Abstract
Growing evidence suggests that the chemokine stromal cell-derived factor-1 (SDF-1) is essential in regulating bone marrow (BM) derived mesenchymal stromal/stem cell (BMSC) survival, and differentiation to either a pro-osteogenic, or pro-adipogenic fate. This study investigates the effects of caloric restriction (CR) and leptin on the SDF-1/CXCR4 axis in bone and BM tissues in the context of age-associated bone loss. For in vivo studies, we collected bone, BM cells and BM interstitial fluid from 12 and 20 month-old C57Bl6 mice fed ad-libitum (AL), and 20 month-old mice on long-term CR with, or without, intraperitoneal injection of leptin for 10 days (10mg/kg). To mimic conditions of CR in vitro, 18 month murine BMSCs were treated with 1) control (Ctrl): normal proliferation medium, 2) Nutrient Restriction (NR): low glucose, low serum medium, or 3) NR+leptin: NR medium + 100 ng/ml Leptin for 6-48 h. In BMSCs both protein and mRNA expression of SDF-1 and CXCR4 were increased by CR and CR + leptin. In contrast, the alternate SDF-1 receptor CXCR7 was decreased, suggesting a nutrient signaling mediated change in SDF-1 axis signaling in BMSCs. However, in bone SDF-1, CXCR4 and 7 gene expression increase with age and this is reversed with CR, while addition of leptin returns this to the “aged” level. Histologically bone formation was lower in the calorically restricted mice and BM adipogenesis increased, both effects were reversed with the 10 day leptin treatment. This suggests that in bone CR and leptin alter the nutrient signaling pathways in different ways to affect the local action of the osteogenic cytokine SDF-1. Studies focusing on the molecular interaction between nutrient signaling by CR, leptin and SDF-1 axis may help to address age-related musculoskeletal changes.
Keywords: Aging, Osteoporosis, CXCR4, CXCR7, Nutrient Signaling, Bone formation
1. Introduction
Aging is a biological process that is accompanied by an increasing prevalence of musculoskeletal disorders that impact a variety of tissues including muscle, cartilage, and bone. Age-related osteoporosis leads to an increased risk of fractures, morbidity and death. It affects 55% of Americans aged 50 and above. Meanwhile, the estimated prevalence of overweight, or obese, adults (≥20 years of age) in the US is 154.7 million, which represents 68.2% of this group as of 2010 (Go, Mozaffarian, Roger et al., 2013). While the mechanisms underlying the age-related defective bone formation remain poorly defined, recent studies suggest that osteoporosis is a stem cell disease (Bonyadi, Waldman, Liu et al., 2003, Egermann, Schneider, Evans et al., 2005,Miura, Miura, Gronthos et al., 2005). Bone marrow (BM) derived mesenchymal stem cells (BMSCs) are multipotent cells that can fully differentiate into mesenchymal lineages, including bone, cartilage, tendon, muscle, and adipose tissue. They decline in the BM niche with age and are thought to be key cells in the development of age-associated bone loss, including osteopenia and osteoporosis (Jiang, Jahagirdar, Reinhardt et al., 2002,Gimble and Nuttall, 2012). Additionally, osteoporosis can be considered to be a disorder of energy metabolism (Cao, 2011,Jeyabalan, Shah, Viollet et al., 2012). As such it is of interest to understand the effect of changes in nutrient signaling on BMSC fate and function.
Both osteoporosis and aging-related bone loss in general are associated with an increase in marrow adipogenesis, which may suggest a commitment of BMSCs, or descendent cells, to differentiate into adipocytes rather than osteoblasts (Meunier, Aaron, Edouard et al., 1971). Cysteine (C)-X-C motif chemokine receptor-4 (CXCR4) is the primary receptor for the chemokine stromal cell-derived factor-1 (SDF-1, also named CXCL12) (Yu, Cecil, Peng et al., 2006). Recent studies from this lab and others demonstrate that SDF-1 is required for CXCR4 mediated BMSCs maintenance (i.e. homing and retention in the BM), Bone Morphogenetic Protein 2 (BMP-2) mediated osteogenic differentiation, and autophagy mediated cell survival of MSCs in the BM (Jiang et al., 2002,Bianco, Robey and Simmons, 2008,Herberg, Shi, Johnson et al., 2013,Herberg, Fulzele, Yang et al., 2013,Wagner and Ho, 2007,Zhang, Khan, Delling et al., 2012). At the mRNA level, both CXCR4 and SDF-1 were shown to decline with aging in musculoskeletal tissues, interestingly however a higher-level of plasma SDF-1 has been reported in aging, suggesting a difference of SDF-1 expression or turnover within different tissue compartments (Guang, Boskey and Zhu, 2013,Subramanian, Liu, Aviv et al., 2014).
Since there are parallels in metabolic dysregulation in aging and obesity, it is possible these conditions share common cellular pathways. Further, stem cell exhaustion (senescence or loss) and deregulated nutrient sensing are proposed to be a consequence in multiple forms of aging-associated dysfunction (Lopez-Otin, Blasco, Partridge et al., 2013). Recently, caloric restriction (CR) has been established as a strategy to extend healthy life span in animals. However, we have shown that CR is also associated with increased loss of muscle and bone mass (Hamrick, Ding, Ponnala et al., 2008). Several bone regulating hormones are altered during CR that negatively influence bone osteoblastic and osteoclastic activity either directly, or indirectly, some due to altered levels of cytokines (Shapses and Sukumar, 2012). Recently, Fenton et al., (2009) demonstrated an increased serum level of SDF-1 in calorically restricted mice, while the opposite has been shown with diet-induced obese mice. During CR there are changes in adipocyte-derived hormones, which play roles in bone metabolism. Additionally, bone loss and fracture risk are increased in older CR humans and rodents compared with younger CR subjects (Redman, Rood, Anton et al., 2008,Talbott, Cifuentes, Dunn et al., 2001,Uusi-Rasi, Rauhio, Kannus et al., 2010).
We have found that circulating levels of leptin decline with age in mice and peripheral leptin treatment can increase muscle mass in aged mice (Hamrick, Ding, Pennington et al., 2006,Hamrick, Della-Fera, Choi et al., 2005,Hamrick, Herberg, Arounleut et al., 2010). As noted the opposite occurs with SDF-1 expression with age in CR mice as a result of feedback when there is an inhibition of CXCR4 receptor signaling, suggest a defect in SDF-1 signaling. Together with a decline in leptin, the change in SDF-1 signaling in BM stem cells niches may be in part responsible for bone loss (Gimble and Nuttall, 2012). Consequently we considered that leptin and SDF-1 might interact to mediate musculoskeletal tissue repair and regeneration. Here we use in vivo and in vitro approaches to determine whether nutrient deficiency and leptin treatment alter SDF-1 secretion and its receptor CXCR4 in aged bone and BM environment.
2. Materials & Methods
2.1. Animals & Treatments
All experiments described were approved by the Institutional Animal Care & Use Committee (IACUC) at Georgia Regents University (formerly Georgia Health Sciences University). Mice aged 12 months (12 M; n=6) and 20 months (20 M) fed ad-libitum (AL) (n=6) and on CR (n=12) were obtained from the aged rodent colony at the National Institute on Aging (Bethesda, MD, USA). Half of the mice in the 20 M CR group were treated with recombinant mouse leptin (intraperitoneal injection; R&D Systems, Minneapolis, MN, USA cat # 498-OB-05M) for 10 days at 10 mg/kg body weight, and the other half of each group treated with vehicle control (saline; VEH). The post-treatment mice were euthanized and the femurs and tibia were excised carefully and all soft tissues were removed from around the bones. The epiphyses of the tibia and femur were removed and the marrow was flushed out with PBS and the cellular material was centrifuged, the supernatant was used as BM interstitial fluid. The remaining diaphyses (bone chips) were washed with PBS, snap-frozen in liquid N2 and stored at − 80 °C.
