Abstract
Purpose
We report the radiographic and clinical response rate of stereotactic body radiation therapy (SBRT) compared with conventional fractionated external beam radiation therapy (CF-EBRT) for renal cell carcinoma (RCC) bone lesions treated at our institution.
Methods and materials
Forty-six consecutive patients were included in the study, with 95 total lesions treated (50 SBRT, 45 CF-EBRT). We included patients who had histologic confirmation of primary RCC and radiographic evidence of metastatic bone lesions. The most common SBRT regimen used was 27 Gy in 3 fractions.
Results
Median follow-up was 10 months (range, 1-64 months). Median time to symptom control between SBRT and CF-EBRT were 2 (range, 0-6 weeks) and 4 weeks (range, 0-7 weeks), respectively. Symptom control rates with SBRT and CF-EBRT were significantly different (P = .020) with control rates at 10, 12, and 24 months of 74.9% versus 44.1%, 74.9% versus 39.9%, and 74.9% versus 35.7%, respectively. The median time to radiographic failure and unadjusted pain progression was 7 months in both groups. When controlling for gross tumor volume, dose per fraction, smoking, and the use of systemic therapy, biologically effective dose ≥80 Gy was significant for clinical response (hazard ratio [HR], 0.204; 95% confidence interval [CI], 0.043-0.963; P = .046) and radiographic (HR, 0.075; 95% CI, 0.013-0.430; P = .004). When controlling for gross tumor volume and total dose, biologically effective dose ≥80 Gy was again predictive of clinical local control (HR, 0.140; 95% CI, 0.025-0.787; P = .026). Toxicity rates were low and equivalent in both groups, with no grade 4 or 5 toxicity reported.
Conclusions
SBRT is both safe and effective for treating RCC bone metastases, with rapid improvement in symptoms after treatment and more durable clinical and radiographic response rate. Future prospective trials are needed to further define efficacy and toxicity of treatment, especially in the setting of targeted agents.
Introduction
In 2015, the estimated number of new kidney and renal pelvis cancers in the United States was 61,560, with approximately 14,080 deaths.1 The role of radiation has been mostly reserved for the metastatic setting as renal cell carcinoma (RCC) has often been considered radioresistant.2 This stems from research in the 1980s demonstrating the need for very high doses of conventionally fractionated radiation therapy to achieve local control of metastatic lesions, which came at the risk of damaging or threatening normal tissue tolerances of surrounding critical structures.3 However, over the past decade, the increased utilization of hypofractionation with stereotactic body radiation therapy (SBRT) has allowed clinicians to accurately target lesions to high doses while minimizing surrounding dose to organs at risk. SBRT's ability to deliver high-dose treatments that are in close proximity to critical structures relies on 3 fundamental principles: (1) accurate and precise stereotactic localization of the tumor (via internal or external references); (2) daily image guidance to visualize and decrease toxicity to critical normal organs; and (3) delivery of therapy in 1 to 5 fractions.4
Several groups have demonstrated promising results with the use of SBRT to target metastatic RCC. Wersall and colleagues5 demonstrated local control rates higher than 90% in their series of RCC-treated sites, including lung, renal bed, lymph nodes, and adrenal gland. Another series published only a 2% documented progression at a median follow-up of 52 months for RCC lesions treated in the lung, renal bed, and adrenal gland.6 The majority of sites in both studies included the lung, with SBRT doses of 30 to 40 Gy given in 3 to 4 fractions. The use of SBRT to metastatic bone lesions has also been studied. Memorial Sloan Kettering Cancer Center compared the tumor control rates of 105 patients with extracranial RCC who were either given single-fraction SBRT (18-24 Gy) or hypofractionation (3-5 fractions to 20-30 Gy). Local control rates were greatest in the high-single-dose (24 Gy), compared with the low-single-dose (<24 Gy) or hypofractionated regimens with 3-year local progression-free survival of 88%, 21%, and 17%, respectively.7 Under multivariate analysis, SBRT 24 Gy was a significant predictor of improved local progression-free survival. Additional studies have shown high rates of local control with minimal toxicity in patients treated with SBRT.8
Based on the results from several single-institutional studies demonstrating high local control rates using SBRT for RCC, we sought to review both radiographic and clinical outcomes of RCC bone lesions treated with conventional fractionated external beam radiation therapy (CF-EBRT) or SBRT in our institution to add to the data on whether RCC is truly radioresistant. Additionally, the goal of this study was to define a minimum dose, in terms of either fraction size or biologically effective dose (BED) cutoff, needed to achieve long-term local control.
