Abstract
High dose cyclophosphamide given after HLA-matched related and unrelated allogeneic bone marrow transplantation (BMT) for patients with hematologic malignancies is effective single agent graft-versus-host disease (GVHD) prophylaxis in adults. Data describing outcomes for pediatric and young adult patients has not been reported. Between the years 2007-2013, 29 pediatric and young adult patients age ≤ 21 years of age treated at our institution for high-risk hematologic malignancies underwent myeloablative HLA-matched related T cell replete BMT. Eleven patients received post-transplant cyclophosphamide (PTCy) as single agent GVHD prophylaxis and were followed prospectively. Eighteen patients received calcineurin inhibitor (CNI)-based standard GVHD prophylaxis and were studied retrospectively as a control group. No acute GVHD developed in patients receiving PTCy, while patients receiving CNI-based GVHD prophylaxis had a cumulative incidence of grade II-IV and grade III-IV acute GVHD of 27% and 5% respectively. No patients receiving PTCy developed chronic GHVD, compared to one in the control group. Two-year overall survival was similar between the two groups (54% PTCy vs 58% CNI-based prophylaxis), as was event-free survival (42% PTCy vs 47% CNI-based). The 5 year cumulative incidence of relapse was 58% for PTCy and 42% for CNI-based GVHD prophylaxis (p=0.45). These results suggest that PTCy is a safe and efficacious method of GVHD prophylaxis following an HLA-matched related BMT in the pediatric and young adult population that affords patients to be off all post-transplant immunosuppression on day +5.
Introduction
Bone marrow transplantation (BMT) is a potentially curative therapy for patients with high risk acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) 1–5. The benefits of this procedure combine the effects of high dose chemo- and/or radiation-therapy in the preparative regimen with a graft-versus-leukemia (GVL) effect. While some separation of GVL and graft-versus-host disease (GVHD) has been achieved in preclinical animal models6–9, for patients undergoing BMT, GVL and GVHD remain linked 3,10,11. Approaches for limiting the morbidity and mortality of GVHD 12,13, while maintaining effective disease control are needed14,15.
Cyclophosphamide has been used in many combinations in BMT for its antitumor and immunosuppressive properties. Utilization of high dose cyclophosphamide in the post-transplant setting has successfully modulated GVHD in preclinical models16–19 as well as in a variety of clinical trials using HLA-matched and haploidentical donors, mostly in the adult population20–28. High dose post-transplant cyclophosphamide (PTCy) targets alloreactive donor T-cells that are highly proliferative early after BMT, thus minimizing the risk of severe GVHD, while still enabling survival of resting memory T cells that can offer protection against infection and a GVL effect 18,29.
Promising clinical trial data using PTCy with or without additional immunosuppressive agents has been demonstrated in the HLA-matched related, unrelated, and haploidentical transplant setting20–28,30. It has been incorporated following myeloablative regimens22,23,27,28 as well as reduced-intensity regimens20,21,24,25, for both malignant and non-malignant disorders24,26,31,32. Prior reports demonstrated the safety and feasibility of PTCy as single agent GVHD prophylaxis after myeloablative HLA-matched T-cell replete BMT in adults22,27,28, with rates of GVHD similar to that of HLA-matched BMT with conventional immunosuppression including a calcineurin inhibitor (CNI) and methotrexate. Specific results for the pediatric and young adult population using PTCy as sole GVHD prophylaxis have not been previously described. Herein, we present our institutional experience using PTCy as single agent GVHD prophylaxis following myeloablative HLA-matched sibling BMT for pediatric and young adult patients, as well as contemporary patients who received historical standard GVHD prophylaxis with methotrexate and a CNI.
Methods
Study Design and Patients
The Institutional Review Board (IRB) of Johns Hopkins University approved retrospective chart analysis of patients ≤ 21 years of age treated at our institution for hematologic malignancies with myeloablative matched related donor BMT using PTCy along with contemporary controls receiving historical standard GVHD prophylaxis. Patients were identified from a departmental database. All participants gave signed informed consent for their treatment. Initial patients (n=8) receiving PTCy were enrolled on a single-institution IRB-approved clinical trial conducted in accordance with the Declaration of Helsinki, open to both adults and pediatric patients (2003-2011; clinicaltrials.gov; no. NCT00134017). Results for adult patients >21 have been previously published22. The primary endpoint of this study was to find the optimal dose of post-grafting immunosuppression with high-dose cyclophosphamide following myeloablative fully HLA-matched related or unrelated BMT for patients with high risk hematologic malignancies. An additional primary objective was to estimate the incidence of acute GVHD and other toxicities using this approach. The phase 1 portion of the study was run according to a Bayesian algorithm, stratified by age (≤ 18 y, > 18 y) to select among predefined regimens of immunosuppression that included PTCy (50 mg/kg/day on Day +3 and +4 or 50 mg/kg on Day +3) only, with options to escalate immune suppression to include MMF and/or tacrolimus or de-escalate to a single dose of PTCy on Day +3. The initial dose level for both adult and pediatric populations, (PTCy 50mg/kg/day on days +3 and +4 only) was the optimal regimen to achieve a rate of grade II-IV aGVHD of approximately 25% plus or minus 20%. Following study closure, subsequent consecutive adult and pediatric patients meeting the same eligibility criteria as required by the clinical trial were treated off-study in the same manner (n=3).
We also retrospectively analyzed pediatric patients with hematologic malignancies transplanted at Johns Hopkins during the same time period using myeloablative conditioning, HLA-matched related donors and historical standard GVHD prophylaxis with post-transplant methotrexate and calcineurin inhibition (CNI-based). These patients were treated on Johns Hopkins University IRB approved collaborative group protocols during the same 2003-2013 time period, including Children's Oncology Group (COG) ASCT0431, AALL0031, AAML03P1 and AAML1031 conducted in accordance with the Declaration of Helsinki or per these protocols as standard of care, following informed consent. Prioritization was given to enroll patients with ALL on TBI-based BMT protocols, based on current pediatric recommendations 33. Patients who did not have clinical trial benefits but met standard BMT eligibility criteria were treated with standard GVHD prophylaxis with methotrexate and a calcineurin inhibitor.