2.2. Isolation and Culture of BMSCs
Six 18-month-old male C57BL/6J mice, purchased from the National Institute on Aging (Bethesda, MD, USA) aged rodent colony, were used to obtain BMSCs at the Georgia Regents University Stem Cell Core Facility as described previously (Zhang, Ou, Hamrick et al., 2008) First, mice were euthanized by CO2 overdose followed by thoracotomy. The femora and tibiae were dissected free of soft tissues and kept in cold PBS on ice. The bones were cut open at both ends and flushed with complete isolation media (CIM) (RPMI-1640 (Cellgro, Mediatech, Manassas, VA, USA), 9% heat-inactivated fetal bovine serum (FBS), 9% horse serum (both from Atlanta Biologicals, Lawrenceville, GA, USA), and 12 μM L-glutamine (Gibco, Invitrogen, Carlsbad, CA, USA) using a 22-gauge syringe followed by filtration through a 70-μm nylon mesh filter. The combined whole bone marrow aspirate was dispersed with a 25-gauge syringe to produce a single cell suspension. Next, BMSCs were isolated using a modified protocol (Gimble, Robinson, Wu et al., 1996,Peister, Mellad, Larson et al., 2004,Tropel, Noel, Platet et al., 2004) by plating the single cell suspension in 175-cm2 flasks at a density of 2×107 cells/flask. After a 3-h incubation at 37°C in 5% CO2, the non-adherent cells were removed and the adherent cells washed two times gently with PBS to reduce the degree of hematopoietic lineage cell contamination. The cells were cultured in CIM for 3-4 weeks with media change every 3-4 days. At 70-80% confluence, the cells were lifted with trypsin/EDTA, washed, and resuspended at a density of 5×106 cells/ml in PBS containing 0.5% bovine serum albumin (BSA) and 2 mM EDTA followed by negative immune-depletion using magnetic microbeads conjugated to anti-mouse CD11b, CD45R/B220 (BD Biosciences Pharmingen, San Diego, CA, USA), CD11c, and PDCA-1 (Miltenyi Biotec, Bergisch Gladbach, Germany) monoclonal antibodies according to the manufacturer's instructions. Resulting CD11b, CD45R/B220, CD11c, PDCA-1-negative cells were subjected to positive immunoselection using anti-Sca-1 microbeads (Miltenyi Biotec) following the manufacturer's recommendations. Enriched BMSCs (0.33% CD45, 0.13% CD11b, 83.18% Sca-1 by FACS analysis(Zhang et al., 2008)), which are depleted of monocytes, granulocytes, macrophages, myeloid-derived dendritic cells (DCs), natural killer cells, B-1 cells, B lymphocytes, T lymphocytes, classical DCs, plasmacytoid DCs, and macrophage progenitors, were maintained in normal proliferation medium comprised of Dulbecco's Modified Eagle Medium (DMEM, 4.5 g/l glucose; Cellgro) with 10% heat-inactivated FBS (Atlanta Biologicals) and used at 70-80% confluence. For all in vitro experiments we used these BMSCs, isolated from 18 month-old mice, at passage 10.
2.3. Nutrient Restriction – in vitro
To mimic conditions of nutrient restriction (NR) in vitro, BMSCs (passage 10) were plated at 1.0×105 cells/cm2 in 6-well plates with normal proliferation medium (Dulbecco's Modified Eagle Medium; DMEM, 4.5 g/l glucose; Cellgro) with 10% heat-inactivated FBS (Atlanta Biologicals). The next day, cells were washed in PBS and then incubated in 1) control (Ctrl): normal glucose & serum medium (DMEM, 4.5 g/l glucose, 10% FBS), 2) NR: low glucose, low serum medium (DMEM, 1.0 g/l glucose, 1% FBS), or 3) NR+leptin (NR+Lep): NR medium + 100 ng/ml recombinant murine leptin (rmLeptin; R&D Systems, Minneapolis, MN, USA) for 6, 24, and 48 h. At the end of the experimental period, conditioned medium from BMSCs were collected and the cell lysates were prepared.
2.4. SDF-1α Enzyme-Linked Immunosorbent Assay (ELISA)
SDF-1α ELISAs (R&D Systems) was performed with either BM interstitial fluid or in medium conditioned by BMSCs cell culture as described previously (Herberg et al., 2013). The anti-SDF-1 capture antibody (R&D Systems) in sodium bicarbonate buffer pH 9.4 was bound to MaxiSorp™ 96-well plates (Nunc, Thermo Fisher Scientific) overnight. Plates were blocked for 2 h with 1% BSA in PBS. Murine SDF-1α (PeproTech, Rocky Hill, NJ, USA) standards and samples (1:2 diluted) were incubated for 2 h prior to incubating with the biotinylated anti-SDF-1α (2 h; R&D Systems). Streptavidin-horseradish peroxidase (HRP) (R&D Systems) was incubated for 20 min followed by the substrate reagent (R&D Systems) for 20 min. Sulfuric acid (2 N) was added to stop the enzymatic color reaction and absorbance was read at 450 nm. SDF-1α protein expression was calculated using standard curves and normalized to total protein, which was quantified using the EZQ® Protein Quantitation Kit (Invitrogen).
2.5. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
For in vivo studies, bone chips were ground in liquid N2 with a pestle and mortar, and the powdered tissue was dissolved in Trizol. For in vitro studies, BMSCs were lysed by TRIzol. RNA isolation and subsequent cDNA synthesis (Bio-Rad, 170-8891) were performed as previously described (Herberg et al., 2013). 50 ng of cDNA was amplified in duplicates according to procedures reported previously (Herberg et al., 2013) with custom-designed qPCR primers (Table 1) (Thermo Fisher Scientific). A melt curve was generated to analyze the purity of amplification products. The expression levels of mRNA were normalized to the average of housekeeping genes β-actin and 18s. Relative expression of mRNA was evaluated by using the comparative CT method (ΔΔCt) (Schmittgen and Livak, 2008). Unless otherwise stated, experimental groups were compared to control groups (20M Ad lib, Control BMSCs).
Table 1.
Oligonucleotide product sizes and accession numbers for qRT-PCR.
| Gene | Product size | Accession number |
|---|---|---|
| CXCR4 | 116 | NM_009911 |
| SDF-1α | 105 | NM_021704 |
| CXCR7 | 196 | NM_001271607 |
| BMP-2 | 83 | NM_007553 |
| PPAR-γ2 | NM_001127330 | |
| 165 | ||
| Housekeeping | ||
| 18S | 90 | V00851 |
| β-actin | 103 | NM_007393 |
2.6. Western Blotting
BMSCs cell lysates were prepared in Complete Lysis-M EDTA-free buffer containing protease inhibitors (Roche Diagnostics, Indianapolis, IN, USA) and western blots performed as described previously (Periyasamy-Thandavan et al. 2008; Periyasamy-Thandavan et al. 2012). The same amounts of protein (25μg) were loaded for each lane for reducing electrophoresis. The resolved proteins were then electroblotted onto polyvinylidene difluoride membranes. The blots were incubated in a blocking buffer with 5% fat-free milk, and exposed to the primary antibodies overnight at 4 °C, and finally incubated with the horseradish peroxidase-conjugated secondary antibody to reveal antigens using an enhanced chemiluminescence kit from Pierce. Primary antibodies were used for actin (Sigma; A2228) diluted 1:50000 and CXCR4 (Abcam; ab2074) diluted 1:250.
2.7. Bone Histomorphometry
Bone histomorphometry was performed at the UAB Center for Metabolic Bone Disease (http://cmbd.path.uab.edu/Bone%20Histopath.htm) on a fee-for-service basis. Femurs were histologically prepared via plastic embedding and thin sections were prepared with Goldner's trichrome stain. Measurements were counted over three fields per section and three sections per specimen. Tetracycline injection was followed by the same dose of calcein 10 days later, and animals were killed 2 days later. Static and dynamic histomorphometry measurements were performed according to protocols recommended by Parfitt et al., (1987,1988). Histomorphometric parameters shown in Figure 6 were measured from plastic-embedded, undecalcified bone specimens. Bone marrow adipocytes were quantified in the distal femoral metaphysis via brightfield histology as the average number of adipocytes per μm2 of tissue in three randomly selected fields per section at 10X magnification (Fig. 7).
Figure 6. Leptin Augments New Bone Formation on a Caloric restricted Background.