Methods and materials
After obtaining institutional review board approval, patients were retrospectively identified by searching an institutional database of patients treated with radiation for RCC at our institution between January 2004 and September 2014. Patients included in the study had a minimum follow-up of 1 month. In total, 46 patients were included, with a total of 95 lesions treated with either CF-EBRT or SBRT. We included patients with histologic confirmation of primary RCC and radiographic evidence of metastatic bone lesions. Patients were treated with SBRT using intensity modulated radiation therapy planning. All patients treated with SBRT underwent treatment simulation previously and all received daily image-guided radiation therapy. Image-guided radiation therapy included cone beam computed tomography scans aligning to the planning tumor volume (PTV) and bony anatomy before each SBRT treatment. For treatment planning, the gross tumor volume (GTV) was considered equal to the clinical target volume. The PTV was typically 5 mm in all directions. Those who underwent conventional fractionation had weekly electronic portal imaging to verify setup. A BED was calculated for each treatment using BED = (TD) [1 + d/(α/β)], where TD = total dose, d = dose per fraction, and α/β = 7, as used in prior studies.9
All clinical notes and imaging were reviewed for each lesion. Patients were evaluated for clinical and radiographic response after treatment. Clinical symptoms were evaluated based on patient report and the Wong–Baker Faces Pain Rating Scale when recorded. Patients with clinically stable (stable disease [SD]), improved (partial response [PR]) or resolution of symptoms (complete response) were recorded as having locoregional control (LRC). Symptom control was defined as SD, PR, or complete response based on patient report and the Wong–Baker Faces Pain Rating Scale when available. Patients with increased pain after radiation therapy (not including treatment-related pain flare) or recurrence of pain, despite initial response, were classified as local failure. Pain flare was defined as documented worsening of pain at the treated site on clinical evaluation within 2 weeks treatment start. Quantity of pain medication use was not included as a measure of symptom control because the majority of patients in both groups continued to use narcotics to control discomfort at additional sites of disease.
Radiographic complete response was defined as no evidence of disease in the treatment volume by interpretation of available (18F)-fluorodeoxyglucose positron emission tomography/computed tomography, magnetic resonance imaging, and computed tomography scans 3 months after SBRT. No response was defined as the absence of marked change or increase in the treated lesion. PR was defined as not meeting the criteria for complete response or no response. Clinical and radiographic LRC was defined as the time from the last day of radiation treatment to local failure or last follow-up in living patients without evidence of recurrence or progression. The Response Evaluation Criteria in Solid Tumors was not used because of difficulty assessing parenchymal changes common after SBRT, especially to bony sites treated at 20 Gy or higher. Subsequent follow-up evaluation was within 2 to 5 weeks with oncology, and was similar between SBRT and CF-EBRT with a median time to first postradiation follow-up of 18 days (range, 7-31 days) and 14 days (range, 6-34 days), respectively.
Log-rank tests and Cox regression models were used to evaluate the association between clinical factors and LRC. The independent variables considered were GTV, PTV, BED, sex, age, total dose, number of fractions, and systemic therapy pre- and postradiation. Logistic regression models were fit for multivariate analysis to evaluate associations between LRC and any of the clinical factors with P < .15. Kaplan–Meier curves were generated to compare radiographic and clinical local control rates between SBRT and CF-EBRT. Toxicity was recording using the Common Terminology Criteria for Adverse Events, version 3.0. Patients were censored at the time of death.
Results
The median follow-up of the study cohort was 10 months (range, 1-64 months). Forty-six patients were included, with 95 total lesions treated (50 SBRT, 45 CF-EBRT). The majority of patients were male (70%) with clear cell histology (93%). The most common SBRT fractionation was 27 Gy given in 3 fractions (9 Gy per fraction); the most common CF-EBRT treatment was 20 Gy in 5 fractions (4 Gy per fraction) (Table 1). The majority of lesions treated were located in the spine. The second most common site was the pelvis. Median BED was 108.00 (range, 54.00-216.66) and 46.67 (range, 29.33-93.33) for SBRT and CF-EBRT, respectively. The majority of patients were on systemic therapy before and after radiation (Table 2).
