Abstract
Introduction
Although epidemiologic evidence points to cardioprotective activity of red wine, the mechanistic basis for antithrombotic activity has not been established. Quercetin and related flavonoids are present in high concentrations in red but not white wine. Quercetin-glycosides were recently shown to prevent thrombosis in animal models through the inhibition of extracellular protein disulfide isomerase (PDI). We evaluated whether red or white wine inhibited PDI activity in vitro.
Methods
Quercetin levels in red and white wines were measured by HPLC analysis. Inhibition of PDI activity by red and white wines was assessed by an insulin reduction turbidity assay at various concentrations of wine. PDI inhibition was confirmed using a reduced peptide that contained a disulfide containing peptide as a substrate. The inhibition of PDI related thiol isomerases ERp5 and ERp57 was also assessed.
Results
We observed a dose-dependent decrease of PDI activity for a variety of red but not white wines. Red wine diluted to 3% final concentration resulted in over 80% inhibition of PDI activity by insulin reductase assay for all varieties tested. This inhibition was also observed in the peptide based assay. Red grape juice yielded similar results but ethanol alone did not affect PDI activity. Interestingly, red wine also inhibited the PDI related thiol isomerases ERp5 and ERp57, albeit to a lesser degree than PDI.
Conclusions
PDI activity is inhibited by red wine and grape juice, identifying a potentially novel mechanism underlying the cardiovascular benefits attributed to wine consumption.
Keywords: protein disulfide isomerase, red wine, quercetin, French Paradox, thiol isomerase
Introduction
Cardiovascular mortality rate is roughly half in France than in the United States despite consuming roughly three-times more animal fat [1]. This phenomena is known as the French Paradox and considerable effort has been made to identify the nutritional/epidemiologic basis for this seemingly incongruous association. A common link among hypothesis is that the cardioprotective activity of red wine is mediated through anti-platelet activity [1–4]. Red wine can inhibit collagen induced, ADP-stimulated or epinephrine-stimulated platelet aggregation, [1, 5, 6] as well as thrombus formation in vivo [7]. However, the mechanism by which red wine inhibits platelet aggregation and thrombus formation is unclear.
Unlike white wine, red wines are produced using the entire grape, included the grape seed and skin. There are a wide variety of phytochemicals found in grape skin and seeds that have been implicated in providing the cardioprotective benefits observed with red wine, including quercetin and its derivatives, which are most associated with the anti-thrombotic actions of wine [8–11]. Quercetin has been previously been associated with the attenuation of reactive oxygen species generation, enhancement of nitric oxide production, and blocking both surface platelet and thromboxane A2 receptors [9, 10, 12], as well as inhibition of p-selectin expression, αiibβ3 activation, and collagen induced ATP release [13]. Subsequently, platelet cell signaling through PI3K, Akt, and MAPK activation was inhibited [13], as well as platelet aggregation, calcium mobilization and thrombus formation [14–16]. Epidemiologic studies have shown that individuals who consume diets high in quercetin have a significantly lower incidence of cardiovascular-related mortality than those who consume less, even when adjusted for standard cardiovascular risk factors [17].
Recently, several related flavonoids (i.e. quercetin-3-glycosides) were identified through a high-throughput screen of small molecules as potent inhibitors of protein disulfide isomerase (PDI) activity [18]. Thiol isomerases such as PDI regulate protein activity through different mechanisms including modification of disulfide bond formation, and while the exact target of PDI in the coagulation cascade is unknown, proteins including tissue factor, VWF and αiibβ3, are known to be regulated by disulfide bond cleavage [19–21]. PDI plays a central role in thrombus formation and platelet aggregation in vivo [8] and in animal studies, quercetin-3- glycosides inhibit thrombus formation via a PDI-dependent mechanism [18]. As quercetin 3-glycosides are abundantly present in red but not white grapes [22], we evaluated whether red versus white wines variably inhibit PDI activity in vitro.