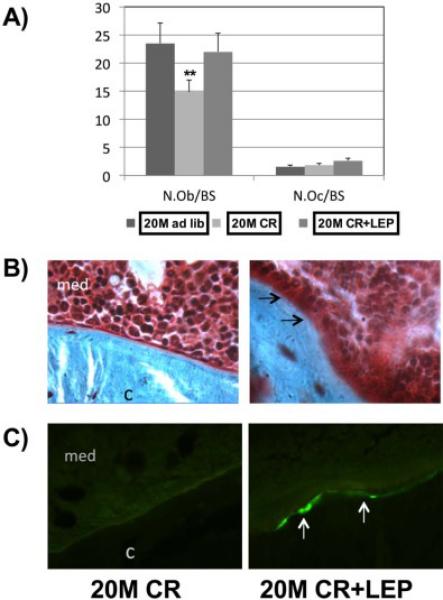
Bone histomorphometry was performed (A) osteoblast Numbere/bone surface (N. Ob./BS), and Osteoclast Number/bone surface (N.Oc./BS). (B) Arrows showing cuboidal osteoblasts lining a partly mineralized osteoid seam. Modified Masson stain, magnification ×250, is shown. (C) Photomicrographs of calcein-labeled areas imaged using a fluorescent microscope. Data represent mean ± SEM. n=6. (**P = .001).
Figure 7. Tibia marrow fat increased with Caloric Restriction diet.
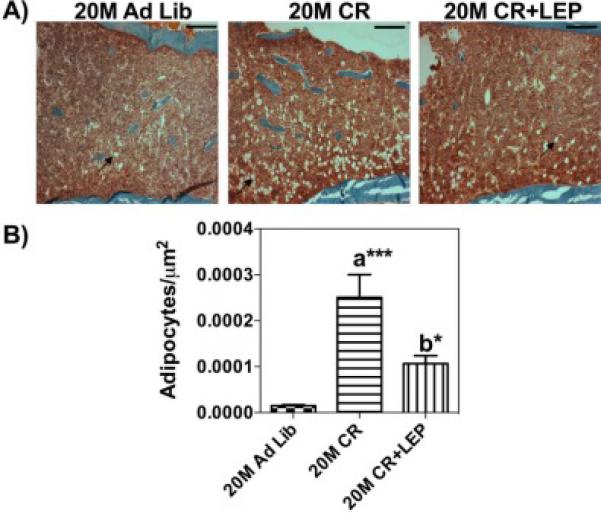
Bone histomorphometry was performed A) Representative H&E-stained coronal section through the center of the diaphysis from each group mentioned. B) Marrow fat cells were calculated as average number of adipocytes per μm2 in three randomly selected fields. (10×, bar 100μm, 1280 × 960 pixels per image, 511463.226μm2 area). Data are shown as means ± S.E.M. *** P<0.0001, * P<0.05; a Vs 20 M AL; b Vs 20 M CR, n=3.
2.8. Transwell Migration Assay
In vitro chemotaxis was assayed using the HTS Transwell® 96-well system (8 μm pore size; Corning®, 3374 and 3583). BM interstitial fluids were loaded in the bottom chamber of the black receiver plate as a source of chemo-attractants. The inserts (upper compartment) of the transwell plate were placed to expose to the lower chamber containing the samples. 18M BMSCs were starved overnight in a Phenol Red Free DMEM supplemented with 1% FBS. To examine the effect of antagonists on SDF-1α– induced chemotactic activity, cells were incubated with the selective SDF-1 receptor (CXCR4) antagonist AMD3100 for 4 hour. After the incubation, the cells were dissociated with trypsin/EDTA and samples each containing 2 × 103 cells in 50 μL of migration buffer (Phenol Red Free DMEM/1% FBS) were added to the transwell inserts (upper chamber) and incubated for 6 h at 37 °C in a 5% CO2 to allow migration across the porous membrane. SDF-1 (100ng/mL) diluted in a migration buffer was used as a positive control and fresh migration buffer was used as a negative control. At the end of the incubation, cells that had migrated into the lower chamber and attached to the lower surface of the filter were detached by trypsin/EDTA, lysed and stained with Cyquant® dye (Molecular probes, C7026) and counted using a fluorescence plate reader (M1000 Infinite®; Tecan). All in vitro experiments were performed three separate times with 3 replicates for each group.
2.9. Statistical Analysis
All data are expressed as means ± S.E.M. One-way analysis of variance (ANOVA) followed by Tukey's post hoc test was used to determine mean differences between groups. The significance level was set at p<0.05. Data were analyzed using GraphPad Prism 5.0 software (GraphPad Software, La Jolla, CA, USA.
3. Results
3.1. Age-Associated Changes in SDF-1 Expression in Bone marrow Interstitial Fluid
We analyzed the effect of aging and CR on SDF-1α in the BM microenvironment, specifically extracted with PBS as the BM interstitial fluid, and whole BM cells harvested from treatment groups. Using custom ELISAs, which employ splice variant specific detection antibody, SDF-1α levels were measured and normalized to total protein. We found no statistical differences in the total BM cell SDF-1α levels (Fig. 1A). In contrast we detected higher secreted SDF-1α levels in the BM interstitial fluid from aged-mice fed AL, suggesting that with age the cells were secreting more SDF-1α protein directly into the interstitial fluid. Further, CR appears to partially decrease interstitial fluid concentration back towards levels seen in young mice. The addition of leptin to CR maintains the level of secreted SDF-1α at the higher age-associated interstitial fluid levels (Fig. 1B). The decrease with CR is not significantly different from the 12 M AL mice, or mice in any of the other 20 M-old groups. These findings prompted us to investigate the soluble leptin receptor (sLR) and BMP-2 in the BM interstitial fluid. In Figure 2A, ELISA analysis did not demonstrate any significant differences between the groups. However, a trend in the circulating levels of sLR in the BM interstitial fluid appeared to show a decrease with age and this was reversed with caloric restriction, but not in response to CR with supplemented leptin. The secretion of BMP-2 did not appear to be affected by age, CR or CR+leptin. (Fig. 2B). Overall, these data show that SDF-1 levels in the bone marrow interstitial fluid increase with age and that CR may alter this increase and that surprisingly BMP-2 levels in the interstitial fluid are not altered with age or nutrient signaling changes.
Figure 1. Caloric Restriction and Leptin Augments SDF-1α Concentration.
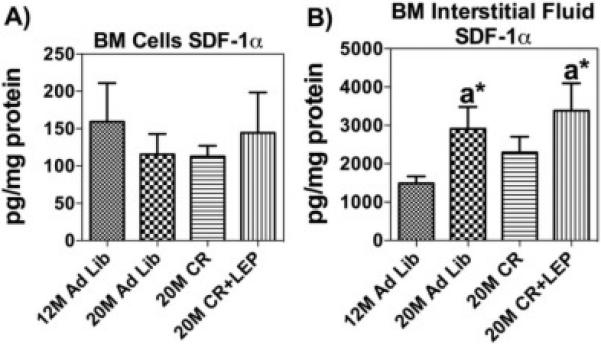
ELISA analysis of SDF-1α was measured for BM cells & BM interstitial fluid. (A) SDF-1α levels in BM cells normalized to protein. (B) SDF-1α levels in BM interstitial fluid normalized to protein. Data are shown as means ± S.E.M, n=6.
Figure 2. Caloric Restriction and Leptin Alters Soluble Leptin Receptor and BMP-2 Levels.
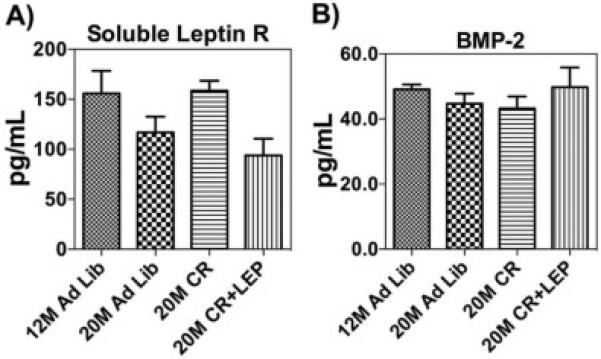
(A) ELISA analysis showed circulating levels of soluble leptin receptor (sLR) in the BM interstitial fluid decrease with age and this was reversed with nutrient restriction, but sLR were reduced in response to addition of exogenous leptin. (B) BMP-2 levels in BM interstitial fluid normalized to protein. ELISA analysis showed circulating levels of BMP-2 were highest in mice on CR treated with leptin. Data are shown as means ± S.E.M, n=6.
