Abstract
Objectives
Zollinger-Ellison Syndrome (ZES) is characterized by hypergastrinemia and gastric acid hypersecretion resulting in peptic ulcer disease, diarrhea and weight loss. Acid secretion can be controlled with medication and biochemical cure is possible with surgery. Data on how these interventions affect patients’ weight are lacking. We aimed to determine how medical and surgical acid control affects weight over time.
Methods
We performed a retrospective cohort study on 60 ZES patients. Acid control was achieved with appropriate dose proton pump inhibitor (PPI) therapy. Surgery was performed for curative intent when appropriate. Weight change was assessed versus pre-acid control or immediate pre-operative weights and expressed as absolute and percent change from baseline at 6, 12, 18 and 24 months.
Results
A total of 30 PPI-controlled patients and 20 surgery-controlled patients were analyzed. Weight gain was noted at all-time points while on appropriate dose PPI therapy (p<0.005). Of patients who had surgery with curative intent, weight gain was noted at 12 months (7.9%, p=0.013) and 18 months (7.1%, p=0.007). There was a trend toward weight gain seen at all-time points in the patients who were surgically cured.
Conclusion
These data represent a novel description of weight gain after acid suppression in ZES.
Keywords: gastrinoma, proton pump inhibitor, neuroendocrine tumor, malabsorption
Introduction
Zollinger-Ellison Syndrome (ZES) is a rare disorder characterized by a gastroenteropancreatic neuroendocrine tumor (GEP-NET), tumoral release of gastrin and gastric acid hypersecretion. It is the result of ectopic production of gastrin from a GEP-NET most commonly found in the duodenum followed by the pancreas.1 ZES was first described in 1955 in a case series of 6 patients and the classic presentation is of refractory peptic ulcer disease complicated by gastrointestinal bleeding, obstruction and perforation.2,3,4 In addition to these complications, hypersecretion results in diarrhea and weight loss.5 Weight loss is related to nutrient malabsorption and steatorrhea. It is a presenting sign of ZES in up to 53% of cases.6
Management of ZES is dependent on multiple endocrine neoplasia type 1 (MEN-1) status but involves medical control of the gastric acid hypersecretion with proton pump inhibitors (PPI) and subsequent surgical resection of the gastrinoma with curative intent in select cases7. PPI therapy is effective in the majority of ZES patients in controlling gastric acid hypersecretion.8,9,10 In appropriately chosen patients, surgical cure is possible in non-MEN-1 related ZES in up to 30% of cases.11,12
Despite data that medical control and surgical cure of ZES is possible, little is known of its resultant effect on patients’ weight. Weight loss is common prior to the diagnosis, but it is unknown what to expect following initiation of therapy. Several case reports note weight gain with acid suppression and expert opinion suggests monitoring for weight gain with regulation of hypersection13,14; however studies to date have not quantified that change. We hypothesized that patients who have effective acid control would gain weight over time.
Materials and Methods
Patients
This is a retrospective cohort study of 60 patients followed for ZES at the Hospital of the University of Pennsylvania from 1994 through 2014. The diagnosis of ZES was consistent with established criteria, using an elevated serum gastrin (>100 pg/mL) in the presence of gastric acid hypersecretion (>15 mEq/h or >10 mEq/h if the patient had previously undergone gastric acid-reducing surgery), as well as positive provocative testing with secretin stimulation (a rise in serum gastrin >200 pg/mL after intravenous injection) or with calcium infusion (a rise in serum gastrin >395 pg/mL), a positive histologic diagnosis of gastrinoma, or a combination of these criteria.15,16 The study was approved by the Institutional Review Board.
Patient level data were abstracted from the medical record, including demographic data, history of MEN-1, extent of disease, type of surgery, surgical outcome, dates of PPI administration and dosage change (if applicable) and weight over time. Appropriate dose PPI therapy was defined as a basal acid output (BAO) < 10 mEq/hr in patients with intact stomachs, a BAO < 5mEq/hr after acid-reducing surgery or the absence of hypersecretory symptoms on at least twice daily full dose PPI if no BAO was performed.