Table 1.
Patient and clinical characteristics
| Variable | No. (%) |
|---|---|
| Total patients | 46 |
| Age (median, range) | 62 (42-78) |
| Sex | |
| Male | 32 (70) |
| Female | 14 (30) |
| Histology | |
| Clear cell | 88 (93) |
| Chromophobe | 4 (4) |
| Not otherwise specified | 3 (3) |
| Number of lesions | 95 |
| Treated with SBRT | 50 (53) |
| 12-20 Gy/1 fx | 14 |
| 21-35 Gy/3 fx | 20 |
| 25-50 Gy/5 fx | 16 |
| Treated with CF-EBRT | 45 (47) |
| 8-10 Gy/1 fx | 6 |
| 20 Gy/5 fx | 23 |
| 24 Gy/8 fx | 1 |
| 30-40 Gy/10 fx | 13 |
| 36 Gy/12 fx | 2 |
CF-EBRT, conventional fractionated external beam radiation therapy; fx, fraction; SBRT, stereotactic body radiation therapy.
Table 2.
Treatment characteristics between SBRT and CF-EBRT
| Variable | SBRT (n = 50) | CF-EBRT (n = 45) |
|---|---|---|
| Location, n (%) | ||
| Spine | 16 (32%) | 18 (40%) |
| Skull | 1 (2%) | 4 (9%) |
| Thorax | 10 (20%) | 7 (16%) |
| Pelvis | 15 (30%) | 8 (18%) |
| Upper extremity | 3 (6%) | 3 (6.7%) |
| Lower extremity | 5 (10%) | 5 (11%) |
| Median dose per fraction (range, Gy) | 9 (5-20) | 4 (1-4) |
| Gross tumor volume (range, cm3) | 35.55 (0.66-208.48) | 30.10 (3.13-259.57) |
| Planned tumor volume (range, cm3) | 93.55 (3.25-385.40) | 84.74 (14.36-874.60) |
| Biologic effective dose (range) | 108.00 (54.00-216.66) | 46.67 (29.33-93.33) |
| Systemic therapy before radiation, n (%) | ||
| Yes | 28 (56%) | 33 (73%) |
| No | 22 (44%) | 12 (27%) |
| Systemic therapy after radiation, n (%) | ||
| Yes | 39 (78%) | 38 (84%) |
| No | 11 (22%) | 7 (16%) |
| Time to symptom relief (range, weeks) | 2 (0-6) | 4 (0-7) |
CF-EBRT, conventional fractionated external beam radiation therapy; SBRT, stereotactic body radiation therapy.
When combining all patients included in the study (SBRT and CF-EBRT), predictive factors for clinical local control under univariate analysis included BED ≥80 Gy (hazard ratio [HR], 0.345; 95% confidence interval [CI], 0.154-0.771; P = .010) and a dose per fraction ≥9 Gy (HR, 0.396; 95% CI, 0.163-0.962; P = .042). Nonsignificant predictive factors for clinical local control included GTV size, age, sex, lesion location, total dose, smoking status, and systemic therapy before or after treatment. Significant predictive factors for combined radiographic local control under univariate analysis were BED ≥80 Gy (HR, 0.140; 95% CI 0.049-0.402; P < .001) and dose per fraction ≥9 Gy (HR, 0.298; 95% CI 0.115-0.777; P = .014). Nonsignificant predictive factors for radiographic local control included GTV, age, sex, lesion location, and systemic therapy before or after treatment. There was a local control trend observed for nonsmokers (P = .067) and higher total dose (P = .060). Under multivariate analysis, BED was predictive for both clinical (HR, 0.204; 95% CI, 0.043-0.963; P = .046) and radiographic (HR, 0.075; 95% CI, 0.013-0.430; P = .004) local control (Table 3).
Table 3.