3.2. Caloric Restriction induces CXCR4 mediated migration
The BM interstitial fluid isolated from treatment groups was tested for its ability to induce the SDF-1 receptor (CXCR4) mediated migration of 18M BMSCs in a transwell plate assay. While it did not reach statistical significance (p<0.16), there appears to be increased migration at 12 M relative to 20 M in the BM interstitial fluid. In contrast, BM interstitial fluid from 20 M mice on CR treatment clearly showed greater chemotactic migration of BMSCs compared with other groups, and this increase was lost with leptin supplementation (Fig. 3). The pretreatment of migratory BMSCs with the selective CXCR4 antagonist AMD3100 significantly reduced chemotaxis suggests that SDF-1 is in part responsible for the activity found in the BM interstitial fluid.
Figure 3. Caloric Restriction Increases CXCR4 Mediated Cell Migration.
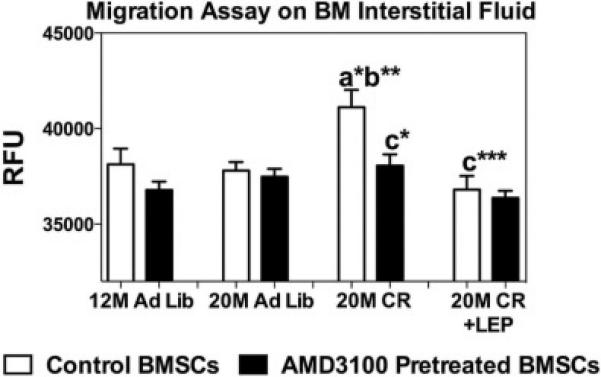
Chemotactic migration of CXC chemokine receptor 4 (CXCR4)-expressing BMSCs ± CXCR4 receptor antagonist AMD3100 groups. Data are shown as means ± S.E.M. * P<0.05, ** P<0.001, *** P<0.0001; a Vs 12 M Ad lib; b Vs 20 M Ad lib; c Vs 20 M CR, n=6.
3.3. Caloric Restriction Reduce PPAR- γ2 Gene Expression
qRT-PCR was performed to analyze the gene expression of the adipogenic and anti-osteogenic transcription factor PPAR-γ2 in BM cells (Fig. 4). PPAR-γ2 at the mRNA level was significantly increased with age and this was reversed with CR and CR + Leptin to the “younger” level.
Figure 4. Caloric Restriction Reduces PPAR-γ2 Gene Expression in Bone Marrow Cells.
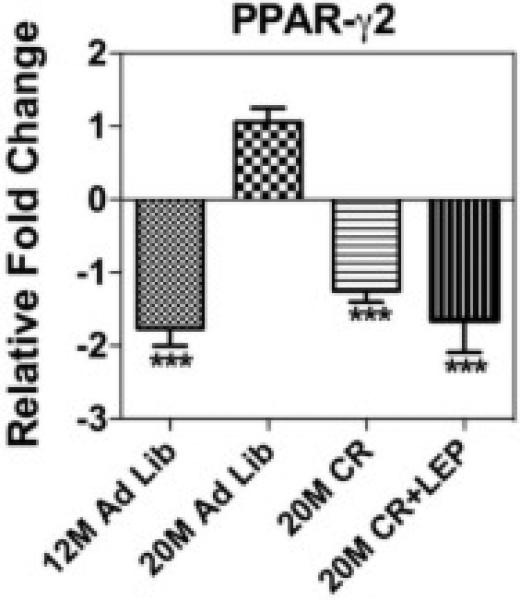
50 ng of cDNA was amplified in each qRT-PCR and specific primers. β-actin and 18s were used as the internal control for normalization. Data are shown as means ± S.E.M. *** P<0.0001; Statistically significant differences are identified between 20M AL Vs other groups, n=6.
3.4. Caloric Restriction and Leptin have Opposite Affects on Bone Formation, Bone Marrow Adipogenesis, and the SDF-1 Axis
qRT-PCR was performed to analyze the gene expression of SDF-1, CXCR4, CXCR7, and BMP-2 from bone (Fig. 5). SDF-1, CXCR4 and CXCR7 at the mRNA level were significantly increased with age and this was reversed with CR to the “younger” level, but the addition of leptin returned it to the “aged” level. However, leptin treatment appeared to increase the BMP-2 at transcription level. To study bone remodeling in this model, bone histomorphometric analysis was performed (Fig. 6). Osteoblast number/ bone surface (N. OB/BS) was significantly decreased in the calorically restricted mice (P = .001) relative to 20 M AL fed mice. Exogenous leptin supplementation reversed the negative effect of CR on osteoblast number. No significant difference was found in the number of osteoclasts between the treatments groups (Fig. 6A) suggesting the effects of CR and leptin were mainly on bone formation rather than bone resorption. Fig. 6B shows a section of an undecalcified bone stained by Goldner's Masson trichrome. The arrow indicates immature new bone matrix stained in red, in a leptin supplemented caloric restricted mouse. Fig. 6C shows the calcein staining for newly calcified bone augmented in leptin supplemented caloric restricted mouse. Altogether, enhanced bone formation was observed in CR mice treated with leptin.Figure 7 shows the bone histomorphometric analysis of adipocytes. The age-associated adipocytes, and the age/CR-associated adipocytes, tended to accumulate around the endosteium and trabecular bone surfaces (Fig. 7A). In the aged mice, the BM adiposity was significantly increased with CR. This increase in adipogenic cells was significantly reduced almost by half with the addition of leptin supplementation for only 10 days (Fig. 7B).
Figure 5. Caloric Restriction Reverses the Effect of Age on SDF-1/CXCR4 Axis Gene Expression in Bone.
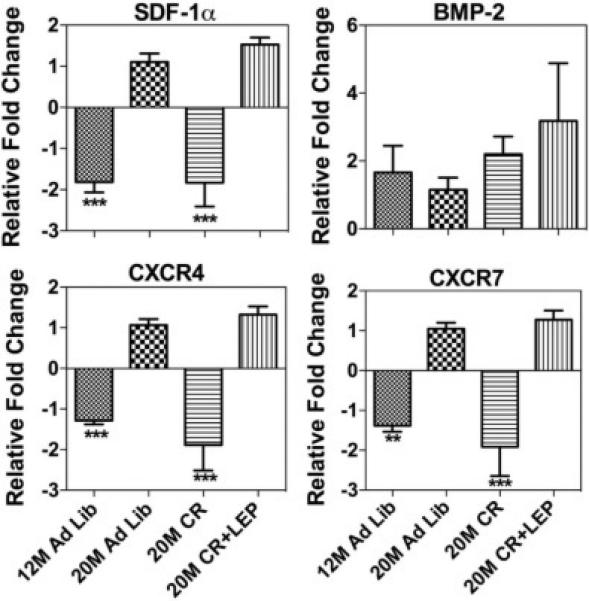
50 ng of cDNA was amplified in each qRT-PCR and specific primers. β-actin and 18s were used as the internal control for normalization. Data are shown as means ± S.E.M. *** P<0.0001; Statistically significant differences are identified between 20 M AL Vs other groups, n=6.
3.5. Effects of nutrient restriction and leptin treatment on the expression of SDF-1 axis in vitro
We quantified in vitro the secreted extracellular SDF-1α and BMP-2 levels in conditioned medium of BMSCs by ELISA and normalized it to total protein (Fig. 8A & B). The results showed increased protein levels of SDF-1α in the conditioned medium collected from the nutrient restricted BMSCs and exogenous leptin treatment with NR further augmented this effect. NR reduced the secretion of BMP-2 in the conditioned medium, but when NR was combined with leptin it increased BMP-2 secretion in the medium. NR also increased the gene expression of SDF-1α and its receptor CXCR4 in BMSCs at 48h, leptin augmented NR further enhanced SDF-1α expression. In contrast, the alternative SDF-1α receptor CXCR7 showed decreased gene expression with both NR and NR + leptin. Additionally, BMP-2 is decreased by NR and this is reversed by the supplementation of NR with leptin (Fig. 9). This matched the protein expression data from the conditioned media. Unlike in vivo this suggests the two SDF-1 receptors, and their downstream signaling, respond differently to nutrient signaling. NR increased the expression of CXCR4 protein early on at the 6h time-point, but this effect was gone by 24h, and leptin decreased CXCR4 levels by 6 hours. (Fig. 10). Figure 11 shows the overall predicted interactions between leptin/SDF-1 from the study.