Patients were excluded from the analysis of PPI effects on weight if pre-PPI initiation or dose optimization weights were unavailable. Patients were excluded from the analysis of surgical effects on weight if their surgery was not with curative intent or if they had a contravening reason for weight change, including the development of decompensated organ failure unrelated to neuroendocrine tumor. All patients in the surgical group were on PPI prior to surgery. Surgical cure was defined as a normal fasting serum gastrin and the absence of imagable tumor postoperatively.
Outcome Measurement
The primary outcome in our study was weight change after control of acid production in the setting of a new diagnosis of ZES or after dose titration of PPI. We evaluated the weight change from baseline, defined as the most proximal weight available prior to surgery or initiation of appropriate dosing of PPI therapy. Weight measurements were extracted from the medical record.
Statistical Analysis
Descriptive statistics were calculated for all variables, including mean, standard deviation and confidence intervals for continuous variables and frequencies for categorical values. Comparisons were made between patients’ baseline weight and those at 6, 12, 18 and 24 months after both optimal medical therapy and surgery with curative intent. Change in weight was measured as an absolute and relative percentage change from baseline. The two-tailed t-test was applied for continuous variables. All statistical analyses were performed with STATA software (version13.0; StataCorp, College Station, Tx). Traditional levels of statistical significance were applied with P values less than 0.05 considered significant.
Results
Patient Characteristics
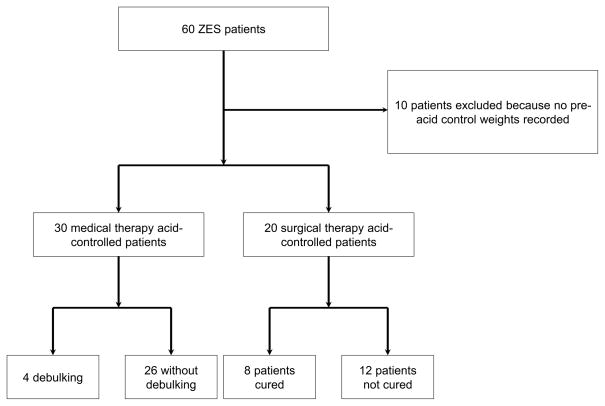
As shown in Figure 1, there were 60 patients with ZES identified in our cohort. Of these, 10 patients were excluded from analysis because they did not have surgery with curative intent or pre-PPI initiation weights were unavailable. There were 30 patients who were not eligible for surgical cure and therefore were treated with PPI exclusively. This constitutes the medical acid control (MAC) group. Four of these patients eventually required surgical debulking for symptom management, but all such operations occurred greater than two years after initial acid control, i.e., beyond the period in this study. A total of 20 patients had surgery with curative intent and were analyzed in the surgical acid control (SAC) group.
Figure 1.
Flow diagram of Study Subjects
The demographic data for our cohort are summarized in Table 1. The median ages of the patients in the MAC and SAC groups were 58.4 ± 16.8 years and 57.2 ± 12.7 years, respectively. There were 17 (56.7%) females in the MAC group and 12 (60.0%) in the SAC group. The majority were white in both groups. MEN-1 syndrome was more common in the MAC group (40%) compared to the SAC group (10%). The maximal extent of disease was restricted to locoregional lymph nodes in 5 (16.7%) versus 15 (75%) patients in the MAC and SAC groups, respectively. Liver metastases were seen in 11 (36.7%) versus 4 (20%) patients in the MAC and SAC groups, respectively. None of the MAC patients were cured of their disease while 8 (40%) in the SAC group were cured.
Table 1.
