Abstract
Up-regulation of proinflammatory cytokines and chemokines in brain (“neuroinflammation”) accompanies neurological disease and neurotoxicity. Previously, we documented a striatal neuroinflammatory response to acute administration of a neurotoxic dose of methamphetamine (METH), i.e. one associated with evidence of dopaminergic terminal damage and activation of microglia and astroglia. When we used minocycline to suppress METH-induced neuroinflammation, indices of dopaminergic neurotoxicity were not affected, but suppression of neuroinflammation was incomplete. Here, we administered the classic anti-inflammatory glucocorticoid, corticosterone (CORT), in an attempt to completely suppress METH-related neuroinflammation. METH alone caused large increases in striatal proinflammatory cytokine/chemokine mRNA and subsequent astrocytic hypertrophy, microglial activation, and dopaminergic nerve terminal damage. Pre-treatment of mice with acute CORT failed to prevent neuroinflammatory responses to METH. Surprisingly, when mice were pre-treated with chronic CORT in the drinking water, an enhanced striatal neuroinflammatory response to METH was observed, an effect that was accompanied by enhanced METH-induced astrogliosis and dopaminergic neurotoxicity. Chronic CORT pre-treatment also sensitized frontal cortex and hippocampus to mount a neuroinflammatory response to METH. Because the levels of chronic CORT used are associated with high physiological stress, our data suggest that chronic CORT therapy or sustained physiological stress may sensitize the neuroinflammatory and neurotoxicity responses to METH.
Keywords: glucocorticoids, methamphetamine, microglial activation, neuroinflammation, neurotoxicity, stress
Neuroinflammation has been associated with a variety of neurological diseases (for a review see: Prinz et al. 2011) and with neurotoxicant-induced damage to the CNS (for reviews see: Block et al. 2007; Sriram and O’Callaghan 2007; Kraft and Harry 2011). Whether these proinflammatory responses represent causes or consequences of neural damage have yet to be elucidated (Graeber and Streit 2010; Streit 2010). Substituted amphetamines are one class of compounds known to cause neurotoxicity (for reviews see: Bowyer and Holson 1995; O’Callaghan and Miller 2005; Riddle et al. 2006; Gudelsky and Yamamoto 2008; Krasnova and Cadet 2009; Silva et al. 2010; Yamamoto et al. 2010). For example, methamphetamine (METH) causes damage to dopaminergic nerve terminals in the striatum, as evidenced by loss of tyrosine hydroxylase (TH) and dopamine (DA), and an accompanying astrogliosis and silver degeneration staining (Ellinwood and Escalante 1970; Seiden et al. 1976; Hotchkiss et al. 1979; Bowyer et al. 1994; Miller and O’Callaghan 1994; O’Callaghan and Miller 1994, 2005; Bowyer and Holson 1995; Ladenheim et al. 2000; Thomas et al. 2004b; Fleckenstein et al. 2007; Krasnova and Cadet 2009). A potential role for neuroinflammation in these neurotoxic effects has been evaluated only recently (Thomas et al. 2004a,b; Sriram et al. 2006). For example, early in the time course of METH-induced damage, we and others (LaVoie et al. 2004; Thomas et al. 2004a,b; Kuhn et al. 2006; Sriram et al. 2006) observed elevations in proinflammatory cytokines and chemokines in striatum, findings consistent with microglial activation in the target region. Suppression of this proinflammatory response by pre-treating with minocycline did not affect METH-induced dopaminergic neurotoxicity and astrogliosis (Sriram et al. 2006), findings that argued for a consequence rather than a causal role of neuroinflammation in METH neurotoxicity. Nevertheless, the enhanced expression of key proinflammatory mediators implicated in neural damage, e.g. Tumor necrosis factor (TNF)-α (Sriram and O’Callaghan 2007; Tansey et al. 2007; McCoy and Tansey 2008), was not completely blocked by pre-treatment with minocycline prior to METH (Sriram et al. 2006). Therefore, the possibility remains for a role of neuroinflammation in METH neurotoxicity.
In this investigation, an effort was made to achieve a greater reduction in the neuroinflammation caused by METH exposure by acute and chronic pre-treatment with the classic anti-inflammatory glucocorticoid, corticosterone (CORT). While acute CORT pre-treatment was able to dampen some of the METH-induced inflammation; chronic (1-week) pre-treatment with CORT in the drinking water caused greatly exacerbated expression of proinflammatory cytokines and chemokines in target and non-target regions. This elevation of neuroinflammation was accompanied by greater gliosis and dopaminergic nerve terminal damage. These findings suggest that chronic exogenous CORT and, perhaps, persistent high physiological stress levels of endogenous CORT, can sensitize the CNS to neuroinflammatory and neurotoxic effects of METH.
Materials and methods
Materials
The following drugs and chemicals were kindly provided by or obtained from the sources indicated: d-METH (Sigma Chemical Co., St. Louis, MO, USA), CORT (Steraloids, Inc., Newport, RI, USA) and bicinchoninic acid protein assay reagent, and bovine serum albumin (Pierce Chemical Co., Rockford, IL, USA). The materials used in the Glial fibrillary acidic protein (GFAP) assay have been described in detail (O’Callaghan 1991, 2002). The materials used in the TH immunoassay have been described previously (O’Callaghan et al. 1990; Sriram et al. 2004, 2006). All other reagents and materials were of at least analytical grade and were obtained from a variety of commercial sources.
Animals
Male C57BL/6J mice (n = 5 mice per group) 4–6 weeks of age were purchased from Jackson Labs (Bar Harbor, ME, USA). All procedures were performed under protocols approved by the Institutional Animal Care and Use Committee of the Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, and the animal colony was certified by the American Association for Accreditation of Laboratory Animal Care. Upon receipt, the mice were housed individually in a temperature-controlled (21 ± 1°C) and humidity-controlled (50 ± 10%) colony room maintained under filtered positive-pressure ventilation on a 12-h light/12-h dark cycle beginning at 0600 EDT. The plastic tub cages were 46 cm in length by 25 cm in width by 15 cm in height; cage bedding consisted of heat-treated pine shavings spread at a depth of approximately 4 cm. Food (Purina rat/mouse chow) and water were available ad libitum.
Dosing
Acute CORT was given as a single s.c. dose of 20 mg/kg 30 min prior to METH (20 mg/kg, s.c.) or vehicle (saline 0.9%) treatment. Chronic CORT was given in the drinking water (400 mg/L in 1.2% EtOH) for 1 week prior to METH (20 mg/kg, s.c.) or vehicle (saline 0.9%) treatment. The latter regimen of CORT was chosen to mimic high physiological stress levels of this hormone, levels known to cause immunosuppression as evidenced by marked involution of the thymus (e.g. O’Callaghan et al. 1991). Mice were killed by decapitation at 12 or 72 h post METH or vehicle injection. These time points correspond to peak increases in expression of proinflammatory cytokines and astrogliosis, respectively, following administration of METH (Sriram et al. 2006).
Core temperature
Rectal temperature was recorded with a Bat-10 thermometer coupled to a RET-3 mouse rectal probe (Physitemp, Inc., Clinton, NJ, USA) lubricated with mineral oil as described previously (Miller and O’Callaghan 1994).
Brain preparation for immunohistochemistry
At 72 h post METH or vehicle injection, mice were anesthetized with sleep away (0.1 mL; Fort Dodge Animal Health, Fort Dodge, IA, USA) and transcardially perfused with 0.9% saline followed by 10% formalin. The brains were then removed, placed in 10% formalin, and kept at 4°C until processed for immunohistochemistry.
Histochemistry and immunohistochemistry
Coronal sections 40-μm thick were cut and collected in 2% formaldehyde with 0.1 M sodium phosphate buffer and stored at 4°C until processing. To visualize dopamine-containing neurons and processes, TH immunolabel was detected using a rabbit antiserum to TH (1 : 2000; Chemicon, Temecula, FL, USA) diluted in 0.1 M phosphate buffer with 0.3% Triton-X. This signal was amplified using the avidin and biotinylated horseradish peroxidase macromolecular complex (ABC; Vector Laboratories, Burlingame, CA, USA), and visualized with 0.4 mg/mL of 3,3′-diaminobenzidine (DAB) in 50 mM Tris buffer. Images were obtained using a SenSys cooled CCD digital camera mounted on a Nikon Eclipse E400 light/epifluorescent microscope and MetaView Imaging Software (advanced Scientific, Inc., Meraux, LA, USA). The isolectin B4-procedure of Streit (1990) was used to label microglia. Sections were incubated overnight at 4°C in a solution of B4 isolectin from Griffoniasimplicifolia (10 μg/mL; Sigma) coupled to horseradish peroxidase, and the binding sites were visualized with DAB and H2O2. GFAP immunolabel was detected using a rabbit antiserum to GFAP (1 : 1000; DAKO) overnight at 4°C, and then the ABC method and DAB were applied as described above.
Brain dissection and tissue preparation
Immediately after decapitation, whole brains were removed from the skull with the aid of blunt curved forceps. Striatum, hippocampus, and frontal cortex (includes motor, insular, and pyriform cortex) were dissected free hand on a thermoelectric cold plate (Model TCP-2, Aldrich Chemical Co., Milwaukee, WI, USA) using a pair of fine curved forceps (Roboz, Washington, DC, USA). Brain regions from one side of the brain were frozen at −85°C and used for subsequent isolation of total RNA; brain regions from the other side of the brain were used for total and specific protein analysis. These regions were weighed, homogenized with a sonic probe (model XL-2005, Heat Systems, Farmingdale, NY, USA) in 10 volumes of hot (90–95°C) 1% sodium dodecyl sulfate, and stored frozen at −70°C before total protein assay and immunoassay of GFAP and TH. A separate set of mice were used to generate striatal sample for analyses of dopamine and metabolites.
