Abstract
Bacillus anthracis lethal toxin (LT) produces symptoms of anthrax in mice and induces rapid lysis of macrophages (Mφ) derived from certain inbred strains. We used nine inbred strains and two inducible nitric oxide synthase (iNOS) knockout C57BL/6J strains polymorphic for the LT Mφ sensitivity Kif1C locus to analyze the role of Mφ sensitivity (to lysis) in LT-mediated cytokine responses and lethality. LT-mediated induction of cytokines KC, MCP-1/JE, MIP-2, eotaxin, and interleukin-1β occurred only in mice having LT-sensitive Mφ. However, while iNOS knockout C57BL/6J mice having LT-sensitive Mφ were much more susceptible to LT than the knockout mice with LT-resistant Mφ, a comparison of susceptibilities to LT in the larger set of inbred mouse strains showed a lack of correlation between Mφ sensitivity and animal susceptibility to toxin. For example, C3H/HeJ mice, harboring LT-sensitive Mφ and having the associated LT-mediated cytokine response, were more resistant than mice with LT-resistant Mφ and no cytokine burst. Toll-like receptor 4 (Tlr4)-deficient, lipopolysaccharide-nonresponsive mice were not more resistant to LT. We also found that CAST/Ei mice are uniquely sensitive to LT and may provide an economical bioassay for toxin-directed therapeutics. The data indicate that while the cytokine response to LT in mice requires Mφ lysis and while Mφ sensitivity in the C57BL/6J background is sufficient for BALB/cJ-like mortality of that strain, the contribution of Mφ sensitivity and cytokine response to animal susceptibility to LT differs among other inbred strains. Thus, LT-mediated lethality in mice is influenced by genetic factors in addition to those controlling Mφ lysis and cytokine response and is independent of Tlr4 function.
Anthrax lethal toxin (LT), a major virulence factor of Bacillus anthracis, consists of two polypeptides, lethal factor (LF) and protective antigen (PA). PA binds to receptors and translocates LF (a protease) into the cytosol (10). LT injection into animals is sufficient to induce symptoms of anthrax (1-3, 7, 8). We recently showed that LT injection in mice results in death through cytokine-independent mechanisms involving vascular collapse and resultant hypoxia (12).
Macrophages (Mφ) from certain inbred strains of mice are uniquely sensitive to rapid lysis by LT, while those from other strains are resistant (4). Three polymorphisms in the LT susceptibility locus (kinesin gene Kif1C on mouse chromosome 11) correlate with Mφ sensitivity to LT (17). Early studies showing that LT-induced death was more rapid in inbred CBA/J mice harboring LT-sensitive (LTs) Mφ than in A/J mice harboring LT-resistant (LTr) Mφ suggested a correlation between Mφ sensitivity and animal susceptibility (18). Studies by Hanna et al. implicated Mφ sensitivity in LT toxicity by showing that depleting BALB/cJ mice of LTs Mφ made them LTr (5). However, new studies comparing BALB/c and DBA/2 mice showed that two additional loci on mouse chromosome 11 contribute to LT susceptibility (11).
Recent studies suggest that Mφ sensitivity to LT may not have the importance previously ascribed to it (12). LT kills both BALB/cJ and C57BL/6J mice, harboring LTs Mφ and LTr Mφ, respectively, although BALB/cJ mice succumb more rapidly to toxin. Hematological analyses of LT-treated mice show that circulating monocytes and Mφ are depleted beginning at 2 h after toxin treatment in BALB/cJ mice, correlating with the lysis induced by LT, and this depletion is complete by 10 h (12). In contrast, C57BL/6J circulating mononuclear cells increase in number after LT treatment (12). Both strains, however, succumb to toxin through similar processes. Histological analyses showed similar pathological manifestations of hypoxia and circulatory collapse in both strains, with these events delayed by 48 to 60 h in the C57BL/6J mice (12). The mice showed none of the classic hallmarks of septic shock. Although the early studies cited above suggested that tumor necrosis factor alpha (TNF-α) production by LTs Mφ could play a role in inducing shock-like symptoms of anthrax (5), we found no induction of this cytokine in either BALB/cJ or C57BL/6J mice (12). Additionally, assessment of over 50 other chemokines and cytokines showed a rapid but transient induction of a small group of factors strictly in the BALB/cJ mice. These factors (which include KC, JE/MCP-1, MIP-2, eotaxin, and interleukin-1β [IL-1β]) were induced within 2 h of toxin injection, peaked at 6 to 12 h after injection, and were no longer present at 24 h (12). Exclusive induction of these factors in BALB/cJ mice led us to hypothesize that Mφ sensitivity (lysis) may be required for their induction and that inbred mice with LTs Mφ die more rapidly due to cytokine-mediated exacerbation of Mφ-independent, LT-mediated pathological processes. In this report we present data indicating that Mφ lysis causes and is required for the rapid, transient induction of cytokines. While this lysis and the associated cytokine cascade are sufficient for normally resistant C57BL/6J mice to show the same rapid mortality as BALB/cJ mice, they are not predictive of LT susceptibility among a larger set of mouse strains. Rather, animal susceptibility to LT is influenced by factors in addition to and independent of Mφ sensitivity and cytokine induction.
MATERIALS AND METHODS
Toxin.
PA and LF were purified as previously described (16). Toxin for injection was prepared in saline. Doses of LT refer to the amount of each component (i.e., 100 μg of LT is 100 μg of PA plus 100 μg of LF).