Univariate and multivariate analysis for clinical and radiographic predictors of local control for all patients
| Clinical |
Radiographic |
|||||
|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
Univariate |
Multivariate |
|||
| Variable | P value | HR (95% CI) | P value | P value | HR (95% CI) | P value |
| BED (≥80 Gy) | .01 | 0.204 (0.043-0.963) | .049 | <.001 | 0.075 (0.013-0.430) | .004 |
| GTV (cm3) | .378 | 1.006 (0.998-1.014) | .155 | .203 | 1.008 (0.999-1.017) | .103 |
| Gender | .291 | - | - | .325 | - | - |
| Age | .417 | - | - | .258 | - | - |
| >5 metastatic lesions treated | .245 | - | - | .280 | - | - |
| Location of lesion | .143 | - | - | .169 | - | - |
| Total dose | .911 | - | - | .060 | - | - |
| Dose per fraction (≥9 Gy) | .042 | 1.007 (0.830-1.222) | .946 | .014 | 1.047 (0.871-1.258) | .627 |
| Smoking history | .385 | 1.323 (0.420-4.209) | .637 | .067 | 1.042 (0.298-3.639) | .949 |
| Systemic therapy before radiation | .438 | - | - | .250 | - | - |
| Systemic therapy after radiation | .934 | 0.891 (0.281-2.827) | .846 | .169 | 0.542 (0.169-1.742) | .306 |
BED, biologic effective dose; CI, confidence interval; GTV, gross tumor volume; HR, hazard ratio.
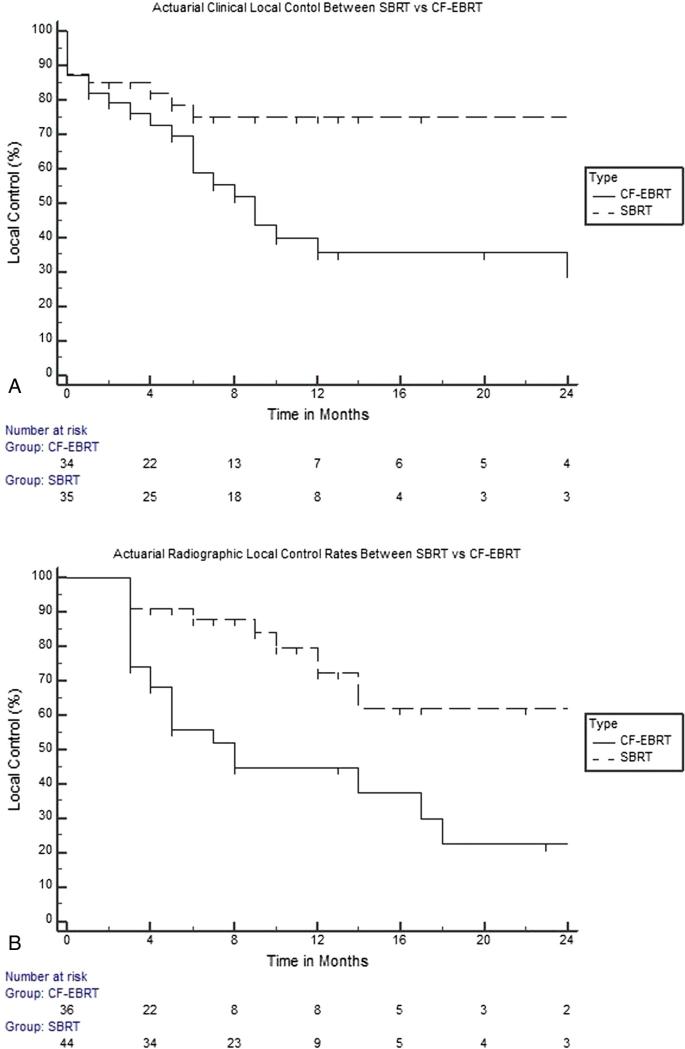
Median time to symptom control between SBRT and CF-EBRT was 2 (range, 0-6 weeks) and 4 weeks (range, 0-7 weeks), respectively. Symptom control rates with SBRT and CF-EBRT were significantly different (P = .020), with control rates at 10, 12, and 24 months of 74.9% versus 44.1%, 74.9% versus 39.9%, and 74.9% versus 35.7%, respectively (Fig 1A). In addition, radiographic local control rates between SBRT and CF-EBRT were significantly different, again favoring SBRT (P < .001). Radiographic control rates at 10, 12, and 24 months for SBRT and CF-EBRT and were 82.5% versus 45.1%, 74.1% versus 45.1%, and 61.4% versus 22.8% accordingly (Fig 1B). Clinical and radiographic response of the treated lesions was further categorized as complete response, PR, SD, and progressive disease (PD). For SBRT, clinical CR, PR, SD, and PD were 14 (36.8%), 17 (44.7%), 2 (5.3%), and 5 (13.2%), respectively; CF-EBRT was 6 (15.4%), 19 (48.7%), 9 (23.1%), and 5 (12.8%), respectively. Radiographic CR, PR, SD, and PD for SBRT was 1 (2.4%), 11 (26.8%), 24 (58.5%), and 5 (12.2%), respectively; CF-EBRT was 1 (2.7%), 6 (16.2%), 16 (43.2%), and 14 (37.8%), respectively.