RNA isolation, cDNA synthesis, and real-time PCR amplification
Total RNA from striatum, hippocampus, and cortex were isolated using Trizol® reagent (Invitrogen, Grand Island, NY, USA) and Phase-lock heavy gel (Eppendorf AG, Hamburg, Germany). The RNA was further cleaned using RNeasy mini spin column (Qiagen, Valencia, CA, USA). Total RNA (1 μg) was reverse transcribed to cDNA using SuperScript™ II RNase H− and oligo (dT)12-18 primers (Invitrogen) in a 20 μL reaction. Real-time PCR analysis of Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), TNF-α, IL-6, CCL-2, IL-1β, Leukemia inhibitory factor (LIF), and Oncostatin M (OSM) was performed in an ABI PRISM 7700 sequence detection system (Applied Biosystems, Carlsbad, CA, USA) in combination with TaqMan® chemistry. Specific primers and dual-labeled internal fluorogenic (FAM/TAMRA) probe sets (TaqMan® Gene Expression Assays) for these genes were procured from Applied Biosystems and used according to the manufacturer’s recommendations. All PCR amplifications (40 cycles) were performed in a total volume of 50 μL, containing 1 μL cDNA, 2.5 μL of the specific Assay of Demand primer/probe mix, and 25 μL of Taqman® Universal master mix. Sequence detection software (version 1.7; Applied Biosystems) results were exported as tab-delimited text files and imported into Microsoft Excel for further analysis. Relative quantification of gene expression was performed using the comparative threshold (CT) method as described by the manufacturer (Applied Biosystems; User Bulletin 2). Changes in mRNA expression levels were calculated after normalization to Glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The ratios obtained after normalization are expressed as fold change over corresponding saline-treated controls.
Protein assay
Total protein was determined by the bicinchoninic acid method (Smith et al. 1985) using bovine serum albumin as standard.
GFAP and TH assays
GFAP was assayed in accordance with a previously described procedure (O’Callaghan 1991, 2002). In brief, a rabbit polyclonal antibody to GFAP was coated on the wells of Immulon-2 microliter plates (Thermo Labsystems, Franklin, MA). The sodium dodecyl sulfate homogenates and standards were diluted in phosphate-buffered saline (pH 7.4) containing 0.5% Triton X-100. After blocking non-specific binding with 5% non-fat dry milk, aliquots of the homogenate and standards were added to the wells and incubated. Following washes, a mouse monoclonal antibody to GFAP (1 : 400; DAKO, Carpenteria, CA, USA) was added to ‘sandwich’ the GFAP between the two antibodies. An alkaline phosphatase-conjugated antibody directed against mouse IgG was then added and a colored reaction product was obtained by subsequent addition of the enzyme substrate p-nitrophenol. Quantification was achieved by spectrophotometry of the colored reaction product at 405 nm in a microplate reader, Spectra Max Plus, and analyzed using Soft Max Pro Plus software (Molecular Devices, Sunnyvale, CA, USA). The amount of GFAP in the samples was calculated as micrograms GFAP per milligram total protein.
TH holoenzyme protein was assessed using a fluorescence-based ELISA developed in the laboratory (Sriram et al. 2004). The protocol was essentially similar to that for the GFAP assay except that a mouse monoclonal antibody to TH (1 : 400; Sigma) was used as the plate capture antibody and a rabbit polyclonal antibody was used to ‘sandwich’ TH protein. The amount of sandwich antibody bound to TH was then detected using a peroxidase-labeled antibody directed against rabbit IgG. Peroxidase activity was detected using the fluorogenic substrate Quantablu (Pierce), which has excitation and emission maxima of 325 and 420 nm, respectively (read at 320/405 nm). The amount of TH in the samples was calculated and expressed as micrograms TH per milligram total protein.
Analysis of dopamine and metabolites
Dopamine and its metabolites were quantified by high-performance liquid chromatography with electrochemical detection (HPLC-EC; Waters) using the method detailed in Nishi et al. (2008). Briefly, tissues were homogenized in 300 μL of ice-cold 0.2 M perchloric acid, containing 1 um dihydroxybenzylamine as internal standard, and centrifuged at 10 000 g for 10 min at 4°C. The supernatant was filtered through a 0.2 μm membrane, and an aliquot (10 μL) was injected from a temperature-controlled (4°C) automatic sample injector (Waters717plus autosampler) connected to a Waters 515 HPLC pump, and catecholamines were separated on a C18 reverse-phase column (LC-18 RP; Waters SYMMETRY, 25 cm × 4.6 mm; 5 μm) and electrochemically detected (Waters 464 Pulsed Electrochemical Detector, Waters, Milford, MA, USA) and analyzed using Millennium software (Waters). Recovery of each analyte was adjusted with respect to the internal standard and quantified from a standard curve. The levels of dopamine and its metabolites were expressed as micrograms per gram of wet tissue.
Statistics
Statistical analysis of data was performed utilizing SigmaPlot (v 11.0). Core temperature data were analyzed using a three-way analysis of variance (ANOVA). PCR data were analyzed using a two-way ANOVA. GFAP and TH protein concentration was analyzed using one-way ANOVA. All ANOVAs were followed by Student-Newman–Keul’s post hoc test for multiple comparisons.
Results
Chronic CORT Enhances METH-induced Expression of Some Proinflammatory Cytokines/Chemokines in Striatum
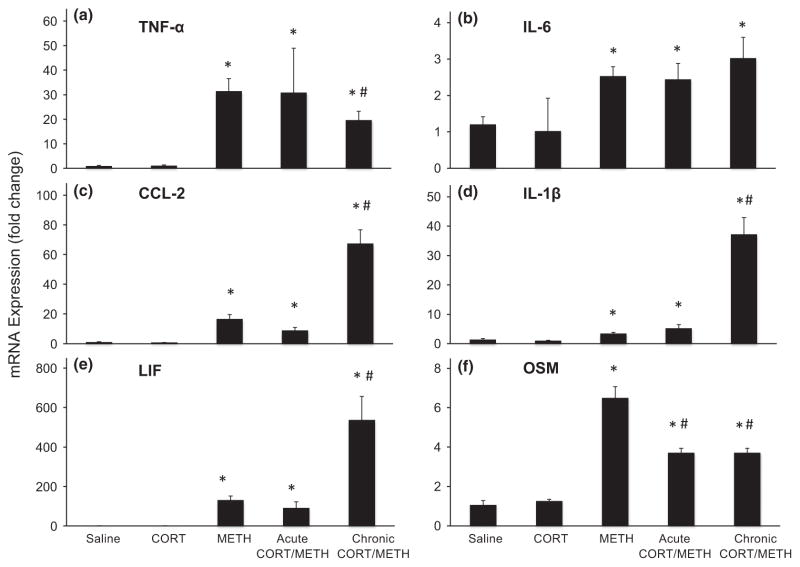
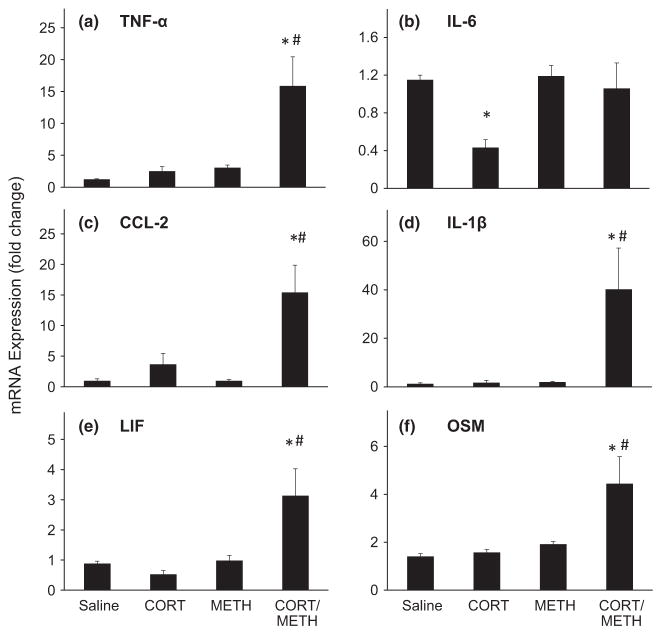
In agreement with our previous findings, a single dose of METH results in enhanced expression of mRNA for a variety of proinflammatory cytokines and chemokines in the mouse striatum at 12 h post dosing (Fig. 1). These effects of METH alone range from 2.5-fold for IL-6 to over 130-fold for LIF. When we attempted to suppress these effects by pre-treating with an acute anti-inflammatory dose of CORT, we were largely unsuccessful. Only the METH-induced expression of CCL-2 and OSM were reduced by pre-treatment with CORT with the maximum suppression reaching only a 40% decrease of the METH-induced increase in OSM.
Fig. 1.
Chronic corticosterone (CORT) enhances methamphetamine (METH)-induced expression of some proinflammatory cytokines/chemokines in striatum. Acute CORT (20 mg/kg, s.c.) was administered 30 min prior to METH (20 mg/kg, s.c.), and chronic CORT (400 mg/L in 1.2% EtOH) was administered for 1 week in the drinking water prior to METH (20 mg/kg, s.c.) or corresponding saline (0.9%, s.c.) controls. At 12 h post METH or saline injection, mice were killed and total RNA was extracted from the striatum. Real-time PCR analysis was performed for TNF-α (a), IL-6 (b), CCL-2 (c), IL-1β (d), LIF (e), and OSM (f). Bars represent mean ± SEM (n = 5 mice/group). Statistical significance was measured by two-way ANOVA with Student-Newman–Keul’s Method of post hoc analysis. Statistical significance of at least p < 0.05 is denoted by * as compared to control and # as compared to METH-treated mice.
