Abstract
Central nervous system (CNS) deficiencies of the monoamine neurotransmitters, dopamine and serotonin, have been implicated in the pathophysiology of neuropsychiatric dysfunction in phenylketonuria (PKU). Increased brain phenylalanine concentration likely competitively inhibits the activities of tyrosine hydroxylase (TH) and tryptophan hydroxylase (TPH), the rate limiting steps in dopamine and serotonin synthesis respectively. Tetrahydrobiopterin (BH4) is a required cofactor for TH and TPH activity. Our hypothesis was that treatment of hyperphenylalaninemic Pahenu2/enu2 mice, a model of human PKU, with sapropterin dihydrochloride, a synthetic form of BH4, would stimulate TH and TPH activities leading to improved dopamine and serotonin synthesis despite persistently elevated brain phenylalanine. Sapropterin (20, 40, or 100 mg/kg body weight in 1% ascorbic acid) was administered daily for 4 days by oral gavage to Pahenu2/enu2 mice followed by measurement of brain biopterin, phenylalanine, tyrosine, tryptophan and monoamine neurotransmitter content. A significant increase in brain biopterin content was detected only in mice that had received the highest sapropterin dose, 100 mg/kg. Blood and brain phenylalanine concentrations were unchanged by sapropterin therapy. Sapropterin therapy also did not alter the absolute amounts of dopamine and serotonin in brain but was associated with increased homovanillic acid (HVA) and 5-hydroxyindoleacetic acid (5-HIAA), dopamine and serotonin metabolites respectively, in both wild type and Pahenu2/enu2 mice. Oral sapropterin therapy likely does not directly affect central nervous system monoamine synthesis in either wild type or hyperphenylalaninemic mice but may stimulate synaptic neurotransmitter release and subsequent metabolism.
Keywords: Phenylketonuria, Tetrahydrobiopterin, Sapropterin dihydrochloride, Dopamine, Serotonin, Monoamine neurotransmitters
Introduction
Phenylketonuria (PKU) due to recessively-inherited deficiency of phenylalanine hydroxylase (PAH, EC 1.14.16.1) is one of the most common inborn errors of metabolism. Dietary therapy of PKU to reduce plasma phenylalanine (Phe) levels prevents the major manifestations of the disease (mental retardation, seizures, and growth failure) yet mild cognitive deficits persist in some treated children (Azen et al. 1991). Additionally, PKU therapy requires lifelong strict adherence to an unpalatable and complicated diet. In our clinical experience, nonadherence to this difficult diet is common in adolescents and adults. Up to 75% of adolescents and adults in some PKU treatment centers are unable to maintain blood Phe levels within the recommended target range (Walter and White 2004). Chronically elevated blood Phe is frequently associated with disturbed executive functioning (VanZutphen et al. 2007; Christ et al. 2010). The proximate central nervous system mechanisms that underlay the effects of hyperphenylalaninemia are incompletely understood, but abundant evidence implicates dysfunction of the dopaminergic and serotonergic neuronal systems, particularly of the prefrontal cortex, as having major roles in the symptoms of anxiety and impaired executive functioning associated with poorly treated PKU (Christ et al. 2010; de Groot et al. 2010; Feillet et al. 2010). Elevated brain Phe but decreased tyrosine and tryptophan, dopamine and serotonin have been measured in brains obtained at autopsy of individuals with untreated PKU (McKean 1972). Elevated blood phenylalanine in individuals with classical PKU has been associated with decreased urinary excretion of dopamine and serotonin or their metabolites (Curtius et al. 1972; Curtius et al. 1981), and in diet treated individuals, a phenylalanine challenge yielded a transient decrease in urine dopamine and serotonin levels along with further functional impairment on neuropsychological measures of higher executive functioning (Krause et al. 1985). In Pahenu2/enu2 mice, a model of human PKU, deficiencies of both dopamine and serotonin have been documented (Puglisi-Allegra et al. 2000; Pascucci et al. 2002; Harding et al. 2014). These deficiencies have been postulated to be involved in the memory deficit exhibited by these animals (Zagreda et al. 1999). Additionally, we have documented decreased exploratory behavior in an open field test in hyperphenylalaninemic Pahenu2/enu2 mice that could also be related to monoamine neurotransmitter deficiency (unpublished data). The likely mechanisms causing monoamine neurotransmitter deficiency in PKU are relative brain tyrosine and tryptophan deficiency due to competition with phenylalanine at the blood brain barrier (Binek-Singer and Johnson 1982; Knudsen et al. 1995; Hoeksma et al. 2009) and phenylalanine-mediated competitive inhibition of tyrosine hydroxylase (TH) and tryptophan hydroxylase (TPH), the rate limiting steps in dopamine and serotonin synthesis respectively (Ikeda et al. 1967; Ogawa and Ichinose 2006; Pascucci et al. 2009; Harding et al. 2014) (Figure 1).
Figure 1. Pathways of dopamine and serotonin synthesis.
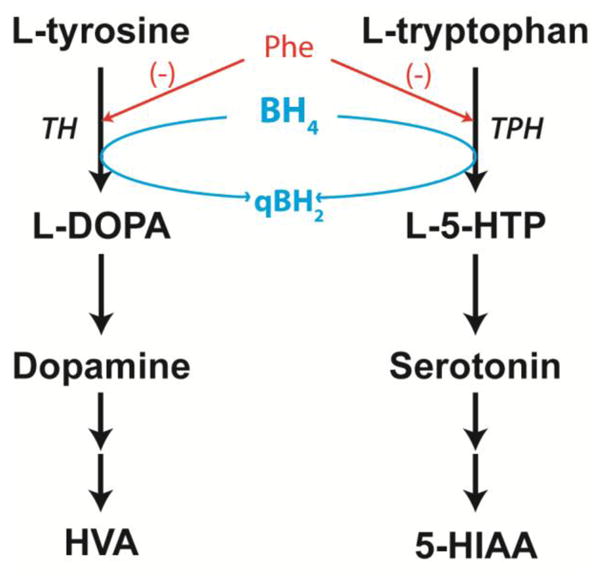
Dopamine and serotonin are synthesized from the amino acids L-tyrosine and L-tryptophan respectively, and after release into the neuronal synapse, metabolized to homovanillic acid (HVA) or 5-hydroxyindoleacetic acid (5-HIAA). The rate limiting steps in these pathways are catalyzed by tyrosine hydroxylase (TH) and tryptophan hydroxylase (TPH) (TPH2 in brain); both reactions require molecular oxygen (not shown) and BH4 cofactor to complete substrate hydroxylation. Elevated brain phenylalanine (Phe) competitively inhibits both enzymes and is associated with deficient brain dopamine and serotonin synthesis. Our hypothesis was that increased brain biopterin content following enteral sapropterin treatment would lead to functionally increased TH and TPH activity and correction of brain dopamine and serotonin content in hyperphenylalaninemic Pahenu2/enu2 mice.