Figure 1.
Kaplan–Meier curve demonstrating actuarial clinical (A) and radiographic (B) control rates between stereotactic body radiation therapy (SBRT) and conventional fractionated external beam radiation therapy (CF-EBRT).
When considering predictive factors for clinical local control for SBRT patients alone, significant variables included BED ≥80 under univariate and multivariate analysis (HR, 0.140; 95% CI, 0.025-0.787; P = .026). BED ≥80 was also predictive under univariate analysis for radiographic local control (P = .008), but not under multivariate analysis (HR, 0.238; 95% CI, 0.038-1.514; P = .134) (Table 4). Toxicity rates were low and equivalent in both groups, with no grade 4 or 5 side effects reported (Table 5). Four spinal and 2 pelvic lesions (N = 6, 6.25%) demonstrated radiographic evidence of fractures secondary to tumor progression after radiation (4 CF [2 pelvic, 2 spine], 2 SBRT [1 pelvic, 1 spine]). Fractures secondary to tumor progression were verified by radiology.
Table 4.
Univariate and multivariate analysis for clinical and radiographic predictors of local control for SBRT patients
| Clinical |
Radiographic |
|||||
|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
Univariate |
Multivariate |
|||
| Variable | P value | HR (95% CI) | P value | P value | HR (95% CI) | P value |
| BED (≥80 Gy) | .053 | 0.140 (0.025-0.787) | .026 | .008 | 0.238 (0.038-1.514) | .13 |
| GTV (cm3) | .91 | 1.003 (0.990-1.016) | .651 | .707 | 1.003 (0.985-1.020) | .767 |
| Sex | .088 | - | - | .136 | - | - |
| Age | .083 | - | - | .094 | - | - |
| >5 metastatic lesions treated | .304 | - | - | .938 | - | - |
| Location of lesion | .397 | - | - | .788 | - | - |
| Total dose | .415 | 1.000 (1.000-1.001) | .829 | .079 | 1.000 (1.000-1.000) | .223 |
| Smoking history | .309 | - | - | .444 | - | - |
| Systemic therapy before radiation | .821 | - | - | .588 | - | - |
| Systemic therapy after radiation | .643 | - | - | .296 | - | - |
BED, biologic effective dose; CI, confidence interval; GTV, gross tumor volume; HR, hazard ratio.
Table 5.
Toxicities
| Grade 1 |
Grade 2 |
Grade 3 |
||||
|---|---|---|---|---|---|---|
| Toxicity | SBRT | CF-EBRT | SBRT | CF-EBRT | SBRT | CF-EBRT |
| Pain | 2 | 1 | 1 | 1 | 0 | 0 |
| Edema | 1 | 1 | 0 | 0 | 0 | 0 |
| Dermatitis | 3 | 2 | 1 | 2 | 1 | 0 |
| Nausea | 3 | 3 | 1 | 1 | 0 | 0 |
| Esophagitis | 2 | 1 | 0 | 0 | 0 | 0 |
| Fatigue | 3 | 4 | 1 | 1 | 0 | 0 |
| Diarrhea | 1 | 0 | 0 | 0 | 0 | 0 |
CF-EBRT, conventional fractionated external beam radiation therapy; SBRT, stereotactic body radiation therapy.
