Abstract
Background
Important therapeutic decisions are made based on the presence or absence of fever in patients with Kawasaki disease (KD), yet no standard method or threshold exists for temperature measurement during the diagnosis and treatment of these patients. We sought to compare surface and internal (rectal or oral) routes of temperature measurement for the detection of fever as a marker of treatment resistance.
Methods
From a randomized, placebo-controlled trial of infliximab as an adjunct to primary intravenous immunoglobulin treatment for acute KD, we collected concurrent (within 5 minutes) axillary and internal temperature measurements and performed receiver-operating characteristic and Bland-Altman analyses. We also determined the ability of surface temperatures to detect treatment resistance defined by internal temperature measurements.
Results
Among 452 oral-axillary and 439 rectal-axillary pairs from 159 patients, mean axillary temperatures were 0.25°C and 0.43°C lower than oral and rectal temperatures and had high receiver-operating characteristic areas-under-curves. However, axillary temperatures ≥38.0°C had limited sensitivity to detect fever defined by internal temperatures. Axillary thresholds of 37.5°C and 37.2°C provided maximal sensitivity and specificity to detect oral and rectal temperatures ≥38.0°C, respectively.
Conclusions
Axillary temperatures are an insensitive metric for fevers defining treatment resistance. Clinical trials should adopt temperature measurement by the oral or rectal routes for adjudication of treatment resistance in KD.
Keywords: Kawasaki Disease, fever measurement methods
Fever is invariably present in children with acute Kawasaki disease (KD) and is the clinical sign that dictates many interventions, including retreatment after initial therapy with intravenous immunoglobulin (IVIG). Despite the importance of fever in KD, no detailed study of best practice for fever measurement has been performed in this patient population, and approaches vary widely across medical centers and between the U.S. and Japan, the country of highest incidence.1,2 Previous clinical studies of KD patients have not specified a standard route of temperature measurement.3 The lack of uniformity in fever definition may contribute to differences in treatment failure rates among sites and studies.1 A Phase III clinical trial of intensification of primary IVIG treatment of acute KD with infliximab4 provided an opportunity to analyze agreement within a large set of temperature readings concurrently measured by different routes and to inform best practices for temperature routes and thresholds in KD research and clinical practice. We postulated that axillary temperatures would differ significantly from oral and rectal temperatures and lack sensitivity for detecting fever in patients with KD.
Methods
Study population
Subjects were patients with KD enrolled and treated in a randomized, placebo-controlled phase III trial of infliximab as an adjunct to IVIG treatment.4 The patients were 4 weeks to 17 years old, had fever for 3 to 10 days (illness day 1: first day of fever ≥38.0° C) at the time of study enrollment, and met the American Heart Association definition of KD.2 The Institutional Review Boards of the University of California San Diego and Nationwide Children's Hospital reviewed and approved this study, and all parents provided signed, informed consent.
Study design and definitions
Nurses measured temperatures using digital thermometers (American Diagnostic Corp, model Adtemp™ II 413) every 6 hours by both surface (axillary) and internal (rectal or oral, based on nurse's assessment of age and developmental level) routes prior to scheduled aspirin doses. Each patient had 2 thermometers, each designated for a specific route. Additional temperatures were measured if clinically indicated. During infusions of IVIG and study medication, nurses obtained hourly measurements by the axillary route only. After discharge, the legal guardian used the same model of thermometer to record temperatures by a single route once daily between 4 and 6 PM for 3 days. We defined treatment resistance as fever occurring between 36 hours and 7 days after completion of the IVIG infusion without another likely source2 and used a threshold of ≥38.0°C by any route.5
We included temperature pairs if a surface and an internal temperature (e.g., oral-axillary or rectal-axillary) were recorded within 5 minutes of each other. If >2 temperature measurements occurred within 5 minutes, only the first 2 qualifying temperatures were included. Among patients with treatment resistance, we determined whether axillary temperatures were sufficient to identify the need for retreatment at any time during the inpatient observation period.
Statistical analysis
We conducted descriptive analyses and generated a scatterplot of surface-internal temperature pairs, calculating Pearson's correlation coefficient for the paired measurements. We analyzed the agreement between the internal and surface temperatures with Bland-Altman plots6 to which we added linear regression lines showing the relationship of temperature differences against mean temperature. We conducted a receiver-operating characteristic (ROC) analysis, calculating areas under the curve (AUCs) as a measure of the ability of surface temperatures to detect fever defined by internal temperatures. Sensitivities and specificities were calculated for surface temperatures of ≥37.5° C and ≥38.0° C with 95% confidence intervals (CIs) computed using the bootstrap approach. We also identified the surface temperature threshold corresponding to the highest sum of sensitivity and specificity. P-values <0.05 were considered statistically significant. Statistical analyses were performed in R (http://cran.r-project.org), version 3.0.2.