The aromatic amino acid hydroxylases, PAH, TH, TPH1, and TPH2, all require (6R)-L-erythro-5,6,7,8-tetrahydrobiopterin (BH4) for catalytic activity. Additionally, BH4 exhibits chaperone activity stabilizing PAH protein (Blau and Erlandsen 2004; Thony et al. 2004), and BH4 supplementation leads to decreased blood phenylalanine in a subset of individuals with PKU (Kure et al. 1999; Levy et al. 2007). BH4 deficiency in mice is associated with decreased TH protein and activity in brain (Brand et al. 1996; Sumi-Ichinose et al. 2001), and BH4 supplementation has been shown to increase striatal TH activity (Nagatsu et al. 1994; Thöny et al. 2008). BH4 has also been shown to stabilize TH protein in vivo, and daily oral administration of BH4 to wild type C57BL/6 mice resulted in increased brain TH protein and activity (Thöny et al. 2008). Sapropterin dihydrochloride (Kuvan™, BioMarin Pharmaceutical Corp., Novato, CA) is a synthetic form of BH4 that is approved for the treatment of BH4 responsive PKU. The purpose of this study was to evaluate the effect of sapropterin treatment upon monoamine neurotransmitter status in Pahenu2/enu2 mice, a model that is known to not be BH4 responsive in terms of blood phenylalanine levels. Our hypothesis was that enteral treatment with sapropterin would lead to improved central nervous system monoamine neurotransmitter status in Pahenu2/enu2 mice even though blood and brain phenylalanine content is unaffected. A study of BH4 penetration into brain following oral sapropterin supplementation was a secondary aim of this project.
Methods
In this experiment, sapropterin dihydrochloride (Kuvan™, BioMarin Pharmaceutical Inc., Novato, CA) was administered by gavage to hyperphenylalaninemic C57BL6-Pahenu2/enu2 mice (Pah−/−) or to wild type C57BL6 mice (Pah+/+) to evaluate the uptake of BH4 into brain and the effect of enteral BH4 administration upon brain monoamine neurotransmitter content. Sapropterin, 100 mg tablets, were dissolved in 1% ascorbic acid immediately prior to administration. The final sapropterin concentration was varied to yield different doses in identical volumes of solution to be administered to the mice. Mice received sapropterin (20, 40, or 100 mg/kg body weight) once daily for four days by gavage. A control group of mice received only 1% ascorbic acid vehicle without added sapropterin. During this period, the animals received standard mouse chow (21% protein by weight) and water ad libitum. On the fifth day, mice were euthanized at various time points following sapropterin administration for tissue harvest. During the time period between sapropterin gavage and euthanasia, mouse chow was withheld in order to minimize variability in blood amino acid concentrations caused by feeding. Animals were sedated using inhaled isoflurane anesthesia. Whole blood was collected by cardiac puncture, allowed to clot in an Eppendorf tube, and serum was separated by centrifugation. The mice were then euthanized by exsanguination and perfused with 20 ml normal saline via the left cardiac ventricle to clear blood from the cerebral circulation. Following decapitation, whole brain was rapidly excised from the cranium, split sagitally, and immediately submerged in liquid nitrogen. Half brains and serum samples were stored at −80°C until processing for amino acid or neurotransmitter analysis.
Amino acid analysis
Amino acid concentrations in sera or brain tissue were measured by precolumn derivitization with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC, Waters AccQ Tag™derivitization system) with separation by ultra high performance liquid chromatography and UV absorbance detection (Waters Acquity™ UPLC, Milford, MA) using the Waters Masstrak Amino Acid Analysis method. Serum samples were deproteinized by adding an equal volume of 10% sulfosalicylic acid containing the nonphysiologic amino acid norvaline (250 μM) as an internal recovery standard. For analysis of amino acid concentrations in brain, half-brains were mechanically homogenized in 5 volumes/weight 10% trichloroacetic acid containing norvaline (150 μM). The homogenates were clarified by centrifugation at 500 × g and supernatants stored at −80°C until analysis. Deproteinized serum or brain homogenate was neutralized by adding 20 μl sample to 60 μl borate buffer (Waters Masstrak kit) plus sodium hydroxide to a final pH = 8. Derivitization was accomplished by adding 20 μl AQC reagent and incubating for 10 minutes at 55°C according to the instructions of the Waters MassTrak amino acid analysis kit (final volume of the derivitization reaction = 100 μl). 1 μl of this final derivitized solution was injected onto the UPLC for analysis. The serum amino acid concentrations were reported as μM and corrected for dilution to reflect the actual concentrations in serum. Brain amino acid concentrations are also reported as μM, but these are the measured amino acid concentrations in the brain homogenate supernatants that have not been corrected for brain weight and are not the actual amino acid concentrations expected in undiluted brain tissue. Because all brain samples were homogenized in a measured amount of homogenizing solution per brain weight wet, these uncorrected amino acid concentrations are suitable for examining differences among experimental groups
Brain biopterin content
Mouse half-brains were mechanically homogenized in ice cold homogenizing buffer (50 mM Tris-HCl, pH 7.5, 0.1 M KCl, 1 mM EDTA, 1 mM dithiothreitol, 0.2 mM phenylmethylsulfonyl fluoride, 1 μM leupeptin, and 1 μM pepstatin), 4 μl/mg tissue and further processed according to a previously published method (Elzaouk et al. 2003). Total biopterin concentration in brain was measured by HPLC and fluorescence detection (Blau and Thöny 2008). Total brain homogenate biopterin content was corrected for the protein content of the homogenate and expressed as pmol/mg protein.
