Ryan TA. Synaptic vesicle mobilization is regulated by distinct synapsin I phosphorylation pathways at different frequencies. Neuron. 2003;38:69–78. doi: 10.1016/s0896-6273(03)00151-x. [DOI] [PubMed] [Google Scholar]
- Coggins MR, Grabner CP, Almers W, Zenisek D. Stimulated exocytosis of endosomes in goldfish retinal bipolar neurons. J.Physiol. 2007;584:853–865. doi: 10.1113/jphysiol.2007.140848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin MA, Malladi CS, Tan TC, Raymond CR, Smillie KJ, Robinson PJ. Synapsin I-associated phosphatidylinositol 3-kinase mediates synaptic vesicle delivery to the readily releasable pool. J.Biol.Chem. 2003;278:29065–29071. doi: 10.1074/jbc.M302386200. [DOI] [PubMed] [Google Scholar]
- Cousin MA, Nicholls DG. Synaptic vesicle recycling in cultured cerebellar granule cells: role of vesicular acidification and refilling. J.Neurochem. 1997;69:1927–1935. doi: 10.1046/j.1471-4159.1997.69051927.x. [DOI] [PubMed] [Google Scholar]
- Dasari S, Cooper RL. Modulation of sensory-CNS-motor circuits by serotonin, octopamine, and dopamine in semi-intact Drosophila larva. Neurosci.Res. 2004;48:221–227. doi: 10.1016/j.neures.2003.10.005. [DOI] [PubMed] [Google Scholar]
- de Lange RP, de Roos AD, Borst JG. Two modes of vesicle recycling in the rat calyx of Held. J.Neurosci. 2003;23:10164–10173. doi: 10.1523/JNEUROSCI.23-31-10164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denker A, Krohnert K, Rizzoli SO. Revisiting synaptic vesicle pool localization in the Drosophila neuromuscular junction. J.Physiol. 2009;587:2919–2926. doi: 10.1113/jphysiol.2009.170985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engisch KL, Nowycky MC. Compensatory and excess retrieval: two types of endocytosis following single step depolarizations in bovine adrenal chromaffin cells. J.Physiol. 1998;506(Pt 3):591–608. doi: 10.1111/j.1469-7793.1998.591bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffield MA, Betz WJ. Synaptic vesicle mobility in mouse motor nerve terminals with and without synapsin. J.Neurosci. 2007;27:13691–13700. doi: 10.1523/JNEUROSCI.3910-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler D, Takagishi Y, Feng J, Ren Y, Rodriguiz RM, Wetsel WC, Greengard P, Augustine GJ. Different presynaptic roles of synapsins at excitatory and inhibitory synapses. J.Neurosci. 2004;24:11368–11380. doi: 10.1523/JNEUROSCI.3795-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler D, Cheng Q, Greengard P, Augustine GJ. Synapsin IIa controls the reserve pool of glutamatergic synaptic vesicles. J.Neurosci. 2008;28:10835–10843. doi: 10.1523/JNEUROSCI.0924-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godenschwege TA, Reisch D, Diegelmann S, Eberle K, Funk N, Heisenberg M, Hoppe V, Hoppe J, Klagges BR, Martin JR, Nikitina EA, Putz G, Reifegerste R, Reisch N, Rister J, Schaupp M, Scholz H, Schwarzel M, Werner U, Zars TD, Buchner S, Buchner E. Flies lacking all synapsins are unexpectedly healthy but are impaired in complex behaviour. Eur.J.Neurosci. 2004;20:611–622. doi: 10.1111/j.1460-9568.2004.03527.x. [DOI] [PubMed] [Google Scholar]
- Goldstein AY, Wang X, Schwarz TL. Axonal transport and the delivery of pre-synaptic components. Curr.Opin.Neurobiol. 2008;18:495–503. doi: 10.1016/j.conb.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P, Valtorta F, Czernik AJ, Benfenati F. Synaptic vesicle phosphoproteins and regulation of synaptic function. Science. 1993;259:780–785. doi: 10.1126/science.8430330. [DOI] [PubMed] [Google Scholar]
- Harris KM, Sultan P. Variation in the number, location and size of synaptic vesicles provides an anatomical basis for the nonuniform probability of release at hippocampal CA1 synapses. Neuropharmacology. 1995;34:1387–1395. doi: 10.1016/0028-3908(95)00142-s. [DOI] [PubMed] [Google Scholar]
- He L, Xue L, Xu J, McNeil BD, Bai L, Melicoff E, Adachi R, Wu LG. Compound vesicle fusion increases quantal size and potentiates synaptic transmission. Nature. 2009 doi: 10.1038/nature07860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser JE, Reese TS. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J.Cell Biol. 1973;57:315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilfiker S, Pieribone VA, Czernik AJ, Kao HT, Augustine GJ, Greengard P. Synapsins as regulators of neurotransmitter release. Philos.Trans.R.Soc.Lond B Biol.Sci. 1999;354:269–279. doi: 10.1098/rstb.1999.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt M, Cooke A, Wu MM, Lagnado L. Bulk membrane retrieval in the synaptic terminal of retinal bipolar cells. J.Neurosci. 2003;23:1329–1339. doi: 10.1523/JNEUROSCI.23-04-01329.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka M, Hammer RE, Sudhof TC. A phospho-switch controls the dynamic association of synapsins with synaptic vesicles. Neuron. 1999;24:377–387. doi: 10.1016/s0896-6273(00)80851-x. [DOI] [PubMed] [Google Scholar]