Baseline characteristics of ZES cohort, separated by medical acid control group and surgical acid control group.
| Characteristics | All Cases (n=60) | MAC Group (n=30) | SAC Group (n=20) |
|---|---|---|---|
| Mean age, years (SD) | 56.9 (14.8) | 58.4 (16.8) | 57.2 (12.7) |
| Female (%) | 33 (55.0) | 17 (56.7) | 12 (60.0) |
| White (%) | 50 (83.3) | 25 (83.3) | 17 (85.0) |
| MEN (%) | 20 (33.3) | 12 (40.0) | 2 (10.0) |
| Maximal extent of disease | |||
| Locoregional lymph nodes (%) | 24 (40.0) | 5 (16.7) | 15 (75) |
| Liver metastases (%) | 20 (33.3) | 11 (36.7) | 4 (20.0) |
| Cured (%) | 8 (13.3) | N/A | 8 (20.0) |
MAC: Medical acid control; SAC: Surgical acid control; SD: Standard deviation; N/A: Not applicable
Medical Acid Control
Of the 30 patient MAC cohort at the time of enrollment: 7 (23%) patients were not on any therapy, 15 (50%) of the cohort were already on once daily PPI therapy that was increased to twice daily dosing and 8 (27%) patients were already on twice daily PPI therapy that was most commonly doubled in strength, ie: omeprazole 20mg twice daily to 40mg twice daily. The median duration of any PPI therapy prior to dose optimization (14 months IQR: 8 mo- 23mo) was available in 13 out of the 23 (56%) patients on therapy at the time of enrollment.
The change in weight in the MAC group is detailed in Table 2. The mean BMI of patients in the MAC group at baseline was 27.78 ± 7.57 kg/m2 with a mean weight of 171 ± 51.3 lbs. The absolute change in weights at 6, 12, 18 and 24 months were 9.5, 11.1, 18.2 and 15.1 pounds, respectively. These changes were highly significant at all-time points.
Table 2.
Absolute change in weight from baseline in the medical acid control group over time.
| Time* | N | Mean weight pre-acid control (SD) | Mean weight post-acid control (SD) | P value |
|---|---|---|---|---|
| 0 | 30 | 171.0 (51.3) | N/A | N/A |
| 6 | 29 | 170.4 (52.1) | 179.9 (51.9) | <0.001 |
| 12 | 16 | 164.1 (48.2) | 175.2 (41.2) | 0.001 |
| 18 | 11 | 161.4 (48.2) | 179.6 (44.8) | <0.001 |
| 24 | 13 | 164.1 (39.7) | 179.2 (40.7) | 0.003 |
Time in months; lbs: Pounds; SD: Standard deviation; N/A: Not applicable
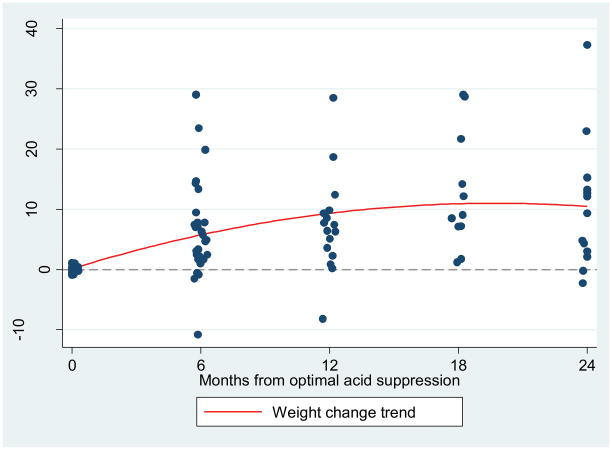
Weights were then measured as a percent change from baseline using each patient as their own reference. Analysis was conducted at the same intervals (Table 3). As seen in Figure 2, patients were noted to gain weight at 6 months (6.4%), 12 months (7.4%), 18 months (13.1%) and 24 months (10.0%) while on appropriate dose PPI therapy. Patient weight increases were highly statistically significant at all measured times (p<0.005). A subgroup analysis was performed by MEN status. All patients who had gastrinoma associated with MEN-1 syndrome also demonstrated significant weight gain at all time points. However, patients with sporadic disease only showed significant increase in body weight at 6 and 12 months after appropriate acid control.