Discussion
The notion that RCC is radioresistant and should not be treated with radiation therapy is based on older, conventionally fractionated data. In the era of hypofractionated SBRT, our findings further support data showing long-term symptomatic and radiographic local control rates for RCC lesions. Additionally, we present a novel BED dose target for use of SBRT in the RCC population. The most common SBRT regimen used for RCC bone metastases at our institution, particularly for vertebral bodies, is 27 Gy in 3 fractions. This fractionation delivers an ablative BED while minimizing the risk of vertebral body fractures, which can be common at high single-fraction doses.10 The results presented here illustrate the rapid and durable response with SBRT, both radiographically and clinically. Predictors for local response included BED ≥80 and fraction size ≥9 Gy. These findings are supported by earlier results evaluating the use of SBRT and doses needed to establish effective control rates for metastatic melanoma and RCC. Stinauer et al11 included 25 RCC lesions treated with 40 to 50 Gy in 5 fractions or 42 to 60 Gy in 3 fractions and had a local control rate of 88% at 18 months. Predictors of in-field local control included BED > 100 Gy and fraction size > 11 Gy, which are comparable to our findings.
Bone is the second most common site of metastatic disease following lung for RCC tumors, with pain being the most common presenting symptom in approximately 75% of all patients. There are several studies published demonstrating high rates of local control using SBRT for RCC lesions. Jhaveri et al9 published their results on pain relief with SBRT for RCC bony metastases, reporting those treated with a BED > 85 Gy compared with < 85 Gy had faster and more durable pain resolution; 78% had symptom control at 10 weeks compared with only 32%. These findings are similar to our results, which showed a median time of 2 weeks compared with 4 weeks for symptom control. M.D. Anderson reported its findings on 55 spinal lesions treated with SBRT (24 Gy in 1 fraction, 27 Gy in 3 fractions, 30 Gy in 5 fractions), demonstrating 1-year spine tumor progression-free survival of 82.1% with approximately half of all patients symptom free at 1 year.12 Additional studies evaluating response rates of spinal metastases have shown similar results with rapid pain relief, low numbers of clinical and radiographic failures, and limited toxicity, as represented in our study.13-15
As discussed previously, Zelefsky et al found similar high rates of local control with SBRT compared with hypofractionation, finding a cutoff of 24 Gy to be predictive.7,16 Another large series including 162 metastases confirmed similar high rates of local control.5 BED has been confirmed to be a strong predictive factor in many studies, including several Radiation Therapy Oncology Group clinical trials evaluating local control rates with SBRT.17-19 In our RCC patient population, a BED ≥80 Gy was found to be significant. Given the overall response rate of 80% to 90% for RCC lesions treated with SBRT, the authors of a recent review article summarized their recommendations of future phase 2 randomized trials to include SBRT in patients with low-volume metastatic disease with 4 potential fraction schedules: 24 Gy in 1 fraction, 36 Gy in 2 or 3 fractions, and 35 Gy in 5 fractions.2 This would be similar to the current NRG Oncology protocol evaluating tolerability of SBRT for 4 or fewer lesions of breast, prostate, or non-small cell lung cancer histology treated with SBRT.
Radiobiologically, the higher dose per fraction with SBRT-based treatments has been shown to provide improved local control over standard fractionation. Because the survival and proliferation of tumor cells are directly dependent on the blood supply, SBRT has been shown to have a direct effect on tumor vasculature. Hypoxia can increase the expression of vascular endothelial growth factors, which is associated with higher grade tumors and metastatic disease.2 High-dose radiation with 10 Gy or higher in a single fraction has been shown to cause severe vascular damage in human tumor xenografts or animal tumors.20,21 Additionally, the vascular injury and ensuing chaotic intratumoral environment (hypoxic, acidic, and deprived of nutrients) caused by high-dose fraction SBRT may significantly hinder the repair of radiation damage.22 There are several hypothesized mechanisms of RCC radiation resistance. One may be a mutation or silencing of the von Hippel-Lindau gene, which is present in more than 50% of cases and is thought to lead in the accumulation of hypoxic-inducible factor 1-alpha, which then creates angiogenesis leading to further tumor growth and potential disease spread.23 As described, the mechanism in which SBRT can cause endothelial apoptosis with single high-dose treatments may help overcome this pathway.