All patients had genotypically HLA-identical first-degree relatives, matched for HLA-A, HLA-B, HLA-Cw, HLA-DRB1, and HLA-DQB1 alleles.
Transplant Regimens
With PTCy, patients received a myeloablative conditioning regimen with intravenous busulfan at age and weight appropriate dosing every 6 hours with pharmacokinetic adjustments for an area under the curve goal of 800-1400 on days -6 through -3, and cyclophosphamide 50 mg/kg/dose per day on days -2 and -1. On day 0, T-cell-replete bone marrow was infused, with a targeted collection of 4 × 108 total nucleated marrow cells (TNC)/kg. GVHD prophylaxis consisted of cyclophosphamide 50 mg/kg/dose daily on days +3 and +4, with the first dose administered 60-72 hours following the completion of marrow infusion.
In order to meet current recommendations to include total body irradiation (TBI) in the conditioning for pediatric patients with ALL2, two patients treated after the study closed received cyclophosphamide 50 mg/kg/dose per day on days -5 and -4, and TBI 1200 cGy provided as 200 cGy fractions twice a day for 3 days on days -3 through -1 with one of the patients also receiving thiotepa 5mg/kg/dose daily on days -6 and -5, per COG ASCT0631.
Patients on CNI-based standard GVHD prophylaxis received myeloablative conditioning with either TBI-based regimens for ALL [cyclophosphamide and TBI (n=7); Cyclophosphamide, TBI and thiotepa (n=3); or cyclophosphamide, TBI, and VP16 (n=1); doses as mentioned above4]; or busulfan-based regimens for AML [busulfan with cyclophosphamide as noted above (n=6); or busulfan and fludarabine (n=1)27]. As noted, GVHD prophylaxis generally included a backbone of a calcineurin inhibitor with additional short-course methotrexate, and some patients (n = 2) also received sirolimus, as outlined in table 1.
Table 1. Patient and disease characteristics.
| PTCy | CNI-Based | p | ||
|---|---|---|---|---|
| N | 11 | 18 | ||
| Median age (Range) | 10 (2.8-19.8) | 11.4 (1.1-20.8) | 0.57 | |
| Male Gender (%) | 5 (45) | 10 (55) | 0.59 | |
| Diagnosis | ALL | 4 | 7 | 0.89 |
| AML | 6 | 5 | ||
| Biphenotypic | 1 | 1 | ||
| NHL | 4 | |||
| Hodgkin | 1 | |||
| Disease status | CR1 | 5 | 8 | 0.52 |
| CR2 | 6 | 6 | ||
| ≥CR3 | 2 | |||
| Not in remission | 2* | |||
| BMT Prep | Bu/Cy | 9 | 6 | |
| Cy/TBI | 1 | 7 | ||
| Cy/TBI/TT | 1 | 3 | ||
| Other | 2** | |||
| GVHD prophylaxis | PTCy | 11 | ||
| CNI + MTX | 16 | |||
| CNI + MTX + Sirolimus | 2 | |||
| MRD status | Overt disease | 0 | 2* | 0.14 |
| Remission, MRD+ | 5 | 2 | ||
| Remission, MRD- | 3 | 13 | ||
| Remission, MRD undetermined | 3 | 1 | ||
| DRI36 | High | 4 | 9 | |
| Intermediate | 5 | 9 | ||
| Low | 2 |
Bulky Hodgkin's disease and primary mediastinal B cell lymphoma
other regimens included fludarabine and busulfan (n=1) and VP16, Cy TBI (n=1).
MRD, minimal residual disease; DRI, disease risk index
Definitions of Disease status and Clinical outcomes
Neutrophil recovery time was defined as the number of days from BMT to the first of 3 consecutive days with an absolute neutrophil count above 0.5 × 109/L. Platelet recovery time was defined as platelet count greater than 20 × 109/L without platelet transfusion in the preceding 7 days. Routine donor chimerism analysis was performed on days 30 and 60 on peripheral blood and bone marrow. Mixed chimerism was defined as >5% and < 95% donor chimerism, and full chimerism as ≥ 95% donor chimerism. Primary graft failure was defined as ≤5% donor chimerism in peripheral blood and/or bone marrow by ∼day 60 without detected bone marrow disease. Secondary graft failure was defined as loss of donor engraftment (<5% donor chimerism) in the absence of progressive malignancy affecting the marrow. Acute GVHD was graded per standard criteria 34, and chronic GVHD was graded per the 2005 National Institutes of Health Working Group Report35 and Seattle standard guidelines. Given the heterogeneity of patients, we also assessed Disease Risk Index (DRI) by the refined criteria, which takes into account disease status, stage, and cytogenetics36.
Progression-free survival (PFS) was the time from BMT to disease relapse, progression, or death from any cause. Overall survival (OS) was the time from HCST to death from any cause. Non-relapse mortality (NRM) was defined as death without disease relapse.
Supportive care
During busulfan administration, patients above the age of 10 years were treated with phenytoin or levetiracetam for seizure prophylaxis. With high dose cyclophosphamide administration (in preparative regimen as well as post-transplant administration) MESNA at a total dose of 80% of the cyclophosphamide dose was administered. Blood products were given according to current standard of care guidelines. All blood products, with exception of the bone marrow graft, were irradiated and leukoreduced. All supportive care measures were administered according to institutional protocols as previously described and included prophylaxis against Pneumocystis jirovecii, fungus, and herpes zoster/simplex infections22. Patients were monitored for CMV reactivation by weekly measurement of CMV pp65 in mononuclear cell smears by immunofluorescent staining or CMV copy number by PCR of serum until day 100. Anti-emetics were used as required. Dexamethasone was not allowed to be used as an anti-emetic after the graft was infused, prior to the administration of PTCy. Patients who were not immunized for varicella but had a history of varicella zoster virus infection were treated with acyclovir prophylaxis for 1 year.