Figure 8. Nutrient Restriction and Leptin Increase Extracellular SDF-1α and BMP-2 Secretion in vitro.
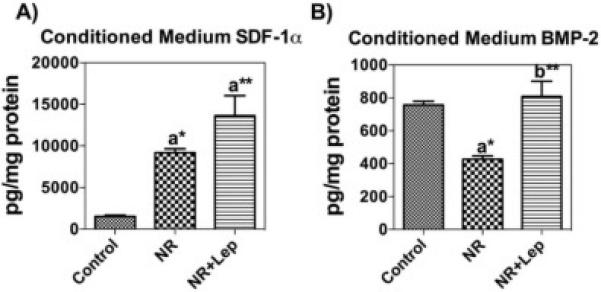
(A) SDF-1α levels in BMSCs normalized to protein. (B) ELISA analysis showed secreted BMP-2 was highest in nutrient restricted cells with leptin. Data are shown as means ± S.E.M. * P<0. 05, ** P<0.001; a Vs Control cells; b Vs Caloric restricted cells, n=3.
Figure 9. Nutrient Restriction and Leptin Differentially Alter the Expression of SDF-1 axis Genes in BMSCs.
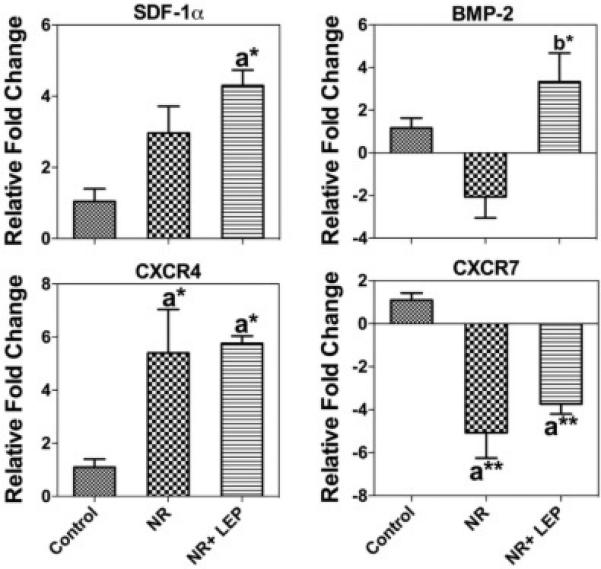
Nutrient restricted cells showed decreased expression of BMP-2, CXCR7 while increasing the expression of SDF-1α and CXCR4 at mRNA level in 48h. Except CXCR7, leptin treatment significantly augmented the gene expression. Data for each sample were normalized with 18s. mRNAs represented as the fold change in expression compared to control cells. Data are shown as means ± S.E.M. * P<0.05, ** P<0.001; a Vs Control cells; b Vs Caloric restricted cells, n=3.
Figure 10. Nutrient Restriction Alter CXCR4 Expression.
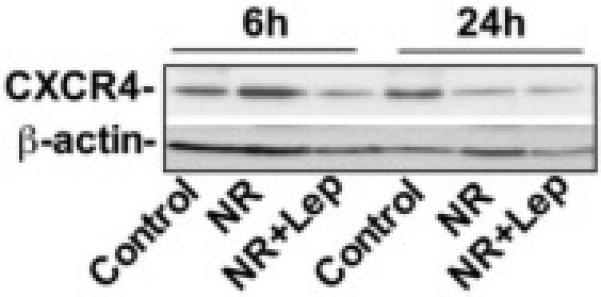
BMSCs lysates were prepared and protein was subjected to SDS/PAGE, followed by immunoblotting using antibodies to CXCR4, and β-actin. Nutrient restriction increased the expression of CXCR4 at the 6h timepoint but this effect is not observed at 24h. Data are representative of at least three separate experiments.
Figure 11. Proposed Model of Interactions between CR, Leptin and SDF-1.
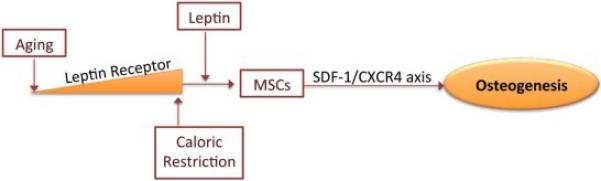
BMSC leptin receptor levels decline with age and reduce sensitivity to leptin. Caloric restriction increases leptin sensitivity, potentially via increasing the expression of Leptin receptor, as well as mediating affects on nutrient signaling pathways that can interact with the SDF-1 axis, such as HIF-1 and mTOR. The addition of peripheral leptin is permissive for BMSCs to move down the osteogenic pathway and reduces BM adiposity, while increasing bone formation. The addition of Leptin enhances CXCR4 downstream signaling.
4. Discussion
In this study, we determined the molecular interaction between leptin and the SDF-1 axis in response to nutrient signaling by CR to alter age-related changes in bone formation and BM adipogenesis. With aging, we detected an increase in SDF-1 levels in BM interstitial fluid, at the same time, no significant differences associated with different treatments with the BM cells, suggesting increased secretion of SDF-1 from the cells as it is synthesized. Increased SDF-1 levels are also thought to be crucial in stimulating the migration of stem and progenitor cells via the CXCR4 receptor to damaged tissues, thereby contributing to repair and regeneration (Takahashi, 2010,Jaerve, Bosse and Muller, 2012,Herberg, Kondrikova, Hussein et al., 2014). At the same time, such rise in SDF-1 levels with age may represent a dysfunctional, or pathogenic state, associated with decreased bone formation. SDF-1 expression is increased under a number of general stressors, irradiation, tissue injury, hypoxia, inflammation, transcription factors e.g. increased HIF-1 activation, NF-kB, SP1, and steroids (Markovic, Uskokovic, Grdovic et al., 2015,Chatterjee, Seizer, Borst et al., 2014). Aging is associated with increases in most of these factors (Herberg et al., 2013,Ierano, Santagata, Napolitano et al., 2014).
Yet, SDF-1 axis is considered to be critical in the survival, and differentiation of BMSCs down an osteogenic path and in permitting and enhancing bone formation (Jiang et al., 2002,Bianco et al., 2008,Herberg et al., 2013,Wagner and Ho, 2007,Zhang et al., 2012,Herberg S, 2012). It has only recently been recognized that there is cross-talk between the mTOR pathway involved in nutrient signaling, autophagy and cell growth decisions and the SDF-1/CXCR4/7 axis. CXCR4 and CXCR7 antagonists inhibited mTOR activation which may affect SDF-1 driven functions (Ierano et al., 2014). Further, a number of factors that CR and leptin can affect may in turn affect the SDF-1/CXCR4/7 axis (Csiszar, Gautam, Sosnowska et al., 2014). It has been shown that BMSCs have leptin receptors and leptin signaling is required for downstream CXCR4 effects (Zhou, Yue, Murphy et al., 2014). Leptin can also indirectly induce the SDF-1 and CXCR4 transcription factor SP1 (Garcia-Ruiz, Gomez-Izquierdo, Diaz-Sanjuan et al., 2012). In a recent study, we are showing CR plus leptin can specifically increase plasma SDF-1 levels in aged mice (Under Review; Davis, Dukes, Periyasamy-Thandavan et al., 2015).