Materials and Methods
All of the wine varieties were purchased from Sutter Home Vineyards (St. Helena, CA) and diluted as indicated. Red and white grape were purchased from Welch Food Inc. (Concord, MA). Recombinant PDI and all other remaining chemicals were purchased from Sigma-Aldrich (St. Louis, MO) except for ERp5 and ERp57, which were purchased from AbCam (Cambridge, MA).
Quercetin levels were determined by HPLC analysis as described previously [23]. The PDI-catalyzed reduction of insulin was assayed by measuring the increase in turbidity as described previously [24]. The PDI-catalyzed oxidation of a disulfide bond was determined with a 9-mer peptide (H3N-VTWCGACKM-NH2) containing a disulfide bond. The peptide was synthesized and analyzed as previously described [25].
Results
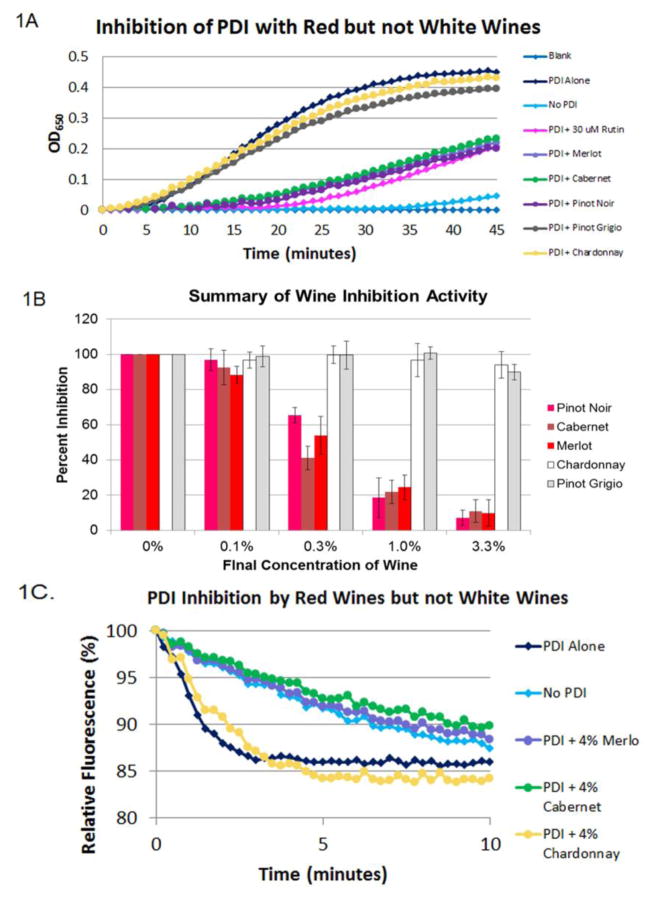
To examine the ability of red and white wines to inhibit PDI, we utilized the insulin turbidity assay to measure PDI reductase activity and the disulfide bond formation assay to measure PDI oxidase activity. All of the red wines but none of the white wines inhibited PDI reducatase activity at a final concentration of 3.3% (Figure 1A). Concentration dependent inhibition of PDI activity was confirmed using endpoint data for the red wines but not the whites (Figure 1B), with the final concentrations of wine varying between 0.1% and 3.3%.
Figure 1. Inhibition of PDI Activity by Red but not White Wine.
(A) The red wines Merlot, Cabernet and Pinot Noir and the white wines Pinot Grigio and Chardonnay were added at a final volume of 3.3% before PDI activity was assessed by the insulin turbidity assay. (B) The PDI-catalyzed reduction of insulin was run for 20 minutes and normalized to a percentage of the control. The 20 minute timepoint is displayed for each of the 5 wines examined over a range of concentrations ranging from 0.1% to 3.3%. (C) Merlot, Cabernet, and Chardonnay were added at a final concentration of 4% and the rate of tryptophan fluorescence change was observed in a disulfide bond containing peptide.
