Abstract
The association between inflammation and the risk of colorectal cancer (CRC) is well documented in animal models and in humans, but the mechanistic role of inflammation in CRC is less well understood. To address this question, the induction of colon tumors was evaluated in (i) wild type (WT) and athymic BALB/c mice treated with the colon carcinogen azoxymethane (AOM) as a single agent, and (ii) in an inflammation model of colon cancer employing AOM and dextran sodium sulfate (DSS) in WT, athymic, TCRβ−/−, TCRδ−/− and TCRβ−/−TCRδ−/− C57Bl/6 mice. The athymic BALB/c mice treated with only AOM developed 90% fewer tumors than the WT mice. The difference in response was not due to metabolic activation of AOM or repair of DNA adducts. In the inflammation model using a standard sequential exposure to AOM followed by DSS treatment, the tumor incidence in WT mice was 58% with 7 adenomas and 6 adenocarcinomas. In contrast, the TCRβ−/−, TCRδ−/− and TCRβ−/−TCRδ−/− C57Bl/6 mice showed adenoma incidences of 10, 33 and 11%, respectively, and none of the immune compromised mice developed adenocarcinomas. When the DSS exposure was increased and the AOM lowered, no difference was observed between WT and TCRβ−/− mice due to an increase in the incidence in the TCR null mice without concomitant increase in the WT mice. No tumors were observed in mice treated with AOM or DSS alone.
Keywords: colorectal cancer, inflammation, immune response, DNA methylation, azoxymethane
Introduction
There exists a complex relationship between the immune system and the etiology of human cancer,[1–5] as different cells of the immune system can play diverse roles in the development, progression and elimination of tumor cells. Moreover, inflammation is well documented to be associated with increased cancer incidence, for example, inflammatory bowel disease and colorectal cancer (CRC).[6–8] Because it is reasonable to assume that mice with defective immune systems would have a different response than wild type (WT) mice to carcinogenesis protocols, including those involving an inflammation component, we compared CRC incidence in BALB/c WT and immune compromised athymic mice treated with azoxymethane (AOM) as a single agent. The BALB/c mice develop CRC after repeated i.p. treatments with AOM as a single agent.[9,10] In addition, we have determined the carcinogenic response of WT, and immune compromised athymic, TCRβ−/−, TCRδ−/− and TCRβ−/−TCRδ−/− C57Bl/6 (B6) mice to sequential treatment with AOM followed by dextran sulfate sodium (DSS).[11] WT B6 mice are relatively refractory to AOM as a single agent but they do develop a high incidence of CRC after a single i.p. injection of AOM followed by exposure to DSS in drinking water.[12,13] The combined AOM+DSS treatment results in colonic adenomas and adenocarcinomas in less than 12 weeks. Also, B6 mice do not develop colon tumors after a single 7-day exposure to DSS. The BALB/c strain is also sensitive to AOM+DSS induced CRC with 100% tumor incidence after a single 10 mg/kg injection of AOM followed by 4 days with 1% DSS.[14] Note the high tumor incidence using a single injection with AOM when combined with DSS.
AOM is metabolized into a DNA methylating agent[15] that generates promutagenic O6-methylguanine lesions and stem cell mutations in the colon.[16–19] DSS, which does not produce stem cell mutations in the colon,[19] causes inflammation in the colon characterized by loss of crypt architecture and disruption of cellular junctions, muscosal ulcerations, hemorrhage and edema resulting from increased serous fluid swelling.[20,21] The colonic epithelial damage causes a substantial influx of immune cell populations, that includes neutrophils, macrophages, and lymphocytes, to mediate both anti-pathogenic and anti-inflammatory functions aiding tissue repair and cell survival.[22,23] The benefit of utilizing the combined AOM+DSS treatment is that it allows for the evaluation of CRC initiated with sporadic mutations produced by the AOM, while inducing a inflammatory response whose severity is dependent on the DSS dose.
The studies reported herein are focused on T-cell deficient athymic BALB/c and B6 mice, and TCRβ−/−, TCRδ−/− and TCRβ−/−TCRδ−/− B6 mice. The TCR null mice do not produce the respective receptors required for normal cytolytic and regulatory α/β and/or γ/δ T-cell functions. We observed a dramatic 90% reduction in the incidence of CRC in athymic BALB/c vs. WT mice after repeated i.p. treatments with AOM. This reduction is not related to the level of promutagenic 6-mG in the colon. Our results in the B6 mice also indicate that the immune compromised TCRβ−/− mice are less sensitive to carcinogenesis produced by AOM+DSS at lower concentrations of DSS. However, this resistance disappears at higher concentrations of DSS. Overall, the results of our studies in immune compromised mice treated with AOM alone or in combination with DSS indicate that T cells enhance CRC incidence.
Materials and Methods
Animals
Male WT and athymic BALB/c mice (Taconic, Hudson, NY), and (Fox n1/J), athymic (Fox n1nu/J), WT, TCRβ−/−, TCRδ−/− and TCRβ−/−δ−/− C57Bl/6 mice (Jackson Laboratory, Bar Harbor, ME) were housed in an immune-compromised mouse facility. Mice, which were 6–8 weeks of age at the start of the treatments, were maintained in microisolator cages in a temperature and humidity controlled barrier facility, and provided autoclaved water and food (Purina rodent chow, St. Louis, MO) ad libitum.
Materials
Fresh stock solutions of AOM (Sigma-Aldridge, St Louis MO) in PBS (ThermoFisher Scientific, Waltham, MA) were prepared on the day of each treatment. The AOM solutions were loaded into individual syringes and the syringes kept cold and sterile until animal treatment. DSS (36–50 kDa) (MP grade colitis grade, white powder, MP Biomedicals LLC, Santa Ana, CA) was dissolved in water to the desired percent concentration (w/v).
Tumorigenicity Studies
BALB/c Mice
One hundred 6-week old male WT and one hundred male athymic BALB/c mice were divided into two groups: 25 were treated with saline and 75 treated with 10 mg/kg AOM by i.p. injection once per week for 6 consecutive weeks. At 26 weeks after the initial injection, five of AOM-treated WT and five of the athymic mice were sacrificed and bladder, colon, duodenum, esophagus, ileum, jejunum, kidney, liver, lung pancreas, spleen, stomach and testes were macroscopically inspected and then histopathologically evaluated after formalin fixation and paraffin embedding. Other than adenomatous polyps observed in the colon in two of the five wild type BALB/c mice, there were no other pathological findings in the animals. At 30 weeks after the initial injection, the remaining mice (treated and controls) were sacrificed by CO2 asphyxiation followed by cervical dislocation as per approved IACUC guidelines (University of Pittsburgh IACUC Protocol #1104674) and their distal colons (terminal 2.5 cm) removed, washed with PBS and cut lengthwise. The colons were excised, their length measured and then placed in 4% formalin. After sitting overnight at 4 °C, the colons were prepared for paraffin embedding following established protocols. Before the tissue dehydration, each colon was Swiss-rolled. Sections were cut at 5 µm and H&E stained using established protocols.