Currently, the role of systemic therapy in RCC continues to improve with more targeted therapies, providing patients with improved survival and quality of life. Because of this, SBRT will continue to play a valuable role in providing not only high local control rates but prolonging duration of symptomatic control from months to years. In addition, new agents that may have a synergistic effect with SBRT are constantly being developed and will need to be evaluated in clinical trials. Currently, the NRG Oncology group has a phase 1 trial evaluating toxicity and efficacy of SBRT in the oligometastatic setting for non-small cell lung, prostate, and breast cancers (NRG-BR001). Future trials evaluating the safety of incorporating SBRT with targeted agents will be important. One present phenomenon that is being studied closely in several cancers, including melanoma and RCC, is the abscopal effect.24 This effect describes an observable event in which 1 site treated with radiation and a biologic agent can trigger the immune system to respond to a distant tumor site that is not being actively targeted with radiation therapy. Whether processes such as the abscopal effect can be used to improve survival for these patients is yet to be fully validated.
Our study is limited by its retrospective nature, short-term follow-up that may have underestimated disease recurrence rates and long-term toxicity, and variability in treatment (specifically, fractionation size and total dosage). A common weakness in these studies is the inherent selection bias that may exist because patients who are treated with SBRT may have had less systemic burden, lower comorbidities, and better overall perfor mance status. However, because our endpoint focuses on local radiographic and symptom control, it is unlikely these factors contribute significantly to our findings. Additionally, because of the relatively small numbers of lesions included in this study, subset analysis of the SBRT group proved challenging. Pain medication use was not included as a final measure of symptom control in either group, although it was initially considered. This was due to the confounding factors of pain medication usage because the majority of our study population required continued medication to control other untreated sites of disease. Last, because our endpoint was local control (radiographic or symptomatic), the unit of analysis was each lesion, not each patient. It is possible that certain factors, including tumor biology, which is difficult to account for without tissue biopsy, could favor one treatment over the other.
Based on our results and other publications, the thought that RCC is truly radioresistant may no longer be true with current era radiation treatment with SBRT. Presently, a phase 1 study of dose escalation using SBRT for primary RCC is being performed, with preliminary findings showing high rates of progression-free survival.25 Future prospective studies are needed to evaluate efficacy and toxicity of SBRT in the setting of oligometastatic disease for RCC. With the current published single-institutional studies available, SBRT appears to significantly improve local control rates and symptom control for metastatic RCC to the bone and should be considered for these patients.
Acknowledgments
Sources of support: This work was supported by the Paul Calabresi Career Development Award for Clinical Oncology (K12); the American Cancer Society Institutional Research Grant #57-001-53; and the National Institutes of Health grant #K12 CA086913. Its contents are solely the responsibility of the authors and do not necessarily represent the views of the National Institutes of Health. We would like to acknowledge Fundación Aramont for their support in M.T.B.'s research activities.
Footnotes
Conflicts of interest: None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.De Meerleer G, Khoo V, Escudier B, et al. Radiotherapy for renal-cell carcinoma. Lancet Oncol. 2014;15:e170–e177. doi: 10.1016/S1470-2045(13)70569-2. [DOI] [PubMed] [Google Scholar]
- 3.Oguchi M, Ikeda H, Watanabe T, et al. Experiences of 23 patients > or = 90 years of age treated with radiation therapy. Int J Radiat Oncol Biol Phys. 1998;41:407–413. doi: 10.1016/s0360-3016(98)00052-2. [DOI] [PubMed] [Google Scholar]
- 4.Kavanagh BD, Timmerman RD. Stereotactic Body Radiation Therapy. Lippincott Williams & Wilkins; Baltimore, MD: 2005. [Google Scholar]
- 5.Wersall PJ, Blomgren H, Lax I, et al. Extracranial stereotactic radiotherapy for primary and metastatic renal cell carcinoma. Radiother Oncol. 2005;77:88–95. doi: 10.1016/j.radonc.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 6.Svedman C, Sandstrom P, Pisa P, et al. A prospective phase II trial of using extracranial stereotactic radiotherapy in primary and metastatic renal cell carcinoma. Acta Oncol. 2006;45:870–875. doi: 10.1080/02841860600954875. [DOI] [PubMed] [Google Scholar]
- 7.Zelefsky MJ, Greco C, Motzer R, et al. Tumor control outcomes after hypofractionated and single-dose stereotactic image-guided intensity-modulated radiotherapy for extracranial metastases from renal cell carcinoma. Int J Radiat Oncol Biol Phys. 2012;82:1744–1748. doi: 10.1016/j.ijrobp.2011.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ranck MC, Golden DW, Corbin KS, et al. Stereotactic body radiotherapy for the treatment of oligometastatic renal cell carcinoma. Am J Clin Oncol. 2013;36:589–595. doi: 10.1097/COC.0b013e31825d52b2. [DOI] [PubMed] [Google Scholar]
- 9.Jhaveri PM, Teh BS, Paulino AC, et al. A dose-response relationship for time to bone pain resolution after stereotactic body radiotherapy (SBRT) for renal cell carcinoma (RCC) bony metastases. Acta Oncol. 2012;51:584–588. doi: 10.3109/0284186X.2011.652741. [DOI] [PubMed] [Google Scholar]
- 10.Rose PS, Laufer I, Boland PJ, et al. Risk of fracture after single fraction image-guided intensity-modulated radiation therapy to spinal metastases. J Clin Oncol. 2009;27:5075–5079. doi: 10.1200/JCO.2008.19.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stinauer MA, Kavanagh BD, Schefter TE, et al. Stereotactic body radiation therapy for melanoma and renal cell carcinoma: Impact of single fraction equivalent dose on local control. Radiat Oncol. 2011;6:34. doi: 10.1186/1748-717X-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen QN, Shiu AS, Rhines LD, et al. Management of spinal metastases from renal cell carcinoma using stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:1185–1192. doi: 10.1016/j.ijrobp.2009.03.062. [DOI] [PubMed] [Google Scholar]
- 13.Gerszten PC, Burton SA, Ozhasoglu C, et al. Stereotactic radiosurgery for spinal metastases from renal cell carcinoma. J Neurosurg Spine. 2005;3:288–295. doi: 10.3171/spi.2005.3.4.0288. [DOI] [PubMed] [Google Scholar]
- 14.Nelson JW, Yoo DS, Sampson JH, et al. Stereotactic body radiotherapy for lesions of the spine and paraspinal regions. Int J Radiat Oncol Biol Phys. 2009;73:1369–1375. doi: 10.1016/j.ijrobp.2008.06.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balagamwala EH, Angelov L, Koyfman SA, et al. Single-fraction stereotactic body radiotherapy for spinal metastases from renal cell carcinoma. J Neurosurg Spine. 2012;17:556–564. doi: 10.3171/2012.8.SPINE12303. [DOI] [PubMed] [Google Scholar]
- 16.Greco C, Zelefsky MJ, Lovelock M, et al. Predictors of local control after single-dose stereotactic image-guided intensity-modulated radiotherapy for extracranial metastases. Int J Radiat Oncol Biol Phys. 2011;79:1151–1157. doi: 10.1016/j.ijrobp.2009.12.038. [DOI] [PubMed] [Google Scholar]
- 17.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: Updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2:S94–S100. doi: 10.1097/JTO.0b013e318074de34. [DOI] [PubMed] [Google Scholar]
- 19.Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27:1572–1578. doi: 10.1200/JCO.2008.19.6329. [DOI] [PubMed] [Google Scholar]
- 20.Chen FH, Chiang CS, Wang CC, et al. Radiotherapy decreases vascular density and causes hypoxia with macrophage aggregation in TRAMP-C1 prostate tumors. Clin Cancer Res. 2009;15:1721–1729. doi: 10.1158/1078-0432.CCR-08-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kioi M, Vogel H, Schultz G, et al. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest. 2010;120:694–705. doi: 10.1172/JCI40283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song CW, Cho LC, Yuan J, et al. Radiobiology of stereotactic body radiation therapy/stereotactic radiosurgery and the linear-quadratic model. Int J Radiat Oncol Biol Phys. 2013;87:18–19. doi: 10.1016/j.ijrobp.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 23.Jonasch E, Futreal PA, Davis IJ, et al. State of the science: An update on renal cell carcinoma. Mol Cancer Res. 2012;10:859–880. doi: 10.1158/1541-7786.MCR-12-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wersall PJ, Blomgren H, Pisa P, et al. Regression of non-irradiated metastases after extracranial stereotactic radiotherapy in metastatic renal cell carcinoma. Acta Oncol. 2006;45:493–497. doi: 10.1080/02841860600604611. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan ID, Redrosa I, Martin C, et al. Results of a phase I dose escalation study of stereotactic radiosurgery for primary renal tumors. Int J Radiat Oncol Biol Phys. 2010;78:S191. [Google Scholar]