We reasoned that a more sustained anti-inflammatory pre-treatment would be required to suppress or block the METH-induced expression of proinflammatory mediators. Therefore, we administered CORT in the drinking water for a week prior to administration of METH. This CORT regimen was known to be immunosuppressive as evidenced by thymic involution, suggesting that this pre-treatment should be sufficient to attenuate expression of proinflammatory mediators. While we did observe a slight attenuation of METH-induced expression of TNF-α and OSM by chronic CORT administration, surprisingly, the dominant effect of this regimen was to markedly exacerbate the proinflammatory effects of METH at 12 h post dosing (Fig. 1). The expression of CCL-2, IL-1β, and LIF in the chronic CORT/METH groups reached 67-, 37-, and 537-fold, respectively. As proinflammatory cytokines and chemokines are released by activated microglia and astroglia, our unexpected findings for the combination of chronic CORT administration with METH prompted us to examine the activation of microglia and astroglia following the chronic CORT/METH exposure.
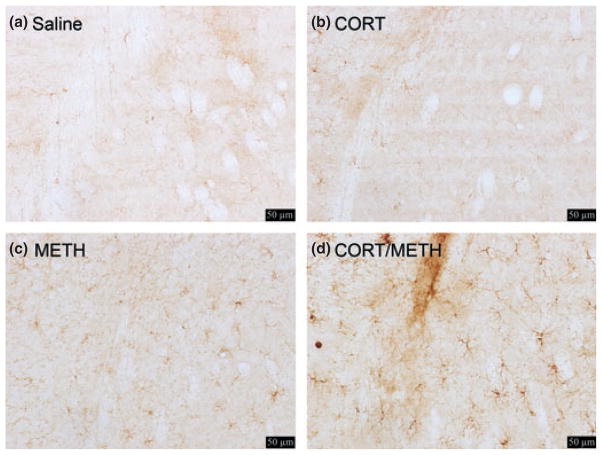
Chronic CORT enhances METH-induced activation of microglia in striatum
Isolectin B staining of activated microglia in the striatum is shown in Figure 2. Enhanced Isolectin B staining is known to reflect an activated state of microglia associated with neuropathology (Streit and Kreutzberg 1987; Kreutzberg 1996). At 72 h post dosing, METH alone (panel c) activated few microglia, whereas pre-treatment with chronic CORT greatly enhanced the activation of microglia because of METH (panel d).
Fig. 2.
Chronic corticosterone (CORT) enhances methamphetamine (METH)-induced activation of microglia in striatum. Mice were treated with Chronic CORT for 1 week prior to METH or saline injection. Photomicrographs are representative images of the striatum at 20× magnification of Saline (a), chronic CORT (b), METH (c), and chronic CORT pre-treated METH (d) mice at 3 days post METH or saline injection. Scale bars: 50 μm.
Chronic CORT enhances METH-induced astrogliosis in striatum
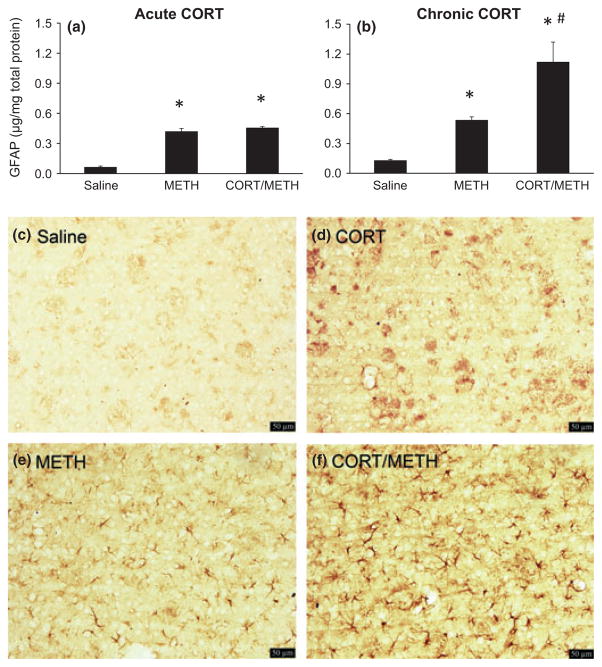
GFAP immunoassay and immunohistochemistry were used to assess striatal astrogliosis quantitatively and qualitatively, respectively. In agreement with our previous findings (e.g. Sriram et al. 2006), METH administration increased striatal concentration of GFAP by over 4-fold at 72 h post dosing (Fig. 3a and b). Acute pre-treatment with CORT (20 mg/kg) did not affect the METH-induced increase in GFAP (Fig. 3a), whereas pre-treatment with chronic CORT greatly enhanced (to eight-fold) the increase in GFAP because of METH (Fig. 3b). Neither acute nor chronic CORT alone affected the concentration of GFAP in striatum (data not shown).
Fig. 3.
Chronic corticosterone (CORT) enhances methamphetamine (METH)-induced astrogliosis in striatum. Astrogliosis was quantified by Glial fibrillary acidic protein (GFAP) ELISA (a and b) and qualitatively demonstrated by GFAP immunostaining (c–f). (a and b) GFAP was assayed 3 days after either acute CORT (a) or chronic CORT (b) pre-treatment of METH-exposed mice. Bars represent mean ± SEM (n = 5 mice/group). Statistical significance was measured using a one-way ANOVA with Student-Newman–Keul’s Method post hoc analysis for GFAP protein concentration. Statistical significance of at least p < 0.05 is denoted by * as compared to control and # as compared to METH-treated mice. (c–f) Mice were pre-treated with chronic CORT and then injected with either METH or Saline. At 3 days post injection of METH or Saline, mice were killed and GFAP immunolabeling was performed. Photomicrographs are representative images of the striatum at 20× magnification of Saline (c), chronic CORT (d), METH (e), and chronic CORT pre-treated METH (f) mice. Scale bars: 50 μm.
Consistent with the known low-level expression of GFAP in striatum relative to other brain areas (Martin and O’Callaghan 1995), very few astrocytic profiles were seen by immunohistochemistry of GFAP in saline or CORT control groups. METH administration resulted in the appearance of numerous astrocytic profiles based on GFAP immunostaining (Fig. 3e). Pre-treatment with chronic CORT increased the size of astroglial somata and processes because of METH (Fig. 3). Thus, GFAP immunoassay and immunohistochemistry data are consistent and confirm that chronic CORT pre-treatment exacerbates METH-induced astrogliosis (panel f). The presence of astrogliosis is indicative of underlying neural damage (O’Callaghan and Sriram 2005) and, in the case of METH; it is consistent with dopaminergic nerve terminal damage in striatum. Therefore, the results obtained for GFAP were suggestive of enhanced METH-induced dopaminergic neurotoxicity following pre-treatment with chronic CORT.
Chronic CORT enhances METH-induced dopaminergic neurotoxicity in striatum
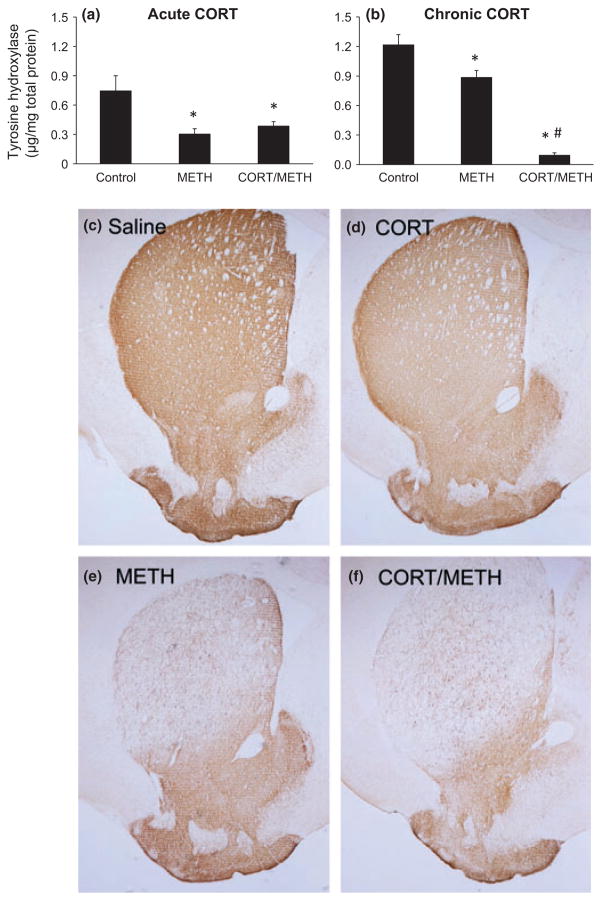
TH immunoassay and immunohistochemistry were used to assess striatal dopaminergic neurotoxicity quantitatively and qualitatively, respectively. In agreement with our previous findings (e.g. Sriram et al. 2006), METH administration decreased striatal concentration of TH by greater than 25% at 72 h post dosing (Fig. 4a and b). Acute pre-treatment with CORT (20 mg/kg) did not affect the METH-induced decrease in TH (Fig. 4a), whereas pre-treatment with chronic CORT greatly enhanced (to 10% of control) the decrease in TH because of METH (Fig. 4b). Neither acute nor chronic CORT alone affected the concentration of TH in striatum (data not shown).
Fig. 4.