The oxidative activity of PDI was determined using a synthetic peptide substrate of nine amino acids [25]. The catalytic reduction of the intra-peptide disulfide bond results in a measurable quenching of tryptophan fluorescence. In the presence of PDI, a 15% percent decrease in relative tryptophan fluorescence occurred during the initial three minutes (Figure 1C). In the absence of PDI, the reaction requires 10 minutes to complete. The kinetics of the PDI + red wine are very similar to those of the non-enzymatic catalyzed reaction, which is consistent with inhibition of PDI activity (Figure 2B). In contrast, the kinetics of the PDI + white wine followed that of the PDI catalyzed reaction, signifying PDI activity was not inhibited (Figure IC). To examine the significance of the difference between the red and white wines, we examined the fluorescence at the 4 minute time point and found that the p-value was <0.01 (data not shown). Taken together, these results confirm that PDI is inhibited by red but not white wine.
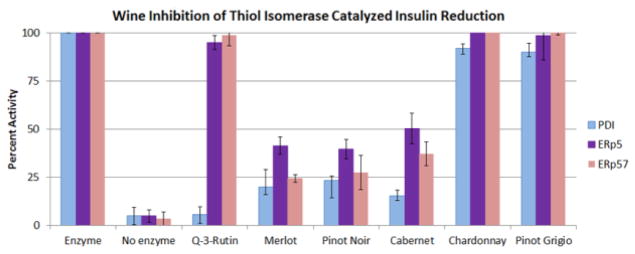
Figure 2. Wine Inhibition of the Activity of PDI, ERp5 and ERp57.
A) The insulin reductase activity assay was used to determine the inhibition of PDI, ERp5 and ERp57 respectively with a final concentration of wine of 3.3%.
We next measured the levels of quercetin in the various wine samples in order to correlate the PDI inhibitory activity to quercetin levels. The levels of the five wines analyzed are shown in Table 1, but as expected the red wines contained more quercetin than the white wines (average 14.58 vs 1.69 μM), which is within the range of previous measurements of quercetin [26].
Table 1.
The concentration of quercetin aglycone for each of the wines examined. Data is presented in μM in the first column and then μg/mL in the second.
| Red Wine | [Q] μmole/L | [Q] μg/mL |
|---|---|---|
| Pinot Noir | 18.97 | 5.73 |
| Merlot | 18.18 | 5.49 |
| Cabernet | 6.60 | 1.99 |
| White Wine | ||
| Chardonnay | 2.50 | 0.76 |
| Pinot Grigio | 0.87 | 0.26 |
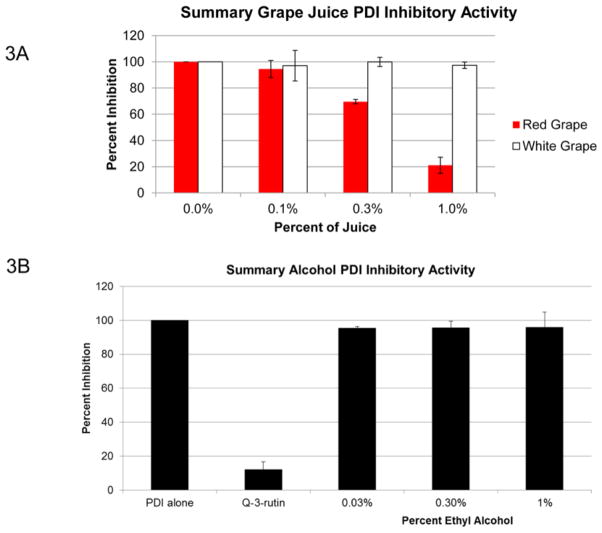
As quercetin-3-glycosides such as quercetin-3-rutin (Q-3-rutin) were specific PDI inhibitors in vitro [18], we examined the ability of red wine to inhibited the PDI related thiol isomerases ERp5 and ERp57. Although most potent for PDI inhibition, red wines also inhibited ERp5 and ERp57 (Figure 2). Thus, there may be other components of red wine that have additional inhibitory effects on these thiol isomerases. There are a wide number of potential thiol isomerase inhibitors in the components of wine that could contribute to its cardioprotective benefits, including alcohol and vitamin C, other flavonoids and phenolics [27]. While a broad examination of all wine components is beyond the scope of this study, we examined the PDI inhibitory effect of non-alcoholic red and white grapes juice. Non-alcoholic red grape juice inhibited PDI activity in a dose-dependent manner whereas no inhibitory activity was observed with white grape juice (Figure 3A). Furthermore, a final concentration of 0.03, 0.3% or 1% ethyl alcohol was added to the PDI catalyzed reaction and no inhibition was observed (Figure 3B).