Earlier work from our lab suggested that there was a high degree of bioactive SDF-1 in the BM interstitial fluid of 3 M-old mice when there is very active bone formation versus 18 M-old mice when there is already age-associated bone loss (Hamrick et al., 2006) (Supplementary Figure 1). Interestingly, while aging increased SDF-1 levels in BM interstitial fluid without increasing its bioactivity, as assessed by a BMSC migration assay, the apparent reduction in BM interstitial fluid SDF-1 with CR showed significantly enhanced chemoattractant activity, possibly due to changes in protease activity targeting SDF-1. CR has been shown to reduce age-related tissue damage by mobilizing stem and progenitor cells from their bone marrow niche into the peripheral blood (De Falco, Porcelli, Torella et al., 2004,Chen, Liu, Chen et al., 2014). In contrast, the high levels of SDF-1 secreted into the BM interstitial fluid with the addition of leptin to the CR mice blocked the increase in migration induced by CR alone. This may be due to leptin altering mTOR and SDF-1 signaling. CR decreases mTOR activation in the mTORC1 complex and enhances autophagy, while leptin may increase activation of both the mTORC 1 & 2 complexes driving cell growth (Schloesser, Campbell, Gluer et al., 2015,Thon, Hosoi, Yoshii et al., 2014,Haissaguerre, Saucisse and Cota, 2014). Recent reports also suggest that SDF-1 at high concentration forms dimers and leads to β-arrestin mediated signaling rather than the canonical G-protein-coupled pathways for CXCR4 signaling (Drury, Ziarek, Gravel et al., 2011,Frolich, Blassfeld, Reiter et al., 2012). In contrast to driving BMSC mobilization and migration, very high levels of SDF-1, potentially present in the CR + leptin samples, may retain the cells in the BM by inhibiting G-coupled protein based cell migration (Drury et al., 2011,Takekoshi, Ziarek, Volkman et al., 2012).
Previous work suggests that in lean subjects leptin circulates mainly in the bound form, whereas in obese subjects the majority of leptin circulates in the free form (Sinha, Opentanova, Ohannesian et al., 1996). A significant decline in the body mass index (BMI) associated with CR depletes the circulating leptin concentration (van Dielen, van 't Veer, Buurman et al., 2002). Thus, CR may induce leptin sensitivity to improve its bioavailability by increasing sLR circulating levels in aged mice and may be occurring here (Lammert, Kiess, Bottner et al., 2001,Huang, Wang and Li, 2001,Lou, Yang, Huang et al., 2010). It is thought that elevated levels of the soluble receptor create a reservoir of bound leptin that helps to maintain a constant pool of readily available leptin. Although it is not clear if this targets the leptin to BMSCs.
Higami et al (2004) and Wasinski (2013) observed a major loss of lipids and adipocytes in peripheral tissue adipocytes as a result of CR, although it is recognized BM adipocytes respond differently. CR has been shown to increase BM fat deposition (Bredella, Fazeli, Daley et al., 2014). Consistent with these data, in the femur, an appreciable number of bone marrow adipocytes were observed in 20 M ad lib animals, but the density of marrow adipocytes was greatly enhanced by long-term CR. Interestingly, leptin administration reduced the number of marrow adipocytes in calorically restricted animals by over 50% over the 10 day course of treatment. These data are consistent with observations that leptin administration reduces marrow adiposity in leptin-deficient mice (Hamrick et al., 2005). Bone marrow adiposity increases with age (Rozman, Feliu, Berga et al., 1989). In our study, aging significantly increased the PPAR-γ2 expression in the BM cells. Despite the large number of marrow adipocytes visible by histology, CR significantly reversed the high PPARγ2 mRNA expression in aged animals, suggesting a shift in their phenotype. Our ongoing studies will directly address the biological nature of these cells and their connection to regulation of metabolism within the BM niche.
Impairment of BMP-2 function has been described as one of the molecular pathogenic mechanisms for osteoporosis and bone metabolism (Kanakaris, Petsatodis, Tagil et al., 2009). In our current study, we found that BMP-2 gene and protein expression appear to decline in aged bone. A low BMI has been identified as an important risk factor for lower bone mineral density and predicts greater bone loss in older age (Ravn, Cizza, Bjarnason et al., 1999). As anticipated, the indices of bone formation were distinctly lower in the calorically restricted mice. However, leptin treatment reversed the CR induced bone growth reduction. We postulate that leptin treatment positively regulates SDF-1 potentially to promote CXCR4 mediated β-arrestin signaling and retain the BMSCs to permit osteoinductive influence on the BM microenvironment including osteoinduction via activating the BMP-2 receptor (Herberg et al., 2013,Herberg et al., 2014,Hosogane, Huang, Rawlins et al., 2010).
We show that NR plus leptin up-regulates SDF-1, CXCR4 and BMP-2 in BMSCs in vitro, while NR appears to down regulate BMP-2 by itself. NR significantly increases SDF-1 both at mRNA and protein levels BMSCs in vitro. This effect appears to be independent of leptin, which is normally very low in the setting of food restriction (Hamrick et al., 2008). At the same time, NR significantly reduced BMP-2 both at mRNA and protein levels BMSCs in vitro. Remarkably, this effect appears to be dependent on leptin, since treatment with leptin significantly reversed it relative to control BMSCs. Age-related decline in the CXCR4 expression in BMSCs, and their reduced capability to differentiate osteogenically, may be a major factor accounting for reduced bone formation (Guang et al., 2013). NR and NR plus leptin reduce in vitro BMSC CXCR4 protein while up-regulating its gene expression suggests increased turnover of CXCR4 as a consequence of NR via β-Arrestin mediated receptor/ligand internalization and degradation through the clatherin mediated endosomal-lysosomal system. This may also contribute to its inhibitory effect on migration (Herberg et al., 2013,Herberg et al., 2013,Ceradini, Kulkarni, Callaghan et al., 2004). The present study documents a significant stimulatory effect of CR and CR supplemented with peripheral leptin on the SDF-1 axis, which may be critical in regulating differentiation and survival of BMSCs with aging.
The interaction of nutrient signaling pathways such as those affected by CR and leptin on the SDF-1 axis has only recently been identified and very little research has focused on this, especially as it relates to bone formation or aging. Here we demonstrate that both CR and leptin can affect SDF-1 and its receptors and that aging alters these outcomes. These novel findings supports further investigation into clarifying the areas of cross-talk and direct mediation of the SDF-1 axis through nutrient signaling pathways including those regulated by mTOR and leptin on BMSC cell fate and function in terms of bone formation. They suggest that manipulation of these pathways during aging may both explain some elements of age-associated musculoskeletal disorders and potential therapeutic interventions.
Supplementary Material
Highlights.
Caloric Restriction induces CXCR4 mediated migration
CR may induce leptin sensitivity
Enhanced bone formation was observed in CR mice treated with leptin
Density of marrow adipocytes was greatly enhanced by long-term CR
Acknowledgements
This publication is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development Program (VA Merit Award 104462, W.D.H.) and the National Institutes of Health (NIA-AG036675-01, M.W.H, C.I and W.D.H.). The contents of this publication do not represent the views of the Department of Veterans Affairs, or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics, C. and Stroke Statistics, S. Executive summary: heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127:143–52. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 2.Bonyadi M, Waldman SD, Liu D, Aubin JE, Grynpas MD, Stanford WL. Mesenchymal progenitor self-renewal deficiency leads to age- dependent osteoporosis in Sca-1/Ly-6A null mice. Proc Natl Acad Sci U S A. 2003;100:5840–5. doi: 10.1073/pnas.1036475100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egermann M, Schneider E, Evans CH, Baltzer AW. The potential of gene therapy for fracture healing in osteoporosis. Osteoporos Int. 16 Suppl. 2005;2:S120–8. doi: 10.1007/s00198-004-1817-9. [DOI] [PubMed] [Google Scholar]
- 4.Miura Y, Miura M, Gronthos S, Allen MR, Cao C, Uveges TE, Bi Y, Ehirchiou D, Kortesidis A, Shi S, Zhang L. Defective osteogenesis of the stromal stem cells predisposes CD18-null mice to osteoporosis. Proc Natl Acad Sci U S A. 2005;102:14022–7. doi: 10.1073/pnas.0409397102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz- Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–9. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 6.Gimble JM, Nuttall ME. The relationship between adipose tissue and bone metabolism. Clin Biochem. 2012;45:874–9. doi: 10.1016/j.clinbiochem.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Cao JJ. Effects of obesity on bone metabolism. J Orthop Surg Res. 2011;6:30. doi: 10.1186/1749-799X-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeyabalan J, Shah M, Viollet B, Chenu C. AMP-activated protein kinase pathway and bone metabolism. J Endocrinol. 2012;212:277–90. doi: 10.1530/JOE-11-0306. [DOI] [PubMed] [Google Scholar]
- 9.Meunier P, Aaron J, Edouard C, Vignon G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res. 1971;80:147–54. doi: 10.1097/00003086-197110000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Yu L, Cecil J, Peng SB, Schrementi J, Kovacevic S, Paul D, Su EW, Wang J. Identification and expression of novel isoforms of human stromal cell-derived factor 1. Gene. 2006;374:174–9. doi: 10.1016/j.gene.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–9. doi: 10.1016/j.stem.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herberg S, Shi X, Johnson MH, Hamrick MW, Isales CM, Hill WD. Stromal cell-derived factor-1beta mediates cell survival through enhancing autophagy in bone marrow-derived mesenchymal stem cells. PLoS One. 2013;8:e58207. doi: 10.1371/journal.pone.0058207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herberg S, Fulzele S, Yang N, Shi X, Hess M, Periyasamy-Thandavan S, Hamrick MW, Isales CM, Hill WD. Stromal cell-derived factor- 1beta potentiates bone morphogenetic protein-2-stimulated osteoinduction of genetically engineered bone marrow-derived mesenchymal stem cells in vitro. Tissue Eng Part A. 2013;19:1–13. doi: 10.1089/ten.tea.2012.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner W, Ho AD. Mesenchymal stem cell preparations--comparing apples and oranges. Stem Cell Rev. 2007;3:239–48. doi: 10.1007/s12015-007-9001-1. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Khan D, Delling J, Tobiasch E. Mechanisms underlying the osteo- and adipo-differentiation of human mesenchymal stem cells. ScientificWorldJournal. 2012;2012:793823. doi: 10.1100/2012/793823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guang LG, Boskey AL, Zhu W. Age-related CXC chemokine receptor-4-deficiency impairs osteogenic differentiation potency of mouse bone marrow mesenchymal stromal stem cells. Int J Biochem Cell Biol. 2013;45:1813–20. doi: 10.1016/j.biocel.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 17.Subramanian S, Liu C, Aviv A, Ho JE, Courchesne P, Muntendam P, Larson MG, Cheng S, Wang TJ, Mehta NN, Levy D. Stromal cell-derived factor 1 as a biomarker of heart failure and mortality risk. Arterioscler Thromb Vasc Biol. 2014;34:2100–5. doi: 10.1161/ATVBAHA.114.303579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamrick MW, Ding KH, Ponnala S, Ferrari SL, Isales CM. Caloric restriction decreases cortical bone mass but spares trabecular bone in the mouse skeleton: implications for the regulation of bone mass by body weight. J Bone Miner Res. 2008;23:870–8. doi: 10.1359/jbmr.080213. [DOI] [PubMed] [Google Scholar]
- 20.Shapses SA, Sukumar D. Bone metabolism in obesity and weight loss. Annu Rev Nutr. 2012;32:287–309. doi: 10.1146/annurev.nutr.012809.104655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fenton JI, Nunez NP, Yakar S, Perkins SN, Hord NG, Hursting SD. Diet-induced adiposity alters the serum profile of inflammation in C57BL/6N mice as measured by antibody array. Diabetes Obes Metab. 2009;11:343–54. doi: 10.1111/j.1463-1326.2008.00974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Redman LM, Rood J, Anton SD, Champagne C, Smith SR, Ravussin E, Pennington Comprehensive Assessment of Long-Term Effects of Reducing Intake of Energy Research, T. Calorie restriction and bone health in young, overweight individuals. Arch Intern Med. 2008;168:1859–66. doi: 10.1001/archinte.168.17.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talbott SM, Cifuentes M, Dunn MG, Shapses SA. Energy restriction reduces bone density and biomechanical properties in aged female rats. J Nutr. 2001;131:2382–7. doi: 10.1093/jn/131.9.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uusi-Rasi K, Rauhio A, Kannus P, Pasanen M, Kukkonen-Harjula K, Fogelholm M, Sievanen H. Three-month weight reduction does not compromise bone strength in obese premenopausal women. Bone. 2010;46:1286–93. doi: 10.1016/j.bone.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Hamrick MW, Ding KH, Pennington C, Chao YJ, Wu YD, Howard B, Immel D, Borlongan C, McNeil PL, Bollag WB, Curl WW, Yu J, Isales CM. Age-related loss of muscle mass and bone strength in mice is associated with a decline in physical activity and serum leptin. Bone. 2006;39:845–53. doi: 10.1016/j.bone.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Hamrick MW, Della-Fera MA, Choi YH, Pennington C, Hartzell D, Baile CA. Leptin treatment induces loss of bone marrow adipocytes and increases bone formation in leptin-deficient ob/ob mice. J Bone Miner Res. 2005;20:994–1001. doi: 10.1359/JBMR.050103. [DOI] [PubMed] [Google Scholar]
- 27.Hamrick MW, Herberg S, Arounleut P, He HZ, Shiver A, Qi RQ, Zhou L, Isales CM, Mi QS. The adipokine leptin increases skeletal muscle mass and significantly alters skeletal muscle miRNA expression profile in aged mice. Biochem Biophys Res Commun. 2010;400:379–83. doi: 10.1016/j.bbrc.2010.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang W, Ou G, Hamrick M, Hill W, Borke J, Wenger K, Chutkan N, Yu J, Mi QS, Isales CM, Shi XM. Age-related changes in the osteogenic differentiation potential of mouse bone marrow stromal cells. Journal of Bone and Mineral Research. 2008;23:1118–28. doi: 10.1359/JBMR.080304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gimble JM, Robinson CE, Wu X, Kelly KA, Rodriguez BR, Kliewer SA, Lehmann JM, Morris DC. Peroxisome proliferator-activated receptor-gamma activation by thiazolidinediones induces adipogenesis in bone marrow stromal cells. Molecular Pharmacology. 1996;50:1087–94. [PubMed] [Google Scholar]
- 30.Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–8. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- 31.Tropel P, Noel D, Platet N, Legrand P, Benabid AL, Berger F. Isolation and characterisation of mesenchymal stem cells from adult mouse bone marrow. Experimental Cell Research. 2004;295:395–406. doi: 10.1016/j.yexcr.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 32.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 33.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 34.Parfitt AM. Bone histomorphometry: standardization of nomenclature, symbols and units. Summary of proposed system. Bone Miner. 1988;4:1–5. [PubMed] [Google Scholar]
- 35.Takahashi M. Role of the SDF-1/CXCR4 system in myocardial infarction. Circ J. 2010;74:418–23. doi: 10.1253/circj.cj-09-1021. [DOI] [PubMed] [Google Scholar]
- 36.Jaerve A, Bosse F, Muller HW. SDF-1/CXCL12: its role in spinal cord injury. Int J Biochem Cell Biol. 2012;44:452–6. doi: 10.1016/j.biocel.2011.11.023. [DOI] [PubMed] [Google Scholar]