Chronic corticosterone (CORT) enhances methamphetamine (METH)-induced dopaminergic neurotoxicity in striatum. Tyrosine hydroxylase was quantified by TH ELISA (a and b) and qualitatively demonstrated by TH immunostaining (c–f). (a and b) TH was assayed 3 days after either acute CORT (a) or chronic CORT (b) pre-treatment of METH-exposed mice. Bars represent mean ± SEM (n = 5 mice/group). Statistical significance was measured using a one-way ANOVA with Student-Newman–Keul’s Method post hoc analysis. Statistical significance of at least p < 0.05 is denoted by * as compared to control and # as compared to METH-treated mice. (c–f) Mice were pre-treated with chronic CORT followed by METH or Saline injection. At 3 days post injection of METH or Saline, mice were killed and TH immunolabeling was performed. Photomicrographs are representative images of the striatum at 2× magnification of Saline (c), chronic CORT (d), METH (e), and chronic CORT pre-treated METH (f) mice.
METH administration resulted in decreased striatal TH immunostaining, consistent with damage to dopaminergic nerve terminals in this brain region (Fig. 4e). Pre-treatment with chronic CORT enhanced the decrements in TH immunostaining in striatum (Fig. 4f). Thus, TH immunoassay and immunohistochemistry data are consistent and suggest that chronic CORT pre-treatment exacerbates METH-induced dopaminergic neurotoxicity (panel f), results in strong agreement with the data already obtained for GFAP levels and staining (Fig. 3b and f). The decrements in TH seen with METH alone and with prior treatment with chronic CORT also were reflected in corresponding decrements in striatal dopamine levels (data not shown).
Chronic CORT sensitizes the hippocampus to METH-induced expression of proinflammatory cytokines/chemokines
We explored the possibility that the effects of chronic CORT pre-treatment prior to METH might be extended to brain regions outside the striatum. Chronic CORT treatment alone did not affect cytokine and chemokine expression in the hippocampus, except for IL-6, which was reduced by over 50% (Fig. 5). At 12 h post dosing, METH treatment alone did not cause a change in proinflammatory cytokine or chemokine expression in the hippocampus, findings consistent with a lack of METH-induced damage to this region using this dosing regimen. In contrast, mice pre-treated with chronic CORT and given METH showed very large increases in mRNA expression for most of the cytokines/chemokines examined. TNF-α, CCL-2, IL-1β, LIF, and OSM showed 15-, 15-, 40-, 3- and 4.5-fold increases, respectively, compared with METH alone or with chronic CORT alone. IL-6 mRNA expression was elevated in the chronic CORT and METH-treated group as well, but only in comparison with the chronic CORT-treated controls. Together these data show that chronic CORT pre-treatment sensitizes the hippocampus to mount a proinflammatory response to METH, findings suggestive of activation of microglia and astrocytes.
Fig. 5.
Chronic corticosterone (CORT) sensitizes the hippocampus to methamphetamine (METH)-induced expression of proinflammatory cytokines/chemokines. Chronic CORT (400 mg/L in 1.2% EtOH) was administered for 1 week in the drinking water prior to METH (20 mg/kg, s.c.) or saline (0.9%, s.c.) exposure. At 12 h post METH or saline injection, mice were killed and total RNA was extracted from the hippocampus. Real-time PCR analysis was performed for TNF-α (a), IL-6 (b), CCL-2 (c), IL-1β (d), Leukemia inhibitory factor (LIF) (e), and OSM (f). Bars represent mean ± SEM (n = 5 mice/group). Statistical significance was measured using a two-way ANOVA with Student-Newman–Keul’s Method post hoc analysis. Statistical significance of at least p < 0.05 is denoted by * as compared to control and # as compared to METH-treated mice.
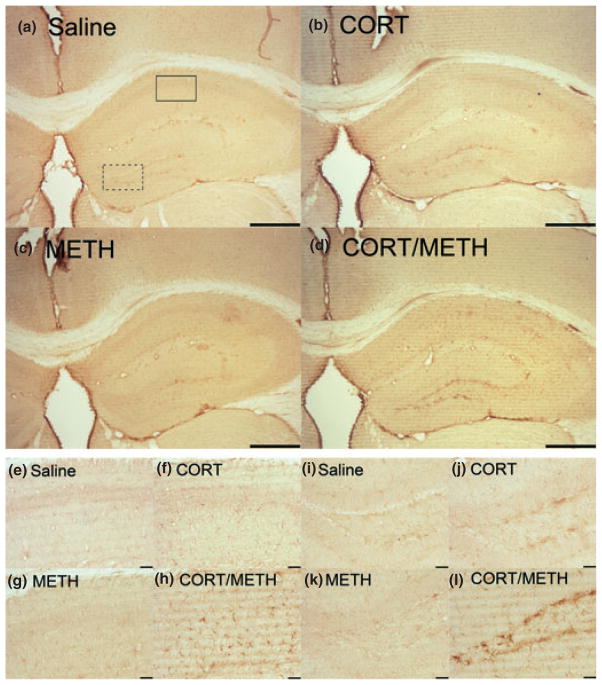
Chronic CORT sensitizes the hippocampus to METH-induced activation of microglia
Isolectin B staining of microglia in the hippocampus is shown in Figure 6. Neither chronic CORT alone, nor METH alone at 72 h post dosing caused a change in microglial staining in comparison to saline controls. However, when mice were pre-treated with chronic CORT and then given METH, there was an increase in activated microglia in multiple layers of the hippocampus (Fig. 6d). In Figure 6e–h and i–l, the CA1 and dentate gyrus (DG) regions, respectively, have been enlarged to show the activated microglia in these regions as a function of chronic CORT pre-treatment and METH exposure.
Fig. 6.
Chronic corticosterone (CORT) sensitizes the hippocampus to methamphetamine (METH)-induced activation of microglia. Mice were treated with chronic CORT for 1 week prior to METH or saline injection. Photomicrographs are representative images of Isolectin B staining in the hippocampus at 4× (a–d). The CA1 region of the hippocampus denoted by the solid box in (a) is shown at 20× magnification in (e–h). The DG region of the hippocampus denoted by the dotted box in (a) is shown at 20× magnification in (i–l). Scale bars: (a–d) 500 μm and (e–l) 50 μm.
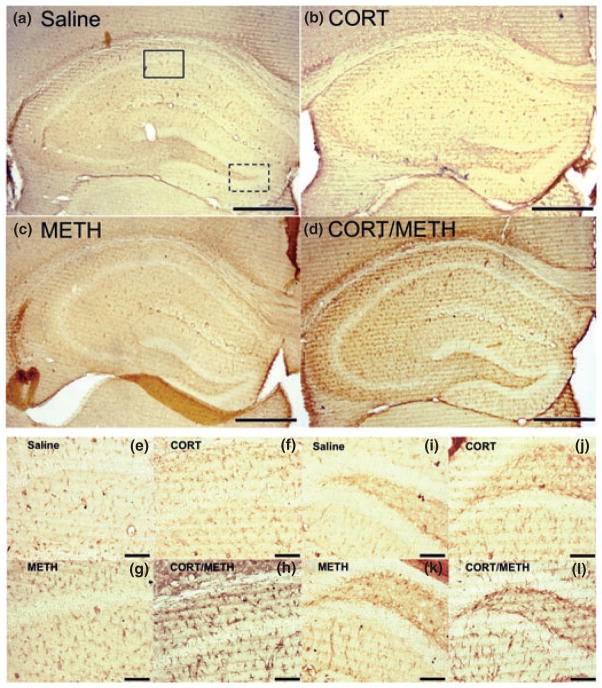
Chronic CORT sensitizes the hippocampus to METH-induced astrogliosis
GFAP immunostaining of astrocytes in the hippocampus is shown in Figure 7. Neither chronic CORT alone, nor METH alone at 72 h post dosing, caused a change in astrocyte staining in comparison to saline controls. However, when mice were pre-treated with chronic CORT and then given METH, there was evidence of astrocytic hypertrophy in multiple layers of the hippocampus (Fig. 7d). In Figure 7e–h and i–l, the CA1 and dentate gyrus (DG) regions, respectively, have been enlarged to show the hypertrophied astrocytes in these regions as a function of chronic CORT pre-treatment and METH exposure. Because microglial activation and astrocytic hypertrophy often occur at sites of CNS damage, these data suggest that chronic CORT pre-treatment prior to METH results in underlying damage to the hippocampus.
Fig. 7.
Chronic corticosterone (CORT) sensitizes the hippocampus to methamphetamine (METH)-induced astrogliosis. Mice were treated with chronic CORT for 1 week prior to METH or saline injection. Photomicrographs are representative images of Glial fibrillary acidic protein (GFAP) staining in the hippocampus at 4× (a–d). The CA1 region of the hippocampus denoted by the solid box in (a) is shown at 20× magnification in (e–h). The DG region of the hippocampus denoted by the dotted box in panel (a) is shown at 20× magnification in (i–l). Scale bars: (a–d) 500 μm and (e–l) 50 μm.