Animals.
AKR/J, A/J, BALB/cJ, B6C3Fe-a/a-Csfmop (Op/Op), C3H/HeJ, C3H/HeOuJ, C57BL/6J, C57BL/6-Nos2tm1Lau (Jackson Laboratories inducible nitric oxide synthase knockout [JAX iNOS KO]), C57BL/10J, C57BL/10ScN, CAST/Ei, CBA/J, C.C3H-Tlr4Lps-d (C.C3H-Tlr4), and DBA/2J mice were purchased from Jackson Laboratories (Bar Harbor, Maine). C57BL/6NTac and C57BL/6Ai N5 (Taconic Farms inducible nitric oxide synthase knockout [TAC iNOS KO]) mice were purchased from Taconic Farms (Germantown, N.Y.). Experiments were with the N5 generation of the TAC iNOS KO mice, backcrossed five times to the C57BL/6J background. This knockout has subsequently been backcrossed by the vendor to C57BL/6NTac to generation N9. Male and female mice (9 to 13 weeks old, 20 to 24 g) were injected with 1.0 ml of toxin intraperitoneally (i.p.). AKR/J and Op/Op mice (each 8 weeks old) and CAST/Ei mice (12 weeks old, 12 to 14 g) were injected with a smaller toxin dose on a per-gram-of-body-weight basis. Some mice were terminally bled for cytokine analyses.
Cells.
Peritoneal Mφ were elicited by injection of 2 ml of filter-sterilized 4% Brewer modified thioglycolate (Becton Dickinson, Cockeysville, Md.) in PBS and harvested 48 to 72 h later by peritoneal lavage. For certain experiments in which Mφ were obtained after LT treatment of animals, thioglycolate pretreatment was not utilized and cells were harvested by peritoneal lavage. The Mφ cell line 23 ScCR[10ScNCr/23] was obtained from American Type Culture Collection (Manassas, Va.). Cells were grown in Dulbecco's modified Eagle's medium with 10% fetal calf serum, 2 mM glutamine, and 50 μg of gentamicin per ml at 37°C with 5% CO2.
Cytotoxicity assays.
Mφ were seeded into 96-well plates at 12 to 16 h prior to LT addition. Cell viability was assayed with MTT [3-(4,5-dimethylthiazo-2-yl)-2,5-diphenyltetrazolium bromide] (Sigma, St. Louis, Mo.) as previously described (16).
Cytokine expression.
Enzyme-linked immunosorbent assay (ELISA) kits for IL-1β, MCP-1/JE, KC, eotaxin, granulocyte colony-stimulating factor (G-CSF) (R&D Systems, Minneapolis, Minn.), MIP-2 (IBL, Fujioka-City, Japan), and TNF-α (Chemicon, Temecula, Calif.) were utilized for assessment of cytokine levels in mouse serum according to the manufacturer's protocols.
Kif1C analysis.
Genomic DNA was isolated from livers of BALB/cJ, C57BL/6J, JAX iNOS KO, and TAC iNOS KO mice by using the DNeasy kit from Qiagen (Valencia, Calif.). Primer pairs 5′-CCTAGGGAGCACCTGCTTATCC-3′-5′-GCTGTAGTTAGGGAGCATGGTGG-3′ and 5′-GCAGTGGGCACCTTCCTCC-3′-5′-GCAGTAAAGGAGATATGCTATGAGGTGGCCCTAGCCG-3′ were used in primary PCR, followed by secondary PCR with 5′-GGTGAGCTGCTGGAAGCAAAGGC-3′-5′-GGAGTCTAACCACAGTACAGCG-3′ and 5′-CCACTCGCTTTGAGGTCAGGGGC-3′-5′-CGAGATTGATGGAAGAGGACCCTGCCTTCCG-3′ to amplify and sequence the Kif1C gene. An additional primer pairs utilized for PCR amplification and sequencing was 5′-TTTCCTGTGCTGCCTGCCACCAGAG-3′-5′-ATGCCCAGAAATCCCTGCCTAGCACTTCC-3′.
Statistical analyses.
All statistical analyses were performed with Prism 3.0 (GraphPad, San Diego, Calif.) software.
RESULTS
LT susceptibility phenotypes of Mφ from two types of iNOS knockout mice.
