Abstract
Background
Adenovirus (ADV) infections after hematopoietic cell transplantation (HCT) range in severity from self-limited to fatal. We have previously reported high mortality rates in CD34+ selected T-cell depleted (TCD) HCT recipients using symptomatic testing and culture methods for ADV detection. We report rates and outcomes of ADV viremia in 215 adult recipients of TCD HCT using CliniMACS® CD34+ selection system.
Methods
This was a prospective observational study of adults transplanted from 3/21/12 through 11/30/14 at Memorial Sloan-Kettering Cancer Center. TCD was performed using CliniMACS® CD34+ cell selection. Patients were monitored for ADV by whole blood PCR assay (Viracor-IBT Laboratories) from +14 to +100 days post-transplant. ADV viremia was defined as ≥1 PCR above the lower limit of quantitation. ADV disease was defined per European Group for Bone Marrow Transplantation guidelines. Treatment for ADV was at the clinician's discretion. Competing risk regression analyses were used to identify predictors for ADV viremia and overall survival.
Results
The median age was 55 years (range 22-72); 215 patients underwent TCD. All patients received myeloablative conditioning. Eighteen patients (8% of cohort) had ADV viremia at a median onset of 57 days [interquartile range (IQR), 23-79] and with a median viral load at first detection of 2.6 log10 copies/mL (IQR, 2.5-4.0). The median maximal viral load was 4.5 log10 copies/mL (IQR, 3.5-5.9). No significant risk factor was indentified for ADV viremia by univariate analysis. Six patients (3% of total cohort, 33% of viremic patients) developed ADV disease (3 colitis, 2 nephritis/cystitis, 1 pneumonitis). ADV viremia preceded onset of ADV disease a median of 11 days from the first positive qPCR (range, +3 to +37) except in one patient with nephritis. Overall, 12 of 18 (67%) viremic patients received antiviral treatment [5 cidofovir only, 7 brincidofovir (+/-cidofovir)]. All patients with ADV disease were treated and 6 patients preemptively treated for ADV. Among the 18 viremic patients, 8 (44%) died during the study period, and of those, 4 (22%) died of ADV.
Conclusions
1) Early ADV viremia was infrequent (8%) among adult HCT recipients of CD34+ selected allografts. 2) Among viremic patients, rate of ADV disease was 33% and ADV attributable mortality was 22%. 3) Further studies are needed to assess the impact of preemptive treatment with brincidofovir on improving outcomes of ADV infections in this patient population.
Introduction
Adenovirus (ADV) infections after hematopoietic cell transplantation (HCT) range in severity from self-limited to fatal [1-11]. We have previously reported high mortality rates in T-cell depleted (TCD) HCT recipients using symptomatic testing for ADV detection [1]. In contrast, low incidence (5-12%) and severity is reported among nonablative HCT, using alemtuzumab for TCD monitored prospectively by quantitative PCR (qPCR) in the plasma [6, 10]. Since March 2012, we implemented routine ADV qPCR monitoring in recipients of TCD allografts using the FDA approved CliniMACS® CD34+ selection system. We report rates and outcomes of ADV viremia in this patient population.
Methods
Study patients
The study was reviewed by the Memorial Sloan-Kettering Cancer Center (MSKCC) Institutional Review Board. The cohort comprised adult patients transplanted between 3/21/2012 and 11/30/2014 at MSKCC with CD34+ selected allograft using the CliniMACS® system (Miltenyi Biotec, Gladbach, Germany) [12]. Data was extracted from institutional databases, medical, microbiology and pharmacy records.
Standards of care
All patients received myeloablative conditioning [13]. TCD was the sole modality for graft-versus-host disease (GVHD) prophylaxis. Supportive care and anti-infective prophylaxis were administered as described [14].
Laboratory methods
Patients were routinely monitored for ADV by plasma qPCR, performed by Viracor-IBT Laboratories (Lee's Summit, MO, USA) weekly from day +14 and day +100. Afterwards, testing was at clinician discretion for symptomatic patients. Urine, stool and respiratory specimens were tested for ADV in symptomatic patients at clinician discretion. QPCR in stool and urine was performed by Viracor IBT. Respiratory specimens were tested by the FilmArray Respiratory Panel (BioFire Diagnostics, Inc., Salt Lake City, UT, USA) and/or performed by viral culture at the MSKCC microbiology laboratory.
Definitions
ADV viremia was defined as ≥1 PCR above the lower limit of quantitation. ADV disease was defined per European Group for Bone Marrow Transplantation guidelines [15]. Death was attributed to ADV if ADV caused or significantly contributed to death.
Statistics
Patients were censored at the date of relapse, second HCT, death or end of study date 2/28/2015, whichever occurred first. Cumulative incidence (CI) and overall survival (OS) were calculated by Kaplan-Meier method. Log-rank test was used for comparisons. Stepwise Forward Cox proportional hazard regression models were used to identify risk factors for ADV viremia. The mortality rate was calculated by divided the total number of deaths from all causes by the sum of days in patients at risk. The standardized mortality ratio was defined as the ratio of observed number of actual deaths to the expected deaths in the study population under the assumption that the mortality rates for the study population are the same as those for the reference population [16]. Statistical analyses were performed using SAS 9.2 (SAS Inc, Cary, NC). A P value of ≤ .05 was considered statistically significant.
Results
ADV viremia
Table 1 shows the baseline characteristics of the cohort. Overall 20 (9.3%) patients developed acute graft- versus-host disease (GVHD) grade 2 and 1 patient acute GVHD grade 3.
Table 1. Baseline demographics and transplant characteristics.
| Characteristic | Number (%) N=215 | |
|---|---|---|
| Age, year, median (range) | 55.1 (21.7-72.1) | |
| Sex, N (%) | ||
| Female/male | 81 (37.7)/134 (62.3) | |
| Underlying disease, N (%) | ||
| Acute leukemia | 93 (43.3) | |
| Multiple myeloma | 58 (27.0) | |
| MDS | 44 (20.5) | |
| MPD* | 12 (5.5) | |
| Lymphoma/Chronic lymphocytic leukemia | 6 (2.8) | |
| Non-hematologic malignancy | 2 (0.9) | |
| Conditioning regimen, N (%) | ||
| Busulfan/Melphalan/Fludarabine | 163 (75.8) | |
| TBI/Thiotepa/Cyclophosphamide | 46 (21.4) | |
| Thiotepa/Melphalan/Clofarabine | 6 (2.8) | |
| Recipient CMV serostatus, N (%) | ||
| Negative | 89 (41.4) | |
| Positive | 126 (58.6) | |
| Donor type, N (%) | ||
| Matched related | 64 (29.8) | |
| Matched unrelated | 107 (49.8) | |
| Mismatched (related or unrelated) | 44 (20.4) | |
| Acute GVHD, N (%) | ||
| Grade 0 | 176 (81.9) | |
| Grade I | 18 (8.4) | |
| Grade II | 20 (9.3) | |
| Grade III | 1 (0.5) | |
| Grade IV | 0 (0.0) |
MPD: Chronic myelomonocytic leukemia 1 patient, essential thrombocytopenia 2 patients, myelofibrosis 7 patients, and polycythemia vera 2 patients.
Abbreviations: MDS, myelodysplastic syndrome; MPD, myeloproliferative disease; MM, multiple myeloma; TBI, total body irradiation; CMV, cytomegalovirus; GVHD, graft-versus-host disease
Eighteen (8.4%) patients developed ADV viremia. The 100-day CI of ADV viremia was 8.5%. The median day of ADV viremia onset was 56.5 days post-HCT [interquartile range (IQR), 23-79]. The median ADV viral load (VL) at detection and median maximum VL were 2.6 log10 copies/mL (IQR, 2.5-4.0) and 4.5 log10 copies/mL (IQR, 3.5-5.9), respectively.
The ADV viral load was a major factor influencing the decision to start treatment. In the early part of the study, ADV viral load of 10,000 copies/mL was used as threshold for treatment based on our previous publication [1]. In the latter part of the study because of our increasing clinical experience and availability of Brincidofovir (BCV) at our Institution, there was a trend for earlier treatment. Other factors taken into consideration were timing of ADV infection post-HCT, presence of symptoms and comorbidities. All patients had more than one positive ADV PCR and the majority of patients had rising ADV viral load prior to starting treatment.
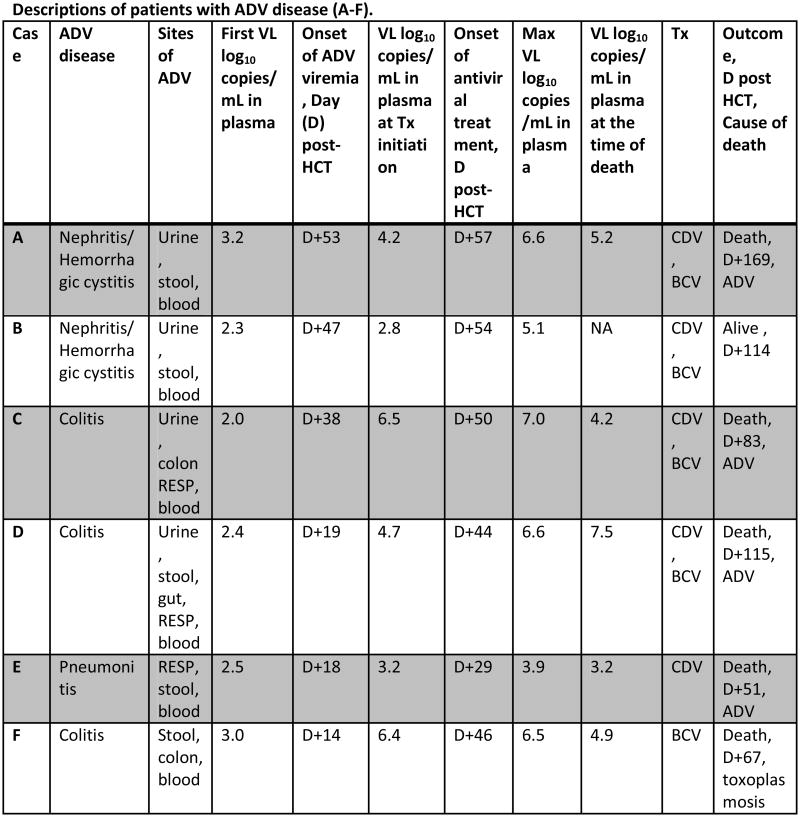
Based on antiviral treatment, patients were categorized into 2 groups: Group I (cases A-L) with treatment and Group II (cases M-R) without treatment (Figure 1).
Figure 1. Duration and outcomes of patients with ADV viremia. Patients (A-F) had ADV disease. Each horizontal line represents a patient.
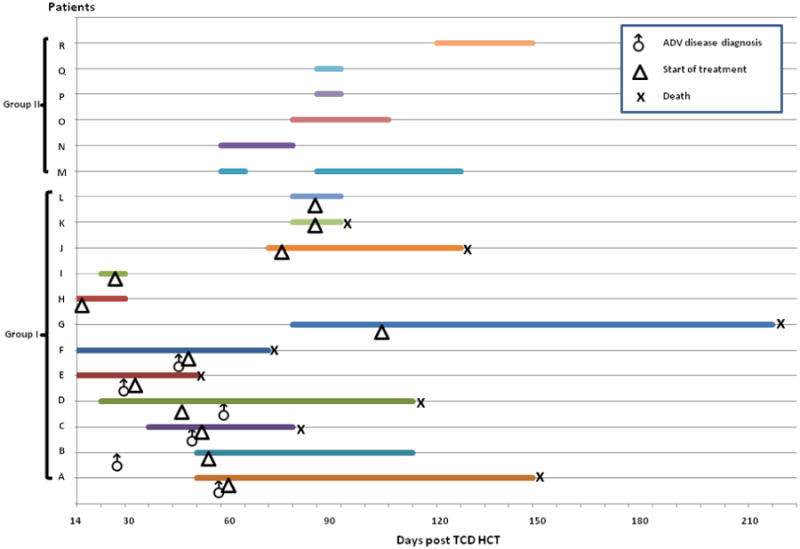
X axis: Days after stem cell infusion. Horizontal line: Duration of ADV viremia. Symbols: ♂, ADV disease diagnosis; Δ, start of treatment; X: death.
All patients had ADV in stool. In addition, 2 patients had ADV in urine, 1 patient had ADV in respiratory specimen, and 2 had ADV in urine and respiratory specimen.
Abbreviations: ADV, adenovirus; TCD, T-cell depleted; HCT, Hematopoietic stem cell transplant; VL, viral load; Max, maximum; Tx, treatment; D, day from transplant; CDV, intravenous cidofovir; BCV, brincidofovir; RESP, respiratory specimens (bronchoalveolar lavage, nasopharyngeal swab)
Among group I (N=12), 5 patients received cidofovir only and 7 patients received brincidofovir (+/- cidofovir). The median VL at initiation of treatment was 4.6 log10 copies/mL (IQR, 3.8-5.7). Treatment was initiated preemptively in 6 patients (cases G-L) and for ADV disease in 6 patients (cases A-F). Among 6 patients preemptively treated for ADV, 4 cleared the viremia [median total duration of treatment 7 days (range, 6-19)] and 2 had persistent viremia at the time of death. Five of 6 with ADV disease died and all had viremia at the time of death (Figure 1). The median ADV viral load at first detection was 2.5 log10 copies/mL (IQR, 2.2-3.0) for patients with ADV disease (cases A-F) whereas the median ADV viral load at first detection was 4.1 log10 copies/mL (IQR, 3.6-4.4) for patients with preemptive antiviral treatment (cases G-L).
Patients in Group II (N=6) had low-grade viremia (Max VL median 3.1 log10 copies/mL, IQR 2.5-3.7), which was transient or intermittent in duration (range, 7-45 days) and cleared without treatment. The ADV viremia onset post-transplant was earlier for group I than Group II [median 43 days (IQR, 18-74) vs 80 days (IQR, 54-96) respectively].
Risk factors for ADV viremia
Host and transplant characteristics listed in Table 1 were examined in univariate models to identify predictors for ADV viremia (Table 2). No statistically significant predictor was identified. Because of a potential association between ADV infection and GVHD, we examined the relationship between onset of ADV viremia and GVHD. Among patients with ADV viremia, 3 (16.7%) patients developed grade 2 GVHD [including 2 patients with lower gastrointestinal (GI) GVHD and 1 with upper GI GVHD]. Two patients developed ADV viremia prior to onset of GVHD. One patient developed mild GI GVHD after ADV infection. Symptoms resolved after oral budesonide treatment for 1 week.
Table 2. Risk factors of ADV viremia during the first 100 days after CD34+ selected hematopoietic cell.
| Variable | HR (95% CI) | P Value | |
|---|---|---|---|
| Age per year | 1.0 (1.0-1.1) | .47 | |
| Sex | |||
| Female | 1.0 | ||
| Male | 1.2 (0.5-3.2) | .72 | |
| Underlying disease* | |||
| Myeloid | 1.0 | ||
| Lymphoid | 1.6 (0.6-4.0) | .37 | |
| Donor type | |||
| Matched related | 1.0 | ||
| Matched unrelated | 0.4 (0.1-1.0) | .06 | |
| Mismatched | 0.5 (0.1-1.7) | .24 | |
| CMV serostatus | |||
| Recipient negative | 1.0 | ||
| Recipient positive | 2.6 (0.9-7.9) | .09 | |
| Acute GVHD grade | |||
| 0-I | 1.0 | ||
| II-IV | 1.9 (0.6-6.7) | .3 |
Myeloid diseases include acute leukemia, myelodysplastic syndrome, myeloproliferative disease and nonhematologic malignancies. Lymphoid diseases include lymphoma, chronic lymphocytic leukemia and multiple myeloma.
Abbreviations: HR, hazard ratio; CI, confidence interval; CMV, cytomegalovirus; GVHD, graft-versus-host disease
CD4+ and CD8+ lymphocyte counts at day +100 post-HCT
Among our cohort, 166 patients had CD4+ and CD8+ lympocyte counts measured at day +100 post-HCT (including 10 patients with ADV viremia and 156 patients without ADV viremia). The median CD4+ count was 29 cells/uL (IQR, 4.3-60) in patients with ADV viremia and 65 cells/uL (IQR, 11-208.3) in patients without ADV viremia (P=.255). The median CD8+ counts was 109 cells/uL (IQR, 30.3-161.5) in patients with ADV viremia and 76 cells/uL (IQR, 3-490) in patients without ADV viremia (P=.9482).
Viral coinfections
Patients with ADV viremia frequently had additional viral infections by double stranded DNA viruses. Twelve (67%) patients had cytomegalovirus (CMV), 4(22%) patients had Ebstein-Barr virus (EBV) and 3 (17%) patients had Human herpesvirus 6 (HHV6) viremia. Eight (44%) patients had ≥2 viruses in addition to ADV.
ADV disease
Among 18 viremic patients, 6 (33%) patients developed disease. The CI of ADV disease was 3.2%. ADV disease was diagnosed a median of 48 days (IQR, 28-56) post-HCT.
The onset of ADV viremia in patients with ADV disease was a median of 29 days (IQR, 17-49) post-transplant. ADV viremia preceded diagnosis of ADV disease by median 11 days (range, +3 to +37) in all but one patient with nephritis/hemorrhagic cystitis (HC). In that patient, the detection of ADV in urine preceded ADV viremia. ADV disease involved the GI tract in 3 patients, the genitourinary tract in 2 patients and the respiratory tract in 1 patient. All patients had ADV in the stool. In addition, 2 patients had ADV in urine, 1 patient had ADV in respiratory specimen, and 2 had ADV in urine and respiratory specimen.
Median Max VL was 6.5 log10 copies/mL (IQR, 4.8-6.7). Five patients received antivirals after diagnosis of ADV disease. Patient B developed ADV nephritis/HC at day +26 post-HCT with negative ADV viremia and received antivirals at day +54 post-HCT when she developed ADV viremia (Figure 1). Median VL at the time of treatment initiation was 4.5 log10 copies/mL (range, 2.8-6.5). Five patients were treated with BCV; 4 discontinued BCV before disease resolution due to diarrhea, ileus or GI bleeding. The median VL at the time of death was 4.9 log10 copies/mL (range, 3.2-7.5).
Attributable mortality
The median follow-up of the cohort is 372 days (range, 29-1,017 days). Patients with ADV viremia had higher mortality compared to patients without ADV viremia (1.21 vs 0.62 per 1,000 person-days respectively). Eight of 18 (44%) patients with ADV viremia died during the study. None of the 8 patients with ADV viremia who died had GVHD at any time post-HCT. ADV disease was the primary cause of death in 4 patients.
The remaining 4 patients died from other infections (toxoplasmosis, CMV pneumonitis, bacterial pneumonia, 1 patient each) and acute fibrinous pericarditis (1 patient). All patients had ongoing ADV viremia at the time of death.
Discussion
We report rates and outcomes of ADV viremia in a large cohort of adult recipients of CD34+ selected HCT. The incidence of ADV viremia (8.5%) was similar to reported incidence after non-ablative conditioning with alemtuzumab [6, 10]. However ADV viremia had major clinical implications and adversely affected overall survival. Among viremic patients, two-thirds required antiviral treatment, one-third developed ADV disease and one-quarter died of ADV. As our patients did not receive exogenous GVHD prophylaxis, reduction of immunosuppression was not possible. The median CD4+ lymphocyte count at day +100 post-HCT was low and similar between patients with and without ADV viremia (median 29 cells/uL and 65 cells/uL respectively; P=NS).
In this cohort of CD34+ selected HCT, we could not identify any risk factor for ADV viremia by univariate analysis. Overall rates of GVHD were low and GVHD of the GI tract was grade 2 in almost all cases. Three (17%) patients with ADV viremia had GI GVHD (grade 2) and ADV infection preceded the onset of mild GVHD in 1 patient only. Thus we could not identify any correlation between ADV and GVHD.
Patients with ADV disease in general had early onset of viremia, at a median of 29 days post-HCT (IQR, 17-49). The interval from detection of viremia to initiation of antiviral treatment varied due to lack of standardized guidelines for managing asymptomatic ADV viremia. Given the short interval from detection of ADV viremia to ADV disease, we advocate aggressive treatment of ADV viremia in recipients of CD34+ selected HCT, especially the first 2 months after transplant. The impact of earlier intervention would need to be evaluated in clinical studies. Although stool testing was not routinely done, all patients with ADV disease had ADV in the stool. Monitoring ADV in stool instead of blood has been of value in predicting viremia in other settings [3]. The utility of this approach merits further study in CD34+ selected HCT.
Because of the limited sample size and heterogeneous antiviral treatment, we cannot conclude on the efficacy of treatment modalities. While the decision to start antiviral therapy was primarily based on ADV viral load, additional factors were taken into consideration including timing of ADV infection post-HCT, presence of symptoms attributable to ADV and overall clinical status and concerns for antiviral treatment related toxicities. The first detected ADV viral load was higher in patients with preemptive antiviral treatment compared to patients treated for ADV disease (median 4.1 log10 copies/mL and 2.5 log10 copies/mL, respectively) and consistent with earlier preemptive treatment for patients with higher viral copy numbers. Because of increasing clinical experience and availability of BCV (under clinical trial) for there was a trend for earlier treatment in the latter part of the study [17]. BCV has shown to be promising for treatment of ADV [18, 19]. BCV was discontinued in 4 patients with established ADV disease due to GI events (profound diarrhea, ileus, bleeding) related to ADV, Clostridium difficile colitis (1 patient) and/or BCV induced gastrointestinal toxicity. Earlier initiation of BCV, prior to gut involvement by ADV, may have improved tolerability. While ongoing clinical trials of BCV will provide valuable data on the efficacy of BCV for preemption of ADV, dedicated trials in adults may be required as tolerability of BCV may be different in adult and pediatric patients.
We have previously shown ADV viremia to be a negative predictor of survival [1]. In the present study after implementation of prospective monitoring for ADV, we show that patients with ADV viremia had higher mortality compared to patients without viremia. It is of note that implementation of prospective monitoring was not coupled with strict guidelines for initiation of treatment. Due to shorter follow up in the current study, it is hard to compare mortality rates before and after implementation of routine monitoring. Additional differences in the patient population including underlying diseases, method of CD34+ selection, age and conditioning regimen may confound comparisons of mortality rates between two non-contemporaneous studies.
Our study has limitations inherent to its observational nature and sample size: First, heterogeneous clinical management makes the interpretation of treatment efficacy challenging. Second, true incidence of ADV disease may be underestimated, as not all symptomatic patients had biopsies. Third, virologic characteristics (serotype, cidofovir resistance genotype) or host genetic or immune predisposition were not examined.
Acknowledging these limitations, to our knowledge this is the first study to examine the outcomes of ADV viremia in adult recipients of CD34+ selected allografts monitored prospectively by ADV qPCR. Given encouraging outcomes of CD34+ selected HCT and FDA approval of the CliniMACS® system for CD34+ selection, the use of CD34+ allografts is likely to increase in the future [12].
In summary, despite the low incidence among adult CD34+ selected HCT (8%), ADV viremia was an important cause of early mortality, adversely affecting early overall survival. Sive et al., using alemtuzumab for TCD, suggests that ADV viral load and infection were responded to reduction in immunosuppression [6] whereas we observed rapidly rising ADV viral load and short interval between onset of ADV viremia and disease in the setting of low CD4+ post-HCT without exogenous immunosuppression. Routine monitoring by ADV qPCR in blood was useful in identifying ADV viremia at low viral loads. Based on our clinical experience, we recommend treatment for confirmed ADV viral load >103 copies/mL, if occurring the first 2 months after CD34+ selected HCT. After 2 months post-HCT, we advocate treatment for rising viral load in consecutive specimens obtained 3-4 days apart, or viral load >103 copies/mL in the presence of coinfections or immunosuppressants. Similar guidelines have been proposed by Lindemans et al. for recipients of cord blood or TCD allografts [5]. Due to the lack of exogenous immunosuppression, recipients of CD34+ selected HCT may benefit from a combined approach of antivirals and cellular therapy to reconstitute immunity against ADV. Further studies on adult recipients of CD34+ selected allografts are needed to improve outcomes of ADV infection in this population.
Highlights.
Adenovirus viremia in adult CD34+-selected HCT is 8% and 33% of viremic patients developed disease.
Acknowledgments
GAP has served as an advisor and received research funding from Chimerix, Inc.
Financial disclosure: RT, SG, EP and AJ are supported in part by National Institutes of Health grant P01 CA023766 Immunobiology for Marrow Allografts for Leukemia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee YJ, Chung D, Xiao K, et al. Adenovirus viremia and disease: comparison of T cell-depleted and conventional hematopoietic stem cell transplantation recipients from a single institution. Biol Blood Marrow Transplant. 2013;19:387–392. doi: 10.1016/j.bbmt.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.La Rosa AM, Champlin RE, Mirza N, et al. Adenovirus infections in adult recipients of blood and marrow transplants. Clin Infect Dis. 2001;32:871–876. doi: 10.1086/319352. [DOI] [PubMed] [Google Scholar]
- 3.Lion T. Adenovirus infections in immunocompetent and immunocompromised patients. Clin Microbiol Rev. 2014;27:441–462. doi: 10.1128/CMR.00116-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soriano G, Perales MA. Adenovirus viremia and infection after reduced-intensity allogeneic hematopoietic stem cell transplant: should we institute a routine screening program? Clin Infect Dis. 2012;55:1371–1372. doi: 10.1093/cid/cis695. [DOI] [PubMed] [Google Scholar]
- 5.Lindemans CA, Leen AM, Boelens JJ. How I treat adenovirus in hematopoietic stem cell transplant recipients. Blood. 2010;116:5476–5485. doi: 10.1182/blood-2010-04-259291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sive JI, Thomson KJ, Morris EC, Ward KN, Peggs KS. Adenoviremia has limited clinical impact in the majority of patients following alemtuzumab-based allogeneic stem cell transplantation in adults. Clin Infect Dis. 2012;55:1362–1370. doi: 10.1093/cid/cis689. [DOI] [PubMed] [Google Scholar]
- 7.Robin M, Marque-Juillet S, Scieux C, et al. Disseminated adenovirus infections after allogeneic hematopoietic stem cell transplantation: incidence, risk factors and outcome. Haematologica. 2007;92:1254–1257. doi: 10.3324/haematol.11279. [DOI] [PubMed] [Google Scholar]
- 8.Runde V, Ross S, Trenschel R, et al. Adenoviral infection after allogeneic stem cell transplantation (SCT): report on 130 patients from a single SCT unit involved in a prospective multi center surveillance study. Bone Marrow Transplant. 2001;28:51–57. doi: 10.1038/sj.bmt.1703083. [DOI] [PubMed] [Google Scholar]
- 9.Chakrabarti S, Mautner V, Osman H, et al. Adenovirus infections following allogeneic stem cell transplantation: incidence and outcome in relation to graft manipulation, immunosuppression, and immune recovery. Blood. 2002;100:1619–1627. doi: 10.1182/blood-2002-02-0377. [DOI] [PubMed] [Google Scholar]
- 10.Ohrmalm L, Lindblom A, Omar H, et al. Evaluation of a surveillance strategy for early detection of adenovirus by PCR of peripheral blood in hematopoietic SCT recipients: incidence and outcome. Bone Marrow Transplant. 2011;46:267–272. doi: 10.1038/bmt.2010.86. [DOI] [PubMed] [Google Scholar]
- 11.Symeonidis N, Jakubowski A, Pierre-Louis S, et al. Invasive adenoviral infections in T-cell-depleted allogeneic hematopoietic stem cell transplantation: high mortality in the era of cidofovir. Transplant Infect Dis. 2007;9:108–113. doi: 10.1111/j.1399-3062.2006.00184.x. [DOI] [PubMed] [Google Scholar]
- 12.Devine SM, Carter S, Soiffer RJ, et al. Low risk of chronic graft-versus-host disease and relapse associated with T cell-depleted peripheral blood stem cell transplantation for acute myelogenous leukemia in first remission: results of the blood and marrow transplant clinical trials network protocol 0303. Biol Blood Marrow Transplant. 2011;17:1343–1351. doi: 10.1016/j.bbmt.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldberg JD, Linker A, Kuk D, et al. T cell-depleted stem cell transplantation for adults with high-risk acute lymphoblastic leukemia: long-term survival for patients in first complete remission with a decreased risk of graft-versus-host disease. Biol Blood Marrow Transplant. 2013;19:208–213. doi: 10.1016/j.bbmt.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bayraktar UD, de Lima M, Saliba RM, et al. Ex vivo T cell-depleted versus unmodified allografts in patients with acute myeloid leukemia in first complete remission. Biol Blood Marrow Transplant. 2013;19:898–903. doi: 10.1016/j.bbmt.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matthes-Martin S, Feuchtinger T, Shaw PJ, et al. European guidelines for diagnosis and treatment of adenovirus infection in leukemia and stem cell transplantation: summary of ECIL-4 (2011) Transplant Infect Dis. 2012;14:555–563. doi: 10.1111/tid.12022. [DOI] [PubMed] [Google Scholar]
- 16.Breslow NE, Day NE. Statistical methods in cancer research Volume II--The design and analysis of cohort studies. IARC Sci Publ. 1987:1–406. [PubMed] [Google Scholar]
- 17.Chimerix; Chimerix. Phase III, Open-labeled, Multicenter Study of the Safety and Efficacy of Brincidofovir (CMX001) in the Treatment of Early Versus Late Adenovirus Infection (CMX001 Adv) Bethesda (MD): National Library of Medicine (US); 2014. ClinicalTrials.giv [Internet] [cited 2015 August 6]. Available from: https://clinicaltrials.gov/ct2/show/NCT02087306 NLM Identifier: NCT02087306. [Google Scholar]
- 18.Grimley MS, Maron GM, Prasad VK, et al. Preliminary Results from the AdVise Study Evaluating Brincidofovir (CMX001, BCV) for the Treatment of Disseminated and High-Risk Adenovirus (AdV) Infection. Biol Blood Marrow Transplant. 2015;21:S108–S109. [Google Scholar]
- 19.Florescu DF, Pergam SA, Neely MN, et al. Safety and efficacy of CMX001 as salvage therapy for severe adenovirus infections in immunocompromised patients. Biol Blood Marrow Transplant. 2012;18:731–738. doi: 10.1016/j.bbmt.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]