C57Bl/6 Mice
The AOM was administered (i.p.) at either 10 mg/kg or 15 mg/kg body weight. DSS was provided ad libitum in the drinking water at 1%, 1.5% or 2% solution (w/v) for the time specified. Animals were euthanized and colonic tissue processed as described above.
Analysis of DNA Damage
Five WT and athymic male BALB/c mice were treated with a single i.p. injection of 20 mg/kg of AOM in PBS, while controls received PBS. Animals were sacrificed by CO2 asphyxiation followed by cervical dislocation at 8 and 24 h after the injection and the distal colon removed, washed with PBS and flash frozen in liquid nitrogen until the DNA was isolated. DNA was extracted using a Qiagen Genomic-tip DNA isolation kit (Qiagen Inc., Valencia, CA) as described by Harrison et al.[24] The Qiagen procedure was followed except the proteinase K incubation was completed overnight at 4 °C, followed by 1 h at 37 °C. DNA concentration and purity were determined by UV absorbance scans between 320 and 220 nm (Perkin Elmer Lambda 40). DNA samples were hydrolyzed in 50 mM BisTris/1 mM MgCl2 (pH 6.5) at 50 °C for 8 h, nuclease P1 (24 units), bacterial alkaline phosphatase (2.4 units), and wheat germ acid phosphatase (0.3 units) per mg of DNA (to a final concentration of 1 mg of DNA/mL). The reactions were stopped by heating at 100 °C for 5 min, and the mixtures were then centrifuged to remove the denatured enzymes. The supernatant was removed and the amount of O6-methylguanine (6-MeG) quantified by HPLC with electrochemical (EC) detection: Varian Star 9050 HPLC; column, Waters YMC ODS-AQ S5 column (4.6 × 250 mm with 120 Å particle size); solvent system, isocratic 100 mM sodium acetate (pH 5.0) with 5% MeOH; flow rate, 1.0 ml/min; detection, Varian UV detector set at 280 nm and a Coulochem II electrochemical detector (ESA, Bedford, MA) with guard cell set at 850 V and the analytical cell at 800 V.
Statistical Analysis of Carcinogenicity Studies
The Mann-Whitney U test was used to compare 6-mG adduct levels in WT and athymic BALB/c mice. Statistical significance was considered at p < 0.05. Statistically significant differences for the cancer incidence among the mice in the different experiments were determined using the Pearson’s Chi Square Test or Fisher’s Exact Test as determined by the number of animals in each experimental group respectively. Statistically significant differences for tumor multiplicity and differences in the numbers of adenomas and carcinomas in the B6 mice were calculated using Student’s T-test after the normality and equivalence of variance was tested.
Results
Carcinogenicity of AOM in BALB/c mice
The results from the study in the BALB/c mice demonstrate a pronounced difference in the susceptibility between WT and athymic BALB/c mice to the carcinogenicity of AOM as a single agent (Table I). The AOM-treated athymic mice had approximately an 11-fold lower tumor incidence than similarly treated WT animals. The decrease occurred at all stages, i.e., adenomatous polyps and adenocarcinomas (Table I, Figure 1). Adenomas were observed in 44 of 70 WT mice (63% incidence), while in athymic animals, only 4 out of 69 (6%) mice had adenomas (p-value < 0.0001). In addition to the higher incidence, multiple adenomas were observed in many of the WT mice, whereas, this was not the case in any of the athymic animals. In WT mice, 15 adenocarcinomas (21%) were observed, while there were only 3 adenocarcinomas (4%) in the athymic animals (p-value < 0.003).
Table I.
Tumorigenicity of azoxymethane (AOM) in WT and athymic BALB/c mice.
| strain | # of mice | treatmenta | adenomasd (%) | total adenomase | adenocarcinomaf (%) |
|---|---|---|---|---|---|
| WT BALB/c | 23 | solventb | 1 (<1) | 1 | 0 |
| athymic BALB/c | 22 | solventb | 1 (<1) | 1 | 0 |
| WT BALB/c | 70 | AOMc | 44 (63)g | 79 | 15 (21)h |
| athymic BALB/c | 69 | AOMc | 4 (6)g | 4 | 3 (4)h |
animals received 6 weekly i.p. doses of solvent or AOM.
200 µL 2% phosphate buffered saline (PBS).
10 mg/kg of AOM dissolved in 200 µL of PBS.
adenoma bearing mice by macroscopic inspection.
total number of macroscopic adenomas.
determined by histopathological examination.
statistically different from each other (p < 0.0001) using Person’s chi-square test.
statistically different from each other (p < 0.003) using T-test.
Figure 1.