Figure 3. The Effect of Ethyl Alcohol and Vitamin C on Thiol Isomerase Activity.
(A) To assess any contribution of the alcohol content of wine to the observed PDI inhibition, non-alcoholic red and white grape juice was examined at 0.1%, 0.3% and 1%. (B) Ethyl alcohol was directed added to the PDI catalyzed reduction of insulin mixture at 0.03%, 0.3%, and 1% to verify no inhibition in enzymatic activity was observed.
Discussion
The antithrombotic activity of red wine is well supported by epidemiological studies [2–4], however, the mechanism by which red wines affect thrombus formation is poorly understood. In this study, we demonstrate that PDI activity is inhibited by red but not white wines in an in vitro assay. Previous studies have found that patients who consume the equivalent of 2–3 glasses a day of purple grape juice had a 77% reduction of whole-blood platelet aggregation [28]. The inhibition of PDI is one potential mechanism that can contribute to the inhibition of platelet aggregation.
The inhibitory concentration for quercetin-glycosides (IC50) on PDI activity in vitro is in the 1–10 micromolar range [26, 29]. The red wines contain 6–19 micromoles of quercetin and derivatives per liter so the consumption of 2–3 glasses of red wine would provide adequate amounts of quercetin. Interestingly, we demonstrated that the PDI inhibitory effect of red wine is likely not exclusively a result of the presence of glycosylated quercetin derivatives, since there is an observed inhibition of the PDI related thiol isomerases ERp5 and ERp57 (Figure 2). Previous studies have determined that quercetin-3-rutinoside has additional anti-platelet functions in addition to PDI inhibition, however in the in vitro assay we examined, quercetin-3-rutinoside has no effect on ERp5 or ERp57 [21]. As platelets are known to have an ability to metabolize quercetin [12], it is possible that quercetin-3-rutinoside is metabolized by the platelet into metabolites that have broad spectrum thiol isomerases activity, which would explain the differences between these results. Nevertheless, considering the wide variety of antioxidants and bioactive compounds found in grape seeds and grape skins, it is probable that additional components of red wine beyond glycosylated quercetin derivatives are also contributing to the observed thiol isomerase inhibitory effect. Based upon our results, alcohol is not a contributing component.
Based on the observed direct PDI, ERp5 and ERp57-inhibitory activity of red wine, we propose a novel inhibitory mechanism by which red wine can mediate antithrombotic activity. While we did not observe any PDI inhibitory activity for alcohol alone, red wine contains a number of bioactive compounds that may modulate thrombotic risk through other mechanisms [10, 11]. Red wine has a high concentration of quercetin-glycosides which likely contribute to, but are not exclusive for the observed PDI inhibitory activity. These data provide initial pre-clinical evidence that red wine inhibits PDI-inhibits activity and rationale to evaluate the PDI inhibitory activity of wine in animal models of thrombosis. Future studies will examine the specific components of red wine that also have thiol isomerases inhibitory activity.
Highlights.
We determine that red wine inhibits the enzymatic activity of protein disulfide isomerase
Quercetin levels in the red wines examined are in the low micromolar range
Red wine also inhibits protein disulfide related thiol isomerases ERp5 and ERp57
Protein disulfide enzymatic activity is not affected by alcohol levels.
Acknowledgments
The authors which to acknowledge the Dr. David Neimann and Mart Pat Meaney from Appalachian State University for their assistance in obtaining the quercetin levels.
This research was supported by funding from Western New England University.