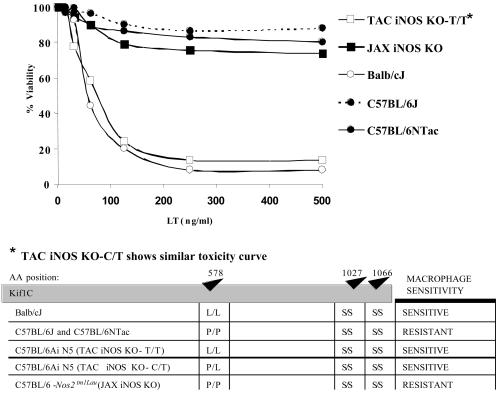
Peritoneal Mφ from BALB/cJ mice are rapidly lysed by LT, whereas Mφ from C57BL/6J are completely resistant (14). To assess the role of iNOS in LT-mediated Mφ lysis, we tested Mφ from TAC iNOS KO mice. Remarkably, TAC iNOS KO Mφ were sensitive to toxin, whereas Mφ from their C57BL/6NTac parents and the similar C57BL/6J strain were, as expected, LTr (Fig. 1, top panel). Extensive investigations into the possible role of iNOS in LT toxicity could not establish any link between LT sensitivity of Mφ and iNOS inhibition or NO induction. It was then found that in contrast to the Mφ of the TAC iNOS KO mouse, Mφ from the presumed identical JAX iNOS KO mouse and its C57BL/6J parent were both LTr (Fig. 1, top panel). The presence of the LT sensitivity locus (Kif1C) on mouse chromosome 11 in proximity to the iNOS gene led us to suspect that the N5 backcrossed TAC iNOS KO mice retained the Kif1C toxin sensitivity allele (17) of the 129 J1 founder iNOS KO mice. Sequencing of this gene in both types of iNOS KO mice showed that a majority of TAC iNOS KO mice carried one or two alleles having a Kif1C polymorphism associated with Mφ sensitivity to LT, whereas the JAX iNOS KO mice were all homozygous for the resistance allele (Fig. 1, bottom panel). Because the Kif1C polymorphism is known to be dominant for sensitivity (17), any mice with a C→T mutation in even one copy of Kif1C, leading to a P→L amino acid change, were expected and subsequently confirmed to have LTs Mφ.
FIG. 1.
LT susceptibility phenotypes and genotypes of Mφ from iNOS knockout mice. (Top) Peritoneal Mφ from BALB/cJ and TAC iNOS KO mice, which are heterozygous at the Kif1c allele, are sensitive to LT. Mφ from JAX iNOS KO mice, which are homozygous at the Kif1c allele, and from C57BL/6J or C57BL/6NTac parents are resistant. (Bottom) Schematic representation of amino acids (AA) at each of the three polymorphic positions in the Kif1C protein.
These two highly similar and essentially isogenic KO mice in the C57BL/6 background, one with LTs Mφ and the other with LTr Mφ, were recognized as offering a unique model for directly assessing the role of Mφ sensitivity in cytokine induction and LT toxicity. Genotyped and age-matched TAC iNOS KO mice harboring one or both LTs alleles (hereafter referred to as TAC iNOS KO-C/T, indicating heterozygous alleles, or as TAC iNOS KO-T/T, indicating homozygous alleles) were both confirmed to have sensitive Mφ and were used as models for C57BL/6 mice with sensitive Mφ. TAC-iNOS KO mice homozygous for the LT resistance allele (hereafter referred to as TAC iNOS KO-C/C) and JAX iNOS KO mice were used as controls having resistant Mφ in the C57BL/6J background.
LT-induced cytokine production in TAC iNOS KO and JAX iNOS KO mice.
We tested the hypothesis that Mφ sensitivity to lysis by LT is required for the rapid, transient cytokine induction observed in BALB/cJ but not C57BL/6J animals (12). KC, JE/MCP-1, IL-1β, eotaxin, MIP-2, and TNF-α in serum were measured at 0, 2.5, 6, 9, 18, 24, 48, 72, and 96 h after LT or PBS injection into TAC iNOS KO-T/T (LTs Mφ) or JAX iNOS KO (LTr Mφ) mice. TAC iNOS KO-T/T (LTs Mφ) mice mounted a remarkable induction of each factor except TNF-α (Fig. 2). The response peaked at around 9 h for each factor except IL-1β, which peaked at 2.5 h and rapidly declined by 6 h after injection (Fig. 2). By 12 to 18 h all factors except JE/MCP-1 were no longer measurable in serum. The iNOS KO mice with LTr Mφ did not show any cytokine response.
FIG. 2.
LT-induced cytokine production in TAC iNOS KO and JAX iNOS KO mice. Levels of KC, JE-MCP-1, IL-1β, eotaxin, MIP-2, and TNF-α in sera from TAC iNOS KO-T/T and JAX iNOS KO mice at different times after injection with PBS or LT (100 μg) are shown. ELISA determinations were done with sera pooled from three mice for each time point and treatment.
LT-induced cytokine production in other inbred mice.