Brain monoamine neurotransmitter analysis
Mouse half-brains were mechanically homogenized in ice cold homogenizing buffer and processed as described above. Monoamine neurotransmitter concentrations (L-DOPA, dopamine, HVA, serotonin, and 5-HIAA) were measured in brain tissue by HPLC and electrochemical detection (Blau et al. 1999). Measured brain homogenate monoamine neurotransmitter concentrations were corrected for the protein content of the homogenate and expressed as pmol/mg protein.
Statistical analysis
All data are reported as means ± standard error of the mean (SEM). Two-way ANOVA with Pah genotype and sapropterin treatment as independent variables was utilized in the statistical analysis of all data. Individual group differences were analyzed using Bonferroni post tests. Means were considered significantly different when p < 0.05. All statistical analyses were carried out with Prism 6.0 software (GraphPad Software, Inc.).
Results
Brain biopterin content
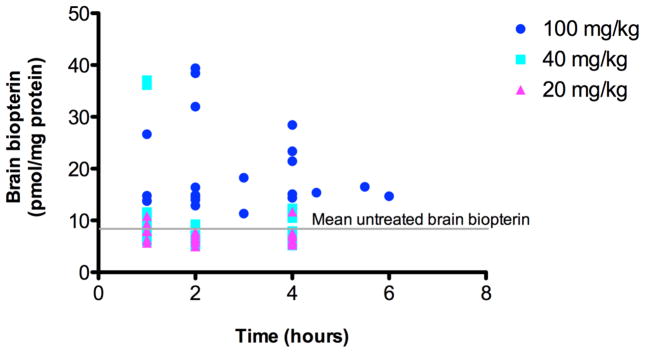
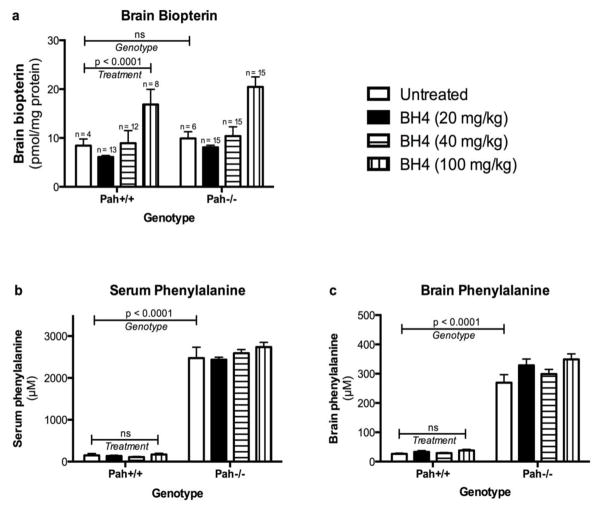
We administered sapropterin dihydrochloride in 1% ascorbic acid at three different doses (20, 40 or 100 mg/kg body weight) to both wild type (Pah+/+) and Pahenu2/enu2 (Pah−/−) mice by daily oral gavage. Separate control animals of both genotypes received only 1% ascorbic acid. On the fourth day of sapropterin treatment, animals were euthanized at various time points following the final gavage and tissues were collected for analysis. Half-brain total biopterin content of sapropterin-treated mice (both genotypes combined) are plotted versus time after sapropterin gavage in Figure 2. Only administration of the highest sapropterin dose (100 mg/kg) yielded a clearly demonstrable increase in brain biopterin content. At 2 hours post sapropterin dose (100 mg/kg), the average brain biopterin content of sapropterin treated mice (22.8 ± 10.9 pmol/mg protein) had increased by more than two fold in comparison to the brain biopterin content of mice that had received only 1% ascorbic acid vehicle (9.9 ± 2.8 pmol/mg protein). Brain biopterin content decreased at later time points but remained above the baseline level for the six hour duration of the experiment. Comparing all treatment groups (Figure 3a), statistical analysis using two-way ANOVA with genotype and sapropterin treatment as independent variables revealed a significant effect of sapropterin treatment upon brain biopterin content (F(3,79) = 14.4, p < 0.0001) but no significant effect of genotype nor any significant interaction between genotype and sapropterin treatment.
Figure 2. Brain biopterin content vs. time following enteral sapropterin administration.
Total biopterin was measured in homogenates of wild type and Pahenu2/enu2 mouse half-brains excised at various times after enteral sapropterin administration at three different doses: 20 mg/kg body weight (n = 28), 40 mg/kg (n = 27), or 100 mg/kg (n = 23). Brain biopterin concentration was corrected for the concentration of protein in the homogenate and expressed as pmol/mg protein. The brain biopterin content in six Pahenu2/enu2 mice that had received only 1% ascorbic acid by gavage feeding (mean untreated brain biopterin, gray line) was 9.2 ± 1.4 pmol/mg protein (mean ± SEM).
Figure 3. Effect of Pah genotype and sapropterin treatment upon brain biopterin, serum and brain phenylalanine.
Brain biopterin content (pmol/mg protein, mean ± SEM) (a) following either sapropterin treatment (BH4) or 1% ascorbic acid by gavage feeding in wild type (Pah +/+) and Pahenu2/enu2 (Pah−/−) mice. The data are the means of brain biopterin content from all animals at all time points up to six hours following gavage. Sapropterin treatment yielded a significant increase in brain biopterin content (two-way ANOVA, p < 0.0001) across all groups but only the 100 mg/kg sapropterin dose yielded a significant increase (p < 0.01) in brain biopterin in comparison to 1% ascorbic acid when analyzed by Bonferroni posttest intergroup analysis. There was no significant effect of genotype (Pah+/+ vs. Pah−/−) upon brain biopterin content. Serum (b) and brain (c) phenylalanine concentration (μM, mean ± SEM) in Pah+/+ or Pah−/− mice treated with sapropterin (BH4) or 1% ascorbic acid. The horizontal bars represent the statistical significance of the effects of either Genotype or sapropterin Treatment upon the brain biopterin, serum phenylalanine and brain phenylalanine concentrations as calculated by two way ANOVA. The Pah genotype had no effect upon brain biopterin content following sapropterin treatment. As expected, both serum and brain phenylalanine were extremely elevated in Pah−/− mice in comparison to Pah+/+ animals. Sapropterin treatment had no significant (ns) effect upon either serum or brain phenylalanine.