Results
Of 196 patients enrolled, 195 completed the study and provided 6869 total temperature measurements. After excluding temperatures taken during medication infusion (35%) and temperatures not paired within 5 minutes, 1782 temperature measurements (25.9%) from 159 patients qualified for analysis, yielding 452 oral-axillary pairs (median patient age: 4.5 years; range 1.9-13.6 years) and 439 rectal-axillary pairs (median patient age: 2.0 years; range 0.2-6.5 years). Strong correlations existed between surface and internal temperatures: for all pairs, 0.852 (p<0.001); for oral-axillary pairs, 0.859 (p<0.001); for rectal-axillary pairs, 0.854 (p<0.001). (Figure 1). The mean oral temperature was higher than the mean surface temperature by 0.25°C (95% CI: -0.78°C, 1.28°C); the mean rectal temperature was higher than the mean surface temperature by 0.43°C (95% CI: -0.66°C, 1.53°C), but the differences were not statistically significant (Figure 2). The axillary-oral temperature difference tended to decrease at higher mean temperatures, but axillary-rectal differences remained constant.
Figure 1.
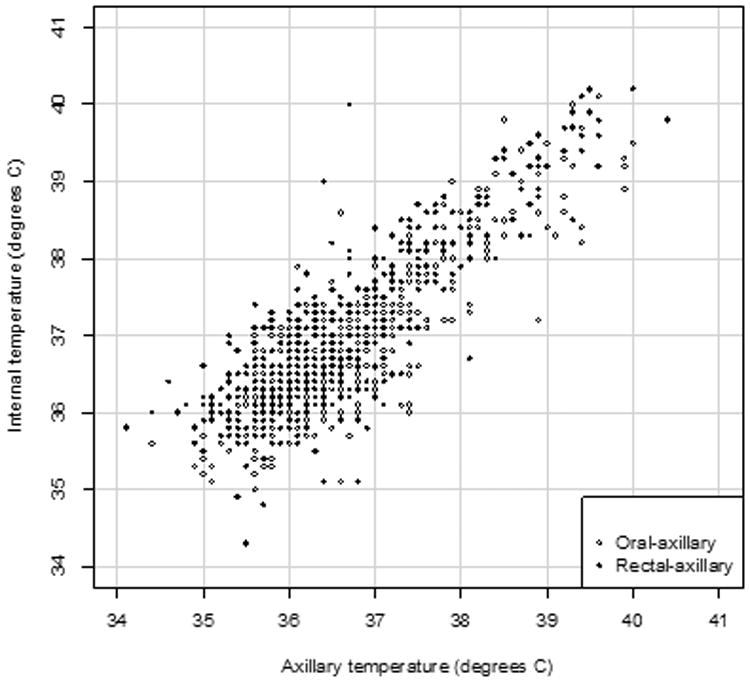
Scatterplot of 452 oral-axillary (open circles) and 439 rectal-axillary (closed circles) temperature pairs from 159 patients during hospital admission for acute KD. Correlation coefficients: all internal-axillary pairs, 0.852 (p<0.001); oral-axillary pairs, 0.859 (p<0.001); rectal-axillary pairs, 0.854 (p<0.001).
Figure 2.
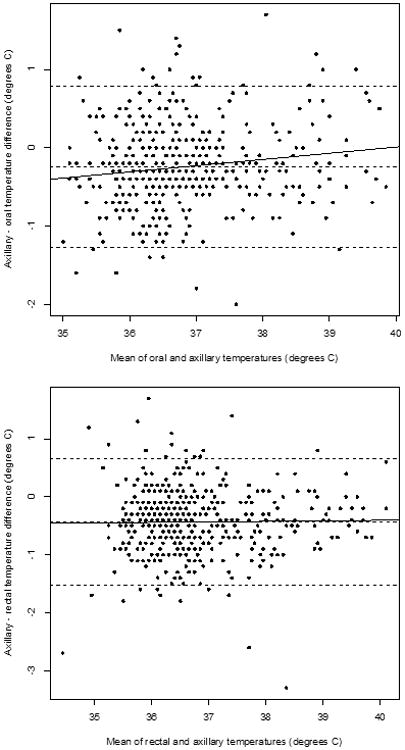
Bland-Altman plots of axillary-internal temperature difference against the mean of axillary and internal temperature for oral-axillary pairs (top) and rectal-axillary pairs (bottom). The horizontal broken lines represent the mean temperature difference with the 95% CIs. The solid line is the regression line of temperature difference against mean temperature.