Table 3.
Percent change from baseline weight in the medical acid control group over time.
| Time* | N | Mean percent change from baseline weight post-acid control (95% CI) | P value |
|---|---|---|---|
| 6 | 29 | 6.4 (3.3, 9.4) | <0.001 |
| 12 | 16 | 7.4 (3.1 11.7) | 0.002 |
| 18 | 11 | 13.1 (6.6, 19.5) | 0.001 |
| 24 | 13 | 10.0 (3.5, 16.4) | 0.006 |
Time in months; CI: Confidence interval
Figure 2.
Percent change in weight over time from baseline in medical acid controlled (MAC) group.
Surgical Acid Control
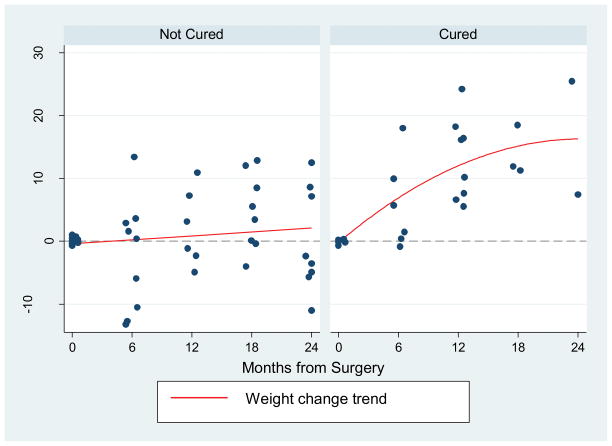
There were 20 patients who had surgery with curative intent and all were on PPI prior to surgery. The mean BMI of patients in the SAC group at baseline was 25.77± 6.88 kg/m2 with a mean weight of 168.4 ± 55.3 lbs. The mean Duodenal wedge resection with lymph node exploration was the primary surgical procedure (n =16, 80%). Among the other patients who had surgery with curative intent, two underwent pancreatic enucleation, one had a partial hepatectomy due to a primary liver tumor and one patient underwent a Billroth type II procedure with lymph node exploration. Weight change was assessed as a percent change from baseline and was significantly increased at 12 and 24 months after surgery. As seen in Table 4, patients had a mean weight increase of 8.4% (p=0.002) at 12 months and 7.1% (p=0.007) at 18 months, respectively. Patient weight change was not significantly different at 6 months post-operatively and the observed weight gain was not durable at 24 months after surgery. These findings were seen in both the surgically cured as well as the non-cured patients. However, as demonstrated in Figure 3, there was a trend toward weight gain seen in the cured group that was not evident in the group with residual disease.
Table 4.
Percent change from baseline weight in surgical acid control group over time.
| Time* | N | Mean percent change from baseline weight post-surgical control (95% CI) | P value |
|---|---|---|---|
| 6 | 15 | 1.2 (−3.9,6.2) | 0.628 |
| 12 | 14 | 8.4 (3.7, 13.1) | 0.002 |
| 18 | 11 | 7.1 (2.4, 11.7) | 0.007 |
| 24 | 10 | 3.3 (−4.7, 11.2) | 0.378 |
Time in months; CI: Confidence interval
Figure 3.
Percent change in weight over time from baseline in surgical acid controlled (SAC) group divided by biochemical cure status
Discussion
This study is the first to examine weight change following the diagnosis of ZES in relation to acid control. We show that once acid control is achieved with appropriate dose PPI based on BAO on therapy or lack of symptoms on at least twice daily PPI, there is significant weight gain over time. The weight gain is durable for at least two years after optimal dosing. Once hormonal secretion was controlled with anti-secretory therapy, patients who underwent surgical intervention with curative intent gained additional weight above that seen with PPI therapy alone.