Statistical analysis
The dataset was locked for analysis on February 1st, 2015. Descriptive statistics were used to summarize baseline patient and transplant characteristics. To assess homogeneity between the standard immune suppression group and the PTCy group, the Chi-Square test was applied for nominal variables such as diagnosis, the Kruskal-Wallis test for ordinal data such as remission number and MRD status, and 2-tailed Mann-Whitney non-parametric test for continuous variables. The probabilities of PFS and OS were estimated using the Kaplan-Meier method with 95% confidence intervals (CIs)37. Cumulative incidences of relapse, NRM, and GVHD were estimated by competing-risk analysis using Gray's method38. Relapse and NRM were competing risks for each other. Relapse, death, and graft failure were competing risks for GVHD. Data were analyzed with the R program, version 2.12 (R Core Development Team, Vienna, Austria) and Prism ver 5.01 (Graphpad software, La Jolla CA).
Results
Patient and Graft Characteristics
Between the years 2005-2014 twenty nine pediatric patients in our institution received a HLA-matched related BMT following myeloablative conditioning for acute hematologic malignancies with either PTCy or standard CNI-based GVHD prophylaxis. Patients' baseline characteristics are outlined in table 1. Generally, patients with AML or biphenotypic leukemia were transplanted in first remission (CR1) unless showing favorable cytogenetics such as inv (16), t (8;21) or t(15;17). Patients with ALL were transplanted in their second or third remission (CR2 / CR3). Eleven patients received PTCy as the sole agent for GVHD prophylaxis with the first eight patients being enrolled on our clinical trial (adult data only previously published)22. Three additional patients received PTCy per protocol after the study closed, with patients with ALL receiving a TBI based regimen2. Eighteen patients received standard CNI-based GVHD prophylaxis. Cyclosporine A with short course of methotrexate were used in 14 patients; tacrolimus with methotrexate, was used in 2 patients, and two other patients received a combination of methotrexate, tacrolimus, and sirolimus on ACST04314. All grafts were a T cell-replete fresh bone marrow product, with a median total nucleated cell dose of 4.97 × 108 per kilogram and a median CD34+ of 7.4 × 106 per kilogram. All patients except for two were categorized as high or intermediate by the DRI.
Engraftment, Chimerism, and Lymphocyte Recovery
No patients evaluable for day 60 donor engraftment rejected their graft. The median time to neutrophil engraftment was 20 days for patients receiving PTCy (range, 17-30 days) and 23.5 days for patients receiving CNI-based GVHD prophylaxis (range, 13-33, p=0.31). The median time to platelet engraftment was 22 days in the PTCy group (range, 13-114) and 19.5 days for CNI-based prophylaxis (range, 15-31 days, p=0.16). Donor chimerism at day 30 was above 95% in all but 1 patient who received PTCy. That patient had 77% donor chimerism at day 30, and at day 46 had evidence of relapse. Bone marrow donor chimerism on day 30 was above 95% for all patients who received standard of care GVHD prophylaxis.
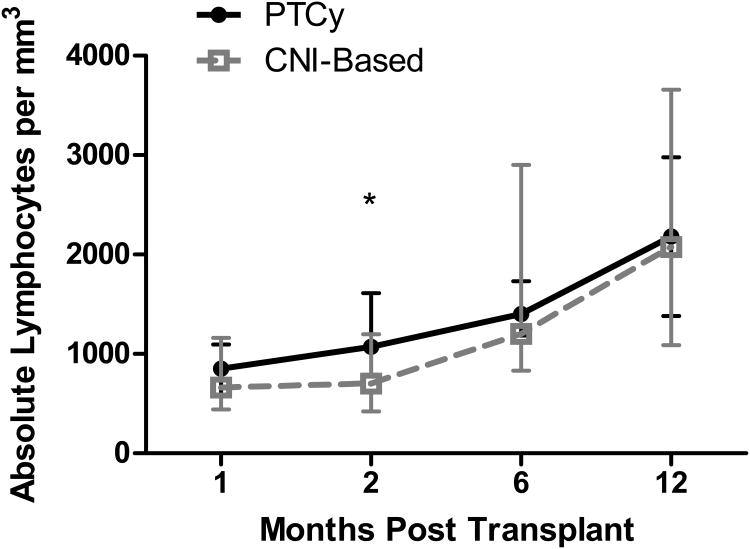
Similar results were obtained on day 60 post BMT (table 2). Recovery of absolute lymphocyte count as a marker of immune reconstitution following PTCy and CNI-based GVHD prophylaxis is depicted in figure 1.
Table 2. Graft properties and engraftment of pediatric patients following myeloablative HLA-matched BMT.
| PTCy | CNI-Based | p | |
|---|---|---|---|
| N | 11 | 18 | |
| Mean graft TNC ×108 (+/- SD) | 5.58 (2) | 4.28 (1.4) | 0.04 |
| Neutrophil engraftment, median (range) | 20 (17-30) | 23.5 (13-33) | 0.31 |
| Platelet engraftment, median (range) | 22 (13-114) | 19.5 (15-31) | 0.16 |
| Day 30 BM chimerism, median | 99.5 | 100 | 0.27 |
| Day 60 BM chimerism, median | 100 | 100 | 0.41 |
TNC, total nucleated cells.
Figure 1.