- Humeau Y, Doussau F, Vitiello F, Greengard P, Benfenati F, Poulain B. Synapsin controls both reserve and releasable synaptic vesicle pools during neuronal activity and short-term plasticity in Aplysia. J.Neurosci. 2001;21:4195–4206. doi: 10.1523/JNEUROSCI.21-12-04195.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan LY, Jan YN. Properties of the larval neuromuscular junction in Drosophila melanogaster. J.Physiol. 1976;262:189–214. doi: 10.1113/jphysiol.1976.sp011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia XX, Gorczyca M, Budnik V. Ultrastructure of neuromuscular junctions in Drosophila: comparison of wild type and mutants with increased excitability. J.Neurobiol. 1993;24:1025–1044. doi: 10.1002/neu.480240804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic JN, Benfenati F, Siow YL, Sihra TS, Sanghera JS, Pelech SL, Greengard P, Czernik AJ. Neurotrophins stimulate phosphorylation of synapsin I by MAP kinase and regulate synapsin I-actin interactions. Proc.Natl.Acad.Sci.U.S.A. 1996;93:3679–3683. doi: 10.1073/pnas.93.8.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic JN, Sihra TS, Nairn AC, Hemmings HC, Jr., Greengard P, Czernik AJ. Opposing changes in phosphorylation of specific sites in synapsin I during Ca2+-dependent glutamate release in isolated nerve terminals. J.Neurosci. 2001;21:7944–7953. doi: 10.1523/JNEUROSCI.21-20-07944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay AR, Alfonso A, Alford S, Cline HT, Holgado AM, Sakmann B, Snitsarev VA, Stricker TP, Takahashi M, Wu LG. Imaging synaptic activity in intact brain and slices with FM1-43 in C. elegans, lamprey, and rat. Neuron. 1999;24:809–817. doi: 10.1016/s0896-6273(00)81029-6. [DOI] [PubMed] [Google Scholar]
- Kidokoro Y, Kuromi H, Delgado R, Maureira C, Oliva C, Labarca P. Synaptic vesicle pools and plasticity of synaptic transmission at the Drosophila synapse. Brain Res.Brain Res.Rev. 2004;47:18–32. doi: 10.1016/j.brainresrev.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Koenig JH, Ikeda K. Synaptic vesicles have two distinct recycling pathways. J.Cell Biol. 1996;135:797–808. doi: 10.1083/jcb.135.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuromi H, Kidokoro Y. Two distinct pools of synaptic vesicles in single presynaptic boutons in a temperature-sensitive Drosophila mutant, shibire. Neuron. 1998;20:917–925. doi: 10.1016/s0896-6273(00)80473-0. [DOI] [PubMed] [Google Scholar]
- Kuromi H, Kidokoro Y. The optically determined size of exo/endo cycling vesicle pool correlates with the quantal content at the neuromuscular junction of Drosophila larvae. J.Neurosci. 1999;19:1557–1565. doi: 10.1523/JNEUROSCI.19-05-01557.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuromi H, Kidokoro Y. Tetanic stimulation recruits vesicles from reserve pool via a cAMP-mediated process in Drosophila synapses. Neuron. 2000;27:133–143. doi: 10.1016/s0896-6273(00)00015-5. [DOI] [PubMed] [Google Scholar]
- Kuromi H, Honda A, Kidokoro Y. Ca2+ influx through distinct routes controls exocytosis and endocytosis at drosophila presynaptic terminals. Neuron. 2004;41:101–111. doi: 10.1016/s0896-6273(03)00815-8. [DOI] [PubMed] [Google Scholar]
- Kuromi H, Kidokoro Y. Exocytosis and endocytosis of synaptic vesicles and functional roles of vesicle pools: lessons from the Drosophila neuromuscular junction. Neuroscientist. 2005;11:138–147. doi: 10.1177/1073858404271679. [DOI] [PubMed] [Google Scholar]
- Levitan ES, Lanni F, Shakiryanova D. In vivo imaging of vesicle motion and release at the Drosophila neuromuscular junction. Nat.Protoc. 2007;2:1117–1125. doi: 10.1038/nprot.2007.142. [DOI] [PubMed] [Google Scholar]
- Levitan ES. Signaling for vesicle mobilization and synaptic plasticity. Mol.Neurobiol. 2008;37:39–43. doi: 10.1007/s12035-008-8014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lnenicka GA, Keshishian H. Identified motor terminals in Drosophila larvae show distinct differences in morphology and physiology. J.Neurobiol. 2000;43:186–197. [PubMed] [Google Scholar]