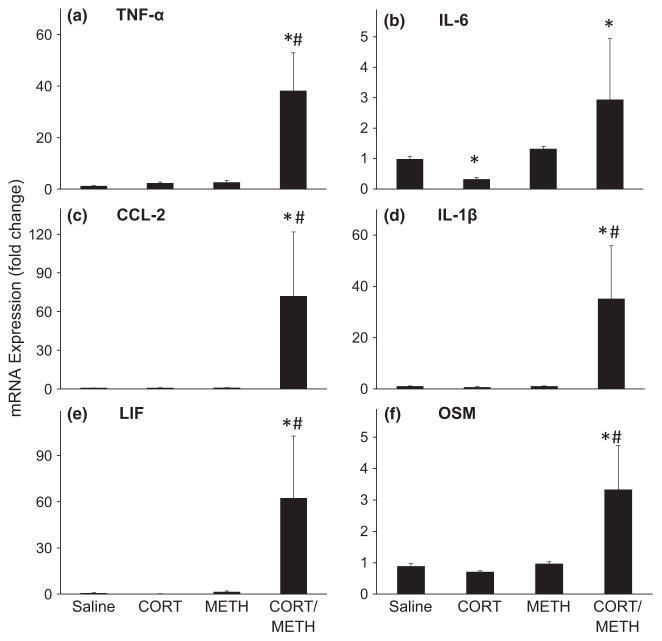
Chronic CORT sensitizes the frontal cortex to METH-induced expression of proinflammatory cytokines/chemokines
The piriform and frontal cortex have been implicated as a target for subtle neurotoxic effects of METH (Schmued and Bowyer 1997; Bowyer et al. 1998, 2004a), therefore, we evaluated the effects of chronic CORT pre-treatment prior to METH in these brain regions (Figs 8 and 9). Chronic CORT treatment alone did not affect cytokine and chemokine expression in the frontal cortex, except for IL-6, which was reduced by over 50% (Fig. 8). At 12 h post dosing, METH treatment alone did not cause a change in proinflammatory cytokine or chemokine expression in the frontal cortex, findings indicative of a lack of METH-induced damage to this region using our dosage regimen. In contrast, mice pre-treated with chronic CORT and given METH showed very large increases in mRNA expression for most of the cytokines/chemokines examined. TNF-α, CCL-2, IL-1β, LIF, and OSM showed 38-, 72-, 35-, 62-, and 3-fold increases, respectively, compared with METH alone or with chronic CORT alone. IL-6 mRNA expression was elevated in the chronic CORT and METH-treated group as well, but only in comparison with the chronic CORT-treated controls. As with our findings for hippocampus, these data show that chronic CORT pre-treatment sensitizes the frontal cortex to mount a proinflammatory response to METH, findings suggestive of activation of microglia and astrocytes
Fig. 8.
Chronic corticosterone (CORT) sensitizes the frontal cortex to methamphetamine (METH)-induced expression of proinflammatory cytokines/chemokines. Chronic CORT (400 mg/L in 1.2% EtOH) was administered for 1 week in the drinking water prior to METH (20 mg/kg, s.c.) or saline (0.9%, s.c.) controls. At 12 h post METH or saline injection, mice were killed and total RNA was extracted from the frontal cortex. Real-time PCR analysis was performed for TNF-α (a), IL-6 (b), CCL-2 (c), IL-1β (d), Leukemia inhibitory factor (LIF) (e), and OSM (f). Bars represent mean ± SEM (n = 5 mice/group). Because of high variance in the cortex sampling, the fold changes were log transformed prior to statistical analysis. Statistical significance was measured using a two-way ANOVA with Student-Newman–Keul’s Method post hoc analysis. Statistical significance of at least p < 0.05 is denoted by * as compared to control and # as compared to METH-treated mice.
Fig. 9.
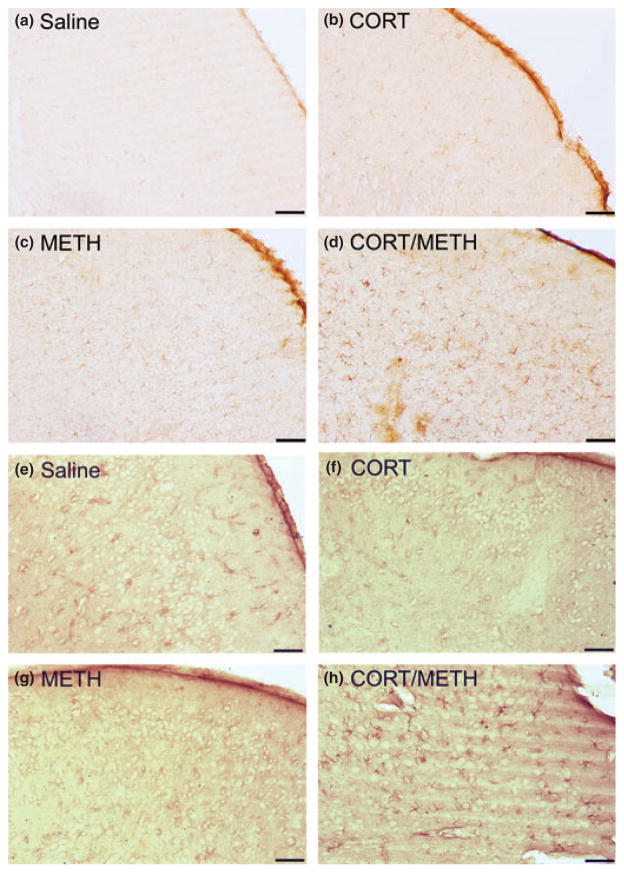
Chronic corticosterone (CORT) Sensitizes the Piriform Cortex to methamphetamine (METH)-induced Microglial Activation and Astrogliosis. Mice were treated with Chronic CORT for 1 week prior to METH or saline injection. Photomicrographs are representative images of Isolectin B (a–d) and GFAP (e–h) staining in the cortex at 20× magnification. Scale bars: 50 μm.
Chronic CORT sensitizes the piriform cortex to METH-induced microglial activation and astrogliosis
Isolectin B staining (a–d) and GFAP immunoreactivity (e–h) in the piriform cortex are shown in Figure 9. These images clearly show the activation of microglia (d) and astrocytic hypertrophy (h) caused by chronic CORT pre-treatment on the effects of METH at 72 h post dosing.
Chronic CORT does not affect METH-induced increase in core temperature
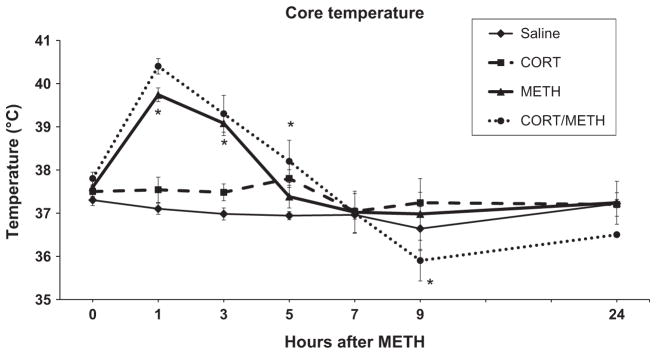
Variations in core temperature can have a marked influence on METH-induced neurotoxicity (Bowyer et al. 1992, 1994; Miller and O’Callaghan 1994; Bowyer and Holson 1995; O’Callaghan and Miller 2005). To determine if core temperature played a role in the effects observed in the present investigation, we examined the effects of CORT pre-treatment on METH-induced hyperthermia. Core temperature was measured via rectal probe at 0, 1, 3, 5, 7, 9, and 24 h (Fig. 10). METH administration alone caused the expected increase in core temperature and CORT pre-treatment alone also caused a slight rise in core temperature based on area under the curve analysis. The increase in core temperature because of METH was not augmented by prior treatment with CORT.
Fig. 10.
Chronic corticosterone (CORT) does not affect methamphetamine (METH)-induced increase in core temperature. Rectal temperature was recorded at 0, 1, 3, 5, 7, 9, and 24 h after METH (20 mg/kg, s.c.) or saline (0.9%, s.c.) injection in mice pre-treated with CORT (400 mg/L in 1.2% EtOH) in the drinking water for 1 week. Points represent mean ± SEM (n = 5 mice/group). Significance was measured using two-way ANOVA with Tukey’s post hoc analysis. * denotes statistical significance of at least p < 0.05 when compared to control mice.
Discussion
Damage to the CNS results in activation of microglia and astrocytes at sites of injury (Kreutzberg 1996; Eng et al. 2000; Norenberg 2005; O’Callaghan and Sriram 2005; Sofroniew 2005, 2009; Streit 2005). These cellular responses often are accompanied by enhanced expression of proinflammatory mediators, i.e., neuroinflammation (Prinz et al. 2011). Microglial and astroglial activation and the elaboration of proinflammatory cytokines and chemokines occur in conjunction with METH-induced damage to striatal dopaminergic nerve terminals (Thomas et al. 2004a; Sriram et al. 2006). These observations are consistent with glial activation and neuroinflammatory responses to METH neurotoxicity. Because these findings also raised the possibility of a contribution of neuroinflammation to METH neurotoxicity, we attempted to suppress neuroinflammatory responses to METH using pre-treatment with the anti-inflammatory tetracycline derivative, minocycline (Sriram et al. 2006). While we were able to dampen the expression of multiple cytokines and chemokines using this approach, METH-induced dopaminergic terminal damage and accompanying astrogliosis were not affected (Sriram et al. 2006). Because suppression of neuroinflammation was incomplete using minocycline, here we turned to acute and chronic administration of the anti-inflammatory glucocorticoid, CORT, as a potentially more effective means of blocking proinflammatory responses to METH. Acute pre-treatment with CORT was not effective in completely suppressing METH-associated neuroinflammation. More surprisingly, however, pre-treatment with chronic high physiological levels of CORT resulted in a marked proinflammatory rather than an anti-inflammatory response to METH-associated neuroinflammation. Moreover, this exaggerated inflammation because of chronic CORT pre-treatment was associated with exacerbated glial responses and exacerbated damage to striatal dopaminergic nerve terminals, deficits that approached those seen in models of Parkinson’s Disease (Jackson-Lewis and Przedborski 2007).