In ZES, the etiology of weight loss is likely multifactorial. Abdominal pain is a symptom of gastric acid hypersecretion in 47– 85% of ZES patients at the time of diagnosis, which may result in reduced caloric intake.5, 6 The hypergastrinemia and resultant gastric acid hypersecretion result in fat and nutrient malabsorption, further confounding the malnourished state.
Accepting that weight loss occurs prior to a diagnosis of ZES, we aimed to determine if weight gain occurs after patients are appropriately managed. There are several proposed explanations for why patients may gain weight once acid control is achieved. With suppression of acid hypersecretion, patients generally feel better and have restoration of protein-fat absorption that accounts for the rapid weight gain seen in the first 6 months. Alternatively, patients may alter their caloric intake during the hypersecretory phase to try to prevent weight loss. Once they are no longer malabsorbing fat, their increased caloric intake continues as a learned behavior resulting in rapid weight gain. This may be the leading candidate explanation as only 53% of patients in the largest ZES cohort to date had weight loss at the time of diagnosis.6 Our patients were actually overweight even at their nadir weights, according to World Health Organization body mass index (BMI) criteria.17 Finally, there is possibly a hormonal basis for the weight gain on top of the simple caloric input and absorption theories. As seen in roux-en-y gastric bypass, there are rapid changes in circulating concentrations of leptin, insulin-like growth factor 1, gastric inhibitory polypeptide and other hormones involved in body weight regulation that are also possibly affected by gastric acid hypersecretion.18
The most rapid weight gain was observed in the MAC group during the first six months of follow-up with a mean increase of 6.4% of body weight (9.5lbs, P < 0.0001). The rate of gain stabilized out to 24 months but was durable throughout this period. At 24 months, patients had a mean increase of 10% of total body weight above time zero (15.1lbs, p = 0.006). This weight gain is far above the expected annual weight gain on a standard American diet (3.35lbs over 4 year increments) as seen in the Nurses’ Health Study.19 The mean percent change in body weight is significant at all-time points when compared to time zero.
In a subgroup analysis, MEN-1 patients had significant weight gain even at 24 months from optimal medical acid control while those with sporadic disease only had significant weight gain at 6 and 12 months. This is more likely on the basis of fundamental differences in disease phenotype rather than reflective of peculiarities of our cohort. Patients with ZES and MEN-1 have lower rates of metastatic disease at the time of diagnosis.20 This is consistent with our findings. MEN-1 patients in our cohort had metastatic disease in 20% of cases versus 40% in the sporadic group. There is debate about whether patients with MEN-1 have improved overall survival compared to sporadic disease. Our previously published data suggest there is at least an improved disease specific mortality in patients with ZES and MEN-1.20,21 In addition, our MAC group only includes sporadic disease patients that were not eligible for curative surgery, which is due to either the presence of metastatic disease or other comorbidities that limited their surgical candidacy. Patients with sporadic tumors may have had a more progressive course than the MEN-1 patients in the MAC group. Among the four patients who ultimately had debulking surgery in the MAC group, all had sporadic disease. Therefore, it is possible that the MEN-1 patients in the MAC group have more indolent disease than the sporadic ZES patients. This fact could account for the weight difference seen at 18 and 24 months.
Our study is not without limitations. The cohort is small, though this represents one of the largest ZES series ever reported. Nonetheless, it may not have had the power to detect a difference in weight among surgical patients two years out from their operation. Given the sample size, we were unable to systematically account for potential confounders that could affect weight, including smoking behavior, socioeconomic status and comorbidities aside from MEN-1. However, we did restrict analysis of patients who developed decompensated organ failure for reasons other than ZES, which should substantially reduce any bias that may be related to comorbid conditions.
While our study included 60 patients, only 30 who did not undergo surgery had pre-acid control weights available (our MAC group). This is largely a result of the widespread use of PPI therapy for many gastrointestinal complaints that can overlap with ZES. Given the frequent use of anti-secretory therapy in the population, there is a delay in diagnosis of 5-8 years of ZES even in patients with established MEN-1 syndrome.3,22 The diagnosis is more difficult to make in the setting of active PPI use.23 Many of our patients had been on some form of anti-secretory therapy for years prior to consideration of ZES and subsequent referral to our center, making it unclear in several cases which weight constituted a true immediately pre-acid control weight. Therefore only patients with clearly documented continued BAO acid hypersecretion on therapy or symptoms that needed dose titration with available weights at that visit were analyzed (N=30). Of those 30 patients, 23 were already on some PPI therapy at the time of enrollment albeit underdosed. Because of this partial therapy, this study may actually underestimate the weight gain seen with medical therapy.
Management of ZES is dependent on control of acid hypersecretion and on growth of the gastrinoma itself. In an era of effective pharmacotherapy, it is the 40–60% of cases20 with malignant gastrinoma that is the major determinant of mortality in ZES.24 Early surgical intervention with attempt at excision of the gastrinoma has been shown to reduce the risk of metastatic disease to the liver25 as well as improve both disease and all-cause mortality.21 Because of the benefits seen with surgical intervention, it is plausible that surgical resection of the gastrinoma would have an independent benefit on weight above that seen with acid secretion therapy alone.
Twenty patients with sporadic ZES were operated on with curative intent and 8 patients (40%) achieved biochemical cure. In the initial post-operative period, there was no significant weight change from baseline. At year one from surgery and for another 6 months there was an increase in weight above that seen alone with acid secretion therapy. It is expected that patients have a recovery time following a major abdominal operation and thus it is reasonable that no change in weight was seen during the first six months. Whereas control of the malabsorptive process and behavioral modifications may explain the weight gain in the MAC group, directly eliminating the gastrinoma may explain the weight gain seen in the SAC group. The etiology of death in 70% of patients in a series of ZES cases was tumor related cachexia.24 It is possible that some tumor related catabolism could lead to weight loss independent of gastric acid hypersecretion that would be eliminated with effective surgical resection. This phenomenon could explain the subsequent weight loss and return to baseline seen at two years, which likely is reflective of tumor growth in a cohort that had only 40% biochemical cure and a 20% rate of metastatic disease to the liver. Evaluating the biochemically cured patients only, there is a trend towards weight gain not seen in the group with residual disease.
In summary, these data represent a novel description of weight gain after gastric acid suppression in ZES. With appropriate antisecretory therapy, patients gain a significant amount of weight with the most rapid increase seen in the first six months. There is an additional weight gain following surgical resection of the gastrinoma with a trend towards significance in those achieving biochemical cure. These findings suggest following weight over time might be a clinically useful surrogate marker for disease control in ZES patients.
Acknowledgments
The authors would like to thank Chris Wirtalla for his help with statistical planning.
DCM and BB conceived the study with significant contributations to study design by BPR and DAL. BPR and DAL were responsible for data management statistical analysis and manuscript preparation. All authors were involved in the interpretation of the data, contributed toward critical revision of the manuscript and approved the final draft.
Funding: This study was in part funded by NIH/NIDDK T32, DK007740
Abbreviations
- BAO
basal acid output
- BMI
body mass index
- GEP- NET
gastroenteropancreatic neuroendocrine tumor
- MAC
medical acid control
- MEN-1
multiple endocrine neoplasia type 1
- PPI
proton pump inhibitor
- SAC
surgical acid control
- ZES
Zollinger-Ellison Syndrome
Footnotes
Statement of Interests: David Metz has received speaker fees from Takeda. The remaining authors have nothing to disclose..
References
- 1.Osefo N, Ito T, Jensen RT. Gastric acid hypersecretory states: recent insights and advances. Curr Gastroenterol Rep. 2009;11:433–441. doi: 10.1007/s11894-009-0067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zollinger RM, Ellison EH. Primary peptic ulcerations of the jejunum associated with islet cell tumors of the pancreas. Ann Surg. 1955;142:709–728. [PMC free article] [PubMed] [Google Scholar]
- 3.Ellison EH, