Abstract
Pediatric hematopoietic stem cell transplantation (HSCT) offers cure for high-risk malignancies and other conditions, but carries a risk of complications. Parental outlook regarding their child’s transplantation course and future health has been largely unexplored. This report presents the Parent Outlook Scale, describes its properties, and examines the outlook of parents embarking on their child’s transplantation course and the associated variables. Parents of children scheduled to undergo HSCT (n = 363) at 8 US transplantation centers completed the Parent Outlook Scale, comprising 4 items assessing frequency of the parent’s thoughts about the potential difficulty of the child’s transplantation (Transplant Diffficult subscale) and worsened health (Health Worse subscale). Item responses were rated on a 5-point Likert scale (ranging from “none” to “all of the time”) and, along with scale/subscale scores, transformed to 100-point scales, with higher scores connoting greater thought frequency. Psychometrics were explored. Multivariable models identified personal and clinical characteristics associated with scale and subscale scores. The Parent Outlook Scale (α = 0.75) and subscales were found to have sound psychometric properties. Factor loading supported the single scale with 2 subscales representing distinct aspects of overall outlook. Mean scores (Parent Outlook, 52.5 ± 21.7; Transplant Difficult, 64.4 ± 25.6; Health Worse, 40.7 ± 25.7) revealed variability within and across scale/subscales. Significantly different mean subscale scores (P < .001) indicated more frequent Transplant Difficult thoughts than Health Worse thoughts. Clinical factors (solid tumor diagnosis and unrelated donor transplant) and a parent factor (worse emotional functioning) were associated with higher scale and subscale scores. Our findings show that the outlook of parents embarking on their child’s HSCT course is varied and not solely a product of clinical factors readily apparent to clinicians. Referring and transplantation clinicians should create opportunities to explore with parents their perspectives and concerns before and during the course of HSCT.
Keywords: Health-related quality of life, Hematopoietic stem cell transplantation, Pediatrics, Supportive care
INTRODUCTION
Hematopoietic stem cell transplantation (HSCT) affords potential cure, often the sole possibility of cure, for children with high-risk malignancies and other life-threatening conditions [1–7]. However, it is intensive therapy posing a risk for serious complications and lasting health sequelae that impair functioning and well-being [8–13]. Parents therefore embark on their child’s transplantation experience holding both hope for their child’s recovery and fear of what the future may bring [14].
Given the risks of HSCT and the serious underlying diagnosis, health outcomes (eg, future health, survival) are difficult to accurately predict for a given child. Parents’ uncertainty and fears are heighted by the unfamiliar and complex nature of HSCT [15]. In this context of high apprehension and unknown outcomes, how parents regard the transplantation course and their child’s potential health outcomes (ie, parent outlook) once they have committed to HSCT is largely unknown.
An understanding of parent outlook provides an important window into a parent’s preparation, perspectives, and concerns regarding HSCT. Knowledge of parent outlook positions clinicians to effectively support and communicate with parents and, in turn, maximize the physical and psychological health of children and parents. On the other hand, assumptions or misperceptions about parent perspectives can impede optimal communication, decision making, and preparation, and in fact contribute to patient/ parent distress [16,17].
Despite its importance, parent outlook has been largely unexplored. In 2 qualitative studies of parents of children undergoing HSCT, parents expressed their fear of the perils of HSCT and described being either incapable or unwilling to think about the situation or their child’s potential outcome [14,18]. Some actively pushed the possibility that their child might die out of their minds, whereas others simply denied this possibility [14]. Additional research building on these 2 relatively small studies is needed to deepen our understanding of parent outlook. It is possible that HSCT parents alternatively have persistent, continuous thoughts (ie, rumination) about the danger that might lie ahead [19], or they may, like other parents of children with cancer, contemplate the future but either not dwell on it or actively focus on positive outcomes or aspects of the situation [15,20–24]. Clearly, parents might think about the upcoming transplantation and their child’s future health in a variety of ways.
The factors shaping the outlook of parents as they embark on their child’s HSCT course are also poorly understood. Studies suggest that parents’ contemplation of HSCT risks and adverse outcomes may be influenced by their sense of culpability for a potentially poor outcome, their lack of control over the situation, and the absence of an alternative treatment offering cure [18,25]. The highly cure-oriented setting of HSCT and the social desirability of positive thinking [26,27] also may promote thoughts focused on positive outcomes.
The need to improve our understanding of HSCT patient/ parent perspectives and their preparation for potential outcomes throughout the course of HSCT is increasingly evident [14,16,28–30]. The Parent Outlook Scale was developed to assess parent outlook. In the present study, we evaluated the properties of this instrument, as well as the association of variables with parent outlook as measured by this scale using data from 2 of the largest health-related quality of life (HRQL) studies conducted in this population to date.
MATERIALS AND METHODS
Participants
Participants (n = 363) were drawn from 2 prospective, multicenter studies evaluating child and parent HRQL over the first year after the child’s HSCT (Figure 1). This analysis focuses on parent-reported outcomes collected just before their child embarked on the course of HSCT. The 2 studies, Journeys to Recovery (JTR) and Hematopoietic Stem Cell Transplant–Comprehensive Health Enhancement Support System (HSCT-CHESS), are described in detail elsewhere [31–36] and briefly summarized here. The 2 studies enrolled child–parent dyads from 8 US transplantation centers and together spanned 2003–2011. Eligible children were aged 5 to 18 years (JTR) or 2 months to 18 years (HSCT-CHESS), were scheduled to undergo HSCT, provided age-appropriate assent, and had an eligible parent who provided consent to participate and informed permission for the child to participate. Parent eligibility criteria included a working knowledge of English (validated study measures were available in English only) and minimum age of 18 years. If more than 1 parent was involved in the child’s care, they were asked to select 1 for participation. The Institutional Review Boards of Tufts Medical Center and all participating transplantation centers approved the studies.
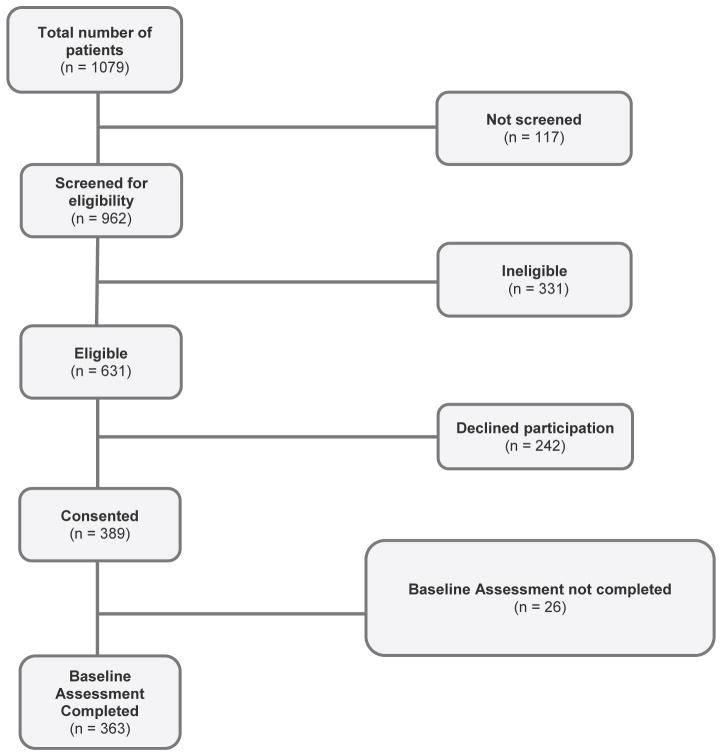
Figure 1.
Flow diagram of study participants. The study sample was derived from 2 studies: Journeys to Recovery, a longitudinal observational study, and HSCT-Comprehensive Health Enhancement Support System, a randomized controlled study that evaluated the efficacy of a web-based intervention providing information and support resources for parents of children post-HSCT.
Measures
The General Health Module of the Child Health Rating Inventories (CHRIs-General) assesses general health and HRQL (physical, emotional, and role functioning, as well as global HRQL) in chronically ill children in the preceding week via child and/or parent-proxy versions that have been validated in the pediatric HSCT population [33,37–39]. The parent version of the CHRIs-General contains a summary item regarding the child’s general health (rated on a 5-point Likert scale ranging from “poor” to “excellent,” converted to a 100-point scale) and items assessing parents’ own HRQL (physical, emotional, and role functioning and global HRQL).
The version administered before HSCT also contains parent outlook items reflecting frequency of the parent’s thoughts about the difficulty of the impending transplantation for the child (“Transplant will be difficult for my child”) and parent (“Transplant will be difficult for me”), worsening of their child’s health (“My child’s future health will be worse than it is now”), and child mortality (“My child might die”). Together the 4 items compose the Parent Outlook Scale, with the 2 transplant difficult and 2 worsened health items forming the Transplant Difficult and Health Worse subscales, respectively.
The primary focus of the present analysis was on parents’ response to the 4 outlook items and resultant Parent Outlook Scale and 2 subscale scores. For each item, parents rated the frequency of their thoughts on a 5-point Likert scale, with response options of “1, none of the time”; “2, a little of the time”; “3, some of the time”; “4, most of the time”; and “5, all of the time.” Both item responses and computed mean scale and subscale scores (range, 1–5) were converted to a 100-point scale (with higher values representing greater frequency of thoughts) to facilitate interpretation of univariate and multivariable analyses, described below.
Data Collection
Parents provided demographic information and completed the CHRIs-General by paper and pencil either before or during the HSCT preparative regimen. Detailed medical information, including the child’s diagnosis, pretransplantation course, transplantation characteristics, and subsequent vital status, was abstracted from the medical record by trained study staff at each site and reviewed by the principal investigator (S.K.P.).
Statistical Analysis
Factor analysis with varimax rotation was used to explore scale properties, with factor loadings for items required to be ≥0.4. We examined scree plots to identify the number of factors with eigenvalues ≥1.00. We forced a 1- and 2-factor solution for the Parent Outlook Scale and the 2 subscales, respectively. Pearson correlations between items were reported for the subscales. Cronbach’s α was calculated to estimate the internal consistency reliability of the Parent Outlook Scale. The minimum acceptable criterion for Cronbach’s α in exploratory scale development is 0.70, whereas for established scales, Cronbach’s α should exceed 0.80 [40].
Descriptive statistics summarized child, parent, and clinical characteristics and parent response frequencies. Associations among child, parent, and clinical variables and the scale and subscale scores were tested with univariate linear regression models. Variables with P ≤ .10 on univariate analysis were considered candidates for inclusion in multivariable linear regression models and then eliminated by backward selection (retention criterion P ≤ .10). Multivariable models controlled for potential confounders, including transplantation center, study/study arm, and timing of baseline assessment relative to the preparative regimen (ie, before versus during the preparative regimen) [41]. Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Study Sample
Data were available from 363 parents who completed the CHRIs-General before transplantation (Figure 1). Table 1 presents characteristics of these parents and their children. The mean CHRIs summary score for parent emotional functioning was 49.6 ± 19.3, indicating significant impairment [42].
Table 1.
Characteristics of Children and Parents
| Characteristic | Value |
|---|---|
| Children (n = 363) | |
| Age, yr, mean ± SD | 9.6 ± 5.1 |
| Female sex, n (%) | 169 (47) |
| Months since diagnosis, median (IQR) | 11 (5–37) |
| Diagnosis, n (%) | |
| Nonmalignant condition | 100 (27) |
| Hematologic malignancy | 206 (57) |
| Solid tumor (peripheral or central nervous system) | 57 (16) |
| Previous treatment unsuccessful, n (%)* | 118 (32) |
| Transplant type, n (%) | |
| Autologous | 83 (23) |
| Allogeneic related donor | 89 (24) |
| Allogeneic unrelated donor | 192 (53) |
| Transplantation center, n (%) | |
| Center A | 4 (1) |
| Center B | 28 (8) |
| Center C | 29 (8) |
| Center D | 39 (11) |
| Center E | 52 (14) |
| Center F | 57 (16) |
| Center G | 62 (17) |
| Center H | 92 (25) |
| Child general health (parent-reported), mean ± SD† | 52.0 ± 28.5 |
| Study/study arm, n (%) | |
| JTR | 165 (45) |
| HSCT-CHESS control arm | 100 (28) |
| HSCT-CHESS intervention arm | 98 (27) |
| Parents (n = 363) | |
| Age, yr, mean ± SD‡ | 38.7 ± 7.5 |
| Female sex, n (%) | 301 (83) |
| Race/ethnicity, n (%)§ | |
| Nonwhite non-Hispanic | 39 (11) |
| Hispanic | 64 (18) |
| White | 252 (71) |
| Postsecondary education, n (%) | 251 (69) |
| Married/living with partner, n (%) | 292 (80) |
| Emotional functioning, mean ± SD|| | 49.6 ± 19.3 |
SD indicates standard deviation; IQR, interquartile range; JTR, Journeys to Recovery study; HSCT-CHESS, Hematopoietic Stem Cell Transplant–Comprehensive Health Enhancement Support System study.
Unsuccessful previous treatment defined as previous disease relapse or unplanned second HSCT.
One parent did not report.
Ten parents did not report.
Eight unknown/missing.
Emotional functioning scale score of the Child Health Rating Inventories General Health Module.
Parent Outlook Scale and Subscales
Table 2 summarizes characteristics of the Parent Outlook Scale, subscales, and individual items. Mean Parent Outlook Scale, Transplant Difficult, and Health Worse values indicated 1 factor with an eigenvalue ≥1.00. For the 1-factor solution, all 4 factor loadings were ≥0.58, indicating that all items contributed substantially to the construct of parent outlook. For the 2-factor solution, loadings were 0.67 and 0.89 for the Transplant Difficult subscale and 0.77 and 0.65 for the Health Worse subscale, supporting the idea that these 2 subscales represent distinct aspects of overall outlook.
Table 2.
Characteristics of the Outlook Scale, Transplant Difficult and Health Worse Subscales, and Individual Outlook Items
| N | N Missing | CC | Mean | SD | Minimum | Maximum | |
|---|---|---|---|---|---|---|---|
| Transplant Difficult subscale* | 363 | 0 | 64.4 | 25.6 | 0 | 100 | |
| Transplant will be difficult for my child. | 362 | 1 | 0.66 | 66.8 | 26.9 | 0 | 100 |
| Transplant will be difficult for me. | 363 | 0 | 0.66 | 70.0 | 29.4 | 0 | 100 |
| Health Worse subscale* | 363 | 0 | 40.7 | 25.7 | 0 | 100 | |
| My child’s future health will be worse than it is now. | 363 | 0 | 0.55 | 39.6 | 29 | 0 | 100 |
| My child might die. | 363 | 0 | 0.55 | 41.9 | 28.2 | 0 | 100 |
| Parent Outlook Scale (a ¼ 0.75)* | 363 | 0 | 52.5 | 21.7 | 0 | 100 |
CC indicates the Pearson correlation coefficient for items within a subscale.
Parents rated the frequency of their thoughts on a 5-point Likert scale, with response options of “1, none of the time”; “2, a little of the time”; “3, some of the time”; “4, most of the time”; and “5, all of the time.” Mean raw scale and subscale scores (range, 1–5) were computed and converted to a 100-point scale (with higher values representing greater frequency of thoughts).
Higher scores reflect greater frequency of parent thoughts about the potential difficulty of the transplantation course or their child’s worsened future health.
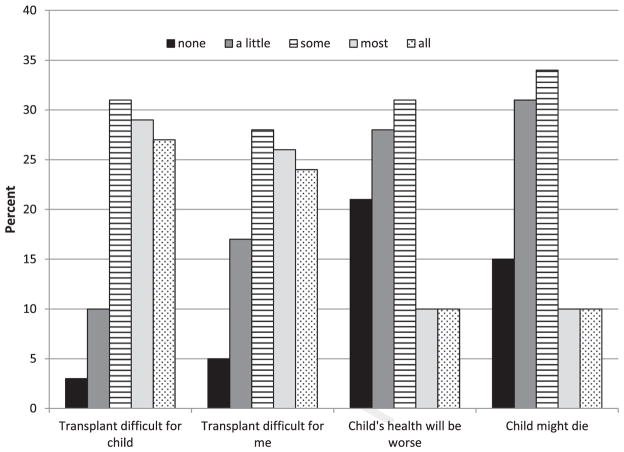
Mean scores revealed variation within and across the Parent Outlook Scale and subscales. Parents had more frequent Transplant Difficult thoughts than Health Worse thoughts, as indicated by significantly higher Transplant Difficult subscale scores than Health Worse subscale scores (P < .001). The plots of percentages of parent responses to individual outlook items shown in Figure 2 also demonstrate that parents’ responses span the full spectrum of response options. Most parents thought frequently about the difficulty of HSCT for their child, with 87% having such thoughts at least “some” or “all” of the time. More than one-half of parents (54%) reported frequent “my child might die” thoughts (“some” or “all” of the time). Interestingly, these parents were no more likely than parents with infrequent thoughts to have a child who died within 12 months (n = 33 [17%] versus n = 30 [18%]; P = .80).
Figure 2.
Distribution of parent responses to outlook items based on frequency of thoughts. For all items, responses ranged from “none of the time” to “all of the time.” At least one-half of all parents reported very frequent (“most” or “all of the time”) thoughts about the anticipated difficulty of transplantation for their child (“Transplant will be difficult for my child”) or themselves (“Transplant will be difficult for me”). Far fewer reported frequent thoughts about adverse child health outcomes (“My child’s future health will be worse than it is now” and “My child might die” items). n = 363 for all except “transplant worse,” with n = 362.
Variables Associated with Parent Outlook Scale and Subscale Scores
In univariate analyses, older parent and child age were associated with lower scores (lower thought frequency), and non-Hispanic white race was associated with higher scores across the Parent Outlook Scale and the 2 subscales (Table 3); however, these variables did not retain significance in the multivariate models. There were no significant differences in scores between mothers and fathers. Mean scores did range widely across the 8 sites (Parent Outlook Scale, 41.7 ± 24.2 to 60.5 ± 22.5; Transplant Difficult, 56.5 ± 27.7 to 70.0 ± 26.1; Health Worse, 25.6 ± 27.6 to 51.0 ± 26.6). Such variation was significant (P > .001 for all).
Table 3.
Univariate and Multivariate Analyses of Parent Outlook Scale and Transplant Difficult and Health Worse Subscale Scores
| Characteristic | Outlook
|
Transplant Difficult
|
Health Worse
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate
|
Multivariate*
|
Univariate
|
Multivariate
|
Univariate
|
Multivariate
|
|||||||
| Beta (SE)† | P | Beta (SE) | P | Beta (SE) | P | Beta (SE) | P | Beta (SE) | P | Beta (SE) | P | |
| Child | ||||||||||||
| Age, yr | 30.7 (0.2) | <.01 | 30.8 (0.3) | <.01 | 30.6 (0.3) | .04 | ||||||
| Female sex | 30.7 (2.3) | .74 | 31.7 (2.7) | .52 | 0.2 (2.7) | .95 | ||||||
| Diagnosis | ||||||||||||
| Solid tumor | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Hematologic malignancy | 37.6 (2.7) | <.001 | 311.0 (4.5) | .01 | 310.1 (3.4) | <.01 | 313.5 (5.7) | .02 | 35.1 (3.5) | .15 | 38.6 (5.4) | .09 |
| Nonmalignancy | 36.5 (3.2) | .04 | 312.5 (4.9) | .01 | 35.8 (3.9) | .14 | 311.2 (6.3) | .08 | 37.2 (4.0) | .07 | 313.7 (5.9) | .02 |
| Months (log) since diagnosis | 1.2 (1.0) | .23 | 1.3 (1.2) | .27 | 1.0 (1.2) | .38 | ||||||
| Previous treatment unsuccessful | 3.3 (2.5) | .18 | 0.49 (2.8) | .86 | 6.0 (2.9) | .04 | ||||||
| HSCT type | ||||||||||||
| Autologous | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Allogeneic related | 37.0 (3.3) | .03 | 4.6 (4.2) | .32 | 36.9 (4.0) | .08 | 5.0 (5.4) | .38 | 36.9 (3.9) | .07 | 4.4 (5.1) | .39 |
| Allogeneic unrelated | 0.1 (2.6) | .96 | 10.8 (4.0) | .01 | 30.7 (3.2) | .83 | 10.6 (5.1) | .04 | 1.0 (3.1) | .75 | 10.9 (4.8) | .02 |
| General health (parent report) | 30.2 (.04) | <.001 | 30.1 (.04) | .03 | 30.2 (.05) | <.001 | 30.1 (.05) | .15 | 30.2 (.04) | <.001 | 30.1 (.04) | .04 |
| Parent | ||||||||||||
| Age, yr | 30.4 (0.2) | .02 | 30.5 (0.2) | .02 | 30.3 (0.19) | .1 | ||||||
| Female sex | 1.4 (2.7) | .61 | 0.3 (3.3) | .92 | 2.4 (3.3) | .45 | ||||||
| White non-Hispanic | 7.9 (2.5) | <.01 | 4.9 (3.1) | .11 | 10.8 (3.0) | <.001 | ||||||
| Postsecondary education | 30.7 (2.5) | .77 | 34.9 (2.9) | .1 | 3.2 (3.0) | .29 | ||||||
| Married/living with partner | 2.2 (3.2) | .49 | 0.6 (3.8) | .87 | 3.6 (3.7) | .33 | ||||||
| Emotional functioning | 30.6 (.06) | <.001 | 30.5 (.05) | <.001 | 30.5 (.07) | <.001 | 30.5 (.07) | <.001 | 30.6 (.06) | <.001 | 30.5 (.06) | <.001 |
HSCT indicates hematopoietic stem cell transplantation; SE, standard error; Ref, reference value. Significant P values are in bold type.
Multivariable models were adjusted for transplantation center, study/study arm, and timing of baseline assessment relative to the start of the preparative regimen.
Higher score (positive estimate) reflects greater frequency of parent thoughts about the potential difficulty of the transplantation course or their child’s worsened future health. The SEs presented are robust SEs.
Multivariable models of the Parent Outlook Scale and subscale scores were largely similar. Unrelated donor allogeneic transplantation and worse parent emotional functioning were significantly associated with higher Parent Outlook Scale and subscale scores. Parents of children with a hematologic malignancy or nonmalignant condition had better Parent Outlook Scale and subscale scores compared with parents of children with a solid tumor. The change in direction of effect (negative to positive) of HSCT type in multivariate models adjusting for diagnosis is likely related to the relationship between solid tumor diagnosis and autologous HSCT; almost all (92%) children with a hematologic malignancy or nonmalignant condition received an allogeneic transplant, whereas all children with a solid tumor received an autologous transplant.
Differences between the multivariate models of the Parent Outlook Scale and subscale scores were observed as well. Whereas the worse parent-reported child general health variable was associated with higher Parent Outlook Scale and Health Worse subscale scores, it had no association with Transplant Difficult subscale score. Another marker of the child’s previous health, unsuccessful previous treatment, also had no association with the Transplant Difficult subscale score.
DISCUSSION
In this paper, we have introduced the Parent Outlook Scale and its subscales and reported scale and subscale scores among parents of children embarking on HSCT and variables associated with these scores. Demonstrated to be psychometrically sound, based on observed factor structure and Cronbach’s α, the Parent Outlook Scale and its subscales permit unique insight into the outlook of parents embarking on their child’s HSCT course with regard to the transplantation and their child’s health outcomes. Variation in frequency of parents’ outlook and thoughts, both within and across Parent Outlook Scale and subscale scores, was notable.
Interestingly, more than one-half of parents reported very infrequent (“a little” or “none of the time”) thoughts that their child’s health might worsen or that their child might die even as the child was about to begin intensive and risky treatment. Because the vast majority of parents acknowledged the potential difficulty of HSCT, incomplete awareness of the rigors and risks of transplantation does not fully explain this finding. Outlook may reflect to some degree a coping strategy for managing fear and lack of control by focusing on immediate challenges over which a parent may feel a greater sense of control as opposed to distant and frightening outcomes, such as deterioration of their child’s health.
Parent outlook was significantly associated with a variety of factors, including clinical factors (eg, diagnosis, type of transplant), parent’s perception of the child’s overall health, and parent’s emotional functioning. These findings show that parent outlook is a complex phenomenon, a product not merely of clinical factors readily apparent to clinicians, but also of parents’ perception of clinical circumstances and previous experience and emotional functioning.
We observed significant variation in Parent Outlook Scale and subscale scores across the 8 study sites. This variation may reflect clinical differences (eg, case mix), or different center-specific practices (eg, communication during HSCT consultation and consent, availability of psychosocial support) [43]. Of note, beyond the observed across-site differences, there is likely within-site variation, based on our observed scale score standard deviations. This may be an interesting topic for future research.
We found a worse outlook in parents of children with a solid tumor compared with parents of children with a hematologic malignancy or nonmalignant condition. This finding was unanticipated, given that most pediatric solid tumors are treated with autologous transplants, which tend to have fewer transplantation-related complications [44]. This discrepancy may be a result of parents facing autologous HSCT thinking beyond short-term transplantation-related outcomes. Even after recovering from the acute effects of transplantation, children with solid tumors remain at very high risk for relapse and, ultimately, mortality. Viewing HSCT through the lens of parents of children with a solid tumor, one can see how they could have very frequent thoughts about the difficult road ahead and adverse child outcomes, even if the procedure itself carries less risk.
Parents of children undergoing unrelated donor allogeneic HSCT also had more frequent thoughts about the difficulty of HSCT and worsened child health. This likely reflects the nature of this type of transplant, which carries a higher likelihood of complications. In general, parents who perceived their child to have better health had lower Parent Outlook Scale and Health Worse subscale scores (ie, fewer thoughts). Interestingly, however, they did not have lower Transplant Difficult subscale scores. The same is true for previous experience of unsuccessful treatment. This may again speak to the widespread view that HSCT will be difficult for their child (and themselves), irrespective of their child’s health status or previous treatment experience.
We found that impaired parent emotional functioning was strongly associated with worse parent outlook, consistent with previous observations [16,19,45–52]. Such emotional stress and poor adjustment has negative consequences for the parent [48,53] and in turn, the child [54–57]. Ongoing psychosocial support to bolster parent emotional functioning, coping, and adjustment to the stressors of HSCT is an essential component of comprehensive transplantation care. Ideally, these supportive interventions are initiated in advance of HSCT, and continue through it and beyond. An important implication of this is that the referring clinicians caring for these children before and after HSCT also must be prepared to take on these issues, as well as personal and system-level barriers to timely care [36].
These findings remind us of the potential pitfall of presuming to know a parent’s outlook, because parents may be thinking about adverse outcomes even when clinical indicators are favorable. Clinicians focusing on the medical complications of transplantation risk may mistakenly believe they share the parents’ outlook, resulting in misunderstanding and empathic failure. To avoid this, clinicians can and should create opportunities to explore with parents their concerns throughout the HSCT trajectory, irrespective of how the clinical course unfolds. High-quality physician communication before HSCT reduces parent psychological distress [16] and builds trust and a therapeutic alliance with parents during HSCT [58]. Parents and patients find that opportunities to communicate with clinicians about the transplantation course help them cope with transplantation [14,47,59,60]. Such efforts may greatly benefit parents and in turn, their children.
This study has several strengths, including self-reported data combined with concomitant detailed clinical data from medical records review. Many patient-reported outcome studies in pediatric oncology/HSCT, including the few that have examined the parent experience, were constrained by single center design or limited sample diversity (eg, constrained to one diagnostic group), limiting their generalizability. The multicenter design and the relatively diverse sample (30% Hispanic or nonwhite), particularly in light of the study population [44,61,62], are other strengths of this study. Finally, this study builds on previous studies that have described parent fear, stress, or impaired psychological functioning during or after HSCT [16,19,45–52], and illustrates specific parental thoughts and thought patterns before their child’s transplantation, deepening our understanding of their lived experience.
We also acknowledge this study’s limitations. First, the fact that the majority of respondents were female might be considered a limitation. Although the proportion of fathers is small (17%), the actual number of fathers (n = 62) surveyed is considerable and at least commensurate with other published studies in this population [47]. In addition, we found no significant differences between mothers’ and fathers’ Parent Outlook Scale and subscale scores. Our overall sample size is robust and likely sufficient to permit detection of a significant difference if it existed. That being said, differences between mothers and fathers with regard to psychological distress and coping before HSCT have been described, and this issue warrants further study [16,49]. Second, the study sample was drawn in part from an intervention study, although the findings presented are from data collected occurred before randomization. Nevertheless, we controlled for study arm in the analyses and found no effect. For these reasons, the intervention is unlikely to influence the results. Given the cross-sectional nature of this analysis, the directionality of association between parent emotional functioning and parent outlook is also unknown. Our findings presented here are limited to the pre-HSCT period. How parent outlook evolves over the course of transplantation and its relationship to personally and clinically meaningful endpoints during this course must be further delineated. Such analyses are underway.
Multiple questions about parent outlook remain. For example, are parents thinking about specific transplantation risks or complications, and if so, over what time frame? What, if any, frequency of parent thoughts about the transplantation course or child outcomes is most adaptive? Finally, to what degree does parent outlook reflect a parent’s prognostic estimate, if it does at all? The Parent Outlook Scale and its subscales hold promise from a psychometric standpoint, and may be useful in future studies addressing these questions.
Implications for Care
This study sheds light on the “inner world” of parents embarking on HSCT for their child, focusing on their thoughts about the difficulty of transplantation or their child’s health worsening despite (or because of) HSCT. It highlights the important roles of both transplantation clinicians and primary oncologists (or other specialty providers) in supporting families before, during, and after HSCT. Through conversations with their primary oncologist or other specialty provider, many patients and families have considered HSCT and even decided to pursue it before the consultation/consent meeting with the HSCT team [63,64]. Primary oncologists/specialists may well have an awareness of the parent’s baseline emotional functioning, and should share such insight with the transplantation team by making them aware of particular family perspectives, worries, and hopes, as well as family strengths and vulnerabilities, to ensure that adequate support is available.
Understanding parent outlook is a cornerstone of clear and compassionate communication throughout the course of HSCT, allowing mutual understanding of hopes, fears, expectations, and goals of care. Further efforts to understand and support dialogue about parent outlook can deepen clinician and parent understanding and are vital to ensuring a clear and shared purpose and optimal support for families throughout the transplantation course.
Acknowledgments
The authors gratefully acknowledge the invaluable support of Susan Stewart, Executive Director of BMT InfoNet, who served as one of the key partners in the development of the HSCT-CHESS intervention. They also thank Doris Hernandez for her assistance in preparing this manuscript.
Footnotes
These data were presented in part in poster form at the International Society for Quality of Life Research Conference, Miami, Florida, October 2013 and the American Society of Clinical Oncology Palliative Care in Oncology Symposium, Boston, MA, October 2014, and also as an oral presentation at the Annual Assembly of the American Academy of Hospice and Palliative Medicine, Philadelphia, PA, February 2015.
Conflict of interest statement: ■ ■ ■
Financial disclosure: Supported by Grant 5R01 CA119196 (S.K.P., PI), American Cancer Society Grant 5RSGPB-02-186 (S.K.P., PI), and Grant 1K23HL107452-01 (C.K.U., PI).
References
- 1.Majhail NS, Chitphakdithai P, Logan B, et al. Significant improvement in survival after unrelated donor hematopoietic cell transplantation in the recent era. Biol Blood Marrow Transplant. 2015;21:142–150. doi: 10.1016/j.bbmt.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacMillan ML, Davies SM, Nelson GO, et al. Twenty years of unrelated donor bone marrow transplantation for pediatric acute leukemia facilitated by the National Marrow Donor Program. Biol Blood Marrow Transplant. 2008;14(9 Suppl):16–22. doi: 10.1016/j.bbmt.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor–recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 4.Eckert C, Henze G, Seeger K, et al. Use of allogeneic hematopoietic stem cell transplantation based on minimal residual disease response improves outcomes for children with relapsed acute lymphoblastic leukemia in the intermediate-risk group. J Clin Oncol. 2013;31:2736–2742. doi: 10.1200/JCO.2012.48.5680. [DOI] [PubMed] [Google Scholar]
- 5.Eapen M, Rubinstein P, Zhang MJ, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: A comparison study. Lancet. 2007;369:1947–1954. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 6.Pai SY, Logan BR, Griffith LM, et al. Transplantation outcomes for severe combined immunodeficiency, 2000–2009. N Engl J Med. 2014;371:434–446. doi: 10.1056/NEJMoa1401177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dedeken L, Lê PQ, Azzi N, et al. Haematopoietic stem cell transplantation for severe sickle cell disease in childhood: A single centre experience of 50 patients. Br J Haematol. 2014;165:402–408. doi: 10.1111/bjh.12737. [DOI] [PubMed] [Google Scholar]
- 8.Parsons SK, Phipps S, Sung L, et al. NCI, NHLBI/PBMTC first international conference on late effects after pediatric hematopoietic cell transplantation: Health-related quality of life, functional, and neuro-cognitive outcomes. Biol Blood Marrow Transplant. 2012;18:162–171. doi: 10.1016/j.bbmt.2011.12.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felder-Puig R, di Gallo A, Waldenmair M, et al. Health-related quality of life of pediatric patients receiving allogeneic stem cell or bone marrow transplantation: Results of a longitudinal, multi-center study. Bone Marrow Transplant. 2006;38:119–126. doi: 10.1038/sj.bmt.1705417. [DOI] [PubMed] [Google Scholar]
- 10.Rusiewicz A, DuHamel KN, Burkhalter J, et al. Psychological distress in long-term survivors of hematopoietic stem cell transplantation. Psychooncology. 2008;17:329–337. doi: 10.1002/pon.1221. [DOI] [PubMed] [Google Scholar]
- 11.Vrijmoet-Wiersma CM, Kolk AM, Grootenhuis MA, et al. Child and parental adaptation to pediatric stem cell transplantation. Support Care Cancer. 2009;17:707–714. doi: 10.1007/s00520-008-0544-8. [DOI] [PubMed] [Google Scholar]
- 12.Barrera M, Atenafu E, Hancock K. Longitudinal health-related quality of life outcomes and related factors after pediatric SCT. Bone Marrow Transplant. 2009;44:249–256. doi: 10.1038/bmt.2009.24. [DOI] [PubMed] [Google Scholar]
- 13.Pallua S, Giesinger J, Oberguggenberger A, et al. Impact of GvHD on quality of life in long-term survivors of haematopoietic transplantation. Bone Marrow Transplant. 2010;45:1534–1539. doi: 10.1038/bmt.2010.5. [DOI] [PubMed] [Google Scholar]
- 14.Forinder U. Bone marrow transplantation from a parental perspective. J Child Health Care. 2004;8:134–148. doi: 10.1177/1367493504041872. [DOI] [PubMed] [Google Scholar]
- 15.De Graves S, Aranda S. Living with hope and fear: The uncertainty of childhood cancer after relapse. Cancer Nurs. 2008;31:292–301. doi: 10.1097/01.NCC.0000305745.41582.73. [DOI] [PubMed] [Google Scholar]
- 16.Dermatis H, Lesko LM. Psychological distress in parents consenting to child’s bone marrow transplantation. Bone Marrow Transplant. 1990;6:411–417. [PubMed] [Google Scholar]
- 17.Valdimarsdóttir U, Kreicbergs U, Hauksdóttir A, et al. Parents’ intellectual and emotional awareness of their child’s impending death to cancer: A population-based long-term follow-up study. Lancet Oncol. 2007;8:706–714. doi: 10.1016/S1470-2045(07)70209-7. [DOI] [PubMed] [Google Scholar]
- 18.Oppenheim D, Valteau-Couanet D, Vasselon S, Hartmann O. How do parents perceive high-dose chemotherapy and autologous stem cell transplantation for their children. Bone Marrow Transplant. 2002;30:35–39. doi: 10.1038/sj.bmt.1703587. [DOI] [PubMed] [Google Scholar]
- 19.Duran B. Developing a scale to measure parental worry and their attitudes toward childhood cancer after successful completion of treatment: A pilot study. J Pediatr Oncol Nurs. 2011;28:154–168. doi: 10.1177/1043454210397755. [DOI] [PubMed] [Google Scholar]
- 20.Bally JM, Duggleby W, Holtslander L, et al. Keeping hope possible: A grounded theory study of the hope experience of parental caregivers who have children in treatment for cancer. Cancer Nurs. 2014;37:363–372. doi: 10.1097/NCC.0b013e3182a453aa. [DOI] [PubMed] [Google Scholar]
- 21.Rini C, Manne S, DuHamel KN, et al. Mothers’ perceptions of benefit following pediatric stem cell transplantation: A longitudinal investigation of the roles of optimism, medical risk, and sociodemographic resources. Ann Behav Med. 2004;28:132–141. doi: 10.1207/s15324796abm2802_9. [DOI] [PubMed] [Google Scholar]
- 22.Barrera M, Granek L, Shaheed J, et al. The tenacity and tenuousness of hope: Parental experiences of hope when their child has a poor cancer prognosis. Cancer Nurs. 2013;36:408–416. doi: 10.1097/NCC.0b013e318291ba7d. [DOI] [PubMed] [Google Scholar]
- 23.Granek L, Barrera M, Shaheed J, et al. Trajectory of parental hope when a child has difficult-to-treat cancer: A prospective qualitative study. Psychooncology. 2013;22:2436–2444. doi: 10.1002/pon.3305. [DOI] [PubMed] [Google Scholar]
- 24.Miedema B, Hamilton R, Fortin P, et al. “You can only take so much, and it took everything out of me”: Coping strategies used by parents of children with cancer. Palliat Support Care. 2010;8:197–206. doi: 10.1017/S1478951510000015. [DOI] [PubMed] [Google Scholar]
- 25.Lesko LM, Dermatis H, Penman D, Holland JC. Patients’, parents’, and oncologists’ perceptions of informed consent for bone marrow transplantation. Med Pediatr Oncol. 1989;17:181–187. doi: 10.1002/mpo.2950170303. [DOI] [PubMed] [Google Scholar]
- 26.Grulke N, Bailer H. Facing haematopoietic stem-cell transplantation: Do patients and their physicians agree regarding the prognosis? Psychooncology. 2010;19:1035–1043. doi: 10.1002/pon.1671. [DOI] [PubMed] [Google Scholar]
- 27.McGrath C, Montgomery K, White K, Kerridge IH. A narrative account of the impact of positive thinking on discussions about death and dying. Support Care Cancer. 2006;14:1246–1251. doi: 10.1007/s00520-006-0083-0. [DOI] [PubMed] [Google Scholar]
- 28.Ullrich CK, Dussel V, Hilden JM, et al. End-of-life experience of children undergoing stem cell transplantation for malignancy: Parent and provider perspectives and patterns of care. Blood. 2010;115:3879–3885. doi: 10.1182/blood-2009-10-250225. [DOI] [PubMed] [Google Scholar]
- 29.Joffe S, Mello MM, Cook EF, Lee SJ. Advance care planning in patients undergoing hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2007;13:65–73. doi: 10.1016/j.bbmt.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 30.Schulmeister L, Quiett K, Mayer K. Quality of life, quality of care, and patient satisfaction: Perceptions of patients undergoing outpatient autologous stem cell transplantation. Oncol Nurs Forum. 2005;32:57–67. doi: 10.1188/05.ONF.57-67. [DOI] [PubMed] [Google Scholar]
- 31.Chang G, Ratichek SJ, Recklitis C, et al. Children’s psychological distress during pediatric HSCT: Parent and child perspectives. Pediatr Blood Cancer. 2012;58:289–296. doi: 10.1002/pbc.23185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pennarola BW, Rodday AM, Mayer DK, et al. Factors associated with parental activation in pediatric hematopoietic stem cell transplant. Med Care Res Rev. 2012;69:194–214. doi: 10.1177/1077558711431460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodday AM, Terrin N, Parsons SK. Measuring global health-related quality of life in children undergoing hematopoietic stem cell transplant: A longitudinal study. Health Qual Life Outcomes. 2013;11:26. doi: 10.1186/1477-7525-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terrin N, Rodday AM, Tighiouart H, et al. Parental emotional functioning declines with occurrence of clinical complications in pediatric hematopoietic stem cell transplant. Support Care Cancer. 2013;21:687–695. doi: 10.1007/s00520-012-1566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mayer DK, Ratichek SJ, Berhe H, et al. Development of a health-related website for parents of children receiving hematopoietic stem cell transplant: HSCT-CHESS. J Cancer Surviv. 2010;4:67–73. doi: 10.1007/s11764-009-0108-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mayer DK, Tighiouart H, Terrin N, et al. A brief report of caregiver needs and resource utilization during pediatric hematopoietic stem cell transplantation. J Pediatr Oncol Nurs. 2009;26:223–229. doi: 10.1177/1043454209340409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parsons SK, Barlow SE, Levy SL, et al. Health-related quality of life in pediatric bone marrow transplant survivors: According to whom? Int J Cancer Suppl. 1999;12:46–51. doi: 10.1002/(sici)1097-0215(1999)83:12+<46::aid-ijc9>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 38.Parsons SK, Shih MC, Mayer DK, et al. Preliminary psychometric evaluation of the Child Health Ratings Inventories (CHRIs) and Disease-Specific Impairment Inventory-Hematopoietic Stem Cell Transplantation (DSII-HSCT) in parents and children. Qual Life Res. 2005;14:1613–1625. doi: 10.1007/s11136-005-1004-2. [DOI] [PubMed] [Google Scholar]
- 39.Parsons SK, Shih MC, Duhamel KN, et al. Maternal perspectives on children’s health-related quality of life during the first year after pediatric hematopoietic stem cell transplant. J Pediatr Psychol. 2006;31:1100–1115. doi: 10.1093/jpepsy/jsj078. [DOI] [PubMed] [Google Scholar]
- 40.Nunnally JC, Bernstein IH. Psychometric theory. 3. New York: McGraw-Hill; 1994. [Google Scholar]
- 41.Parsons SK, Shih MC, Ratichek S, et al. The Journeys to Recovery study: Establishing the baseline in longitudinal evaluation of health-related quality of life (HRQL): The pediatric hematopoietic stem cell transplantation (HSCT) example. Presentation at the Patient-Reported Outcomes Assessment in Cancer Trials; National Cancer Institute; 2006. [Google Scholar]
- 42.Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 health survey manual and interpretation guide. Boston: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 43.Lee SJ, Astigarraga CC, Eapen M, et al. Variation in supportive care practices in hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14:1231–1238. doi: 10.1016/j.bbmt.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.National Marrow Donor program, a contractor for the C.W. Bill Young Cell Transplantation Program operated through the US Department of Health and Human Services, Health Resources and Services Administration, Healthcare Systems Bureau.
- 45.Virtue SM, Manne SL, Mee L, et al. Psychological distress and psychiatric diagnoses among primary caregivers of children undergoing hematopoietic stem cell transplant: An examination of prevalence, correlates, and racial/ethnic differences. Gen Hosp Psychiatry. 2014;36:620–626. doi: 10.1016/j.genhosppsych.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lindahl NA, Mellgren K, Winiarski J, Forinder U. Relationship between problems related to child late effects and parent burnout after pediatric hematopoietic stem cell transplantation. Pediatr Transplant. 2014;18:302–309. doi: 10.1111/petr.12228. [DOI] [PubMed] [Google Scholar]
- 47.Rodrigue JR, MacNaughton K, Hoffmann RG, III, et al. Transplantation in children: A longitudinal assessment of mothers’ stress, coping, and perceptions of family functioning. Psychosomatics. 1997;38:478–486. doi: 10.1016/S0033-3182(97)71425-7. [DOI] [PubMed] [Google Scholar]
- 48.Manne S, DuHamel K, Ostroff J, et al. Anxiety, depressive, and post-traumatic stress disorders among mothers of pediatric survivors of hematopoietic stem cell transplantation. Pediatrics. 2004;113:1700–1708. doi: 10.1542/peds.113.6.1700. [DOI] [PubMed] [Google Scholar]
- 49.Barrera M, Atenafu E, Doyle J, et al. Differences in mothers’ and fathers’ health-related quality of life after pediatric SCT: A longitudinal study. Bone Marrow Transplant. 2012;47:855–859. doi: 10.1038/bmt.2011.190. [DOI] [PubMed] [Google Scholar]
- 50.Manne S, Duhamel K, Ostroff J, et al. Coping and the course of mother’s depressive symptoms during and after pediatric bone marrow transplantation. J Am Acad Child Adolesc Psychiatry. 2003;42:1055–1068. doi: 10.1097/01.CHI.0000070248.24125.C0. [DOI] [PubMed] [Google Scholar]
- 51.Nelson AE, Gleaves L, Nuss S. Mothers’ responses during the child’s stem cell transplantation: Pilot study. Pediatr Nurs. 2003;29:219–223. [PubMed] [Google Scholar]
- 52.Phipps S, Dunavant M, Lensing S, Rai SN. Patterns of distress in parents of children undergoing stem cell transplantation. Pediatr Blood Cancer. 2004;43:267–274. doi: 10.1002/pbc.20101. [DOI] [PubMed] [Google Scholar]
- 53.Kazak AE, Barakat LP. Brief report: Parenting stress and quality of life during treatment for childhood leukemia predicts child and parent adjustment after treatment ends. J Pediatr Psychol. 1997;22:749–758. doi: 10.1093/jpepsy/22.5.749. [DOI] [PubMed] [Google Scholar]
- 54.Kronenberger WG, Carter BD, Stewart J, Morrow C. Psychological adjustment of children in the pretransplant phase of bone marrow transplantation: Relationships with parent distress, parent stress, and child coping. J Clin Psychol Med Set. 1996;3:319–335. doi: 10.1007/BF01994017. [DOI] [PubMed] [Google Scholar]
- 55.Colletti CJ, Wolfe-Christensen C, Carpentier MY, et al. The relationship of parental overprotection, perceived vulnerability, and parenting stress to behavioral, emotional, and social adjustment in children with cancer. Pediatr Blood Cancer. 2008;51:269–274. doi: 10.1002/pbc.21577. [DOI] [PubMed] [Google Scholar]
- 56.Hile S, Erickson SJ, Agee B, Annett RD. Parental stress predicts functional outcome in pediatric cancer survivors. Psychooncology. 2014;23:1157–1164. doi: 10.1002/pon.3543. [DOI] [PubMed] [Google Scholar]
- 57.Jobe-Shields L, Alderfer MA, Barrera M, et al. Parental depression and family environment predict distress in children prior to stem-cell transplantation. J Dev Behav Pediatr. 2009;30:140–146. doi: 10.1097/DBP.0b013e3181976a59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Benedict JM, Simpson C, Fernandez CV. Validity and consequence of informed consent in pediatric bone marrow transplantation: The parental experience. Pediatr Blood Cancer. 2007;49:846–851. doi: 10.1002/pbc.21073. [DOI] [PubMed] [Google Scholar]
- 59.Nelson AE, Miles MS, Belyea MJ. Coping and support effects on mothers’ stress responses to their child’s hematopoietic stem cell transplantation. J Pediatr Oncol Nurs. 1997;14:202–212. doi: 10.1177/104345429701400404. [DOI] [PubMed] [Google Scholar]
- 60.Cohen MZ, Ley CD. Bone marrow transplantation: The battle for hope in the face of fear. Oncol Nurs Forum. 2000;27:473–480. [PubMed] [Google Scholar]
- 61.Majhail NS, Nayyar S, Santibañez ME, et al. Racial disparities in hematopoietic cell transplantation in the United States. Bone Marrow Transplant. 2012;47:1385–1390. doi: 10.1038/bmt.2011.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Radeva JI, VanScoyoc E, Smith FO, et al. National estimates of the use of hematopoietic stem-cell transplantation in children with cancer in the United States. Bone Marrow Transplant. 2005;36:397–404. doi: 10.1038/sj.bmt.1705077. [DOI] [PubMed] [Google Scholar]
- 63.D’Souza A, Pasquini M, Spellecy R. Is “informed consent” an “understood consent” in hematopoietic cell transplantation? Bone Marrow Transplant. 2015;50:10–14. doi: 10.1038/bmt.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chauvenet AR, Smith NM. Referral of pediatric oncology patients for marrow transplantation and the process of informed consent. Med Pediatr Oncol. 1988;16:40–44. doi: 10.1002/mpo.2950160110. [DOI] [PubMed] [Google Scholar]