Dr. Zwicker is supported by a research grant from NHLBI (U54HL112302)
Footnotes
The authors declare there are no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lippi G, Franchini M, Favaloro EJ, Targher G. Moderate red wine consumption and cardiovascular disease risk: beyond the “French paradox”. Seminars in thrombosis and hemostasis. 2010;36:59–70. doi: 10.1055/s-0030-1248725. [DOI] [PubMed] [Google Scholar]
- 2.Gronbaek M, Deis A, Sorensen TI, Becker U, Schnohr P, Jensen G. Mortality associated with moderate intakes of wine, beer, or spirits. Bmj. 1995;310:1165–9. doi: 10.1136/bmj.310.6988.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Renaud SC, Gueguen R, Siest G, Salamon R. Wine, beer, and mortality in middle-aged men from eastern France. Archives of internal medicine. 1999;159:1865–70. doi: 10.1001/archinte.159.16.1865. [DOI] [PubMed] [Google Scholar]
- 4.St Leger AS, Cochrane AL, Moore F. Factors associated with cardiac mortality in developed countries with particular reference to the consumption of wine. Lancet. 1979;1:1017–20. doi: 10.1016/s0140-6736(79)92765-x. [DOI] [PubMed] [Google Scholar]
- 5.Pikaar NA, Wedel M, van der Beek EJ, van Dokkum W, Kempen HJ, Kluft C, et al. Effects of moderate alcohol consumption on platelet aggregation, fibrinolysis, and blood lipids. Metabolism: clinical and experimental. 1987;36:538–43. doi: 10.1016/0026-0495(87)90163-6. [DOI] [PubMed] [Google Scholar]
- 6.Polagruto JA, Gross HB, Kamangar F, Kosuna K, Sun B, Fujii H, et al. Platelet reactivity in male smokers following the acute consumption of a flavanol-rich grapeseed extract. Journal of medicinal food. 2007;10:725–30. doi: 10.1089/jmf.2007.402. [DOI] [PubMed] [Google Scholar]
- 7.Demrow HS, Slane PR, Folts JD. Administration of wine and grape juice inhibits in vivo platelet activity and thrombosis in stenosed canine coronary arteries. Circulation. 1995;91:1182–8. doi: 10.1161/01.cir.91.4.1182. [DOI] [PubMed] [Google Scholar]
- 8.Flaumenhaft R. Protein disulfide isomerase as an antithrombotic target. Trends in cardiovascular medicine. 2013;23:264–8. doi: 10.1016/j.tcm.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guerrero JA, Navarro-Nunez L, Lozano ML, Martinez C, Vicente V, Gibbins JM, et al. Flavonoids inhibit the platelet TxA(2) signalling pathway and antagonize TxA(2) receptors (TP) in platelets and smooth muscle cells. British journal of clinical pharmacology. 2007;64:133–44. doi: 10.1111/j.1365-2125.2007.02881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hubbard GP, Wolffram S, Lovegrove JA, Gibbins JM. Ingestion of quercetin inhibits platelet aggregation and essential components of the collagen-stimulated platelet activation pathway in humans. Journal of thrombosis and haemostasis: JTH. 2004;2:2138–45. doi: 10.1111/j.1538-7836.2004.01067.x. [DOI] [PubMed] [Google Scholar]
- 11.Wright B, Moraes LA, Kemp CF, Mullen W, Crozier A, Lovegrove JA, et al. A structural basis for the inhibition of collagen-stimulated platelet function by quercetin and structurally related flavonoids. British journal of pharmacology. 2010;159:1312–25. doi: 10.1111/j.1476-5381.2009.00632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright B, Gibson T, Spencer J, Lovegrove JA, Gibbins JM. Platelet-mediated metabolism of the common dietary flavonoid, quercetin. PloS one. 2010;5:e9673. doi: 10.1371/journal.pone.0009673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh WJ, Endale M, Park SC, Cho JY, Rhee MH. Dual Roles of Quercetin in Platelets: Phosphoinositide-3-Kinase and MAP Kinases Inhibition, and cAMP-Dependent Vasodilator-Stimulated Phosphoprotein Stimulation. Evidence-based complementary and alternative medicine: eCAM. 2012;2012:485262. doi: 10.1155/2012/485262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chakrabarti S, Freedman JE. Review: Nutriceuticals as antithrombotic agents. Cardiovascular therapeutics. 2010;28:227–35. doi: 10.1111/j.1755-5922.2010.00161.x. [DOI] [PubMed] [Google Scholar]
- 15.Freedman JE, Parker C, 3rd, Li L, Perlman JA, Frei B, Ivanov V, et al. Select flavonoids and whole juice from purple grapes inhibit platelet function and enhance nitric oxide release. Circulation. 2001;103:2792–8. doi: 10.1161/01.cir.103.23.2792. [DOI] [PubMed] [Google Scholar]
- 16.Gresele P, Cerletti C, Guglielmini G, Pignatelli P, de Gaetano G, Violi F. Effects of resveratrol and other wine polyphenols on vascular function: an update. The Journal of nutritional biochemistry. 2011;22:201–11. doi: 10.1016/j.jnutbio.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Hertog MG, Kromhout D, Aravanis C, Blackburn H, Buzina R, Fidanza F, et al. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Archives of internal medicine. 1995;155:381–6. [PubMed] [Google Scholar]
- 18.Jasuja R, Passam FH, Kennedy DR, Kim SH, van Hessem L, Lin L, et al. Protein disulfide isomerase inhibitors constitute a new class of antithrombotic agents. The Journal of clinical investigation. 2012;122:2104–13. doi: 10.1172/JCI61228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahamed J, Versteeg HH, Kerver M, Chen VM, Mueller BM, Hogg PJ, et al. Disulfide isomerization switches tissue factor from coagulation to cell signaling. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13932–7. doi: 10.1073/pnas.0606411103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butera D, Cook KM, Chiu J, Wong JW, Hogg PJ. Control of blood proteins by functional disulfide bonds. Blood. 2014;123:2000–7. doi: 10.1182/blood-2014-01-549816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim K, Hahm E, Li J, Holbrook LM, Sasikumar P, Stanley RG, et al. Platelet protein disulfide isomerase is required for thrombus formation but not for hemostasis in mice. Blood. 2013;122:1052–61. doi: 10.1182/blood-2013-03-492504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novak I, Janeiro P, Seruga M, Oliveira-Brett AM. Ultrasound extracted flavonoids from four varieties of Portuguese red grape skins determined by reverse-phase high-performance liquid chromatography with electrochemical detection. Analytica chimica acta. 2008;630:107–15. doi: 10.1016/j.aca.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Jin F, Nieman DC, Shanely RA, Knab AM, Austin MD, Sha W. The variable plasma quercetin response to 12-week quercetin supplementation in humans. European journal of clinical nutrition. 2010;64:692–7. doi: 10.1038/ejcn.2010.91. [DOI] [PubMed] [Google Scholar]
- 24.Khodier C, VerPlank L, Nag PP, Pu J, Wurst J, Pilyugina T, et al. Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD): 2010. Identification of ML359 as a Small Molecule Inhibitor of Protein Disulfide Isomerase. [PubMed] [Google Scholar]
- 25.Cline DJ, Thorpe C, Schneider JP. Structure-based design of a fluorimetric redox active peptide probe. Analytical biochemistry. 2004;325:144–50. doi: 10.1016/j.ab.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Careri M, Corradini C, Elviri L, Nicoletti I, Zagnoni I. Direct HPLC analysis of quercetin and trans-resveratrol in red wine, grape, and winemaking byproducts. Journal of agricultural and food chemistry. 2003;51:5226–31. doi: 10.1021/jf034149g. [DOI] [PubMed] [Google Scholar]
- 27.Liang Z, Cheng L, Zhong GY, Liu RH. Antioxidant and antiproliferative activities of twenty-four Vitis vinifera grapes. PloS one. 2014;9:e105146. doi: 10.1371/journal.pone.0105146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keevil JG, Osman HE, Reed JD, Folts JD. Grape juice, but not orange juice or grapefruit juice, inhibits human platelet aggregation. The Journal of nutrition. 2000;130:53–6. doi: 10.1093/jn/130.1.53. [DOI] [PubMed] [Google Scholar]
- 29.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. The American journal of clinical nutrition. 2005;81:230S–42S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]