Serum and brain phenylalanine
Sapropterin treatment had no significant effect upon either serum (Figure 3b) or brain (Figure 3c) phenylalanine content in Pah+/+ or Pah−/− mice. By two way ANOVA, only genotype had a significant effect upon serum (F(1,77) = 1073, p < 0.0001) or brain (F(1,74) = 445.2, p < 0.0001) phenylalanine. There was no significant interaction between genotype and sapropterin treatment with regards to either serum or brain phenylalanine. In the cohorts of Pah−/− animals that had received sapropterin (any dose), the mean serum phenylalanine at euthanasia (2591 ± 367 μM) was not different than the serum phenylalanine concentrations in Pah−/− mice that had received only 1% ascorbic acid (2476 ± 634 μM) confirming that this animal model is not responsive to BH4 administration in terms of blood phenylalanine concentrations (Figure 3b). Likewise, neither blood nor brain phenylalanine concentrations in wild type mice were affected by sapropterin treatment.
Serum and brain tyrosine and tryptophan content
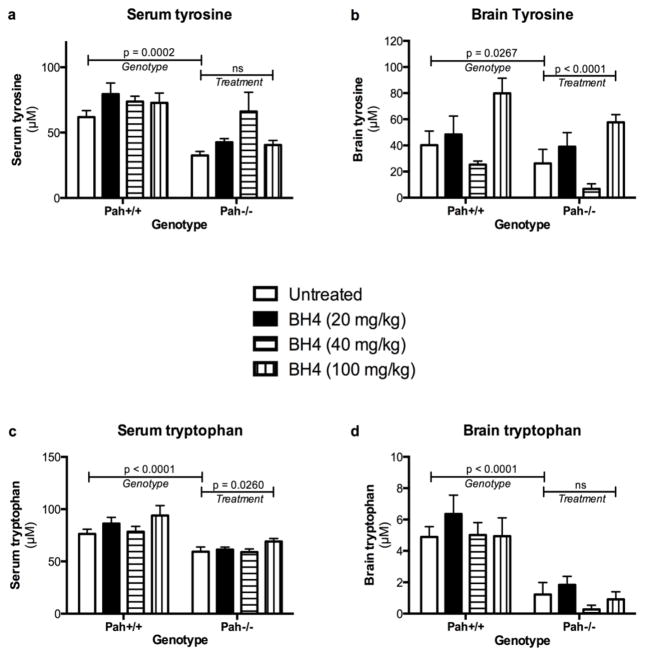
PAH deficiency causes decreased tyrosine synthesis, and in our experiment both serum and brain tyrosine concentrations were significantly decreased in Pah−/− mice relative to Pah+/+ mice (Figures 4a and 4b). By two way ANOVA, the variance in serum tyrosine concentrations among the experimental groups was entirely attributed to the Pahenu2/enu2 genotype (F(1;78) = 15.1, p = 0.0002); there was no effect of sapropterin treatment (F(3,78) = 1.795) and no significant interaction between genotype and treatment. The mean serum tyrosine of Pah−/− mice in all treatment groups (47.8 ± 4.7 μM) was 65% of that in Pah+/+ mice (74.0 ± 3.5 μM). Likewise, genotype had a significant effect upon brain tyrosine content (F(1,75) = 5.108, p = 0.0267), but surprisingly sapropterin treatment also had a statistically significant but inconsistent effect upon brain tyrosine (F(3,75) = 12.49, p < 0.0001). There was no significant interaction between genotype and sapropterin treatment. This apparent effect of sapropterin treatment upon brain tyrosine (predominantly at the highest sapropterin dose) remains unexplained and was not consistently seen in measurement of other large neutral amino acids (data not shown).
Figure 4. Effect of Pah genotype and sapropterin treatment upon serum and brain tyrosine and tryptophan.
The concentrations (μM, mean ± SEM) of tyrosine in serum (a) and brain (b) and of tryptophan in serum (c) and brain (d) from Pah+/+ and Pah−/− mice following gavage with sapropterin or 1% ascorbic acid are displayed. The data are the means from all animals obtained at all time points up to six hours after gavage. The horizontal bars represent the statistical significance of the effects of either Genotype or sapropterin Treatment upon the analytes as calculated by two way ANOVA. Pah−/− mice consistently exhibit tyrosine and tryptophan deficiency in both serum and brain relative to Pah+/+ animals. Sapropterin treatment had no effect upon serum tyrosine or brain tryptophan concentrations but did have apparent effects upon brain tyrosine and serum tryptophan, primarily in the highest sapropterin dose groups.
PAH deficiency was also associated with effects upon blood tryptophan content with mean Pah−/− serum tryptophan (62.7 ± 1.6 μM) measuring 74% of mean serum tryptophan in Pah+/+ mice (84.2 ± 3.4 μM). Two way ANOVA revealed a significant effect of genotype upon serum tryptophan (F(1,78) = 34.99, p < 0.0001) with a modest effect from sapropterin treatment (F(3,78) = 3.257, p = 0.026); there was no significant interaction between genotype and sapropterin treatment. The mechanism behind serum tryptophan deficiency in Pahenu2/enu2 mice is unknown; dietary phenylalanine likely competes against dietary tryptophan for uptake into circulation across the intestinal enterocyte, but it is difficult to explain how hyperphenylalaninemia would interfere with intestinal tryptophan absorption. Phenylalanine is also known to compete against uptake of other large neutral amino acids across the blood brain barrier. Accordingly, the brain tryptophan concentration in Pahenu2/enu2 mice (1.0 ± 0.2 μM) was approximately 1/5 of the concentration in wild type mice (5.4 ± 0.5 μM). Two way ANOVA demonstrated a significant effect of genotype upon brain tryptophan (F(1,75) =52.03, p < 0.0001) but no significant effect from sapropterin treatment (F(3,75) = 1.676, p = 0.1794) nor any significant interaction between genotype and treatment.
Brain monoamine neurotransmitter content
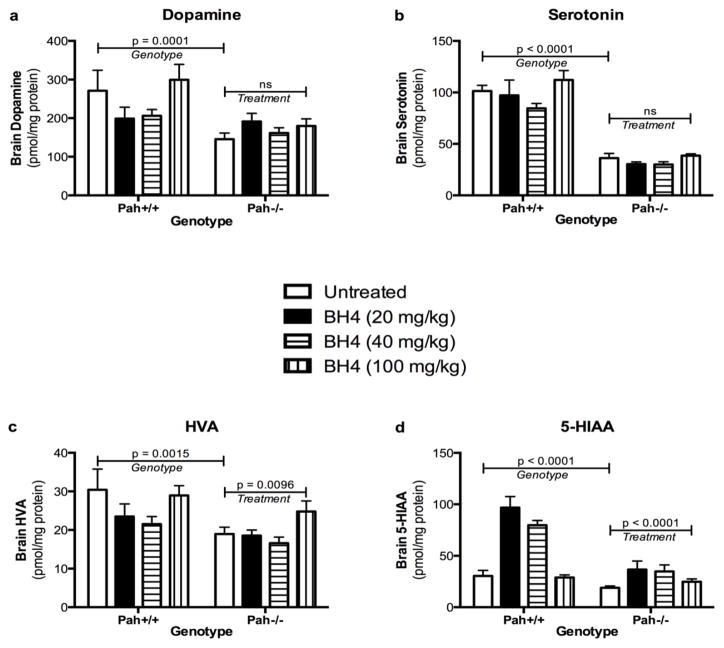
Both dopamine (Figure 4a) and serotonin (Figure 4b) content are depressed in brain of Pah−/− mice in comparison to wild type mice as we and others have been reported previously (Harding et al. 2014). Additionally, the concentrations of homovanillic acid (HVA) (Figure 4c) and 5-hydroxyindoleacetic acid (5-HIAA) (Figure 4d), markers of dopamine and serotonin turnover respectively, are significantly depressed in Pah−/− mice. Brain dopamine content in untreated Pah−/− mice (145.4 ± 16.1 pmol/mg protein, n = 6) was reduced to approximately 54% of that in Pah+/+ mice (271.0 ± 15.2 pmol/mg, n = 4), while Pah−/− HVA (19.0 ± 1.8 pmol/mg) was only 62% of HVA in wild type mice (30.4 ± 5.4 pmol/mg). Brain serotonin was more severely reduced with Pah−/− serotonin content (36.2 ± 4.5 pmol/mg) measuring only 36% of wild type brain serotonin (101.5 ± 5.4 pmol/mg). Similarly, Pah−/− brain 5-HIAA (19.0 ± 1.8 pmol/mg) was reduced to 62% of Pah+/+ levels (30.4 ± 5.4 pmol/mg).
Sapropterin treatment had no effect upon brain dopamine or serotonin content in either wild type or Pahenu2/enu2 mice (Figure 4). Two-way ANOVA of brain dopamine in all experimental groups revealed a significant effect of genotype only (F(1,79) = 15.54, p < 0.0001) with no significant effect from sapropterin treatment (F(3,79) = 2.182, p = 0.0967) and no significant interaction between genotype and sapropterin treatment. Similarly, genotype significantly affected brain serotonin (F(1,79) = 144.9, p <0.0001), but sapropterin treatment did not (F(3,79) = 2.602, p = 0.0578). Again, no significant interaction between genotype and sapropterin treatment was detected. Interestingly, brain HVA and 5-HIAA contents were significantly affected by both genotype and sapropterin treatment. For HVA, two way ANOVA revealed that both genotype (F(1,79) = 10.8, p = 0.0015) and sapropterin treatment (F(3,79) = 4.077, p = 0.0096) contributed equally to the variance among the experimental groups. Similar results were obtained for brain 5-HIAA content with genotype (F(1,79) = 2.37, p < 0.0001) and sapropterin treatment (F(3,79) = 15.97, p < 0.0001) having significant effects. Overall these results suggest that sapropterin treatment did not alter de novo dopamine or serotonin synthesis but did increase the metabolism of both dopamine and serotonin to HVA and 5-HIAA respectively.
Conclusions
Short term enteral administration by oral gavage of sapropterin dihydrochloride at 20, 40, or 100 mg/kg once per day for four days was well tolerated in both wild type and Pahenu2/enu2 mice. As expected, sapropterin treatment had no effect upon serum or brain phenylalanine concentration as the Pahenu2/enu2 model of human PKU is known to not be responsive to biopterin treatment in terms of lowering blood phenylalanine. Sapropterin treatment was associated with a statistically significant increase in brain total biopterin content but the greatest and most consistent effect was seen only in the animals that had received 100 mg sapropterin/kg. In these mice, brain biopterin content 2 hours after sapropterin gavage was more than double the brain biopterin content of untreated mice. This dose however is five fold higher than the oral dose typically prescribed to humans with BH4-responsive PKU. These data are consistent with previous work demonstrating rather poor penetration of peripherally administered BH4 across the blood-brain barrier of rodents except at high dosages (Kapatos and Kaufman 1981; Levine et al. 1987; Brand et al. 1996; Fiege and Blau 2006; Thöny et al. 2008) (summarized in Table 1) with better penetration into brain following parenteral BH4 injection. Our previous experience demonstrated that BH4 administered by intravenous injection to mice was primarily and rapidly taken up by liver and kidney with very little increase in BH4 content of other tissues (Harding et al. 2004). Data concerning biopterin uptake into human brain following BH4 administration is extremely limited. Oral BH4 administration of 7.5–20 mg/kg/day to humans is associated with a significant increase in biopterin content of cerebrospinal fluid (CSF) (Kaufman et al. 1982; Ponzone et al. 2006) but with no or only partial correction of CSF HVA and 5-HIAA concentrations in patients with inherited defects in BH4 synthesis suggesting minimal functional correction of brain monoamine neurotransmitter metabolism following oral BH4 administration at these lower doses (Ponzone et al. 2006).
Table 1.