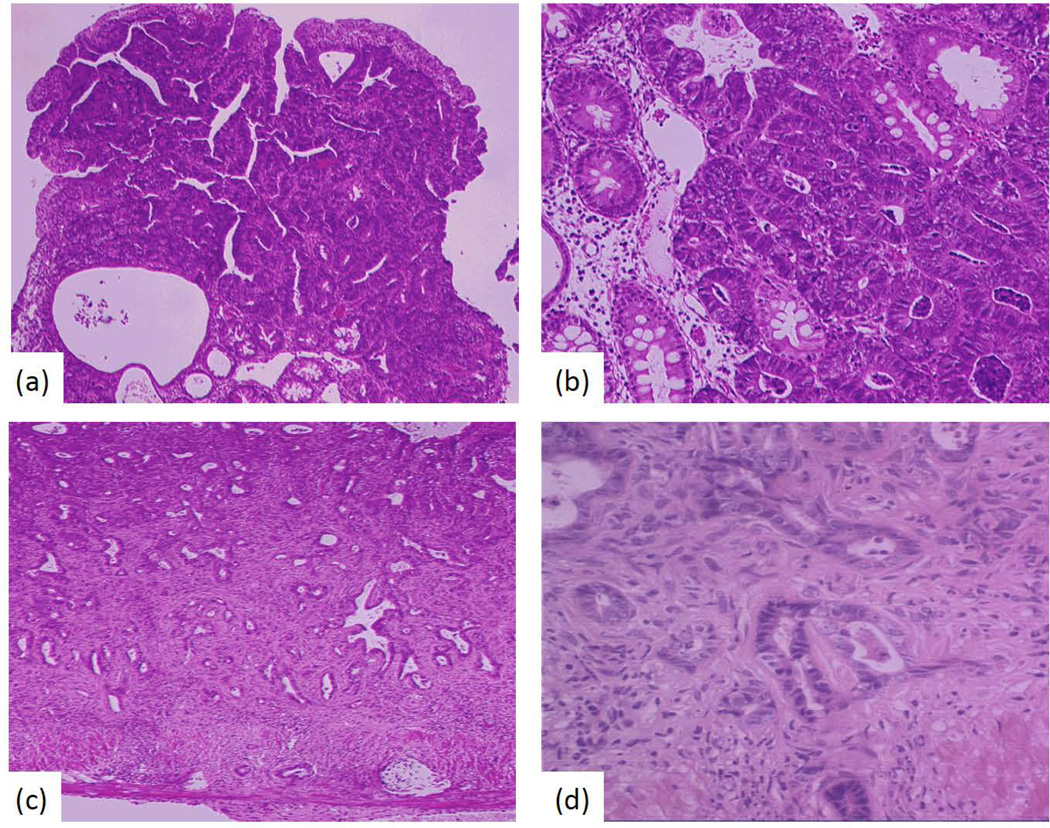
Azoxymethane induced colon tumors produced in wild type and athymic mice: (a) WT BALB/c mice - a colonic mucosal adenomatous polyp is present containing numerous neoplastic glands, some with high grade dysplasia, characterized by mucin depletion, nuclear pseudostratification, marked cytologic atypia, increased mitotic activity, and lack of surface maturation. Focal intramucosal adenocarcinoma is present, where glands exhibit back-to-back architectural arrangement, and cells filtrate into the lamina propria. This animal was negative for submucosal tumor invasion (magnification 40×) (b) Higher magnification (100×) of 1a. (c) Athymic BALB/c mice - colonic submucosa and muscularis propria are infiltrated by invasive adenocarcinoma, characterized by irregular, malignant glands lined by dysplastic, hyperchromatic cells with high nucleus to cytoplasm ratios. Some glands contain karyorrhectic cell debris. Background stromal desmoplasia is characteristic of submucosal tumor invasion, and mild background chronic inflammation is noted (magnification 40×). (d) Higher magnification of tumor in 1c (100×).
Adducts levels in WT and athymic BALB/c mice after treatment with AOM
To determine if the difference in tumor incidence between WT and athymic mice (Table I) could arise from differences in the metabolic activation of AOM into a DNA methylating agent and/or differences in DNA repair, the levels of 6-MeG, the critical promutagenic lesion,[25,26] in the distal colons of WT and athymic mice were quantified at 8 and 24 h after i.p. injection of 20 mg/kg AOM. Analysis by HPLC with electrochemical detection showed a slightly higher level of adducts in the athymic mice (Table II); however, the differences were not statistically significant, i.e., p-value ≥ 0.05. Therefore, differences in the efficiency of AOM metabolism and/or DNA repair of 6-MeG do not explain why athymic mice are refractory to AOM induced colon tumors. This result is consistent with previous reports of similar adduct levels in sensitive and insensitive rodent strains.[26]
Table II.
O6-Methylguanine levels in the distal colon of wild type and athymic BALB/c mice after i.p. injection with 20 mg/kg of azoxymethane (AOM) were determined at 8 and 24 h by HPLC with electrochemical detection.
| mouse strain | treatmenta | time (h) | O6-methylguanine (pmol/µmol DNA)b,c |
|---|---|---|---|
| wild type BALB/c | solvent | 24 | 0 |
| 20 mg/kg AOM | 8 | 26.30 ± 8.50c | |
| 20 mg/kg AOM | 24 | 23.30 ± 6.12d | |
| athymic BALB/c | solvent | 24 | 0 |
| 20 mg/kg AOM | 8 | 43.65 ± 14.43c | |
| 20 mg/kg AOM | 24 | 40.27 ± 12.63d |
5 male mice were i.p. injected with 100 µL of phosphate buffered saline or AOM in phosphate buffered saline.
Animals were sacrificed at 8 or 24 h and DNA from the distal colon isolated, depurinated with 0.1 N HCl at 80 °C for 30 minutes and analyzed by HPLC separation with electrochemical detection.
The Mann-Whitney U test was used to compare 6-mG adduct levels in WT and athymic BALB/c mice.
The p-values were all > 0.05 and not considered significant.
Carcinogenicity of AOM+DSS in C57Bl/6 mice
The initial attempt to compare the carcinogenicity of AOM (10 mg/kg, i.p.) followed 7 days later with DSS (2% in the drinking water for 7 days) in WT (Foxn1/J) and athymic (Foxn1nu/J) C57Bl/6 mice provided some insight into the sensitivity of athymic mice to the effect of DSS. In this study 19 of 20 athymic mice died or had to be euthanized by 7 days after initiation of the exposure to DSS. The mice suffered severe rectal bleeding and diarrhea, and appeared moribund. In contrast, the WT mice all survived and 70% of the animals developed a mixture of colon adenomas (6/20) and adenocarcinomas (16/20) (Table III, Figure 2). As expected, none of the untreated control mice showed tumors by 120 days.
Table III.
Tumorigenicity of azoxymethane (AOM) followed by dextran sulfate sodium (DSS) in WT and immune compromised C67Bl/6 mice
| Treatment | Mouse Strain | # of mice | Incidence | Adenomas | Carcinomas | Multiplicity |
|---|---|---|---|---|---|---|
| 10 mg/kg AOM + 2% DSS | Foxn1/Ja | 20 | 14 | 6 | 16 | 22 |
| Foxn1nu/Jb | 20 | n/ac | n/a | n/a | n/a | |
| 10 mg/kg AOM + 1% DSS | Foxn1/Ja | 20 | 0 | - | - | - |
| Foxn1nu/Jb | 20 | 0 | - | - | - | |
| 15 mg/kg AOM + 1.5% DSS | Foxn1/Ja | 14 | 12 | 11d | 22e,j | 32 |
| Foxn1nu/Jb | 12 | 11 | 19d | 7e | 26 | |
| 15 mg/kg AOM + 1.5% DSS | WT | 12 | 7f,g | 7 | 6j | 13h,i |
| TCRβ−/− | 10 | 1f,k | 1l | 0 | 1h,m | |
| TCRδ−/− | 9 | 3 | 3 | 0 | 3 | |
| TCRβ−/−δ−/− | 9 | 1g | 1 | 0 | 1i | |
| 10 mg/kg AOM + 2% DSS | WT | 12 | 10 | 11 | 10 | 21 |
| TCRβ−/− | 12 | 11k | 10l | 12 | 22m |
heterozygote B6.
homozygote (athymic) B6.
animals died or had to be sacrificed immediately after exposure to DSS.
p-value: 0.020 (T-test).
p-value: 0.046 (T-test).
p-value: 0.026 (Fisher’s exact test).
p-value: 0.038 (Fisher’s exact test).
p-value: 0.024 (T-test).
p-value: 0.025 (T-test).
p-value: 0.027 (T-test).
p-value: 0.015 (Fisher’s exact test).
p-value: 0.012 (T-test).
p-value 9.250e−05(T-test).
Figure 2.
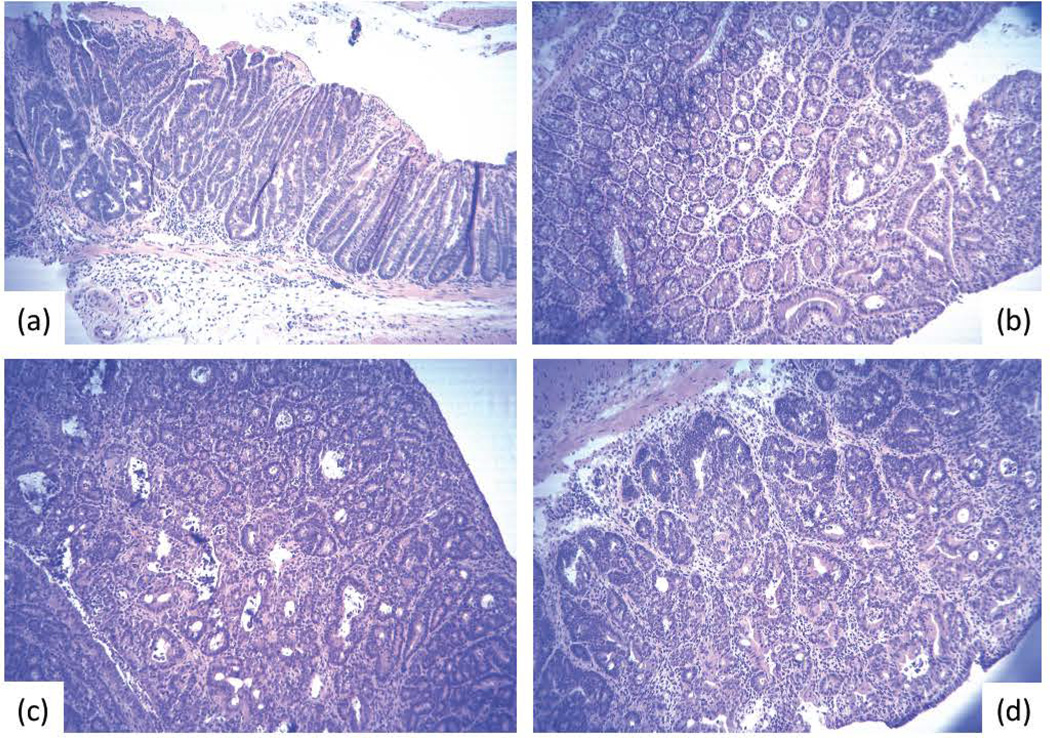
Representative adenomas and adenocarcinomas formed in C57BL/6 mice treated with 10 mg/kg (I.P.) AOM followed 7 days later with exposure to 2% DSS in the drinking water for 7 days: (a) adenoma and (c) carcinoma in WT mice; (b) adenoma and (d) carcinoma in TCRβ−/− mice.
Because of intolerance of athymic mice to 2% DSS in the drinking water, the DSS concentration was reduced from 2% to 1% and the duration of treatment from 7 to 5 days. All mice survived for the 120 day experiment. However, the tumor response was eliminated. The dose of AOM was then increased to 15 mg/kg and DSS to 1.5% in an attempt to amplify tumor incidence. After 120 days this resulted in a tumor incidence of 13 of 15 in the WT vs. 11 of 13 in the athymic mice (Table III). While the overall tumor incidence in the two strains of mice was not statistically different, there was a significant difference in the ratio of adenomas to adenocarcinomas. Nearly twice as many adenomas occured in the athymic mice while nearly three times as many adenocarcinomas developed in the WT mice, indicating a slower progression of CRC in the athymic mice (Table III).
Due to difficulties in breeding athymic (Foxn1nu/J) C57Bl/6 mice and their sensitivity to DSS, tumor studies were initiated in WT, TCRβ−/−, TCRδ−/− and TCRβ−/−TCRδ−/− C57Bl/6 mice. The TCRβ−/− and TCRδ−/− mice do not produce the respective receptors required for normal cytolytic and regulatory α/β and γ/δ T-cell functions. The mice received a single i.p. injection of 15 mg/kg AOM followed 7 days later with exposure to 1.5% DSS in the drinking water for 7 days. The mice were sacrificed at 90 days after AOM injection, unless earlier euthanasia was indicated, and tumor incidence determined (Table III). The TCR null strains tolerated the AOM and DSS treatments similarly to WT mice. The results show a significant reduction of tumors in the TCRβ−/− mice as only one of 10 mice developed a single adenoma (10% incidence). This compared to a 60% incidence in WT animals with a mixture of 7 adenomas and 6 adenocarcinomas (Table III, Figure 2). The TCRδ−/− mice also had a lower incidence (33%) with a total of 3 adenomas but the difference did not quite reach a p-value of < 0.05. The double null TCRβ−/−/TCRδ−/− animals responded similar to the TCRβ−/− mice: 11% incidence and only 1 adenoma. None of the TCR null animals developed adenocarcinomas.
To determine how the DSS dose affected the immune compromised mice, in a final set of carcinogenicity studies, WT and TCRβ−/− were treated with either 10 mg/kg of AOM, 2.0% DSS or a combination of both. Neither AOM or DSS alone yielded any colon tumors in either strain. Note that in the AOM +DSS study a higher concentration of DSS (0.5% higher) and a lower concentration of AOM (5 mg/kg lower) was employed than in the experiment described above (Table III). Tumor incidence in the two strains was the same as was the ratio of adenomas to adenocarcinomas (Table III). The similarity between the WT and TCR null mice results from a significant increase in the tumor incidence in the TCRβ−/− mice treated with 10 mg/kg AOM+2% DSS without a concomitant increase in the tumorigenic response in the WT strain. This result strongly contrasted with the experimental results in the WT mice and TCRβ−/− mice treated with 15 mg/kg AOM+1.5% DSS where a clear difference was observed in cancer incidence, multiplicity, and progression between the two strains.
Discussion
The goal of this study was to determine how T-cell mediated responses affected CRC induction in both BALB/c and B6 mouse strains using AOM alone and AOM+DSS. The AOM+DSS combination in the B6 mouse is generally considered an inflammation associated model of CRC. The six weekly injections of AOM without DSS in the BALB/c strain does not produce frank inflammation.[28] However, repeated exposure to AOM causes toxicity in the colon, as indicated by reduction in the number of crypts, and probably elicits an immune response, albeit, significantly different from that produced by the severe physical damage occurring in the epithelium as a result of DSS treatment. [20–23]
There have been a number of reports on the relationship of CRC induction after treatment with AOM+DSS in immune-altered mice. These studies addressed the role of plasma membrane-associated toll-like receptors (TLRs) and cytosolic Nod/NACHT-LRR (NLR) proteins that recognize pathogen-associated molecular patterns (PAMPs) and initiate inflammatory responses.[29–34] These receptors affect the unique relationship that the colon exhibits with commensal microbiota, as it is through TLR/NRL signaling that natural tolerance for microbiota is both established and broken.[35–40] DSS treatment disrupts the colon architecture allowing bacteria access to subepithelial regions of the colon, which induces inflammation.[35,37] It is interesting that different TLR/NLR knockouts impacted colon carcinogenesis resulting from AOM+DSS treatment in different ways. For example, TLR2−/− and NLR3−/− B6 deficient mice had a significant increase in CRC development over WT after AOM+DSS treatment.[41–43] In contrast, TLR4−/− B6 mice had a significantly decreased CRC incidence vs. WT mice.[42] These results suggest that how the immune response initiates and progresses after AOM and/or DSS treatments determines whether or not the immune response will potentiate cancer development.
TLR/NRL receptors are initiators of inflammation, but what would occur in an immune deficient mouse lacking the cells that mediate the adaptive immune response? The results of our studies in athymic Balb/c treated with AOM, and in TCRβ−/− and TCRβ−/−/ TCRδ−/− B6 mice treated with AOM+DSS support an important role for T cells in mediating colon carcinogenesis (Tables I, III).
The dramatic reduction in CRC in the athymic BALB/c mice is not a function of metabolic activation or repair of the AOM derived 6-mG DNA lesions based on the adduct studies reported herein. Moreover, the 6-mG adduct level in the colon of WT BALB/c mice (~25 pmol/µmol DNA) is very similar to that observed in WT B6 mouse (20 pmol/µmol DNA)[44] yet BALB/c mice are significantly more sensitive to AOM alone and to AOM+DSS than B6 mice.[45,46] Adduct measurements in the colon are not equivalent to mutations in the critical stem cell population so we recently measured the mutation frequency in the X-linked glucose-6-phosphate dehydrogenase (G6PD) gene in colonic stem cells using AOM, DSS and AOM+DSS.[19] The mutation frequency is significantly reduced by 75% in TCRβ−/− vs. WT B6 mice after treatment with a single i.p. dose of AOM.[19] A similar reduction was also observed in TCRβ−/− vs. WT mice after AOM+DSS.[19] As 6-mG levels do not differ in WT and athymic mice, it is reasonable to assume that the difference in the mutation frequency is related to reduced death of AOM damaged/mutagenized stem cells in WT vs. TCRβ−/− B6 mice. The more damaged stem cells that survive, the higher the mutation frequency, and presumably the higher the cancer incidence.
In WT B6 mice, the effect of α/β T-cells on the stem cell mutation frequency and tumorigenicity, could be partially mediated through IEL γ/δ T-cells that display a phenotype strongly associated with wound repair and regeneration,[47] as well as tissue resident macrophages and dendritic cells whose activation should involve TLR and/or NRL pathways. It is known that IEL γ/δ T-cells are activated by colonic epithelial damage generated by DSS and that γ/δ T-cells express keratinocyte growth factor (KGF)-1 and -2 in response to DSS.[48] By providing KGF-1 and -2 to the colonic epithelium, IEL γ/δ T-cells promote colonic stem cell survival and, therefore, would inadvertently lead to the survival of stem cells with oncogenic mutations.[49,50] It has been reported that both KGF−/− and TCRδ−/− mice share an impaired recovery to DSS colitis damage in comparison to WT mice.[48] Experiments with TLR/NRL B6 null mice treated with AOM+DSS strongly suggest that the way the immune response is initiated can impact colon carcinogenesis, at least with AOM+DSS treated mice.
AOM toxicity causes apoptotic and necrotic cell death in colonic epithelial cells.[51] Although apoptotic cells can initially maintain epithelial integrity and, therefore, sterile conditions, this pathway still initiates tissue repair.[52] Once AOM damage occurs in colonic stem cells of WT mice, epithelial macrophages, dendritic cells and activated IEL γ/δ T-cell express cytokines and chemokines directed toward infiltrating immune cells. These infiltrating immune cells will include α/β effector T-cells and potentially cytolytic α/β T-cells. If the initiation of the immune response is driven toward tissue repair it could result in macrophage, and IEL γ/δ T-cell mediated signals enhancing the survival of AOM damaged stem cells due to a bypass of cell cycle arrest signals and subsequent apoptosis by directing α/β T-cells toward growth/survival responses. For example; α/β T-cell signaling may effect γ/δ T-cell mediated expression of KGF. Consistent with this scenario is the lower tumor incidence and/or progression of the immune compromised athymic BALB/c and, at lower DSS concentrations, in TCRβ−/− B6 mice.
The stem cell mutations generated by AOM in both WT and TCRβ−/− B6 mice, are not sufficient to produce tumors; DSS inflammation is required in B6 mice. Since DSS does not produce stem cell mutations in B6 mice,[19] what does the DSS do that transforms a mutagen, e.g., AOM, into a carcinogen in the B6 mouse? The effect of DSS on the colon includes a dose-dependent disruption of colonic architecture.[23] It is tempting to hypothesize that treatment with AOM results in critical stem cell mutations in the β-catenin oncogene, a mutation that is observed in virtually all AOM+DSS induced tumors in mice.[12,53] However, nuclear localization of mutant β-catenin is still regulated through its sequestering by membrane associated E-cadherin.[54–57] DSS disruption of the colonic cell-cell junctions[23,57] would liberate E-cadherin associated mutant β-catenin and allow it to enter into the nucleus where it initiates the transcription of its target genes.[58,59] In support of this proposal is that in mice with a stable mutation of β-catenin, polyps and some microadenomas were observed.[60] However, despite the present of an activated β-catenin gene in every cell in the intestine, macroscopic tumors similar to that in AOM+DSS were not observed. This again argues that activating mutations in oncogenes or inactivating mutations in tumor suppressor genes are required but not sufficient to produce CRC in the B6 mouse.
In our studies, the TCRδ−/− B6 mice showed a tumor incidence that was not statistically different from either WT or TCRβ−/− mice treated with AOM+DSS (Table III). In contrast, when TCRδ−/− B6 mice were treated with 5 weekly injections of 10 mg/kg AOM (without DSS) the tumor incidence was 50% at 7 months, while WT and TCRα−/− B6 mice did not develop CRC.[61] B6 mice are relatively resistant to AOM as a single agent; therefore, this result in the TCRδ null animals indicates that γ/δ T-cells may play a role in the resistance of WT B6 mice to CRC development. It is unclear why TCRδ null mice are susceptible to AOM and AOM+DSS initiated colon carcinogenesis while WT B6 mice require DSS treatment after AOM exposure. As above, it is suggested that IEL γ/δ T-cells could support colonic tissue repair and potentially enhance the survival of AOM damaged colonic stem cells. These different results in AOM induced carcinogenesis reinforce the suggestion that how the immune response is initiated (e.g., by toxicity and/or inflammation) and how specific immune cells are activated, could significantly impact colon carcinogenesis induced by AOM+DSS treatment.
While there was a clear reduction in susceptibility of the TCRβ−/− mice at the lower exposure to DSS (1.5%), this difference disappeared when mice were treated with the higher 2% DSS in combination with the lower 10 mg/kg AOM dose. This change in susceptibility results from a statistically significant increase in the incidence of adenomas and carcinomas in the TCRβ−/− mice at 2% DSS relative to the response observed at 1.5% DSS in the TCRβ−/− animals. At the same time, the values for the WT B6 mice remained statistically unchanged. It is possible that the severity of the inflammation induced by the higher 2% DSS dose resulted in compensation by other immune cells for the deficient α/β T-cell mediated signaling in the TCR β−/− mice. This would potentiate CRC development in what is normally a resistant mouse. It is interesting that tumor response increased in the null mice even though the exposure to the AOM mutagen was lowered. This further supports the contention that mutations are not limiting in the AOM+DSS CRC model.
Acknowledgements
We thank Dr. Shih-Fan Kuan (Department of Pathology, University of Pittsburgh) for his pathological analysis of the colon tumors.
Source of support: NIH RO1 CA29088
Abbreviations
- AOM
azoxymethane
- B6
C57Bl/6
- CRC
colorectal cancer
- DSS
dextran sulfate sodium
- NLR
NOD-like receptor
- PAMPs
pathogen-associated molecular patterns
- TCR
T-cell receptor
- TLR
toll-like receptor
- WT
wild type
Footnotes
Conflict of Interest Statement: none declared
References
- 1.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [PMID: 11229684] [DOI] [PubMed] [Google Scholar]
- 2.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [PMID: 12490959] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mladenova D, Kohonen-Corish MR. Review: Mouse models of inflammatory bowel disease--insights into the mechanisms of inflammation-associated colorectal cancer. In Vivo. 2012;26:627–646. [PMID: 22773577] [PubMed] [Google Scholar]
- 4.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology. 2002;16:217–226. [PMID: 11866137] [PubMed] [Google Scholar]
- 6.Stewart BW, et al. World Cancer Report, I.A.R.C., International Agency on Research in Cancer. 2003 http://monographs.iarc.fr/ENG/Classification/index.php. [Google Scholar]
- 7.Jess T, Gamborg M, Matzen P, Munkholm P, Sørensen TI. Increased risk of intestinal cancer in Crohn’s disease: A meta-analysis of population-based cohort studies. Am J Gastroenterol. 2005;100:2724–2729. doi: 10.1111/j.1572-0241.2005.00287.x. [PMID: 16393226] [DOI] [PubMed] [Google Scholar]
- 8.Itzkowitz SH, Yio X. Inflammation, Cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–G17. doi: 10.1152/ajpgi.00079.2004. [PMID: 15194558] [DOI] [PubMed] [Google Scholar]
- 9.Zedeck MS. Hydrazine derivatives, azo and azoxy compounds, and methylazoxymethanol and cycasin. In: Searle CE, editor. Chemical Carcinogens. Vol. 2. Washington, D.C.: American Chemical Society Monographs; 1984. pp. 915–944. [Google Scholar]
- 10.Rosenberg DW, Giardina C, Tanaka T. Mouse models for the study of colon carcinogenesis. Carcinogenesis. 2009;30:183–196. doi: 10.1093/carcin/bgn267. [PMID: 19037092] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka T. Animal models of carcinogenesis in inflamed colorectum: potential use in chemoprevention study. Curr Drug Targets. 2012;13:1689–1697. doi: 10.2174/138945012804545452. [PMID: 23140280] [DOI] [PubMed] [Google Scholar]
- 12.Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94:965–973. doi: 10.1111/j.1349-7006.2003.tb01386.x. [PMID: 14611673] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suzuki R, Kohno H, Sugie S, Tanaka T. Dose-dependent promoting effect of dextran sodium sulfate on mouse colon carcinogenesis initiated with azoxymethane. Histol Histopathol. 2005;20:483–492. doi: 10.14670/HH-20.483. [PMID: 15736053] [DOI] [PubMed] [Google Scholar]
- 14.Suzuki R, Kohno H, Sugie S, Nakagama H, Tanaka T. Strain differences in the susceptibility to azoxymethane and dextran sodium sulfate-induced colon carcinogenesis in mice. Carcinogenesis. 2006;27:162–169. doi: 10.1093/carcin/bgi205. [PubMed: 16081511] [DOI] [PubMed] [Google Scholar]
- 15.Megaraj V, Ding X, Fang C, Kovalchuk N, Zhu Y, Zhang QY. Role of hepatic and intestinal p450 enzymes in the metabolic activation of the colon carcinogen azoxymethane in mice. Chem Res Toxicol. 2014;27:656–662. doi: 10.1021/tx4004769. [PMID: 24552495] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loechler EL, Green CL, Essigmann JM. In vivo mutagenesis by O6-methylguanine built into a unique site in a viral genome. Proc Natl Acad. Sci USA. 1984;81:6271–6275. doi: 10.1073/pnas.81.20.6271. [PMID: 6093094] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carpentino JE, Hynes MJ, Appelman HD, Zheng T, Steindler DA, Scott EW, Huang EH. Aldehyde dehydrogenase-expressing colon stem cells contribute to tumorigenesis in the transition from colitis to cancer. Cancer Res. 2009;69:8208–8215. doi: 10.1158/0008-5472.CAN-09-1132. [PMID: 19808966] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fiala ES, Kulakis C, Christiansen G, Weisburger JH. Inhibition of the metabolism of the colon carcinogen, azoxymethane, by pyrazole. Cancer Res. 1978;38:4515–4521. [PMID: 719636] [PubMed] [Google Scholar]
- 19.Whetstone RD, Gold B. T-Cells enhance stem cell mutagenesis in the mouse colon. Mutat Res. 2015;774:1–5. doi: 10.1016/j.mrfmmm.2015.02.004. (in press) [PMID: 25770826] [DOI] [PubMed] [Google Scholar]
- 20.Solomon L, Mansor S, Mallon P, Donnelly E, Hoper M, Loughrey M, et al. The dextran sulphate sodium (DSS) model of colitis: an overview. Compar Clin Pathol. 2010;19:235–239. [Google Scholar]
- 21.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [PMID: 1688816] [DOI] [PubMed] [Google Scholar]
- 22.Kitajima S, Takoma S, Morimoto M. Changes in colonic mucosal permeability in mouse colitis induced with dextran sulfate sodium. Exp Anim. 1999;48:137–143. doi: 10.1538/expanim.48.137. [PMID:] [DOI] [PubMed] [Google Scholar]
- 23.Rose WA, 2nd1, Sakamoto K, Leifer CA. Multifunctional role of dextran sulfate sodium for in vivo modeling of intestinal diseases. BMC Immunol. 2012;13:13–41. doi: 10.1186/1471-2172-13-41. [PMID: 22853702] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrison KL, Jukes R, Cooper DP, Shuker DE. Detection of concomitant formation of O6-carboxymethyl- and O6-methyl-2'-deoxyguanosine in DNA exposed to nitrosated glycine derivatives using a combined immunoaffinity/HPLC method. Chem Res Toxicol. 1999;12:106–111. doi: 10.1021/tx980057n. [PMID: 9894025] [DOI] [PubMed] [Google Scholar]
- 25.Bugni JM, Meira LB, Samson LD. Alkylation-induced colon tumorigenesis in mice deficient in the Mgmt and Msh6 proteins. Oncogene. 2009;28:734–741. doi: 10.1038/onc.2008.426. [PMID: 19029948] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wirtz S, Nagel G, Eshkind L, Neurath MF, Samson LD, Kaina B. Both base excision repair and O6-methylguanine-DNA methyltransferase protect against methylation-induced colon carcinogenesis. Carcinogenesis. 2010;31:2111–2117. doi: 10.1093/carcin/bgq174. [PMID: 20732909] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papanikolaou A, Shank RC, Delker DA, Povey A, Cooper DP, Rosenberg DW. Initial levels of azoxymethane-induced DNA methyl adducts are not predictive of tumor susceptibility in inbred mice. Toxicol Appl Pharmacol. 1998;150:196–203. doi: 10.1006/taap.1998.8393. [PMID: 9630469] [DOI] [PubMed] [Google Scholar]
- 28.Venning FA, Claesson MH, Kissow H. The carcinogenic agent azoxymethane (AOM) enhances early inflammation-induced colon crypt pathology. J Cancer Sci Ther. 2013;5:377–383. [Google Scholar]
- 29.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–820. doi: 10.1016/j.cell.2010.01.022. [PMID: 20303872] [DOI] [PubMed] [Google Scholar]
- 30.Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9:57–63. doi: 10.1038/nrc2541. [PMID: 19052556] [DOI] [PubMed] [Google Scholar]
- 31.Pradere JP, Dapito DH, Schwabe RF. The Yin and Yang of Toll-like receptors in cancer. Oncogene. 2014;33:3485–3495. doi: 10.1038/onc.2013.302. [PMID: 23934186] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinon F, Tschopp J. NLRs join TLRs as innate sensors of pathogens. Trends Immunol. 2005;26:447–454. doi: 10.1016/j.it.2005.06.004. [PMID: 15967716] [DOI] [PubMed] [Google Scholar]
- 33.Ting JP, Kastner DL, Hoffman HM. CATERPILLERs, pyrin and hereditary immunological disorders. Nat Rev Immunol. 2006;6:183–195. doi: 10.1038/nri1788. [PMID: 16498449] [DOI] [PubMed] [Google Scholar]
- 34.Delbridge LM, O'Riordan MX. Innate recognition of intracellular bacteria. Curr Opin Immunol. 2007;19:10–16. doi: 10.1016/j.coi.2006.11.005. [PMID: 17126540] [DOI] [PubMed] [Google Scholar]
- 35.Richman PI, Tilly R, Jass JR, Bodmer WF. Colonic pericrypt sheath cells: characterisation of cell type with new monoclonal antibody. J Clin Pathol. 1987;40:593–600. doi: 10.1136/jcp.40.6.593. [PMID: 3301906] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Henderson P, van Limbergen JE, Schwarze J, Wilson DC. Function of the intestinal epithelium and its dysregulation in inflammatory bowel disease. Inflamm. Bowel Dis. 2011;17:382–395. doi: 10.1002/ibd.21379. [PMID: 20645321] [DOI] [PubMed] [Google Scholar]
- 37.Saleh M, Trinchieri G. Innate immune mechanisms of colitis and colitis associated colorectal cancer. Nat Rev Immunol. 2011;11:9–20. doi: 10.1038/nri2891. [PMID: 21151034] [DOI] [PubMed] [Google Scholar]
- 38.Hill DA, Artis D. Intestinal bacteria and the regulation of immune cell homeostasis. Annu Rev Immunol. 2010;28:623–667. doi: 10.1146/annurev-immunol-030409-101330. [PMID: 20192812] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jump RL, Levine AD. Mechanisms of natural tolerance in the intestine: implications for inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:462–478. doi: 10.1097/00054725-200407000-00023. [PMID: 15475760] [DOI] [PubMed] [Google Scholar]
- 40.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [PMID: 15260992] [DOI] [PubMed] [Google Scholar]
- 41.Lowe EL, Crother TR, Rabizadeh S, Hu B, Wang H, Chen S, et al. Toll-like receptor 2 signaling protects mice from tumor development in a mouse model of colitis-induced cancer. PLoS One. 2010;5:e13027. doi: 10.1371/journal.pone.0013027. [PMID: 20885960] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fukata M, Chen A, Vamadevan AS, Cohen J, Breglio K, Krishnareddy S, et al. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007;133:1869–1881. doi: 10.1053/j.gastro.2007.09.008. [PMID: 18054559] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allen IC, TeKippe EM, Woodford RM, Uronis JM, Holl EK, Rogers AB, et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med. 2010;207:1045–1056. doi: 10.1084/jem.20100050. [PMID: 20385749] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Megaraj V, Ding X, Fang C, Kovalchuk N, Zhu Y, Zhang QY. Role of hepatic and intestinal p450 enzymes in the metabolic activation of the colon carcinogen azoxymethane in mice. Chem Res Toxicol. 2014;27:656–662. doi: 10.1021/tx4004769. [PMID: 24552495] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suzuki R, Kohno H, Sugie S, Nakagama H, Tanaka T. Strain differences in the susceptibility to azoxymethane and dextran sodium sulfate-induced colon carcinogenesis in mice. Carcinogenesis. 2006;27:162–169. doi: 10.1093/carcin/bgi205. [PMID: 16081511] [DOI] [PubMed] [Google Scholar]
- 46.De Robertis M, Massi E, Poeta ML, Carotti S, Morini S, Cecchetelli L, Signori E, Fazio VM. The AOM/DSS murine model for the study of colon carcinogenesis: From pathways to diagnosis and therapy studies. J Carcinog. 2011;10:9. doi: 10.4103/1477-3163.78279. [PMID: 21483655] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheroutre H, Lambolez F, Mucida D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat Rev Immunol. 2011;11:445–456. doi: 10.1038/nri3007. [PMID: 21681197] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Y, Chou K, Fuchs E, Havran WL, Boismenu R. Protection of the intestinal mucosa by intraepithelial γδ T cells. Proc Natl Acad Sci USA. 2002;99:14338–14343. doi: 10.1073/pnas.212290499. [PMID: PMC137885] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang H, Antony PA, Wildhaber BE, Teitelbaum DH. Intestinal intraepithelial lymphocyte gamma delta-T cell-derived keratinocyte growth factor modulates epithelial growth in the mouse. J Immunol. 2004;172:4151–4158. doi: 10.4049/jimmunol.172.7.4151. [PMID: 15034027] [DOI] [PubMed] [Google Scholar]
- 50.Booth D, Potten CS. Protection against mucosal injury by growth factors and cytokines. J Natl Cancer Inst. 2001;29:16–20. doi: 10.1093/oxfordjournals.jncimonographs.a003433. [PMID: 11694560] [DOI] [PubMed] [Google Scholar]
- 51.Hong MY, Chapkin RS, Wild CP, Morris JS, Wang N, Carroll RJ, Turner ND, Lupton JR. Relationship between DNA adduct levels, repair enzyme, and apoptosis as a function of DNA methylation by azoxymethane. Cell Growth Differ. 1999;10:749–758. [PMID: 10593651] [PubMed] [Google Scholar]
- 52.Rock KL, Kono H. The inflammatory response to cell death. Annual Review of Pathology. 2008;3:99–126. doi: 10.1146/annurev.pathmechdis.3.121806.151456. (2008). [PMID: 18039143] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takahashi M, Nakatsugi S, Sugimura T, Wakabayashi K. Frequent mutations of the beta-catenin gene in mouse colon tumors induced by azoxymethane. Carcinogenesis. 2000;21:1117–1120. (2000). [PMID: 10836998] [PubMed] [Google Scholar]
- 54.Fearnhead NS, Britton MP, Bodmer WF. The ABC of APC. Human Mol Genet. 2001;10:721–733. doi: 10.1093/hmg/10.7.721. (2001). [PMID: 11257105] [DOI] [PubMed] [Google Scholar]
- 55.Orsulic S, Huber O, Aberle H, Arnold S, Kemler R. E-cadherin binding prevents beta-catenin nuclear localization and beta-catenin/LEF-1-mediated transactivation. J Cell Sci. 1999;112:1237–1245. doi: 10.1242/jcs.112.8.1237. [PMID: 10085258] [DOI] [PubMed] [Google Scholar]
- 56.Brembeck FH, Rosário M, Birchmeier W. Balancing cell adhesion and Wnt signaling, the key role of beta-catenin. Curr Opin Genet Dev. 2006;16:51–59. doi: 10.1016/j.gde.2005.12.007. [PMID: 16377174] [DOI] [PubMed] [Google Scholar]
- 57.Maher MT, Flozak AS, Stocker AM, Chenn A, Gottardi CJ. Activity of the beta-catenin phosphodestruction complex at cell-cell contacts is enhanced by cadherin-based adhesion. J. Cell Biol. 2009;186:219–228. doi: 10.1083/jcb.200811108. [PMID: 19620634] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poritz LS, Garver KI, Green C, Fitzpatrick L, Ruggiero F, Koltun WA. Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J Surg Res. 2007;140:12–19. doi: 10.1016/j.jss.2006.07.050. [PMID: 17418867] [DOI] [PubMed] [Google Scholar]
- 59.Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, Brenner MB. Adhesion between epithelial cells and T lymphocytes mediated by E cadherin and the alpha E beta 7 integrin. Nature. 1994;372:190–193. doi: 10.1038/372190a0. [PMID: 7969453] [DOI] [PubMed] [Google Scholar]
- 60.Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–42. doi: 10.1093/emboj/18.21.5931. [PMID: 10545105] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matsuda S, Kudoh S, Katayama S. Enhanced formation of azoxymethane induced colorectal adenocarcinoma in gammadelta T lymphocyte-deficient mice. Jpn J Cancer Res. 2001;92:880–885. doi: 10.1111/j.1349-7006.2001.tb01176.x. [PMID: 11509121] [DOI] [PMC free article] [PubMed] [Google Scholar]