Immune reconstitution following allogeneic BMT as shown in median values of absolute lymphocyte count at 1, 2, 6 and 12 months. Grey dashed line: CNI-based GVHD prophylaxis. Black line: PTCy. *=p<0.05
Regimen Related toxicities
The incidence of veno-occlusive disease (VOD) in children following HSCT ranges from 11 to 60%, giving a mean incidence in children of approximately 25% compared with an incidence of 13.7% across all age groups, as recently summarized by Coppell et al39,40. VOD as defined by the Baltimore criteria41 was diagnosed in a total of 6 of our patients. Two of the six patients developed severe VOD39,42 following myeloablative BMT with PTCy; both had Bu/Cy preparative regimens and several prior intensive chemotherapy regimens. Both were treated with defibrotide, with resolution of VOD. Four of the patients diagnosed with VOD received standard GVHD prophylaxis with MTX + CNI with 2 classified as moderate and 2 as mild. Three of the four had a Cy/TBI based prep and 1 had Bu/Cy based prep. Of note, 1 of these patients had a prior autologous-BMT and another had prior mediastinal radiation. Two of these four received defibrotide, and all four had resolution of VOD.
No CMV reactivations were seen in the PTCy treated group despite 3 patient-donor pairs at risk (patient and/or donor CMV positive). There were 3 cases (16%/27% at risk) of CMV reactivation in patients receiving CNI-based GVHD prophylaxis, none contributing to mortality.
The incidence of hemorrhagic cystitis in pediatric patients is widely variable, reported anywhere between 1.6-30% after matched related donor BMT43,44. Hemorrhagic cystitis was diagnosed in 3 patients receiving PTCy (27%), all associated with BK virus reactivation and treated with cidofovir. One of these patients had grade IV hemorrhagic cystitis45, ultimately required cystoscopy and cauterization, as well as hyperbaric oxygen, after which he made a complete recovery. The other two patients had grade 1 and 2 cystitis, respectively. Two patients (11%) receiving standard GVHD prophylaxis with MTX +CNI also had BK virus associated hemorrhagic cystitis, grades 1 and 3, respectively.
Idiopathic pneumonia syndrome (IPS), as previously defined46, was noted in 2 patients; both were patients with NHL and had received the standard CNI-based GVHD prophylaxis with CsA/MTX. They were treated with steroids and etanercept47 but developed secondary infectious complications [bacterial (n=1) and viral (n=1)] ultimately leading to their death. Significant regimen related toxicities are summarized in table 3.
Table 3. Regimen-related toxicities following myeloablative HLA-matched BMT for pediatric patients with hematologic malignancies.
| PTCy (n=11) | CNI-Based (n=18) | P | |
|---|---|---|---|
| CMV reactivation | 0 | 3 (17%) | 0.15 |
| Veno-occlusive disease | 2 (18%) | 4 (17%) | 0.79 |
| Hemorrhagic cystitis | 3 (23%) | 2 (9%) | 0.26 |
| Idiopathic pneumonia syndrome | 0 | 3 (13%) | 0.15 |
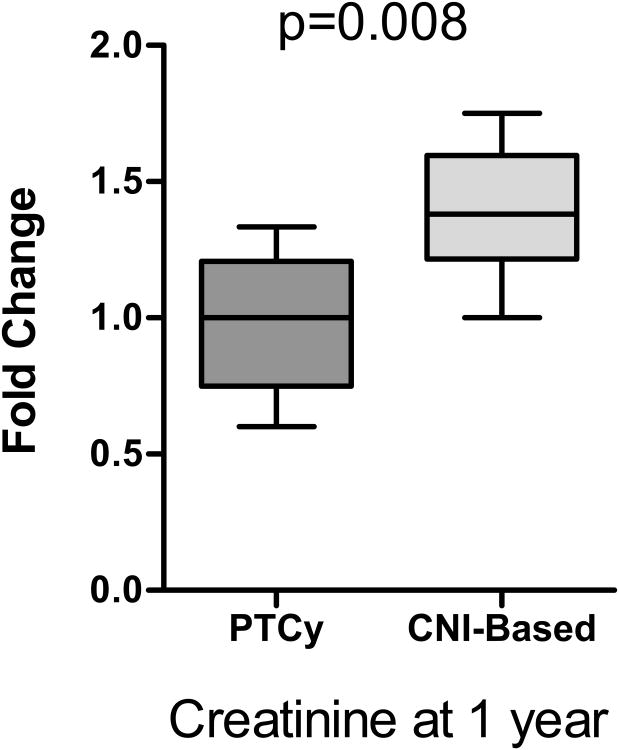
Renal dysfunction has multifactorial causes following allogeneic BMT, including toxicities from CNIs48,49. To assess renal function, we measured serum creatinine at 1 year post transplantation and compared it to baseline pre-BMT creatinine. reatinine at the one year mark remained persistently elevated over baseline in patients receiving CNI-based regimens, while it had largely normalized by one year in the PTCy patients (median fold change 1 vs 1.38, p=0.008, figure 2).
Figure 2.
Assessment of renal dysfunction following BMT was performed by calculating the fold change of the maximum creatinine at 1 year post transplant compared to pre-BMT creatinine.
Non-relapse mortality (NRM)
There were no non-relapse mortalities in patients receiving PTCy while the cumulative incidence for NRM was 11% for patients receiving CNI-based GVHD prophylaxis (figure 3A). The two patients with NRM had IPS and eventually died from pulmonary toxicities (IPS in one and radiation pneumonitis in the other) combined with secondary viral/bacterial infections.
Figure 3.
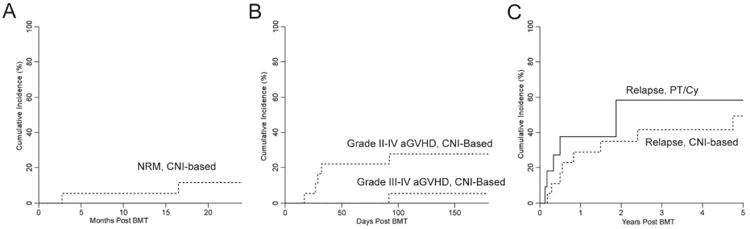
Transplantation outcomes for CNI-based GVHD prophylaxis (dashes) and PTCy (full lines). Cumulative incidence of non-relapse mortality (A), acute GVHD (B) and relapse (C) are shown.
GVHD
In the cohort of patients receiving PTCy, no patients developed acute GVHD. Five patients receiving CNI-based regimens developed grade 2-4 aGVHD, and one patient developed grade 3-4 aGVHD of the gut and liver, leading to cumulative incidences for grades 2-4 and 3-4 of 27% and 6% respectively (figure 3B). No patients receiving PTCy developed chronic GVHD, while only one patient receiving CNI-based GVHD prophylaxis developed cGVHD of the eyes, requiring topical therapy only.
Relapse and Survival
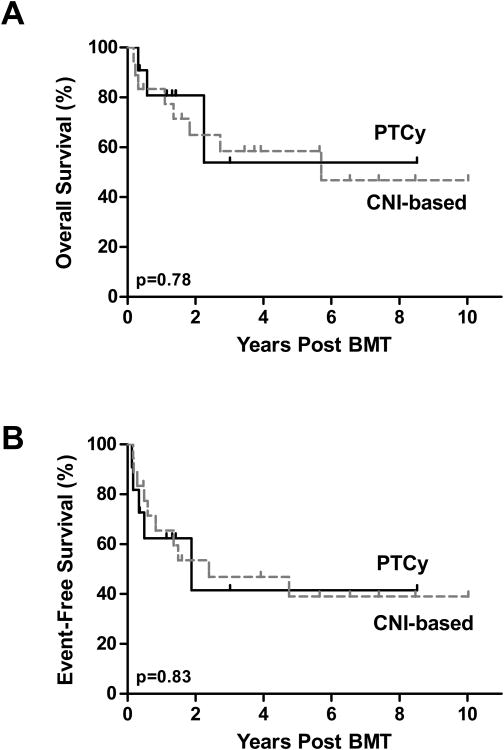
With a median follow-up of 584 days for all patients and 1178 days for survivors, the 5 year cumulative incidence of relapse in patients that received PTCy or CNI-based immunosuppression did not differ (p=0.454, figure 3C). Five of 11 patients receiving PTCy relapsed, with a median time to relapse of 121 days (range, 46-685). One of the five patients received chemotherapy, donor lymphocyte infusion (DLI) andlocal irradiation for testicular relapse of ALL. He is disease free 2 years post-relapse. A second patient with ALL had persistent disease despite one high dose re-induction chemotherapy regimen and enrollment on a Phase I toll like receptor 9 agonist trial before receiving CD19-CAR T cells on a clinical trial50, now with ongoing remission 11 months after CD19-CAR T cells. One patient relapsed with AML two years after BMT, received a second myeloablative BMT after achieving a CR2 but died of NRM. Two patients who had an early relapse of ALL (day 46) and AML (day 60), received palliative care and ultimately died of disease. For the CNI-based GVHD prophylaxis cohort, 8 of 18 patients relapsed, with a median time to relapse of 251 days (range 65-1734 days). DLI alone induced long term remission with chronic GVHD in one of these patients with AML. Six patients received further chemotherapy with 3 failing to achieve a remission and ultimately dying of their disease, and three patients with ALL receiving a second transplant, one who is disease free 3 years post relapse and two who died from transplant-related complications. One patient received palliative care alone and ultimately died of disease. As shown in figure 4, the 2-year event free survival and overall survival were similar among patients receiving either PTCy (42% and 54%, respectively) or CNI-based GVHD prophylaxis (47% and 58%, respectively).
Figure 4.
Kaplan-Meier survival curves of patients undergoing BMT with CNI-based GVHD prophylaxis (dashed lines) or PTCy (Full lines). (A) Probability of overall survival. (B) Probability of event-free survival.
Discussion
Consistent with our previous work in adults this study shows that PTCy is effective and safe as single agent GVHD prophylaxis for HLA-matched sibling BMT in this small pediatric population with advanced hematologic malignancies. We saw no aGVHD in patients receiving PTCy. The incidence of grade 2-4 aGVHD following PTCy as sole GVHD prophylaxis is lower than the published adult experience of 38-42% grade 2-4 aGVHD following myeloablative conditioning with an HLA-matched related allogeneic BMT22,27,28. While older age at time of BMT has been correlated with an increased risk of aGVHD51, the small sample size here needs to be considered. Our contemporaneous control group, receiving historical standard CNI-based GVHD prophylaxis, had a grade 2-4 aGVHD rate of 27% with a grade 3-4 aGVHD of 5%, numbers similar to reported literature in pediatric BMT for ALL and AML3,4,52–54. PTCy has also consistently prevented cGVHD, as seen in this series. We did not observe any significant increase in other regimen related toxicities in the group receiving PTCy, and no NRM.
Although small numbers do not allow conclusive analysis, patients receiving standard immunosuppression trended towards having more NRM, while patients receiving PTCy trended towards higher rates of relapse. It is difficult to make any definitive conclusions regarding disease or risk category and relapse with small numbers. Additionally, some of our patients were transplanted at a time when available molecular tests were limited and/or specific restaging pre-BMT did not standardly include MRD evaluation or molecular reassessment for patients who had a molecular marker that could be followed. Thus some patients who truly had MRD (i.e. by molecular testing) may not have been recognized, and flow cytometric MRD is missing on several subjects. Ideally, a larger study would allow for further analysis by MRD status, high versus low risk cytogenetics, and/or disease risk index. Our entire cohort showed a 2 year OS and EFS of 64% and 46% respectively regardless of the GVHD prophylaxis method. The recently published adult cohort using PTCy in HLA-matched BMT showed a 3 year OS of 58% and event-free survival of 46%, similar to our results28. Results from pediatric published data is also similar for HLA-matched related BMT for leukemia with standard immunosuppressive regimens including a CNI, with progression free survival ranging between 45-70%3,4,53,54.
It is important to note that we are unable to assess the optimal dosing of PTCy in our small pediatric study population. As described, the initial dose level (PTCy 50mg/kg/day on days +3 and +4 only) for adult and pediatric patients, enrolled on our institutional trial was selected to achieve a rate of grade II-IV aGVHD of approximately 25% plus or minus 20%. Further study of Cy pharmacokinetics and pharmacogenomics are needed and will be performed on the Pediatric Blood and Marrow Transplantation (PBMTC) multi-institutional trial of myeloablative haploidentical BMT with PTCy, as well as studying this regimen in larger numbers of younger patients.
Several important benefits of single agent GVHD prophylaxis with post-transplantation cyclophosphamide are noteworthy. Firstly, PTCy is inexpensive, easy to administer, and readily available, making this regimen feasible in less developed countries. Secondly, limiting post-transplant immunosuppression, specifically by eliminating prolonged calcineurin inhibition, permits reconstitution of the immune system in an environment free of ongoing pharmacologic regulation. As previously described55, recovery of absolute lymphocyte numbers following PTCy was earlier and relatively rapid compared to CNI based prophylaxis. Rapid immune reconstitution would be predicted to reduce the risk of transplant related infections. There were no CMV reactivations in patients who received PTCy, and no NRM. Despite high cyclophosphamide doses, only 1 patient receiving PTCy required significant intervention for higher grade hemorrhagic cystitis beyond administration of fluids and cidofovir in the case of BK viruria or viremia. Finally, relapse remains the most significant problem after BMT for hematologic malignancies. Several trials of various immunologic and pharmacologic post-transplant maintenance approaches are being studied at our institution and others. Using PTCy as sole GVHD prophylaxis allows early incorporation of additional chemo- and/or immuno-therapy to further prevent relapse in high-risk patients.
In conclusion, we have shown that PTCy is effective in preventing GVHD following BMT from HLA-matched related donors in a small population of pediatric patients with high-risk hematologic malignancies. Despite having small numbers, the high engraftment rate, low cumulative incidence of acute and chronic GVHD, lack of significant infections and NRM associated with PTCy as sole GVHD prophylaxis are significant findings for this high risk pediatric and young adult population that warrant further study. PTCy provides an ideal platform for the addition of novel approaches to prevent relapse in patients who receive no post-transplant immunosuppressive therapy after Day +4. Given these promising outcomes, studying PTCy as sole immunoprophylaxis for pediatric patients in a larger multi-institutional clinical trial with both chemotherapy and TBI based myeloablative conditioning is warranted to make any further comparisons to more standard CNI-based approaches.
Single agent PTCy provided effective GVHD prophylaxis for pediatric HLA-matched BMT
No differences in OS or EFS between PTCy and CNI-based GVHD prophylaxis
Improved 1 year post-BMT renal function following PTCy
Early cessation of immunosuppression provides a platform for post-BMT immunotherapy
Acknowledgments
This work was supported by National Institutes of Health, National Cancer Institute grants P01 CA015396 and P30 CA006973, and the Giant Food Children's Cancer Research Fund. EJ was also supported by the joint American Physicians Fellowship/Israel Medical Association Fellowship Grant.
Footnotes
Trial registration: clinicaltrials.gov identifier: NCT00134017
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wayne AS, Baird K, Egeler RM. Hematopoietic stem cell transplantation for leukemia. Pediatr Clin North Am. 2010;57(1):1–25. doi: 10.1016/j.pcl.2009.11.005. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed9&NEWS=N&AN=2010231679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oliansky DM, Camitta B, Gaynon P, et al. Role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of pediatric acute lymphoblastic leukemia: update of the 2005 evidence-based review. Biol Blood Marrow Transplant. 2012;18(4):505–522. doi: 10.1016/j.bbmt.2011.12.585. [DOI] [PubMed] [Google Scholar]
- 3.Neudorf S, Sanders J, Kobrinsky N, et al. Allogeneic bone marrow transplantation for children with acute myelocytic leukemia in first remission demonstrates a role for graft versus leukemia in the maintenance of disease-free survival. Blood. 2004;103(10):3655–3661. doi: 10.1182/blood-2003-08-2705. [DOI] [PubMed] [Google Scholar]
- 4.Pulsipher MA, Langholz B, Wall DA, et al. The addition of sirolimus to tacrolimus / methotrexate GVHD prophylaxis in children with ALL : a phase 3 Children's Oncology Group / Pediatric Blood and Marrow Transplant Consortium trial. 2014;123(13):2017–2025. doi: 10.1182/blood-2013-10-534297. Presented. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pulsipher MA, Peters C, Pui CH. High-risk pediatric acute lymphoblastic leukemia: to transplant or not to transplant? Biol Blood Marrow Transplant. 2011;17(1 Suppl):S137–S148. doi: 10.1016/j.bbmt.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teshima T, Hill GR, Pan L, et al. IL-11 separates graft-versus-leukemia effects from graft-versus-host disease after bone marrow transplantation. [Accessed July 8, 2015];J Clin Investig. 1999 104(3):317–325. doi: 10.1172/JCI7111. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC408425/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooke KR, Gerbitz A, Crawford JM, et al. LPS antagonism reduces graft-versus-host disease and preserves graft-versus-leukemia activity after experimental bone marrow transplantation. J Clin Investig. 2001;107(12):1581–1589. doi: 10.1172/JCI12156. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC200193/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddy P, Maeda Y, Hotary K, et al. Histone deacetylase inhibitor suberoylanilide hydroxamic acid reduces acute graft-versus-host disease and preserves graft-versus-leukemia effect. Proc Natl Acad Sci U S A. 2004;101(11):3921–3926. doi: 10.1073/pnas.0400380101. http://www.pnas.org/content/101/11/3921.short. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capitini CM, Herby S, Milliron M, Anver MR, Mackall CL, Fry TJ. Bone marrow deficient in IFN-{gamma} signaling selectively reverses GVHD-associated immunosuppression and enhances a tumor-specific GVT effect. Blood. 2009;113(20):5002–5009. doi: 10.1182/blood-2008-11-187385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiden PL, Flournoy N, Thomas ED, et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med. 1979;300(19):1068–1073. doi: 10.1056/NEJM197905103001902. [DOI] [PubMed] [Google Scholar]