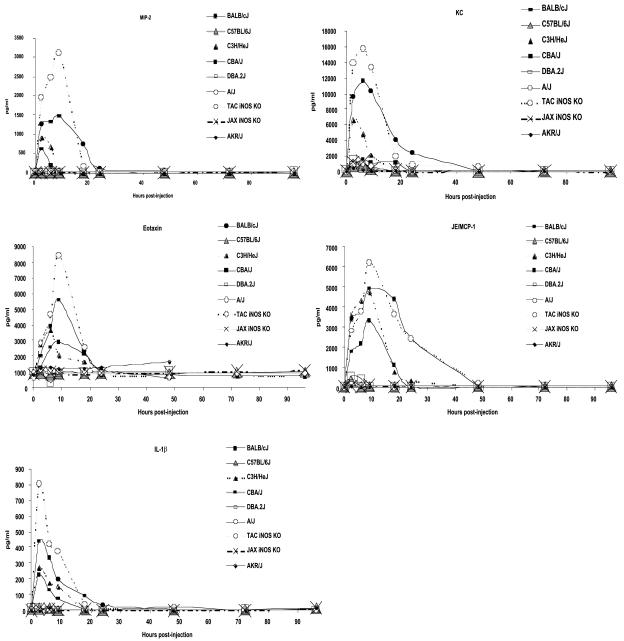
To confirm that cytokine production required LT-mediated Mφ lysis, we compared a number of different inbred mice having either LTr or LTs Mφ to the TAC iNOS KO-T/T (LTs Mφ) and JAX iNOS KO (LTr Mφ) mice. AKR/J, A/J, and DBA/2J mice harbor LTr Mφ, whereas C3H/HeJ, CAST/Ei, and CBA/J mice have LTs Mφ. BALB/cJ (LTs Mφ) and C57BL/6J (LTr Mφ) mice have previously been shown to be cytokine-producing and nonresponsive strains, respectively (12). Additionally lysis of circulating Mφ occurs in vivo in mice harboring LTs Mφ, as shown by their depletion in BALB/cJ mice as early as 2 h after LT administration, while C57BL/6J circulating mononuclear cells increase in number with LT treatment (12). The results clearly indicate that the cytokine production previously seen in the BALB/cJ mice also occurs in all other strains harboring sensitive Mφ but in none of the strains with LTr Mφ. We conclude that Mφ lysis is a requirement for induction of KC, JE/MCP-1, MIP-2, and eotaxin and for release of IL-1β (Fig. 3). A limited number of samples for fewer time points from each strain were also tested for G-CSF and TNF-α. G-CSF production was associated with LTs Mφ only in BALB/cJ and TAC iNOS KO-T/T (LTs Mφ) mice (data not shown). TNF-α production was not observed in any mouse (data not shown). Among strains producing LT-mediated cytokines, CBA/J mice consistently had the lowest response and TAC iNOS KO mice had the highest (Fig. 3). As previously observed (12), all factors were induced by 2.5 h, peaked at 6 to 12 h, and were not measurable by 24 h, with the exception of JE/MCP-1. Because the LT-induced IL-1β increase is not accompanied by mRNA synthesis (12), we suggest that it must occur by lysis-induced release of intracellular stores. Testing for early appearance of factors was done by sampling sera at 30, 60, 90, 120, and 150 min after LT injection. Only IL-1β achieved 40% of its maximal release by 30 min (data not shown). All other factors were at <15% of their maximal levels even 90 min after LT treatment. Rapid increases in factors were seen after 120 min (data not shown).
FIG. 3.
LT-induced cytokine production in inbred mice. Cytokine profiles for BALB/cJ, C57BL/6J, C3H/HeJ, CBA/J, DBA2/J, A/J, and AKR/J mice compared to TAC iNOS KO and JAX iNOS KO mice at intervals after injection of PBS or LT (100 μg) are shown. Results for all time points were collected for all strains, except for the 72- and 96-h time points, which were assessed only for selected strains. ELISA determinations were done with sera pooled from two or three mice for each time point and treatment.
Role of Mφ sensitivity and associated cytokine response in animal susceptibility to LT.
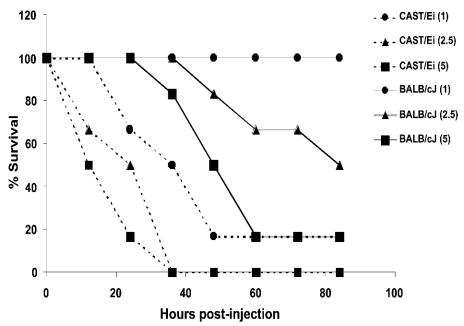
We investigated the correlation between Mφ sensitivity to LT and animal susceptibility to toxin. Age-matched A/J (LTr Mφ), BALB/cJ (LTs Mφ), C3H/HeJ (LTs Mφ), C57BL/6J (LTr Mφ), C57BL/6NTac (LTr Mφ), CBA/J (LTs Mφ), DBA/2J (LTr Mφ), JAX iNOS KO (LTr Mφ), TAC iNOS KO-C/T (LTs Mφ), and TAC iNOS KO-C/C (LTr Mφ) mice were injected i.p. with 100 μg of LT. AKR/J (LTr Mφ) and CAST/Ei (LTs Mφ) mice were injected with 5 μg of LT/g of body weight, as they had weights of above 25 g (AKR/J) or below 15 g (CAST/Ei) when age matched to the other mice. We also tested the mutant B6C3Fe-a/a-Csfmop (Op/Op) mice, which have a spontaneous knockout of the gene for CSF-1 and are considered Mφ deficient due to severe monocytopenia and marked reduction and abnormal differentiation of tissue Mφ (19). Figure 4A shows that knocking out the iNos gene had no significant effect on the susceptibility of C57BL/6 mice when the Kif1C gene was homozygous for the LT resistance allele (P = 0.7166 by log rank test comparing C57BL/6NTac and TAC iNOS KO-C/C, and P = 0.6980 by log rank test comparing C57BL/6J and JAX iNOS KO). However, the TAC iNOS KO mice heterozygous for the sensitivity allele had a susceptibility indistinguishable from that of the prototype sensitive BALB/cJ strain (P = 0.980 by log rank test), indicating that Mφ sensitivity in the C57BL/6J background was sufficient to exacerbate Mφ-independent LT-mediated events so as to confer a BALB/cJ-like mortality (Fig. 4A). Surprisingly, however, Mφ sensitivity and the associated cytokine production did not correlate with LT susceptibility in other strains (Fig. 4B). Although some cytokine-responsive mouse strains harboring LTs Mφ had greater susceptibility to toxin, there were distinct exceptions. C3H/HeJ mice had a significantly higher degree of resistance than most strains harboring LTr Mφ when compared to BALB/cJ mice (P = 0.0001 for C3H/HeJ compared to BALB/cJ; P = 0.0010 for C57BL/6J compared to BALB/cJ) (Fig. 4B). There was no statistical significance in the differences between CBA/J mice (LTs Mφ) and A/J mice (LTr Mφ) (P = 0.4706 by log rank test) or between CBA/J and C57BL/6J mice (P = 0.7250 by log rank test). The only entirely LTr strain was DBA/2J. CAST/Ei mice were uniquely susceptible to the toxin. Comparison of this strain to BALB/cJ showed that the 50% lethal dose (LD50) for CAST/Ei mice was fivefold lower than that for BALB/cJ mice (Fig. 5). The Mφ-deficient Op/Op mice showed a rapid early mortality similar to that of BALB/cJ mice, but a higher number of these mice survived LT treatment (Fig. 4B). Although the two most resistant strains had LTr Mφ and the two most susceptible strains had LTs Mφ, and despite the BALB/cJ-like mortality seen with the TAC-iNOS KO-C/T mice, a correlation between mouse susceptibility and Mφ sensitivity was not evident within the entire set of strains.
FIG. 4.
LT-mediated mortality in inbred mice. Survival of mice injected with 5 μg of LT/g was monitored. Survival percentages are based on the following numbers for each strain: C57BL/6J, n = 60; BALB/cJ, n = 60; TAC iNOS KO-CT (sensitive Mφ), n = 18; TAC iNOS KO-CC (resistant Mφ), n = 15; JAX iNOS KO, n = 35; C57BL/6NTac, n = 18; Op/Op, n = 10; CAST/Ei, n = 26; AKR/J, n = 10; C3H/HeJ, n = 47; CBA/J, n = 30; DBA/2J, n = 37; and A/J, n = 30. The BALB/cJ and C57BL/6J data from panel A are replotted in panel B for comparison to other strains. R and S indicate that the mouse strain has LTr or LTs Mφ, respectively.
FIG. 5.
LT susceptibilities of BALB/cJ and CAST/Ei mice. Three doses of LT were compared in BALB/cJ (n = 6) or CAST/Ei (n = 6) mice. Survival of mice injected with 5, 2.5, or 1 μg of LT/g of body weight was monitored every 12 h postinjection.
Role of Tlr4 in susceptibility to LT.
The most resistant strain harboring LTs Mφ was the C3H/HeJ lipopolysaccharide (LPS)-nonresponsive strain. This strain has a point mutation in Toll-like receptor 4 (Tlr4), the endotoxin receptor. Recent studies show that LPS pretreatment sensitizes C57BL/6J Mφ to LT (6, 13). Although the C3H/HeJ strain already harbors LTs Mφ and it was unlikely that sensitization of its Mφ due to LPS played a role in its increased resistance, we decided to test the potential role of an LPS-nonresponsive state in LT susceptibility. We measured the LT sensitivity of the LPS-responsive control C3H/HeOuJ mice compared to that of C3H/HeJ mice and the sensitivity of two other Tlr4 KO strains and their parental strains. The BALB/cJ congenic C.C3H-Tlr4 mouse, harboring the same point mutation as the C3H/HeJ strain, was compared to its BALB/cJ LPS-responsive control. C57BL/10ScN mice have a deletion of the Tlr4 gene that results in the absence of both mRNA and protein. The LPS-responsive control for these mice is the C57BL/10J strain. There were no statistically significant differences in susceptibility between any of the Tlr4 mutant mice and their LPS-responsive controls (Fig. 6). The contrast between the BALB/cJ-C.C3H-Tlr4 pair and the other four strains was the only statistically significant difference, indicating that Tlr4 function does not play a role in LT susceptibility.
FIG. 6.
LT-mediated mortality in Tlr4 mutant mice. Survival of BALB/cJ, C.C3-Tlr4, C57BL/10J, C57BL/10ScN, C3H/HeOuJ, C3H/HeJ, and C57BL/6J mice injected with 100 μg of LT was monitored every 12 h postinjection. Survival percentages are based on 22 mice for all strains except C.C3-Tlr4, C57BL/10ScN, and C57BL/10J (n = 10). No statistically significant differences were found between any of the Tlr4 mutant mice and their LPS-responsive controls (BALB/cJ versus C.C3-Tlr4, P = 0.7075; C57BL/10J versus C57BL/10ScN, P = 0.9070; C3H/HeOuJ versus C3H/HeJ, P = 0.9911). All P values were calculated by the log rank test.
Role of Mφ sensitization in LT-mediated toxicity.
As noted above, recent reports have indicated that LPS or TNF pretreatment of C57BL/6J Mφ sensitizes them to LT (6, 13). To see whether LT treatment of C57BL/6J mice sensitizes their Mφ, we isolated Mφ from LT-injected C57BL/6J mice every 24 h and measured Mφ sensitivity. C57BL/6J Mφ remained resistant at all times after toxin injection (data not shown). However, while Mφ from C57BL/10ScN mice were similarly resistant to LT treatment, we were surprised to find that the immortalized Tlr4-deficient Mφ line 23 ScCR[10ScNCr/23], derived from the same mouse, is completely sensitive to LT. This Mφ line is known to produce TNF-α, which is sufficient to sensitize resistant C57BL/6J Mφ to LT (6), explaining this cell line's sensitivity.
DISCUSSION
This study exploited the serendipitous discovery that iNOS knockout mice in the C57BL/6J background available from two different sources differed absolutely in the sensitivity of their peritoneal Mφ to lysis by LT. We showed that most TAC iNOS KO mice retained one allele encoding the dominant Mφ sensitivity allele of Kif1C (37.0 cM, chromosome 11) located near the iNOS locus (45.6 cM, chromosome 11). We genotyped and thereby identified iNOS knockout mice that were heterozygous for the Kif1C allele. The two types of mice differing in the Kif1C gene and therefore in Mφ sensitivity to lysis provided a unique opportunity to assess the contribution of Mφ sensitivity to LT function in mice. We had shown previously that C57BL/6J mice, which normally harbor LTr Mφ, are more resistant to LT treatment than BALB/cJ mice, which have LTs Mφ (12). We also showed that rapid induction of a panel of cytokines, including KC, JE/MCP-1, MIP-2, eotaxin, G-CSF, and release of IL-1β in response to LT, occurred only in BALB/cJ mice (12). In this study we tested the hypothesis that the cytokine response seen in BALB/cJ mice required LT-induced Mφ lysis. This was done by comparing the response of the TAC iNOS KO mice having the heterozygous Kif1C alleles (and LTs Mφ) with that of mice having homozygous Kif1C toxin resistance alleles (and LTr Mφ). The cytokine response clearly required Mφ sensitivity. Mφ are not the source of the released cytokines, however, with the exception of IL-1β (12). It seems the lysis of Mφ induces synthesis of the factors by a variety of other cell types and organs (12). Our analysis of seven additional inbred strains with LTs Mφ and LTr Mφ confirmed that the cytokine response required Mφ sensitivity.
Because BALB/cJ mice die more rapidly and in higher numbers than C57BL/6J mice, we investigated the contribution of Mφ lysis and cytokine production to LT susceptibility in the C57BL/6J mouse. The exacerbation of toxic events by Mφ lysis and cytokine release was clear in the C57BL/6J mice harboring LTs Mφ. In the C57BL/6J background, we showed that knockout of iNOS alone had no significant effect on susceptibility to LT in either knockout model when LTr Mφ were present, while the presence of the Kif1C sensitivity allele (and thus Mφ sensitivity to lysis) in the iNOS KO background was sufficient to confer a sensitivity identical to that of BALB/cJ mice. Although this result seems to indicate that a single locus is sufficient to determine animal susceptibility, a recent study comparing recombinant congenic mice with segments of chromosome 11 derived from resistant DBA/2 and sensitive BALB/c mice shows that susceptibility to LT in those mice is linked to two additional loci on chromosome 11, Ltxs2 (35 to 37 cM) and Ltxs3 (45 to 47 cM) (11). The authors postulated that iNOS could potentially be Ltxs3. In our studies, the knockout of iNOS had no effect on mortality in the C57BL/6J background, and a single Kif1C sensitivity allele was sufficient for anthrax susceptibility in iNOS-deficient mice. Sequencing and/or functional analysis of the iNOS locus (or of any other potential Ltxs3 candidate) in C57BL/6J and BALB/cJ mice may, however, show that both strains carry the same Ltxs3 allele as DBA/2J mice, explaining the apparent lack of contribution of iNOS to their relative susceptibilities. Similarly, the Ltxs2 allele is likely to be the same susceptible allele in C57BL/6J and BALB/cJ mice, because the high resistance seen in DBA/2 mice (which is almost absolute, in striking contrast to the moderate resistance of C57BL/6J mice) was shown to require the presence of the resistance alleles at all three loci (11). The presence of resistance alleles at only one of these three loci (Ltxs1/Kif1c) in C57BL/6J would be in agreement with the mortality we observe in DBA/2J, BALB/cJ, and C57BL/6J mice. Complete analysis of the contributions of the Ltxs loci to LT susceptibility will require identification of the genes and their functionally significant polymorphisms.
The relative contributions of each of these identified LT mouse susceptibility loci and of other potential loci may differ widely among different inbred mice. While Mφ resistance clearly explains the higher resistance of the C57BL/6J mouse compared to the BALB/cJ mouse, our comparison of many different inbred strains with sensitive and resistant Mφ showed that certain strains with LTs Mφ and a strong cytokine response are more resistant than strains harboring LTr Mφ (e.g., C3H/HeJ versus C57BL/6J mice). This suggests that Mφ lysis and the cytokine burst can exacerbate lethality in certain mice (e.g., C57BL/6J) but do not play a direct role in lethality caused by LT. Alternatively, other resistance loci in the C3H/HeJ and CBA/J mice may contribute to their higher resistance despite the sensitivity of their Mφ to lysis. It is unlikely that Ltxs2 is such a locus, because resistance alleles at Ltxs2 in combination with sensitivity alleles at the other two LT susceptibility loci result in 80% mortality (11). Ltxs3 or additional loci may represent better candidates for resistance alleles in these mice.
Interestingly, the Op/Op mutant mouse, which have very low numbers of mononuclear cells and are considered Mφ deficient (19), resembled BALB/cJ mice in their initial rate of dying and resembled C57BL/6J mice in their final mortality. These mice also clearly indicate that Mφ sensitivity (to lysis by LT) is not necessarily correlated with the rate of animal death. Op/Op mice are TNF-α deficient and IL-1β response deficient and have increased resistance to endotoxin and septic shock (15), consistent with our previous evidence that TNF-α is not involved in LT toxicity to mice (12). The lack of an IL-1β response in these mice and their rapid BALB/cJ-like death indicate that factors other than cytokine response contribute to their higher susceptibility.
Therefore, although LTs Mφ are not necessary for LT toxicity in mice, they are sufficient to make some strains (C57BL/6J) more susceptible to LT. Other genetic elements, however, contribute at a higher degree in other mice, as comparison of the C3H/HeJ or CBA/J (LTs Mφ) with A/J or C57BL/6J (LTr Mφ) mice makes clear. Whether the three currently identified loci on chromosome 11 will be sufficient to explain the high resistance of C3H/HeJ mice remains to be seen. It will be interesting to see if the C3H/HeJ mouse, which carries a sensitive Ltxs1 allele, has resistant alleles at both Ltxs2 and Ltxs3, allowing it to overcome the consequences of Mφ lysis.
One factor that might be expected to influence toxin susceptibility is responsiveness to LPS. Recent reports indicated that endotoxin or TNF treatment sensitizes LTr C57BL/6J Mφ (6, 13). Although the relatively LTr, LPS-nonresponsive C3H/HeJ strain has LTs Mφ, we considered that LPS responsiveness might possibly affect LT susceptibility in this animal through other loci. We compared LT mortalities in this strain and its LPS-responsive control, C3H/HeOuJ, and found no significant difference. We also tested the LPS-nonresponsive, Tlr4-deficient C57BL10/ScN mouse and its LPS-responsive parent C57BL/10J, both of which have LTr Mφ, as well as the C.C3-Tlr4 knockout mouse and its BALB/cJ LT-susceptible parent, both with LTs Mφ. No statistically significant differences in LT susceptibility were found between Tlr4 knockout mice and their parents, demonstrating that Tlr4 function does not play a role in LT susceptibility. The C.C3-Tlr4 knockout clearly shows that other factors in the BALB/cJ background contribute to markedly greater LT susceptibility independent of Tlr4 and Kif1C function.
Although BALB/cJ mice had been shown to be susceptible to LT (5), very few studies have compared susceptibilities of different inbred strains to LT. One study compared A/J and CBA/J mice, representing LTr Mφ- and LTs Mφ-containing mice, respectively (18). Although LD50s and cumulative mortalities for both strains were similar, CBA/J animals died more rapidly (18). In our studies we find no significant difference between these strains and no more than 60% mortality in either at the LT dose used. LT was administered intravenously (i.v.) in the previous studies and i.p. in our study. Our comparison of i.v. and i.p. routes of LT injection in mice showed that a 50-μg dose killed more rapidly when given i.v. versus i.p., but this difference disappeared at the 100-μg dose (12). Interestingly, the LD50s of toxin reported for A/J and CBA/J mice in the earlier study (11.0 μg of PA and 2.2 μg of LF for A/J mice and 12.4 μg of PA and 2.5 μg of LF for CBA/J mice) are doses at which we see no mortality in these mice or even in the more susceptible BALB/cJ strain (12). A dose of 25 μg of LT (25 μg of PA and 25 μg of LF) does not kill A/J or CBA/J mice when administered by the i.p. route (data not shown). Other recent reports also showed no mortality in BALB/cJ mice administered 50 μg of PA and 10 μg of LF (11) and a very prolonged time for 100% mortality (9 days) in BALB/cJ mice treated with 125 μg PA and 25 μg LF. These apparent differences in LT potency in mice may be due to variations in toxin preparations.
The high susceptibility of CAST/Ei mice to LT is very interesting. This strain is genetically distant from most other classical inbred strains, with the largest variation in single sequence repeats from classical inbred strains, reaching 98% with C57BL/6J mice. It also ranks at the extremes on the scales of 80% of the behavioral and phenotypic characteristics by which numerous inbred strains have been compared (9). A survey of the mouse phenome database (http://aretha.jax.org/pub-cgi/phenome/mpdcgi?rtn=docs/home) also indicates that this strain has a very high rank as an outlier for many measurements. Understanding CAST/Ei hypersensitivity to LT is an interesting future area of study which will no doubt yield additional loci controlling toxin susceptibility. In addition, this mouse may offer a convenient bioassay for testing antitoxin therapies because of the lower lethal toxin dose and more rapid death.
The results presented in this paper establish that Mφ sensitivity to lysis by LT is not required for whole animal sensitivity to toxin and does not necessarily correlate with relative animal susceptibility to LT, but it can certainly exacerbate toxin susceptibility in some inbred strains. Although TNF-α and LPS responsiveness do not play a direct role in LT toxicity, the sensitization of Mφ to LT by these factors could clearly exacerbate toxicity were they to be present, as might occur during an infection. A rapid transient cytokine response is associated with LT-induced Mφ lysis but does not necessarily correlate with higher animal susceptibility to toxin. This could indicate that Mφ play a relatively minor role in LT pathology, less than has been attributed to them, or that other resistance loci usually have a more dominant role, obscuring any contribution of Mφ. Ultimately, understanding the mechanism of LT-induced toxicity will be crucial in providing clues to strain-based differences, which are clearly more complicated than previously recognized.
Editor: J. D. Clements
REFERENCES
- 1.Beall, F. A., and F. G. Dalldorf. 1966. The pathogenesis of the lethal effect of anthrax toxin in the rat. J. Infect. Dis. 116:377-389. [DOI] [PubMed] [Google Scholar]
- 2.Beall, F. A., M. J. Taylor, and C. B. Thorne. 1962. Rapid lethal effect in rats of a third component found upon fractionating the toxin of Bacillus anthracis. J. Bacteriol. 83:1274-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fish, D. C., F. Klein, R. E. Lincoln, J. S. Walker, and J. P. Dobbs. 1968. Pathophysiological changes in the rat associated with anthrax toxin. J. Infect. Dis. 118:114-124. [DOI] [PubMed] [Google Scholar]
- 4.Friedlander, A. M., R. Bhatnagar, S. H. Leppla, L. Johnson, and Y. Singh. 1993. Characterization of macrophage sensitivity and resistance to anthrax lethal toxin. Infect. Immun. 61:245-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanna, P. C., D. Acosta, and R. J. Collier. 1993. On the role of macrophages in anthrax. Proc. Natl. Acad. Sci. USA. 90:10198-10201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim, S. O., Q. Jing, K. Hoebe, B. Beutler, N. S. Duesbery, and J. Han. 2002. Sensitizing anthrax lethal toxin-resistant macrophages to lethal toxin-induced killing by tumor necrosis factor-alpha. J. Biol. Chem. 278:7413-7421. [DOI] [PubMed] [Google Scholar]
- 7.Klein, F., R. Dean, D. R. Hodges, B. G. Mahlandt, W. I. Jones, W B. Haines, and R. Lincoln. 1962. Anthrax toxin: causative agent in the death of rhesus monkeys. Science 138:1331-1334. [DOI] [PubMed] [Google Scholar]
- 8.Klein, F., J. S. Walker, D. F. Fitzpatrick, R. E. Lincoln, B. G. Mahlandt, W. I. Jones, Jr., J. P. Dobbs, and K. J. Hendrix. 1966. Pathophysiology of anthrax. J. Infect. Dis. 116:123-138. [DOI] [PubMed] [Google Scholar]
- 9.Le, R., I, P. L. Roubertoux, L. Jamot, F. Maarouf, S. Tordjman, S. Mortaud, C. Blanchard, B. Martin, P. V. Guillot, and V. Duquenne. 1998. Neuronal and behavioral differences between Mus musculus domesticus (C57BL/6JBy) and Mus musculus castaneus (CAST/Ei). Behav. Brain Res. 95:135-142. [DOI] [PubMed] [Google Scholar]
- 10.Leppla, S. H. 1999. The bifactorial Bacillus anthracis lethal and oedema toxins, p. 243-263. In J. E. Alouf and J. H. Freer (ed.), Comprehensive sourcebook of bacterial protein toxins. Academic Press, London, United Kingdom.
- 11.McAllister, R. D., Y. Singh, W. D. du Bois, M. Potter, T. Boehm, N. D. Meeker, P. D. Fillmore, L. M. Anderson, M. E. Poynter, and C. Teuscher. 2003. Susceptibility to anthrax lethal toxin is controlled by three linked quantitative trait loci. Am. J. Pathol. 163:1735-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moayeri, M., D. Haines, H. A. Young, and S. H. Leppla. 2003. Bacillus anthracis lethal toxin induces TNF-alpha independent hypoxia-mediated toxicity in mice. J. Clin. Investig. 112:670-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park, J. M., F. R. Greten, Z. W. Li, and M. Karin. 2002. Macrophage apoptosis by anthrax lethal factor through p38 MAP kinase inhibition. Science 297:2048-2051. [DOI] [PubMed] [Google Scholar]
- 14.Roberts, J. E., J. W. Watters, J. D. Ballard, and W. F. Dietrich. 1998. Ltx1, a mouse locus that influences the susceptibility of macrophages to cytolysis caused by intoxication with Bacillus anthracis lethal factor, maps to chromosome 11. Mol. Microbiol. 29:581-591. [DOI] [PubMed] [Google Scholar]
- 15.Szperl, M., A. A. Ansari, E. Urbanowska, P. Szwech, P. Kalinski, and W. Wiktor-Jedrzejczak. 1995. Increased resistance of CSF-1-deficient, macrophage-deficient, TNF alpha-deficient, and IL-1 alpha-deficient op/op mice to endotoxin. Ann. N. Y. Acad. Sci. 762:499-501. [PubMed] [Google Scholar]
- 16.Varughese, M., A. Chi, A. V. Teixeira, P. J. Nicholls, J. M. Keith, and S. H. Leppla. 1998. Internalization of a Bacillus anthracis protective antigen-c-Myc fusion protein mediated by cell surface anti-c-Myc antibodies. Mol. Med. 4:87-95. [PMC free article] [PubMed] [Google Scholar]
- 17.Watters, J. W., K. Dewar, J. Lehoczky, V. Boyartchuk, and W. F. Dietrich. 2001. Kif1C, a kinesin-like motor protein, mediates mouse macrophage resistance to anthrax lethal factor. Curr. Biol. 11:1503-1511. [DOI] [PubMed] [Google Scholar]
- 18.Welkos, S. L., T. J. Keener, and P. H. Gibbs. 1986. Differences in susceptibility of inbred mice to Bacillus anthracis. Infect. Immun. 51:795-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiktor-Jedrzejczak, W., A. Bartocci, A. W. Ferrante, Jr., A. Ahmed-Ansari, K. W. Sell, J. W. Pollard, and E. R. Stanley. 1990. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc. Natl. Acad. Sci. USA 87:4828-4832. [DOI] [PMC free article] [PubMed] [Google Scholar]