- 37.Herberg S, Kondrikova G, Hussein KA, Periyasamy-Thandavan S, Johnson MH, Elsalanty ME, Shi X, Hamrick MW, Isales CM, Hill WD. Total Body Irradiation Is Permissive for Mesenchymal Stem Cell- Mediated New Bone Formation Following Local Transplantation. Tissue Eng Part A. 2014 doi: 10.1089/ten.tea.2013.0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Markovic J, Uskokovic A, Grdovic N, Dinic S, Mihailovic M, Jovanovic JA, Poznanovic G, Vidakovic M. Identification of transcription factors involved in the transcriptional regulation of the CXCL12 gene in rat pancreatic insulinoma Rin-5F cell line. Biochem Cell Biol. 2015;93:54–62. doi: 10.1139/bcb-2014-0104. [DOI] [PubMed] [Google Scholar]
- 39.Chatterjee M, Seizer P, Borst O, Schonberger T, Mack A, Geisler T, Langer HF, May AE, Vogel S, Lang F, Gawaz M. SDF-1alpha induces differential trafficking of CXCR4-CXCR7 involving cyclophilin A, CXCR7 ubiquitination and promotes platelet survival. FASEB J. 2014;28:2864–78. doi: 10.1096/fj.14-249730. [DOI] [PubMed] [Google Scholar]
- 40.Ierano C, Santagata S, Napolitano M, Guardia F, Grimaldi A, Antignani E, Botti G, Consales C, Riccio A, Nanayakkara M, Barone MV, Caraglia M, Scala S. CXCR4 and CXCR7 transduce through mTOR in human renal cancer cells. Cell Death Dis. 2014;5:e1310. doi: 10.1038/cddis.2014.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herberg S FS, Yang N, Shi X, Hess M, Periyasamy-Thandavan S, Hamrick MW, Isales CM, Hill WD. Enhanced Osteogenic Differentiation In Genetically Engineered Bone Marrow-Derived Mesenchymal Stem Cells Conditionally Overexpressing Stromal Cell-Derived Factor-1β. Tissue Engineering, Part A. 2012 doi: 10.1089/ten.tea.2012.0085. under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Csiszar A, Gautam T, Sosnowska D, Tarantini S, Banki E, Tucsek Z, Toth P, Losonczy G, Koller A, Reglodi D, Giles CB, Wren JD, Sonntag WE, Ungvari Z. Caloric restriction confers persistent anti-oxidative, pro angiogenic, and anti-inflammatory effects and promotes anti-aging miRNA expression profile in cerebromicrovascular endothelial cells of aged rats. Am J Physiol Heart Circ Physiol. 2014;307:H292–306. doi: 10.1152/ajpheart.00307.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ. Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014;15:154–68. doi: 10.1016/j.stem.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garcia-Ruiz I, Gomez-Izquierdo E, Diaz-Sanjuan T, Grau M, Solis-Munoz P, Munoz-Yague T, Solis-Herruzo JA. Sp1 and Sp3 transcription factors mediate leptin-induced collagen alpha1(I) gene expression in primary culture of male rat hepatic stellate cells. Endocrinology. 2012;153:5845–56. doi: 10.1210/en.2012-1626. [DOI] [PubMed] [Google Scholar]
- 45.Davis C, Dukes A, Periyasamy-Thandavan S, Upadhayaya S, Mork S, Herberg S, Kondrikova G, Johnson M, Isales CM, Hill WD, Hamrick M. Nutrient Restriction Induces the Chemokine SDF-1 (CXCL12) in Aged Skeletal Muscle and in Myoprogenitor Cells. PLoS One. 2015 [Google Scholar]
- 46.De Falco E, Porcelli D, Torella AR, Straino S, Iachininoto MG, Orlandi A, Truffa S, Biglioli P, Napolitano M, Capogrossi MC, Pesce M. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood. 2004;104:3472–82. doi: 10.1182/blood-2003-12-4423. [DOI] [PubMed] [Google Scholar]
- 47.Chen H, Liu X, Chen H, Cao J, Zhang L, Hu X, Wang J. Role of SIRT1 and AMPK in mesenchymal stem cells differentiation. Ageing Res Rev. 2014;13:55–64. doi: 10.1016/j.arr.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Schloesser A, Campbell G, Gluer CC, Rimbach G, Huebbe P. Restriction on an Energy-Dense Diet Improves Markers of Metabolic Health and Cellular Aging in Mice Through Decreasing Hepatic mTOR Activity. Rejuvenation Res. 2015;18:30–9. doi: 10.1089/rej.2014.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thon M, Hosoi T, Yoshii M, Ozawa K. Leptin induced GRP78 expression through the PI3K-mTOR pathway in neuronal cells. Sci Rep. 2014;4:7096. doi: 10.1038/srep07096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haissaguerre M, Saucisse N, Cota D. Influence of mTOR in energy and metabolic homeostasis. Mol Cell Endocrinol. 2014;397:67–77. doi: 10.1016/j.mce.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 51.Drury LJ, Ziarek JJ, Gravel S, Veldkamp CT, Takekoshi T, Hwang ST, Heveker N, Volkman BF, Dwinell MB. Monomeric and dimeric CXCL12 inhibit metastasis through distinct CXCR4 interactions and signaling pathways. Proc Natl Acad Sci U S A. 2011;108:17655–60. doi: 10.1073/pnas.1101133108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frolich D, Blassfeld D, Reiter K, Giesecke C, Daridon C, Mei HE, Burmester GR, Goldenberg DM, Salama A, Dorner T. The anti-CD74 humanized monoclonal antibody, milatuzumab, which targets the invariant chain of MHC II complexes, alters B-cell proliferation, migration, and adhesion molecule expression. Arthritis Res Ther. 2012;14:R54. doi: 10.1186/ar3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takekoshi T, Ziarek JJ, Volkman BF, Hwang ST. A locked, dimeric CXCL12 variant effectively inhibits pulmonary metastasis of CXCR4- expressing melanoma cells due to enhanced serum stability. Mol Cancer Ther. 2012;11:2516–25. doi: 10.1158/1535-7163.MCT-12-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sinha MK, Opentanova I, Ohannesian JP, Kolaczynski JW, Heiman ML, Hale J, Becker GW, Bowsher RR, Stephens TW, Caro JF. Evidence of free and bound leptin in human circulation. Studies in lean and obese subjects and during short-term fasting. J Clin Invest. 1996;98:1277–82. doi: 10.1172/JCI118913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Dielen FM, van 't Veer C, Buurman WA, Greve JW. Leptin and soluble leptin receptor levels in obese and weight-losing individuals. J Clin Endocrinol Metab. 2002;87:1708–16. doi: 10.1210/jcem.87.4.8381. [DOI] [PubMed] [Google Scholar]
- 56.Lammert A, Kiess W, Bottner A, Glasow A, Kratzsch J. Soluble leptin receptor represents the main leptin binding activity in human blood. Biochem Biophys Res Commun. 2001;283:982–8. doi: 10.1006/bbrc.2001.4885. [DOI] [PubMed] [Google Scholar]
- 57.Huang L, Wang Z, Li C. Modulation of circulating leptin levels by its soluble receptor. J Biol Chem. 2001;276:6343–9. doi: 10.1074/jbc.M009795200. [DOI] [PubMed] [Google Scholar]
- 58.Lou PH, Yang G, Huang L, Cui Y, Pourbahrami T, Radda GK, Li C, Han W. Reduced body weight and increased energy expenditure in transgenic mice over-expressing soluble leptin receptor. PLoS One. 2010;5:e11669. doi: 10.1371/journal.pone.0011669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Higami Y, Pugh TD, Page GP, Allison DB, Prolla TA, Weindruch R. Adipose tissue energy metabolism: altered gene expression profile of mice subjected to long-term caloric restriction. FASEB J. 2004;18:415–7. doi: 10.1096/fj.03-0678fje. [DOI] [PubMed] [Google Scholar]
- 60.Wasinski F, Bacurau RF, Moraes MR, Haro AS, Moraes-Vieira PM, Estrela GR, Paredes-Gamero EJ, Barros CC, Almeida SS, Camara NO, Araujo RC. Exercise and caloric restriction alter the immune system of mice submitted to a high-fat diet. Mediators Inflamm. 2013;2013:395672. doi: 10.1155/2013/395672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bredella MA, Fazeli PK, Daley SM, Miller KK, Rosen CJ, Klibanski A, Torriani M. Marrow fat composition in anorexia nervosa. Bone. 2014;66:199–204. doi: 10.1016/j.bone.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rozman C, Feliu E, Berga L, Reverter JC, Climent C, Ferran MJ. Age-related variations of fat tissue fraction in normal human bone marrow depend both on size and number of adipocytes: a stereological study. Exp Hematol. 1989;17:34–7. [PubMed] [Google Scholar]
- 63.Kanakaris NK, Petsatodis G, Tagil M, Giannoudis PV. Is there a role for bone morphogenetic proteins in osteoporotic fractures? Injury. 40 Suppl. 2009;3:S21–6. doi: 10.1016/S0020-1383(09)70007-5. [DOI] [PubMed] [Google Scholar]
- 64.Ravn P, Cizza G, Bjarnason NH, Thompson D, Daley M, Wasnich RD, McClung M, Hosking D, Yates AJ, Christiansen C. Low body mass index is an important risk factor for low bone mass and increased bone loss in early postmenopausal women. Early Postmenopausal Intervention Cohort (EPIC) study group. J Bone Miner Res. 1999;14:1622–7. doi: 10.1359/jbmr.1999.14.9.1622. [DOI] [PubMed] [Google Scholar]
- 65.Hosogane N, Huang Z, Rawlins BA, Liu X, Boachie-Adjei O, Boskey AL, Zhu W. Stromal derived factor-1 regulates bone morphogenetic protein 2-induced osteogenic differentiation of primary mesenchymal stem cells. Int J Biochem Cell Biol. 2010;42:1132–41. doi: 10.1016/j.biocel.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–64. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