By surveying the expression of mRNA for key proinflammatory cytokines and chemokines, we confirmed that even a single dose of METH (20 mg/kg) results in striatal neuroinflammation involving multiple proinflammatory mediators. As we previously observed for pre-treatment with minocycline (Sriram et al. 2006), TNF-α also remained resistant to suppression following pre-treatment with acute CORT. Moreover, with the exception of minor suppression of expression of CCL-2 and OSM, none of the METH-induced inflammatory mediators were altered by CORT. These findings with acute CORT pre-treatment followed by METH stand in contrast to those observed with acute pre-treatment with CORT followed by administration of the known inflammogen, lipopolysaccharide (LPS). LPS-induced neuroinflammation, brain wide, was markedly attenuated by acute CORT pre-treatment (Kelly et al. 2011). These distinctions between the effects of acute CORT on METH versus LPS-related neuroinflammation suggest that the former involves damage-related microglial activation resistant to anti-inflammatory therapy, whereas the latter involves an acute phase response amenable to the anti-inflammatory effects of CORT.
By moving to chronic administration of CORT in the drinking water, we felt that a more sustained immunosuppression offered a better means to prevent striatal neuroinflammatory responses to METH. Not only did the week-long pre-treatment with CORT either fail (IL-6) or only marginally suppress (TNF-α, OSM) the expression of cytokines induced by METH, but extraordinarily large enhancements in METH-related cytokine (IL-1β, LIF) and chemokine (CCL-2) expression were caused by chronic CORT. Thus, the classic anti-inflammatory agent served as a proinflammatory stimulus for METH-related neuroinflammation. These data emphasize the complex nature of the regulation of proinflammatory responses to CNS injury (Sriram et al. 2006; O’Callaghan et al. 2008), as with systemic inflammogens (Frank et al. 2010), against a background of chronic immune suppression (Dhabhar 2009).
In the CNS, microglial activation is the most widely recognized cellular response associated with neuroinflammation (Kreutzberg 1996; Graeber and Streit 2010; Streit 2010; Prinz et al. 2011). Staining for activated microglia with isolectin confirmed such activation in striatum with METH alone and, consistent with qPCR data for IL-1β, LIF, and CCL-2, microglial activation was markedly enhanced with chronic CORT pre-treatment prior to METH. Activation of microglia and the enhanced expression of proinflammatory mediators constitute components of an acute phase response to systemic inflammation (Quan et al. 1999), effects distinct from, and that do not require, underlying CNS damage. Moreover, as we observed previously (Sriram et al. 2006), suppression of neuroinflammation by minocycline administration prior to METH does not protect against dopaminergic terminal loss or the induction of astrogliosis, two key features of METH neurotoxicity. Therefore, enhanced neuroinflammation and microglial activation because of chronic CORT prior to METH did not necessarily predict enhanced damage to dopaminergic terminals and accompanying enhancement of astrogliosis. The fact that we indeed did observe a linkage among enhanced microglia activation, enhanced astroglial activation, and increased damage to dopaminergic terminals because of chronic (but not acute) CORT suggested that the neuroinflammatory component of these responses constituted elements of a damage response. Thus, the priming/sensitization caused by chronic CORT appears to involve neural damaging aspects of neuroinflammation distinct from those merely associated with acute phase responses (Sugama et al. 2009; Frank et al. 2010). The mechanisms through which CORT acts chronically to exert a proinflammatory effect in the intact or injured CNS have been proposed (Sorrells et al. 2009), but largely remain to be identified and characterized (Sorrells and Sapolsky 2007; Sorrells et al. 2009).
CNS neurotoxicity because of METH is most pronounced in the neostriatum, but damage to frontal, amygaloid, piriform, and parietal cortex, as well as hippocampus, has been reported in both mice and rats (Bowyer et al. 1994, 1998, 2004a,b, 2008; O’Callaghan and Miller 1994; Schmued and Bowyer 1997; Eisch et al. 1998). These observations raised the possibility that our chronic CORT regimen would sensitize areas outside of striatum to mount a neuroinflammatory response to METH. The lack of an effect of METH alone on expression of proinflammatory mediators in frontal cortex or hippocampus is consistent with a lack of damage to either area with the single dosage of METH employed (20 mg/kg). In contrast, the emergence of neuroinflammation, microglial activation, and astrogliosis in both areas as a consequence of chronic CORT pre-treatment prior to METH, suggests that chronic CORT can make an otherwise non-vulnerable region into a target of METH neurotoxicity. Such effects with METH alone only are seen with much higher doses that result in significant blood–brain barrier damage and seizures (Bowyer and Ali 2006). Chronic CORT does not affect mouse brain levels of the METH analog, MDMA, given at high multiple doses (Johnson et al. 2004), making it unlikely to have affected METH brain levels in this study. Whether other areas of the CNS can be sensitized to METH damage by chronic CORT pre-treatment remains to be explored. The large decreases in TH and DA seen in striatum after pre-treatment with chronic CORT and METH, i.e., decrements equivalent to those seen in mouse models of Parkinson’s disease (Jackson-Lewis and Przedborski 2007), were suggestive of the possibility of nigral damage. While no overt loss of TH-positive neurons or accompanying astrogliosis was observed, further examination of this region by unbiased stereological cell counts and TH and GFAP immunoassays remains a priority.
While the classic view of glucocorticoids is one associated with their anti-inflammatory properties, a proinflammatory role for these adrenal steroids is not without precedent (see review by Sorrells and Sapolsky 2007; Dhabhar 2009). Prior administration of CORT sensitizes the neuroinflammatory response to systemically administered LPS (Munhoz et al. 2006; Frank et al. 2010). Glucocorticoids also may sensitize/prime the neuroinflammatory response to CNS disease and potentially chemically induced injury (Sorrells and Sapolsky 2007). These prior observations, taken together with the present data, are suggestive of a role of high physiological stress in enhancing expression of proinflammatory mediators in the CNS because of systemic inflammogens or CNS injury. Indeed, a variety of stress paradigms have been shown to activate microglia (Sugama et al. 2007; Kwon et al.2008) and potentiate CNS proinflammatory responses (De Pablos et al. 2006; Frank et al. 2006, 2011). Acute and chronic stress paradigms can have dramatically different effects at the cellular and molecular levels (Bowers et al. 2008; Dhabhar 2009). Thus, establishing any role for stressors and endogenous glucocorticoids in the effects we report here for exogenous CORT likely will require evaluation of multiple chronic stress paradigms implicated in priming CNS neuroinflammation (Johnson et al. 2002; Wohleb et al. 2011).
The data we have presented largely are descriptive with as yet no mechanistic underpinnings. Core temperature is a dominant determinant of METH-induced neurotoxicity, with any moderation likely influencing neurotoxic outcomes (Bowyer et al. 1992, 1994, 1998; Miller and O’Callaghan 1994; O’Callaghan and Miller 1994; Cappon et al. 1996). While we have ruled out this variable in this investigation, effectors of the actions of both endogenous as well as exogenous glucocorticoids have yet to be explored. These include targets implicated in chronic stress, microglial activation, and neuroinflammation (Nair and Bonneau 2006; Wohleb et al. 2011) that also may influence METH-induced dopaminergic neurotoxicity, such glutamate and NMDA receptors (Bowyer et al. 1991, 2004b; Nash and Yamamoto 1992; Mark et al. 2004; Brown et al. 2005; Tata and Yamamoto 2008; Yamamoto et al. 2010). Redistribution of cellular mediators of inflammation (Dhabhar 2009)and alteration in components of the toll signaling pathway (Watkins et al. 2009; Munhoz et al. 2010) remain as potential candidates. Nevertheless, our preliminary findings (Kelly et al. 2011) suggest that striatal toll receptor expression and activation of NF-κB, a key downstream effector in this pathway, do not play a role in the chronic CORT priming of METH-induced neuroinflammation. We also note that while the “bad actor” moniker often is associated with neuroinflammation (Watkins et al. 2007), the neuropoeitic/neuroinflammatory cytokine family also can play a restorative role in CNS injury (Bauer et al. 2007). Our findings of enhanced expression of these mediators with chronic CORT and METH are only associational. Moreover, our prior data showing suppression of METH-induced neuroinflammation without altering neurotoxicity (Sriram et al. 2006) serve as an illustration of this point. Therefore, we do not yet have causal data for an adverse role for neuroinflammation in the enhanced neurotoxic effects of METH.
In summary, we have demonstrated that chronic administration of high physiological levels of CORT primes multiple areas of the CNS to mount an enhanced neuroinflammatory response to METH administered at a neurotoxic dose. Molecular and cellular evidence of enhanced METH neurotoxicity and astrogliosis also were observed. These data add to the view that CORT administered chronically can have a proinflammatory effect, and also show that such an effect can be associated with enhanced neurotoxicity.
Acknowledgments
We appreciate the excellent technical assistance provided by Brenda K. Billig, Christopher M. Felton, and Karen M. Tranter.
Funding source
Intramural funds from the Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health.