ROC analysis revealed high AUCs for the axillary route of detection of fever defined by internal temperature ≥38.0°C: 0.983 (95% CI: 0.970 – 0.997) against oral measurements; 0.967 (95% CI: 0.949 – 0.985) against rectal measurements; and 0.972 (95% CI: 0.959 - 0.984) against any internal method. However, the axillary temperature threshold of 38.0°C had limited sensitivity for detection of fever defined by internal temperatures (Table). An axillary temperature threshold of 37.5°C had highest sensitivity and specificity (0.94 for both) for the detection of fever defined by oral temperatures ≥38.0°C, and axillary threshold of 37.2°C was optimal to detect rectal temperatures ≥38.0°C (Table).
Table 1.
Performance of axillary temperature measurement in detecting fever defined by concurrent oral or rectal temperature measurement in hospitalized children with acute Kawasaki disease.
| Sensitivity | Specificity | |
|---|---|---|
| Ability of axillary temperature ≥37.5°C to detect concurrent: | ||
| Oral temperature ≥38.0°Ca | 0.94 (0.88, 0.98) | 0.94 (0.92, 0.97) |
| Rectal temperature ≥38.0°C | 0.80 (0.71, 0.89) | 0.96 (0.94, 0.98) |
| Ability of axillary temperature ≥38.0°C to detect concurrent: | ||
| Oral temperature ≥38.0°C | 0.73 (0.63, 0.84) | 0.99 (0.98, 1.0) |
| Rectal temperature ≥38.0°C | 0.59 (0.47 0.70) | 0.99 (0.99, 1.0) |
| Ability of optimal axillary temperature ≥37.2°C to detect fever defined by rectal temperatures ≥38.0°Ca, b | 0.88 (0.80, 0.95) | 0.94 (0.91, 0.96) |
Data presented as proportions with 95% CI in parentheses.
With rounding, the values of sensitivity and specificity for the optimal axillary temperature threshold (37.45°C) to detect oral temperature ≥38.0°C are the same as for the axillary temperature threshold of 37.5°C.
Optimal axillary temperature to detect rectal temperature ≥38.0°C rounded from 37.25°C.
In this clinical trial, 22 subjects had a rectal or oral temperature ≥38.0°C at least 36 hours after completion of their initial IVIG infusion and received a second treatment with IVIG as rescue therapy (17 during the initial admission and 5 during a readmission). If we had used axillary temperatures alone, 2 of the 17 subjects re-treated during their initial admission may not have received additional therapy. This omission would have changed the conclusions of our clinical trial regarding the intensification of initial therapy with infliximab to prevent IVIG-resistance. An axillary temperature threshold of 37.5°C would have detected the fever which prompted re-treatment in only 1 of the patients, but a threshold of 37.2°C would not have improved detection.
Discussion
In this first large-scale study of paired temperatures obtained during the same phase of illness and treatment from a cohort of patients with acute KD, surface temperatures were, on average, lower than oral and rectal temperatures. However, the temperature differences were highly variable and lacked statistical significance. Despite high ROC AUCs, axillary temperatures ≥38.0° C had limited sensitivity to detect fever defined by concurrent oral and rectal temperatures. The axillary temperature threshold of ≥37.5° C, commonly used by Japanese investigators, was more sensitive but still misclassified as afebrile 6% and 20% of fevers defined by oral and rectal temperatures.
The diagnosis and treatment of KD rely on accurately identifying fever. IVIG resistance, with its increased risk for CA and need for additional treatment, is defined by the persistence or recurrence of fever. However, the 2004 American Heart Association statement,2 the Centers for Disease Control case definition,7,8 and the American Academy of Pediatrics Committee on Infectious Diseases Red Book9 provide no definitions for fever in KD. Published temperature criteria for the adjudication of fever and thus treatment resistance in the United States include thresholds of 37.8°C,10 38.0°C,5 38.1°C,11 and 38.3°C.3,12 Japanese investigators have defined fever for initial diagnosis as a temperature of >38°C1,13 and for treatment resistance as ≥37.5°C1,13-16 and have used the axillary route of measurement.16 Non-uniform routes and temperature definitions compromise reliability of fever detection in KD and may be a cause of differing results in studies of treatment failure.1,3
The surface and internal mean differences we observed are consistent with previously reported differences between rectal and axillary17-19 and between oral and axillary temperatures.19,20 Our observation that the mean difference between axillary and rectal temperatures was greater than between axillary and oral temperatures is consistent with previous studies of hospitalized pediatric patients with various clinical diagnoses.19 Axillary temperature measurement poorly detects elevated temperatures among hospitalized infants, even with an axillary threshold of 37.2°C.21 A simple conversion between surface and internal temperature18,20 would be useful for clinical care and research in KD. However, the wide variability in surface-internal temperature differences in our study and others17,19,21 precludes development of a formula to predict oral or rectal temperatures from axillary measurements.