- Lohr R, Godenschwege T, Buchner E, Prokop A. Compartmentalization of central neurons in Drosophila: a new strategy of mosaic analysis reveals localization of presynaptic sites to specific segments of neurites. J.Neurosci. 2002;22:10357–10367. doi: 10.1523/JNEUROSCI.22-23-10357.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marxen M, Volknandt W, Zimmermann H. Endocytic vacuoles formed following a short pulse of K+ -stimulation contain a plethora of presynaptic membrane proteins. Neuroscience. 1999;94:985–996. doi: 10.1016/s0306-4522(99)00351-6. [DOI] [PubMed] [Google Scholar]
- McGuinness TL, Brady ST, Gruner JA, Sugimori M, Llinas R, Greengard P. Phosphorylation-dependent inhibition by synapsin I of organelle movement in squid axoplasm. J.Neurosci. 1989;9:4138–4149. doi: 10.1523/JNEUROSCI.09-12-04138.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegon A, Bonanomi D, Albertinazzi C, Lotti F, Ferrari G, Kao HT, Benfenati F, Baldelli P, Valtorta F. Protein kinase A-mediated synapsin I phosphorylation is a central modulator of Ca2+-dependent synaptic activity. J.Neurosci. 2006;26:11670–11681. doi: 10.1523/JNEUROSCI.3321-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron. 1998;20:389–399. doi: 10.1016/s0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- Nunes P, Haines N, Kuppuswamy V, Fleet DJ, Stewart BA. Synaptic vesicle mobility and presynaptic F-actin are disrupted in a N-ethylmaleimide-sensitive factor allele of Drosophila. Mol.Biol.Cell. 2006;17:4709–4719. doi: 10.1091/mbc.E06-03-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Palay SL, Webster FN. The fine structure of the nervous system. 1991 [Google Scholar]
- Richards DA, Rizzoli SO, Betz WJ. Effects of wortmannin and latrunculin A on slow endocytosis at the frog neuromuscular junction. J.Physiol. 2004;557:77–91. doi: 10.1113/jphysiol.2004.062158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoli SO, Betz WJ. Synaptic vesicle pools. Nat.Rev.Neurosci. 2005;6:57–69. doi: 10.1038/nrn1583. [DOI] [PubMed] [Google Scholar]
- Rosahl TW, Spillane D, Missler M, Herz J, Selig DK, Wolff JR, Hammer RE, Malenka RC, Sudhof TC. Essential functions of synapsins I and II in synaptic vesicle regulation. Nature. 1995;375:488–493. doi: 10.1038/375488a0. [DOI] [PubMed] [Google Scholar]
- Samigullin D, Bill CA, Coleman WL, Bykhovskaia M. Regulation of transmitter release by synapsin II in mouse motor terminals. J.Physiol. 2004;561:149–158. doi: 10.1113/jphysiol.2004.073494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schikorski T, Stevens CF. Morphological correlates of functionally defined synaptic vesicle populations. Nat.Neurosci. 2001;4:391–395. doi: 10.1038/86042. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Sakaba T, Neher E. Vesicle pools and short-term synaptic depression: lessons from a large synapse. Trends Neurosci. 2002;25:206–212. doi: 10.1016/s0166-2236(02)02139-2. [DOI] [PubMed] [Google Scholar]
- Siksou L, Rostaing P, Lechaire JP, Boudier T, Ohtsuka T, Fejtova A, Kao HT, Greengard P, Gundelfinger ED, Triller A, Marty S. Three-dimensional architecture of presynaptic terminal cytomatrix. J.Neurosci. 2007;27:6868–6877. doi: 10.1523/JNEUROSCI.1773-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert JR, Kuromi H, Hellwig A, Knirr M, Wyatt AW, Kidokoro Y, Schuster CM. Experience-dependent formation and recruitment of large vesicles from reserve pool. Neuron. 2006;50:723–733. doi: 10.1016/j.neuron.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Takei K, Mundigl O, Daniell L, De Camilli P. The synaptic vesicle cycle: a single vesicle budding step involving clathrin and dynamin. J.Cell Biol. 1996;133:1237–1250. doi: 10.1083/jcb.133.6.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng H, Wilkinson RS. Clathrin-mediated endocytosis near active zones in snake motor boutons. J.Neurosci. 2000;20:7986–7993. doi: 10.1523/JNEUROSCI.20-21-07986.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreken P, Ly CV, Venken KJ, Koh TW, Zhou Y, Bellen HJ. Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron. 2005;47:365–378. doi: 10.1016/j.neuron.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Wong MY, Shakiryanova D, Levitan ES. Presynaptic Ryanodine Receptor-CamKII Signaling is Required for Activity-dependent Capture of Transiting Vesicles. J.Mol.Neurosci. 2008 doi: 10.1007/s12031-008-9080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Xu J, Wu XS, Wu LG. Activity-dependent acceleration of endocytosis at a central synapse. J.Neurosci. 2005;25:11676–11683. doi: 10.1523/JNEUROSCI.2972-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wucherpfennig T, Wilsch-Brauninger M, Gonzalez-Gaitan M. Role of Drosophila Rab5 during endosomal trafficking at the synapse and evoked neurotransmitter release. J.Cell Biol. 2003;161:609–624. doi: 10.1083/jcb.200211087. [DOI] [PMC free article] [PubMed] [Google Scholar]