Abbreviations used
- CORT
corticosterone
- DAB
diaminobenzidine
- GFAP
Glial fibrillary acidic protein
- LIF
Leukemia inhibitory factor
- LPS
Lipopolysaccharides
- METH
methamphetamine
- OSM
Oncostatin M
- TH
tyrosine hydroxylase
- TNF
Tumor necrosis factor
Footnotes
Conflict of interest
None
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
References
- Bauer S, Kerr BJ, Patterson PH. The neuropoietic cytokine family in development, plasticity, disease and injury. Neuroscience. 2007;8:221–232. doi: 10.1038/nrn2054. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Bowers SL, Bilbo SD, Dhabhar FS, Nelson RJ. Stressor-specific alterations in corticosterone and immune responses in mice. Brain Behav Immun. 2008;22:105–113. doi: 10.1016/j.bbi.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowyer JF, Ali S. High doses of methamphetamine that cause disruption of the blood-brain barrier in limbic regions produce extensive neuronal degeneration in mouse hippocampus. Synapse. 2006;60:521–532. doi: 10.1002/syn.20324. [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Holson RR. Methamphetamine and amphetamine neurotoxicity. In: Chang LW, Dyer RS, editors. Handbook of Neurotoxicity. Marcel Dekker; New York: 1995. pp. 845–870. [Google Scholar]
- Bowyer JF, Scallet AC, Holson RR, Lipe GW, Slikker W, Jr, Ali SF. Interactions of MK-801 with glutamate-, glutamine- and methamphetamine-evoked release of [3H]dopamine from striatal slices. J Pharmacol Exp Ther. 1991;257:262–270. [PubMed] [Google Scholar]
- Bowyer JF, Tank AW, Newport GD, Slikker W, Jr, Ali SF, Holson RR. The influence of environmental temperature on the transient effects of methamphetamine on dopamine levels and dopamine release in rat striatum. J Pharmacol Exp Ther. 1992;260:817–824. [PubMed] [Google Scholar]
- Bowyer JF, Davies DL, Schmued L, Broening HW, Newport GD, Slikker W, Jr, Holson RR. Further studies of the role of hyperthermia in methamphetamine neurotoxicity. J Pharmacol Exp Ther. 1994;268:1571–1580. [PubMed] [Google Scholar]
- Bowyer JF, Peterson SL, Rountree RL, Tor-Agbidye J, Wang GJ. Neuronal degeneration in rat forebrain resulting from D-amphetamine-induced convulsions is dependent on seizure severity and age. Brain Res. 1998;809:77–90. doi: 10.1016/s0006-8993(98)00846-4. [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Harris AJ, Delongchamp RR, Jakab RL, Miller DB, Little AR, O’Callaghan JP. Selective changes in gene expression in cortical regions sensitive to amphetamine during the neurodegenerative process. Neurotoxicology. 2004a;25:555–572. doi: 10.1016/j.neuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Delongchamp RR, Jakab RL. Glutamate N-methyl-D-aspartate and dopamine receptors have contrasting effects on the limbic versus the somatosensory cortex with respect to amphetamine-induced neurodegeneration. Brain Res. 2004b;1030:234–236. doi: 10.1016/j.brainres.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Thomas M, Schmued LC, Ali SF. Brain region-specific neurodegenerative profiles showing the relative importance of amphetamine dose, hyperthermia, seizures, and the blood-brain barrier. Ann N Y Acad Sci. 2008;1139:127–139. doi: 10.1196/annals.1432.005. [DOI] [PubMed] [Google Scholar]
- Brown JM, Quinton MS, Yamamoto BK. Methamphetamine-induced inhibition of mitochondrial complex II: roles of glutamate and peroxynitrite. J Neurochem. 2005;95:429–436. doi: 10.1111/j.1471-4159.2005.03379.x. [DOI] [PubMed] [Google Scholar]
- Cappon GD, Broening HW, Pu C, Morford L, Vorhees CV. alpha-Phenyl-N-tert-butyl nitrone attenuates methamphetamine-induced depletion of striatal dopamine without altering hyperthermia. Synapse. 1996;24:173–181. doi: 10.1002/(SICI)1098-2396(199610)24:2<173::AID-SYN9>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- De Pablos RM, Villaran RF, Arguelles S, Herrera AJ, Venero JL, Ayala A, Cano J, Machado A. Stress increases vulnerability to inflammation in the rat prefrontal cortex. J Neurosci. 2006;26:5709–5719. doi: 10.1523/JNEUROSCI.0802-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implicatons for immuosprotection and immunopathology. Neuro Immuno Modulation. 2009;16:300–317. doi: 10.1159/000216188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Schmued LC, Marshall JF. Characterizing cortical neuron injury with fluorojade labeling after a neurotoxic regimen of methamphetamine. Synapse. 1998;30:329–333. doi: 10.1002/(SICI)1098-2396(199811)30:3<329::AID-SYN10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Ellinwood EH, Jr, Escalante O. Behavior and histopathological findings during chronic methedrine intoxication. Biol Psychiatry. 1970;2:27–39. [PubMed] [Google Scholar]
- Eng LF, Ghirnikar RS, Lee YL. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000) Neurochem Res. 2000;25:1439–1451. doi: 10.1023/a:1007677003387. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrata MV, Sprunger DB, Watkins LR, Maier SF. Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain Behav Immun. 2006;21:47–59. doi: 10.1016/j.bbi.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Frank MG, Miguel ZD, Watkins LR, Maier SF. Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses to E. coli lipopolysaccharide. Brain Behav Immun. 2010;24:19–30. doi: 10.1016/j.bbi.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Frank MG, Watkins LR, Maier SF. Stress- and gluco-corticoid-induced priming of neuroinflammatory responses: potential mechanisms of stress-induced vulnerability to drugs of abuse. Brain Behav Immun. 2011;25:S21–S28. doi: 10.1016/j.bbi.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeber MB, Streit WJ. Microglia: biology and pathology. Acta Neuropathol. 2010;119:89–105. doi: 10.1007/s00401-009-0622-0. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA, Yamamoto BK. Actions of 3,4-methylene-di-oxymethamphetamine (MDMA) on cerebral dopaminergic, serotonergic and cholinergic neurons. Pharmacol Biochem Behav. 2008;90:198–207. doi: 10.1016/j.pbb.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss AJ, Morgan ME, Gibb JW. The long-term effects of multiple doses of methamphetamine in neostriatal tryptophan hydroxylase, tyrosine hydroxylase, choline acetyltransferase and glutamate decarboxylase activities. Life Sci. 1979;25:1373–1379. doi: 10.1016/0024-3205(79)90414-4. [DOI] [PubMed] [Google Scholar]
- Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat Protoc. 2007;2:141–151. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- Johnson JD, O’Connor KA, Deak T, Stark M, Watkins LR, Maier SF. Prior stressor exposure sensitizes LPS-induced cytokine production. Brain Behav Immun. 2002;16:461–476. doi: 10.1006/brbi.2001.0638. [DOI] [PubMed] [Google Scholar]
- Johnson EA, O’Callaghan JP, Miller DB. Brain concentrations of d-MDMA are increased after stress. Psychopharmacology. 2004;173:278–286. doi: 10.1007/s00213-003-1740-3. [DOI] [PubMed] [Google Scholar]
- Kelly KA, Miller DB, Lasley SM, O’Callaghan JP. Chronic Exposure to Glucocorticoids, Pb and DEET Primes the Neuroinflammatory Response to the Nerve Agent DFP in a Potential Model for Gulf War Illness. Program No. 676.01. 2011 Neuroscience Meeting Planner; Washington, DC: Society for Neuroscience Abstracts; 2011. [Google Scholar]
- Kraft AD, Harry GJ. Features of microglia and neuroinflammation relevant to environmental exposure and neurotoxicity. Int J Environ Res Public Health. 2011;8:2980–3018. doi: 10.3390/ijerph8072980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova IN, Cadet JL. Methamphetamine toxicity and messengers of death. Brain Res Rev. 2009;60:379–407. doi: 10.1016/j.brainresrev.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Kuhn DM, Francescutti-Verbeem DM, Thomas DM. Dopamine quinones activate microglia and induce a neurotoxic gene expression profile: relationship to methamphetamine-induced nerve ending damage. Ann N Y Acad Sci. 2006;1074:31–41. doi: 10.1196/annals.1369.003. [DOI] [PubMed] [Google Scholar]
- Kwon MS, Seo YS, Lee JK, Jung JS, Jang JE, Park SH, Suh HW. The repeated immobilization stress increases IL-1β immunoreactivites in only neuron, but not astrocyte or microglia in hippocampal CA1 region, striatum and paraventricular nucleus. Neurosci Lett. 2008;430:258–263. doi: 10.1016/j.neulet.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Ladenheim B, Krasnova IN, Deng X, Oyler JM, Polettini A, Moran TH, Huestis MA, Cadet JL. Methamphetamine-induced neurotoxicity is attenuated in transgenic mice with a null mutation for interleukin-6. Mol Pharmacol. 2000;58:1247–1256. doi: 10.1124/mol.58.6.1247. [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Card JP, Hastings TG. Microglial activation precedes dopamine terminal pathology in methamphetamine-induced neurotoxicity. Exp Neurol. 2004;187:47–57. doi: 10.1016/j.expneurol.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Mark KA, Soghomonian JJ, Yamamoto BK. High-dose methamphetamine acutely activates the striatonigral pathway to increase striatal glutamate and mediate long-term dopamine toxicity. J Neurosci. 2004;24:11449–11456. doi: 10.1523/JNEUROSCI.3597-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PM, O’Callaghan JP. A direct comparison of GFAP immunocytochemistry and GFAP concentration in various regions of ethanol-fixed rat and mouse brain. J Neurosci Methods. 1995;58:181–192. doi: 10.1016/0165-0270(94)00175-g. [DOI] [PubMed] [Google Scholar]
- McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflamm. 2008;17:5–45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DB, O’Callaghan JP. Environment-, drug- and stress-induced alterations in body temperature affect the neurotoxicity of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther. 1994;270:752–760. [PubMed] [Google Scholar]
- Munhoz CD, Lepsch LB, Kawamoto EM, Malta MB, de Lima LS, Avellar MC, Sapolsky RM, Scavone C. Chronic unpredictable stress exacerbates lipopolysaccharide-induced activation of nuclear factor-kappaB in the frontal cortex and hippocampus via glucocorticoid secretion. J Neurosci. 2006;26:3813–3820. doi: 10.1523/JNEUROSCI.4398-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munhoz CD, Sorrells SF, Caso JR, Scavone C, Sapolsky RM. Glucocorticoids exacerbate lipopolysaccharide-induced signaling in the frontal cortex and hippocampus in a dose-dependent manner. J Neurosci. 2010;30:13690–13698. doi: 10.1523/JNEUROSCI.0303-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Bonneau RH. Stress-induced elevation of gluco-corticoids increases microglia proliferation through NMDA receptor activation. J Neuroimmunol. 2006;171:72–85. doi: 10.1016/j.jneuroim.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Nash JF, Yamamoto BK. Methamphetamine neurotoxicity and striatal glutamate release: comparison to 3,4-methylenedioxy-methamphetamine. Brain Res. 1992;581:237–243. doi: 10.1016/0006-8993(92)90713-j. [DOI] [PubMed] [Google Scholar]
- Nishi A, Kuroiwa M, Miller D, et al. Distinct roles of PDE4 and PDE10A in the regulation of cAMP/PKA signaling in the striatum. J Neurosci. 2008;28:10460–10471. doi: 10.1523/JNEUROSCI.2518-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norenberg MD. The reactive astrocyte. In: Aschner M, Costa LG, editors. The Role of Glia in Neurotoxicity. CRC Press; Boca Raton, FL: 2005. pp. 73–92. [Google Scholar]
- O’Callaghan JP. Quantification of glial fibrillary acidic protein: comparison of slot-immunobinding assays with a novel sandwich ELISA. Neurotoxicol Teratol. 1991;13:275–281. doi: 10.1016/0892-0362(91)90073-6. [DOI] [PubMed] [Google Scholar]
- O’Callaghan JP. Measurement of glial fibrillary acidic protein. John Wiley & Sons; New York: 2002. pp. 12.8.1–12.8.12. [DOI] [PubMed] [Google Scholar]
- O’Callaghan JP, Miller DB. Neurotoxicity profiles of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther. 1994;270:741–751. [PubMed] [Google Scholar]
- O’Callaghan JP, Miller DB. Neurotoxic effects of substituted amphetamines in rats and mice. In: Massaro EJ, editor. Handbook of Neurotoxicology. Human Press, Inc; Totowa, NJ: 2005. pp. 269–301. [Google Scholar]
- O’Callaghan JP, Sriram K. Glial fibrillary acidic protein and related glial proteins as biomarkers of neurotoxicity. Expert Opin Drug Saf. 2005;4:433–442. doi: 10.1517/14740338.4.3.433. [DOI] [PubMed] [Google Scholar]
- O’Callaghan JP, Miller DB, Reinhard JF., Jr Characterization of the origins of astrocyte response to injury using the dopaminergic neurotoxicant, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Brain Res. 1990;521:73–80. doi: 10.1016/0006-8993(90)91526-m. [DOI] [PubMed] [Google Scholar]
- O’Callaghan JP, Brinton RE, McEwen BS. Glucocoritcoids regulate the synthesis of glial fibrillary acidic protein in intact and adrenalectomized rats but do not affect its expression following brain injury. J Neurochem. 1991;57:860–869. doi: 10.1111/j.1471-4159.1991.tb08230.x. [DOI] [PubMed] [Google Scholar]
- O’Callaghan JP, Sriram K, Miller DB. Defining “Neuro-inflammation”: Lessons from MPTP- and Methamphetamine-Induced Neurotoxicity. Annal NY Acad Sci. 2008;1139:318–330. doi: 10.1196/annals.1432.032. [DOI] [PubMed] [Google Scholar]
- Prinz M, Priller J, Sisodia SS, Ransohoff RM. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci. 2011;14:1227–12235. doi: 10.1038/nn.2923. [DOI] [PubMed] [Google Scholar]
- Quan N, Stern EL, Whiteside MB, Herkenham M. Induction of pro-inflammatory cytokine mRNAs in the brain after peripheral injection of subseptic doses of lipopolysaccharide in the rat. J Neuroimmunol. 1999;93:72–80. doi: 10.1016/s0165-5728(98)00193-3. [DOI] [PubMed] [Google Scholar]
- Riddle EL, Fleckenstein AE, Hanson GR. Mechanisms of methamphetamine-induced dopaminergic neurotoxicity. AAPS J. 2006;8:E413–E418. doi: 10.1007/BF02854914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmued LC, Bowyer JF. Methamphetamine exposure can produce neuronal degeneration in mouse hippocampal remnants. Brain Res. 1997;759:135–140. doi: 10.1016/s0006-8993(97)00173-x. [DOI] [PubMed] [Google Scholar]
- Seiden LS, Fischuman MW, Schuster CR. Long-term methamphetamine induced in brain catecholamines in tolerant rhesus monkeys. Drug Alcohol Depend. 1976;1:215–229. doi: 10.1016/0376-8716(76)90030-2. [DOI] [PubMed] [Google Scholar]
- Silva AP, Martins T, Baptista S, Gonçalves J, Agasse F, Malva JO. Brain Injury associated with widely abused amphetamines: neuroinflammation, neurogenesis and blood-brain barrier.Curr. Drug Abuse Rev. 2010;3:239–354. doi: 10.2174/1874473711003040239. [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist. 2005;11:400–407. doi: 10.1177/1073858405278321. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells SF, Sapolsky RM. An inflammatory review of glucocorticoid actions in the CNS. Brain Behav Immun. 2007;21:259–272. doi: 10.1016/j.bbi.2006.11.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrells SF, Caso JR, Munhoz CD, Sapolsky RM. The stressed CNS: when glucocoritcoids aggravate inflammation. Neuron. 2009;64:33–39. doi: 10.1016/j.neuron.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram K, O’Callaghan JP. Divergent roles for tumor necrosis factor-α in the brain. J Neuroimmune Pharmacol. 2007;2:140–153. doi: 10.1007/s11481-007-9070-6. [DOI] [PubMed] [Google Scholar]
- Sriram K, Benkovic SA, Hebert MA, Miller DB, O’Callaghan JP. Induction of gp130-related cytokines and activation of JAK2/STAT3 pathway in astrocytes precedes up-regulation of glial fibrillary acidic protein in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of neurodegeneration. J Biol Chem. 2004;279:19936–19947. doi: 10.1074/jbc.M309304200. [DOI] [PubMed] [Google Scholar]
- Sriram K, Miller DB, O’Callaghan JP. Minocycline attenuates microglial activation but fails to mitigate striatal dopaminergic neurotoxicity: role of tumor necrosis factor-α. J Neurochem. 2006;96:706–718. doi: 10.1111/j.1471-4159.2005.03566.x. [DOI] [PubMed] [Google Scholar]
- Streit WJ. An improved staining method for rat microglial cells using the lectin from Griffoniasimplicifolia (GSA I-B4) J Histochem Cytochem. 1990;38:1683–1686. doi: 10.1177/38.11.2212623. [DOI] [PubMed] [Google Scholar]
- Streit WJ. The role of microglia in neurotoxicity. In: Aschner M, Costa LG, editors. The Role of Glia in Neurotoxicity. 2. CRC Press; Boca Raton, FL: 2005. pp. 29–40. [Google Scholar]
- Streit WJ. Microglia activation and neuroinflammation in Alzheimer’s disease: a critical examination of recent history. Front Aging Neurosci. 2010;2:1–5. doi: 10.3389/fnagi.2010.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Kreutzberg GW. Lectin binding by resting and reactive microglia. J Neurocytol. 1987;16:249–260. doi: 10.1007/BF01795308. [DOI] [PubMed] [Google Scholar]
- Sugama S, Fujita M, Hashimoto M. Stress induced morphological microglial activation in the rodent brain: involvement of interleukin-18. Neuroscience. 2007;146:1388–1399. doi: 10.1016/j.neuroscience.2007.02.043. [DOI] [PubMed] [Google Scholar]
- Sugama S, Takenouchi T, Fujita M, Conti B, Hashimoto M. Differential microglial activation between acute stress and lipopolysaccharide treatment. J Neuroimmunol. 2009;207:24–31. doi: 10.1016/j.jneuroim.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Tansey M, McCoy MK, Frank-Cannon TC. Neuroinflammatory mechanisms in Parkinson’s disease: Potential environmental triggers, pathways, and targets for early therapeutic intervention Exp. Neurology. 2007;208:1–25. doi: 10.1016/j.expneurol.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata DA, Yamamoto BK. Chronic stress enhances methamphetamine-induced extracellular glutamate and excitotoxicity in the rat striatum. Synapse. 2008;62:325–326. doi: 10.1002/syn.20497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DM, Dowgiert J, Geddes TJ, Frtancescutti-Verbeem D, Liu X, Kuhn DM. Microglial activation is a pharmacologically specific marker for the neurotoxic amphetamines. Neurosci Lett. 2004a;367:349–354. doi: 10.1016/j.neulet.2004.06.065. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM. Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. J Pharmacol Exp Ther. 2004b;311:1–7. doi: 10.1124/jpet.104.070961. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, Maier SF. glia as the “bad guys”: Implications for improving clinical pain control and clinical utility of opioids. Brain Behav Immun. 2007;21:131–146. doi: 10.1016/j.bbi.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Rice KC, Maier SF. The “toll” of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol Sci. 2009;30:581–591. doi: 10.1016/j.tips.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, Nelson RJ, Godbout JP, Sheridan JF. β-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31:6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto BK, Moszczynska A, Gudelsky GA. Amphetamine toxicities: classical and emerging mechanisms. Ann N Y Acad Sci. 2010;1187:101–121. doi: 10.1111/j.1749-6632.2009.05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]