We recognize several strengths and limitations of this study. We address an important controversy in KD clinical care and research using a large cohort of subjects at the same stage of treatment. This study did not incorporate the methodology of thermometry studies and did not specify the order of temperature measurements, the choice of internal temperature route, or ambient temperature or clothing conditions. However, thermometry by oral, rectal, and axillary routes is standard practice for pediatric nurses, who used the same model of thermometer for all patients. Therefore, although our intention was to inform temperature measurement in KD, our findings are relevant to general pediatric care and to special populations such as young infants22 and patients with cancer,23 for whom specific temperature thresholds dictate clinical care and research and for whom differences among axillary, oral, and rectal temperature may affect fever detection. Although nurses were not blinded to the results of temperature pairs or to clinical condition, they were not aware of the study objective and were blinded to the treatment allocation. Therefore, the knowledge of initial temperature should not have influenced the second measurement. Because our subjects were under treatment for an illness characterized by changes in body temperature, an unknown number of paired temperature measurements may have been obtained during rapid temperature changes. Thus, surface-internal temperature differences may reflect true changes in body temperature as well as differences in route of measurement, particularly if the separation in measurements was greater than the 5 minutes documented. Other temperature routes warrant study in KD, as data from other acutely ill pediatric populations suggest that the temporal artery route is superior to the tympanic and axillary routes in detecting rectal temperature elevation.24,25 Finally, because the first detected temperature ≥38.0°C led to prompt IVIG retreatment, our data cannot determine whether a single temperature elevation by any route predicts sustained fever or whether fever undetected by a single axillary measurement is readily detected during the ensuing hours or days.
Axillary temperature measurement at thresholds of 37.5°C or 38.0°C inadequately detects fevers identified by oral or rectal routes during the treatment of acute KD. If axillary temperature measurement must be used because of hospital protocol, a threshold of 37.2°C may be a better predictor of fever for KD patients. Because of the importance of fever in the diagnosis and management of acute KD, temperatures should preferably be measured by the rectal or oral route with a standardized threshold to define fever for clinical care and research.
Acknowledgments
Funding: This work was supported in part by the Food and Drug Administration Orphan Drug Program (FD 003514 to JCB and AT); National Heart, Lung, Blood Institute at the National Institutes of Health (RO1 HL69413 to JCB and JTK); and The Harold Amos Medical Faculty Development Program from the Robert Wood Johnson Foundation (to AHT).
Footnotes
Disclosure: The authors have no conflicts of interest to disclose.
References
- 1.Inoue Y, Okada Y, Shinohara M, et al. A multicenter prospective randomized trial of corticosteroids in primary therapy for Kawasaki disease: clinical course and coronary artery outcome. J Pediatr. 2006;149:336–341. doi: 10.1016/j.jpeds.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 2.Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110:2747–2771. doi: 10.1161/01.CIR.0000145143.19711.78. [DOI] [PubMed] [Google Scholar]
- 3.Newburger JW, Sleeper LA, McCrindle BW, et al. Randomized trial of pulsed corticosteroid therapy for primary treatment of Kawasaki disease. N Engl J Med. 2007;356:663–675. doi: 10.1056/NEJMoa061235. [DOI] [PubMed] [Google Scholar]
- 4.Tremoulet AH, Jain S, Jaggi P, et al. Infliximab for intensification of primary therapy for Kawasaki disease: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet. 2014;383:1731–1738. doi: 10.1016/S0140-6736(13)62298-9. [DOI] [PubMed] [Google Scholar]
- 5.Newburger JW, Takahashi M, Beiser AS, et al. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med. 1991;324:1633–1639. doi: 10.1056/NEJM199106063242305. [DOI] [PubMed] [Google Scholar]
- 6.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 7.Kawasaki syndrome--United States. MMWR Morb Mortal Wkly Rep. 1983;32:98–100. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Kawasaki syndrome. [cited 2013 November 5];2011 Available from: http://www.cdc.gov/kawasaki/
- 9.American Academy of Pediatrics. Kawasaki disease. In: Pickering LK, Baker CJ, Kimberlin DW, editors. Red Book: 2012 Report of the Committee on Infectious Diseases. 29. Elk Grove Village, IL: American Academy of Pediatrics; 2012. pp. 454–460. [Google Scholar]
- 10.Wallace CA, French JW, Kahn SJ, Sherry DD. Initial intravenous gammaglobulin treatment failure in Kawasaki disease. Pediatrics. 2000;105:E78. doi: 10.1542/peds.105.6.e78. [DOI] [PubMed] [Google Scholar]
- 11.Sundel RP, Burns JC, Baker A, Beiser AS, Newburger JW. Gamma globulin retreatment in Kawasaki disease. J Pediatr. 1993;123:657–659. doi: 10.1016/s0022-3476(05)80972-2. [DOI] [PubMed] [Google Scholar]
- 12.Burns JC, Capparelli EV, Brown JA, Newburger JW, Glode MP. Intravenous gamma-globulin treatment and retreatment in Kawasaki disease. US/Canadian Kawasaki Syndrome Study Group. Pediatr Infect Dis J. 1998;17:1144–1148. doi: 10.1097/00006454-199812000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi T, Inoue Y, Takeuchi K, et al. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. 2006;113:2606–2612. doi: 10.1161/CIRCULATIONAHA.105.592865. [DOI] [PubMed] [Google Scholar]
- 14.Hashino K, Ishii M, Iemura M, Akagi T, Kato H. Re-treatment for immune globulin-resistant Kawasaki disease: a comparative study of additional immune globulin and steroid pulse therapy. Pediatr Int. 2001;43:211–217. doi: 10.1046/j.1442-200x.2001.01373.x. [DOI] [PubMed] [Google Scholar]
- 15.Ogata S, Bando Y, Kimura S, et al. The strategy of immune globulin resistant Kawasaki disease: a comparative study of additional immune globulin and steroid pulse therapy. J Cardiol. 2009;53:15–19. doi: 10.1016/j.jjcc.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Ogata S, Ogihara Y, Honda T, et al. Corticosteroid pulse combination therapy for refractory Kawasaki disease: a randomized trial. Pediatrics. 2012;129:e17–23. doi: 10.1542/peds.2011-0148. [DOI] [PubMed] [Google Scholar]
- 17.Craig JV, Lancaster GA, Williamson PR, Smyth RL. Temperature measured at the axilla compared with rectum in children and young people: systematic review. BMJ. 2000;320:1174–1178. doi: 10.1136/bmj.320.7243.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edelu BO, Ojinnaka NC, Ikefuna AN. A comparison of axillary with rectal thermometry in under 5 children. Niger Med J. 2011;52:207–210. doi: 10.4103/0300-1652.93789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Falzon A, Grech V, Caruana B, Magro A, Attard-Montalto S. How reliable is axillary temperature measurement? Acta Paediatr. 2003;92:309–313. doi: 10.1080/08035250310009220. [DOI] [PubMed] [Google Scholar]
- 20.Lodha R, Mukerji N, Sinha N, Pandey RM, Jain Y. Is axillary temperature an appropriate surrogate for core temperature? Indian J Pediatr. 2000;67:571–574. doi: 10.1007/BF02758482. [DOI] [PubMed] [Google Scholar]
- 21.Morley CJ, Hewson PH, Thornton AJ, Cole TJ. Axillary and rectal temperature measurements in infants. Arch Dis Child. 1992;67:122–125. doi: 10.1136/adc.67.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider C, Blumberg S, Crain EF. A survey of the management of febrile infants in pediatric emergency departments. Pediatr Emerg Care. 2012;28:1022–1026. doi: 10.1097/PEC.0b013e31826caa94. [DOI] [PubMed] [Google Scholar]
- 23.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52:427–431. doi: 10.1093/cid/ciq147. [DOI] [PubMed] [Google Scholar]
- 24.Batra P, Goyal S. Comparison of rectal, axillary, tympanic, and temporal artery thermometry in the pediatric emergency room. Pediatr Emerg Care. 2013;29:63–66. doi: 10.1097/PEC.0b013e31827b5427. [DOI] [PubMed] [Google Scholar]
- 25.Greenes DS, Fleisher GR. Accuracy of a noninvasive temporal artery thermometer for use in infants. Arch Pediatr Adolesc Med. 2001;155:376–381. doi: 10.1001/archpedi.155.3.376. [DOI] [PubMed] [Google Scholar]