- 11.Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75(3):555–562. http://www.ncbi.nlm.nih.gov/pubmed/2297567. [PubMed] [Google Scholar]
- 12.Lee SE, Cho BS, Kim JH, et al. Risk and prognostic factors for acute GVHD based on NIH consensus criteria. Bone Marrow Transplant. 2013;48(4):587–592. doi: 10.1038/bmt.2012.187. [DOI] [PubMed] [Google Scholar]
- 13.Baird K, Cooke K, Schultz KR. Chronic Graft-Versus-Host Disease (GVHD) in children. Pediatr Clin North Am. 2010;57(1):297–322. doi: 10.1016/j.pcl.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li JM, Giver CR, Lu Y, Hossain MS, Akhtari M, Waller EK. Separating graft-versus-leukemia from graft-versus-host disease in allogeneic hematopoietic stem cell transplantation. Immunotherapy. 2009;1(4):599–621. doi: 10.2217/imt.09.32. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2827928&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller JS, Warren EH, van den Brink MRM, et al. NCI First International Workshop on The Biology, Prevention, and Treatment of Relapse After Allogeneic Hematopoietic Stem Cell Transplantation: Report from the Committee on the Biology Underlying Recurrence of Malignant Disease following Allogeneic HSCT. Biol Blood Marrow Transplant. 2010;16(5):565–586. doi: 10.1016/j.bbmt.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luznik L, Jalla S, Engstrom LW, Iannone R, Fuchs EJ. Durable engraftment of major histocompatibility complex – incompatible cells after nonmyeloablative conditioning with fludarabine, low-dose total body irradiation, and posttransplantation cyclophosphamide. Blood. 2001;98(12):3456–3465. doi: 10.1182/blood.v98.12.3456. [DOI] [PubMed] [Google Scholar]
- 17.Luznik L, Engstrom L, Iannone R, Fuchs EJ. Posttransplantation cyclophosphamide facilitates engraftment of major histocompatibility complex-identical allogeneic marrow in mice conditioned with low-dose total body irradiation. [Accessed June 21, 2015];Biol Blood Marrow Transplant. 2002 8:131–138. doi: 10.1053/bbmt.2002.v8.pm11939602. http://www.sciencedirect.com/science/article/pii/S1083879102500471. [DOI] [PubMed] [Google Scholar]
- 18.Luznik L, O'Donnell PV, Fuchs EJ. Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical bone marrow transplantation. Semin Oncol. 2012;39(6):683–693. doi: 10.1053/j.seminoncol.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ganguly S, Ross D. Donor CD4 Foxp3 regulatory T cells are necessary for post-transplantation cyclophosphamide-mediated protection against GVHD in mice. Blood. 2014;124(13):2131–2142. doi: 10.1182/blood-2013-10-525873. The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Donnell PV, Luznik L, Jones RJ, et al. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2002;8(7):377–386. doi: 10.1053/bbmt.2002.v8.pm12171484. http://www.ncbi.nlm.nih.gov/pubmed/16333862. [DOI] [PubMed] [Google Scholar]
- 21.Luznik L, O'Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14(6):641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luznik L, Bolaños-Meade J, Zahurak M, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115(16):3224–3230. doi: 10.1182/blood-2009-11-251595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Symons HJ, Chen AR, Luznik L, et al. Myeloablative Haploidentical Bone Marrow Transplantation with T Cell Replete Grafts and Post-Transplant Cyclophosphamide: Results of a Phase II Clinical Trial. Blood. 2011;118 [Google Scholar]
- 24.Bolaños-Meade J, Fuchs E, Luznik L, et al. HLA-haploidentical bone marrow transplantation with posttransplant cyclophosphamide expands the donor pool for patients with sickle cell disease. Blood. 2012;120(22):4285–4291. doi: 10.1182/blood-2012-07-438408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brunstein CG, Fuchs EJ, Carter SL, et al. Alternative donor transplantation after reduced intensity conditioning : results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118(2):282–288. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brodsky RA, Luznik L, Bolaños-Meade J, Leffell MS, Jones RJ, Fuchs EJ. Reduced intensity HLA-haploidentical BMT with post transplantation cyclophosphamide in nonmalignant hematologic diseases. Bone Marrow Transplant. 2008;42(8):523–527. doi: 10.1038/bmt.2008.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanakry CG, O'Donnell PV, Furlong T, et al. Multi-institutional study of post-transplantation cyclophosphamide as single-agent graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. J Clin Oncol. 2014;32(31):3497–3505. doi: 10.1200/JCO.2013.54.0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanakry CG, Tsai H, Bolaños-Meade J, et al. Single-agent GVHD prophylaxis with posttransplantation cyclophosphamide after myeloablative, HLA-matched BMT for AML, ALL, and MDS. Blood. 2014;124(25):3817–3828. doi: 10.1182/blood-2014-07-587477. The. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ross D, Jones M, Komanduri K, Levy R. Antigen and lymphopenia-driven donor T cells are differentially diminished by post-transplantation administration of cyclophosphamide after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2013;19(10):1430–1438. doi: 10.1016/j.bbmt.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCurdy S, Kanakry J, Showel M, et al. Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood. 2015;125(19):3024–3032. doi: 10.1182/blood-2015-01-623991. Presented. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thakar MS, Bonfim C, Sandmaier BM, et al. Cyclophosphamide-based in vivo T-cell depletion for HLA-haploidentical transplantation in Fanconi anemia. Pediatr Hematol Oncol. 2012;29(6):568–578. doi: 10.3109/08880018.2012.708708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raiola AM, Dominietto A, Ghiso A, et al. Unmanipulated haploidentical bone marrow transplantation and posttransplantation cyclophosphamide for hematologic malignancies after myeloablative conditioning. Biol Blood Marrow Transplant. 2013;19(1):117–122. doi: 10.1016/j.bbmt.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 33.Davies S, Ramsay N, Klein JP, Weisdorf DJ, Bolwell B, Cahn JY. Comparison of preparative regimens in transplants for children with acute lymphoblastic leukemia. [Accessed July 7, 2015];J Clin Oncol. 2000 18(2):340–347. doi: 10.1200/JCO.2000.18.2.340. http://jco.ascopubs.org/content/18/2/340.short. [DOI] [PubMed] [Google Scholar]
- 34.Przepiokra D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 35.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 36.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123(23):3664–3671. doi: 10.1182/blood-2014-01-552984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–480. [Google Scholar]
- 38.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 39.Coppell Ja, Richardson PG, Soiffer R, et al. Hepatic veno-occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biol Blood Marrow Transplant. 2010;16(2):157–168. doi: 10.1016/j.bbmt.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corbacioglu S, Kernan N, Lehmann L, et al. Defibrotide for the treatment of hepatic veno-occlusive disease in children after hematopoietic stem cell transplantation. Expert Rev Hematol. 2012;5(3):291–302. doi: 10.1586/ehm.12.18. [DOI] [PubMed] [Google Scholar]
- 41.Jones RJ, Lee KS, Beschorner WE, et al. Venooclusive Disease of the Liver Following Bone Marrow Transplantation. Transpl antation. 1987;44(6):778–783. doi: 10.1097/00007890-198712000-00011. [DOI] [PubMed] [Google Scholar]
- 42.Carreras E, Díaz-Beyá M, Rosiñol L, Martínez C, Fernández-Avilés F, Rovira M. The incidence of veno-occlusive disease following allogeneic hematopoietic stem cell transplantation has diminished and the outcome improved over the last decade. Biol Blood Marrow Transplant. 2011;17(11):1713–1720. doi: 10.1016/j.bbmt.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 43.El-zimaity M, Saliba R, Chan K, et al. Hemorrhagic cystitis after allogeneic hematopoietic stem cell transplantation : donor type matters. Blood. 2004;103(12):4674–4680. doi: 10.1182/blood-2003-08-2815. Reprints. [DOI] [PubMed] [Google Scholar]
- 44.Riachy E, Krauel L, Rich B, et al. Risk factors and predictors of severity score and complications of pediatric hemorrhagic cystitis. J Urol. 2014;191(1):186–192. doi: 10.1016/j.juro.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 45.Droller M, Saral R, Santos G. Prevention of cyclophosphamide-induced hemorrhagic cystitis. Urology. 1982;20(3):256–258. doi: 10.1016/0090-4295(82)90633-1. [DOI] [PubMed] [Google Scholar]
- 46.Clark JG, Hansen JA, Hertz MI, Parkman R, Jensen L, Peavy HH. Idiopathic pneumonia syndrome after bone marrow transplantation. Am Rev Respir Dis. 1993;147(6):1601–1606. doi: 10.1164/ajrccm/147.6_Pt_1.1601. [DOI] [PubMed] [Google Scholar]
- 47.Yanik Ga, Grupp Sa, Pulsipher Ma, et al. TNF-receptor inhibitor therapy for the treatment of children with idiopathic pneumonia syndrome. A joint Pediatric Blood and Marrow Transplant Consortium and Children's Oncology Group Study (ASCT0521) Biol Blood Marrow Transplant. 2015;21(1):67–73. doi: 10.1016/j.bbmt.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parikh CR, McSweeney PA, Korular D, et al. Renal dysfunction in allogeneic hematopoietic cell transplantation. Kidney Int. 2002;62:566–573. doi: 10.1046/j.1523-1755.2002.00455.x. [DOI] [PubMed] [Google Scholar]
- 49.Wolff D, Wilhelm S, Hahn J, et al. Replacement of calcineurin inhibitors with daclizumab in patients with transplantation-associated microangiopathy or renal insufficiency associated with graft-versus-host disease. Bone Marrow Transplant. 2006;38(January):445–451. doi: 10.1038/sj.bmt.1705454. [DOI] [PubMed] [Google Scholar]
- 50.Maude SL, Frey N, Shaw Pa, et al. Chimeric Antigen Receptor T Cells for Sustained Remissions in Leukemia. N Engl J Med. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jacobsohn DA. Acute graft-versus-host disease in children. Bone Marrow Transplant. 2008;41(2):215–221. doi: 10.1038/sj.bmt.1705885. [DOI] [PubMed] [Google Scholar]
- 52.Bunin N, Aplenc R, Kamani N, Shaw K, Cnaan a, Simms S. Randomized trial of busulfan vs total body irradiation containing conditioning regimens for children with acute lymphoblastic leukemia: a Pediatric Blood and Marrow Transplant Consortium study. Bone Marrow Transplant. 2003;32(6):543–548. doi: 10.1038/sj.bmt.1704198. [DOI] [PubMed] [Google Scholar]
- 53.Peters C, Schrappe M, von Stackelberg a, et al. Stem-Cell Transplantation in Children With Acute Lymphoblastic Leukemia: A Prospective International Multicenter Trial Comparing Sibling Donors With Matched Unrelated Donors--The ALL-SCT-BFM-2003 Trial. J Clin Oncol. 2015 Mar; doi: 10.1200/JCO.2014.58.9747. [DOI] [PubMed] [Google Scholar]
- 54.Klusmann JH, Reinhardt D, Zimmermann M, et al. The role of matched sibling donor allogeneic stem cell transplantation in pediatric high-risk acute myeloid leukemia: results from the AML-BFM 98 study. Haematologica. 2012;97(1):21–29. doi: 10.3324/haematol.2011.051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holtick U, Chemnitz JM, Shimabukuro-Vornhagen A, et al. OCTET-CY: a phase II study to investigate the efficacy of post-transplant cyclophosphamide as sole graft-versus-host prophylaxis after allogeneic peripheral blood stem cell transplantation. Eur J Hematol. 2015 doi: 10.1111/ejh.12541. [DOI] [PMC free article] [PubMed] [Google Scholar]