Effect of peripherally administered BH4 upon biopterin content in rodent brain as summarized from the literature
| Reference | Species | Route of BH4 administration | Dose (mg/kg) | Fold increase in brain biopterin |
|---|---|---|---|---|
| Kapatos and Kaufman 1981 | Rat | IP | 24 | 1.8 |
| Levine et al 1987 | Rat | IP | 10 20 |
1 3 |
| Brand et al 1996 | Mouse | SQ | 30 | 2 |
| Hayashi et al 1992 | Rat | Oral | 10 100 |
1 1.4 |
| Thöny et al 2008 | Mouse | Oral | 20 100 |
1.3 1.6 |
| This report | Mouse | Oral | 20 40 100 |
1 1 2.1 |
This table lists the available literature reports of brain biopterin content following peripheral administration of BH4 to rodents. ‘Fold increase in brain biopterin’ reports the ratio of brain biopterin following BH4 administration to the brain biopterin content of untreated animals in each individual study. A ratio of 1 reflects no measureable increase in brain biopterin of BH4 treated animals above untreated controls. The data from Hayashi T, Ogata A, Takehisha M, Komoridani K, Oonuma N, Studies on metabolism and disposition of sapropterine hydrochloride (SUN-0588) L-erythro-tetrahydrobiopterin dichloride in rats, Clin Report 26:3471–95, 1992 were translated for and reported in Fiege and Blau, 2006.
CNS dopamine and serotonin deficiencies have been previously documented in Pahenu2/enu2 mice (Puglisi-Allegra et al. 2000; Pascucci et al. 2002; Harding et al. 2014) and were again confirmed in this study. Chronic deficiency of these critical monoamine neurotransmitters are thought to contribute to the neurobehavioral abnormalities associated with human hyperphenylalaninemia (Feillet et al. 2010). Given that oral BH4 treatment has been shown to increase TH protein abundance and activity in rodent brain (Thöny et al. 2008), we hypothesized that sapropterin treatment of Pah−/− mice would improve brain dopamine status despite persistent hyperphenylalaninemia. Our data suggest however that the absolute brain dopamine content was unaffected by sapropterin treatment in our experiment. Likewise, sapropterin administration had no significant positive effect upon brain serotonin content. It is likely that phenylalanine-mediated inhibition of tyrosine hydroxylase and tryptophan hydroxylase activities was sustained even in the presence of increased brain biopterin. This result is consistent with previously published in vitro data showing that the abundance and activity of TPH are unaffected by increased BH4 concentration (Calvo et al. 2010). Alternately, brain tyrosine and tryptophan content in Pahenu2/enu2 brain may have been insufficient to fully support dopamine and serotonin synthesis even if TH and TPH activities were increased via sapropterin-mediated stimulation. We did not attempt to directly measure brain TH or TPH activities in this experiment as these assays, carried out ex vivo, are performed under optimal conditions with the addition of BH4 to the assay; measurement of TH or TPH activity in brain homogenate would likely not accurately reflect the in vivo status of dopamine and serotonin synthesis.
Sapropterin treatment was associated however with statistically significant increases in brain HVA and 5-HIAA content. These results suggest that dopamine and serotonin turnover had increased in sapropterin treated mice, both wild type and Pahenu2/enu2 mice, despite unchanged brain dopamine and serotonin content. BH4-stimulated synaptic release of dopamine or serotonin followed by metabolism to HVA and 5-HIAA respectively is a possible mechanistic explanation for our result. Previous investigators have documented, using brain microdialysis, that BH4 added to the perfusate of microdialysis probes placed in wild type rat striatum was associated with significantly increased synaptic dopamine release and HVA production (Koshimura et al. 1990; Tsukada et al. 1994; Koshimura et al. 1995), although a prior similar experiment had failed to show this association (Nakahara et al. 1988). The existence of a presynaptic BH4 receptor that stimulated monoamine release into the synaptic cleft upon BH4 binding has been proposed (Koshimura et al. 1995). Our data suggest that sapropterin treatment can affect CNS monoamine neurotransmitter metabolism status in a manner that is independent of any effect of BH4 treatment upon blood and brain phenylalanine concentrations and would be consistent with a BH4-mediated effect upon synaptic monoamine neurotransmitter release.
This experiment did not examine possible sapropterin effects upon specific brain regions such as the striatum or prefrontal cortex, areas that are rich in dopaminergic and serotonergic neurons, but rather relied upon measurements of monoamine neurotransmitters in half-brain extracts. We have initiated experiments using brain microdialysis to directly measure the effect of sapropterin therapy upon neurotransmitters specifically in those brain regions. Also, microdialysis will allow us to evaluate whether sapropterin treatment has any effect upon the synaptic release of dopamine or serotonin independent of any effects upon dopamine or serotonin synthesis.
Ultimately, regardless of measurable changes in brain monoamine status in sapropterin treated mice, any effects upon the neurobehavioral phenotype of Pahenu2/enu2 mice remain to be studied. For instance, hyperphenylalaninemic mice exhibit consistent and quantifiable deficits of memory and exploratory behavior; reduction of blood phenylalanine through restriction of dietary phenylalanine intake is associated with a measurable improvement in exploratory behavior (unpublished data). We plan to evaluate whether oral sapropterin treatment will alter behavior in the animals in the face of persistent hyperphenylalaninemia. In summary, oral sapropterin therapy in both wild type C57BL/6 and C57BL/6-Pahenu2/enu2 mice is associated with increased brain biopterin content, but sapropterin doses that are much larger than have typically been used in clinical practice are necessary to measure a significant increase in brain biopterin content. At the largest dose of 100 mg/kg/day, we have documented evidence of increased HVA and 5-HIAA content in brain but no effect upon the absolute amounts of dopamine or serotonin. Any behavioral effects associated with increased sapropterin-induced monoamine neurotransmitter turnover need yet to be evaluated.
Figure 5. Effect of Pah genotype and sapropterin treatment upon brain monoamine neurotransmitters.
The concentrations (pmol/mg protein, mean ± SEM) of the monoamine neurotransmitters dopamine (a) and serotonin (b), their metabolites homovanillic acid (HVA) (c) and 5-hydroxyindoleacetic acid (5-HIAA) (d) in half-brain homogenates from wild type (Pah+/+) and Pahenu2/enu2 (Pah−/−) mice following gavage with sapropterin or 1% ascorbic acid are displayed. The data are the means of brain neurotransmitters from all animals obtained at all time points up to six hours after gavage. The horizontal bars represent the statistical significance of the effects of either Genotype or sapropterin Treatment upon the brain monoamine neurotransmitter concentrations as calculated by two way ANOVA.
Acknowledgments
The authors would like to thank Melanie Gillingham, PhD and Aurora Martinez, PhD for critical review of the manuscript. This project was supported by NINHS grant R01-NS080866 and a sponsored research agreement with BioMarin Pharmaceutical Corp., Novato, CA. Pharmaceutical grade sapropterin dihydrochloride was provided by BioMarin for this project.
Abbreviations
- BH4
Tetrahydrobiopterin
- TH
Tyrosine hydroxylase
- TPH
Tryptophan hydroxylase
- HVA
Homovanillic acid
- 5-HIAA
5-hydroxyindoleacetic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azen CG, Koch R, et al. Intellectual development in 12-year-old children treated for phenylketonuria. Amer J Dis Child. 1991;145(1):35–39. doi: 10.1001/archpedi.1991.02160010037012. [DOI] [PubMed] [Google Scholar]
- Binek-Singer P, Johnson TC. The effects of chronic hyperphenylalaninaemia on mouse brain protein synthesis can be prevented by other amino acids. Biochem J. 1982;206(2):407–414. doi: 10.1042/bj2060407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau N, Erlandsen H. The metabolic and molecular bases of tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency. Mol Genet Metab. 2004;82(2):101–111. doi: 10.1016/j.ymgme.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Blau N, Thöny B. Pterins and Related Enzymes. In: Blau N, Duran M, Gibson KM, editors. Laboratory Guide to the Methods in Biochemical Genetics. Berlin: Springer-Verlag; 2008. pp. 669–682. [Google Scholar]
- Blau N, Thony B, et al. Variant of dihydropteridine reductase deficiency without hyperphenylalaninaemia: effect of oral phenylalanine loading. J Inherit Metab Dis. 1999;22(3):216–220. doi: 10.1023/a:1005584627797. [DOI] [PubMed] [Google Scholar]
- Brand MP, Hyland K, et al. Neurochemical effects following peripheral administration of tetrahydropterin derivatives to the hph-1 mouse. J Neurochem. 1996;66(3):1150–1156. doi: 10.1046/j.1471-4159.1996.66031150.x. [DOI] [PubMed] [Google Scholar]
- Calvo AC, Scherer T, et al. Effect of pharmacological chaperones on brain tyrosine hydroxylase and tryptophan hydroxylase 2. J Neurochem. 2010;114(3):853–863. doi: 10.1111/j.1471-4159.2010.06821.x. [DOI] [PubMed] [Google Scholar]
- Christ S, Huijbregts S, et al. Executive function in early-treated phenylketonuria: Profile and underlying mechanisms. Mol Genet Metab. 2010;99(Suppl 1):S22–S32. doi: 10.1016/j.ymgme.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Curtius HC, Baerlocher K, et al. Pathogenesis of phenylketonuria: inhibition of DOPA and catecholamine synthesis in patients with phenylketonuria. Clin Chim Acta. 1972;42(1):235–239. doi: 10.1016/0009-8981(72)90406-8. [DOI] [PubMed] [Google Scholar]
- Curtius HC, Niederwieser A, et al. Serotonin and dopamine synthesis in phenylketonuria. Adv Exp Med Biol. 1981;133:277–291. doi: 10.1007/978-1-4684-3860-4_16. [DOI] [PubMed] [Google Scholar]
- de Groot MJ, Hoeksma M, et al. Pathogenesis of cognitive dysfunction in phenylketonuria: review of hypotheses. Mol Genet Metab. 2010;99(Suppl 1):S86–S89. doi: 10.1016/j.ymgme.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Elzaouk L, Leimbacher W, et al. Dwarfism and low insulin-like growth factor-1 due to dopamine depletion in Pts−/− mice rescued by feeding neurotransmitter precursors and H4-biopterin. J Biol Chem. 2003;278(30):28303–28311. doi: 10.1074/jbc.M303986200. [DOI] [PubMed] [Google Scholar]
- Feillet F, van Spronsen FJ, et al. Challenges and pitfalls in the management of phenylketonuria. Pediatrics. 2010;126(2):333–341. doi: 10.1542/peds.2009-3584. [DOI] [PubMed] [Google Scholar]
- Fiege B, Blau N. Pharmacokinetics of Tetrahydrobiopterin in Humans and Rat. In: Blau N, editor. PKU and BH4 - Advances in Phenylketonuria and Tetrahydrobiopterin. Heilbronn, Germany: SPS Verlagsgesellschaft; 2006. pp. 638–651. [Google Scholar]
- Harding CO, Neff M, et al. The fate of intravenously administered tetrahydrobiopterin and its implications for heterologous gene therapy of phenylketonuria. Mol Genet Metab. 2004;81(1):52–57. doi: 10.1016/j.ymgme.2003.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding CO, Winn SR, et al. Pharmacologic inhibition of L-tyrosine degradation ameliorates cerebral dopamine deficiency in murine phenylketonuria (PKU) J Inherit Metab Dis. 2014;37(5):735–743. doi: 10.1007/s10545-013-9675-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeksma M, Reijngoud DJ, et al. Phenylketonuria: High plasma phenylalanine decreases cerebral protein synthesis. Mol Genet Metab. 2009;96(4):177–182. doi: 10.1016/j.ymgme.2008.12.019. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Levitt M, et al. Phenylalanine as substrate and inhibitor of tyrosine hydroxylase. Arch Biochem Biophys. 1967;120(2):420–427. doi: 10.1016/0003-9861(67)90259-7. [DOI] [PubMed] [Google Scholar]
- Kapatos G, Kaufman S. Peripherally administered reduced pterins do enter the brain. Science. 1981;212(4497):955–956. doi: 10.1126/science.7233193. [DOI] [PubMed] [Google Scholar]
- Kaufman S, Kapatos G, et al. Use of tetrahydropterins in the treatment of hyperphenylalaninemia due to defective synthesis of tetrahydrobiopterin: evidence that peripherally administered tetrahydropterins enter the brain. Pediatrics. 1982;70(3):376–380. [PubMed] [Google Scholar]
- Knudsen GM, Hasselbalch S, et al. Blood-brain barrier transport of amino acids in healthy controls and in patients with phenylketonuria. J Inherit Metab Dis. 1995;18(6):653–664. doi: 10.1007/BF02436753. [DOI] [PubMed] [Google Scholar]
- Koshimura K, Miwa S, et al. Enhancement of dopamine release in vivo from the rat striatum by dialytic perfusion of 6R-L-erythro-5,6,7,8-tetrahydrobiopterin. J Neurochem. 1990;54(4):1391–1397. doi: 10.1111/j.1471-4159.1990.tb01974.x. [DOI] [PubMed] [Google Scholar]
- Koshimura K, Takagi Y, et al. Characterization of a dopamine-releasing action of 6R-L-erythro-tetrahydrobiopterin: comparison with a 6S-form. J Neurochem. 1995;65(2):827–830. doi: 10.1046/j.1471-4159.1995.65020827.x. [DOI] [PubMed] [Google Scholar]
- Krause W, Halminski M, et al. Biochemical and neuropsychological effects of elevated plasma phenylalanine in patients with treated phenylketonuria. A model for the study of phenylalanine and brain function in man. J Clin Invest. 1985;75(1):40–48. doi: 10.1172/JCI111695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kure S, Hou DC, et al. Tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency. J Pediatr. 1999;135(3):375–378. doi: 10.1016/s0022-3476(99)70138-1. [DOI] [PubMed] [Google Scholar]
- Levine RA, Zoephel GP, et al. Entrance of tetrahydropterin derivatives in brain after peripheral administration: effect on biogenic amine metabolism. J Pharmacol Exp Ther. 1987;242(2):514–522. [PubMed] [Google Scholar]
- Levy HL, Milanowski A, et al. Efficacy of sapropterin dihydrochloride (tetrahydrobiopterin, 6R-BH4) for reduction of phenylalanine concentration in patients with phenylketonuria: a phase III randomised placebo-controlled study. Lancet. 2007;370(9586):504–510. doi: 10.1016/S0140-6736(07)61234-3. [DOI] [PubMed] [Google Scholar]
- McKean CM. The effects of high phenylalanine concentrations on serotonin and catecholamine metabolism in the human brain. Brain Res. 1972;47(2):469–476. doi: 10.1016/0006-8993(72)90653-1. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Nakahara D, et al. Peripherally administered (6R)-tetrahydrobiopterin increases in vivo tyrosine hydroxylase activity in the striatum measured by microdialysis both in normal mice and in transgenic mice carrying human tyrosine hydroxylase. Neurosci Lett. 1994;182(1):44–46. doi: 10.1016/0304-3940(94)90201-1. [DOI] [PubMed] [Google Scholar]
- Nakahara D, Ozaki N, et al. Intracerebrally administered (6r)-l-erythro-tetrahydrobiopterin does not affect extracellular levels of dopamine and serotonin metabolites in rat striatum in vivo during measurement by brain micro-dialysis system. Neurochem Int. 1988;12(2):121–124. doi: 10.1016/0197-0186(88)90118-0. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Ichinose H. Effect of metals and phenylalanine on the activity of human tryptophan hydroxylase-2: comparison with that on tyrosine hydroxylase activity. Neurosci Lett. 2006;401(3):261–265. doi: 10.1016/j.neulet.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Pascucci T, Andolina D, et al. 5-Hydroxytryptophan rescues serotonin response to stress in prefrontal cortex of hyperphenylalaninaemic mice. Int J Neuropsychopharmacol. 2009;12(8):1067–1079. doi: 10.1017/S1461145709990381. [DOI] [PubMed] [Google Scholar]
- Pascucci T, Ventura R, et al. Deficits in brain serotonin synthesis in a genetic mouse model of phenylketonuria. Neuroreport. 2002;13(18):2561–2564. doi: 10.1097/00001756-200212200-00036. [DOI] [PubMed] [Google Scholar]
- Ponzone A, Ferraris S, et al. Treatment of Tetrahydrobiopterin Deficiencies. In: Blau N, editor. PKU and BH4 - Advances in Phenylketonuria and Tetrahydrobiopterin. Heilbronn, Germany: SPS Verlagsgesellschaft; 2006. pp. 612–637. [Google Scholar]
- Puglisi-Allegra S, Cabib S, et al. Dramatic brain aminergic deficit in a genetic mouse model of phenylketonuria. Neuroreport. 2000;11(6):1361–1364. doi: 10.1097/00001756-200004270-00042. [DOI] [PubMed] [Google Scholar]
- Sumi-Ichinose C, Urano F, et al. Catecholamines and serotonin are differently regulated by tetrahydrobiopterin. A study from 6-pyruvoyltetrahydropterin synthase knockout mice. J Biol Chem. 2001;276(44):41150–41160. doi: 10.1074/jbc.M102237200. [DOI] [PubMed] [Google Scholar]
- Thöny B, Calvo AC, et al. Tetrahydrobiopterin shows chaperone activity for tyrosine hydroxylase. J Neurochem. 2008;106(2):672–681. doi: 10.1111/j.1471-4159.2008.05423.x. [DOI] [PubMed] [Google Scholar]
- Thony B, Ding Z, et al. Tetrahydrobiopterin protects phenylalanine hydroxylase activity in vivo: implications for tetrahydrobiopterin-responsive hyperphenylalaninemia. FEBS Lett. 2004;577(3):507–511. doi: 10.1016/j.febslet.2004.10.056. [DOI] [PubMed] [Google Scholar]
- Tsukada H, Lindner KJ, et al. Effect of 6R-L-erythro-5,6,7,8-tetrahydrobiopterin on the extracellular levels of dopamine and serotonin in the rat striatum: a microdialysis study with tyrosine or tryptophan infusion. Brain research. 1994;635(1–2):59–67. doi: 10.1016/0006-8993(94)91423-0. [DOI] [PubMed] [Google Scholar]
- VanZutphen KH, Packman W, et al. Executive functioning in children and adolescents with phenylketonuria. Clin Genet. 2007;72(1):13–18. doi: 10.1111/j.1399-0004.2007.00816.x. [DOI] [PubMed] [Google Scholar]
- Walter JH, White FJ. Blood phenylalanine control in adolescents with phenylketonuria. Int J Adolesc Med Health. 2004;16(1):41–45. doi: 10.1515/ijamh.2004.16.1.41. [DOI] [PubMed] [Google Scholar]
- Zagreda L, Goodman J, et al. Cognitive deficits in a genetic mouse model of the most common biochemical cause of human mental retardation. J Neurosci. 1999;19(14):6175–6182. doi: 10.1523/JNEUROSCI.19-14-06175.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]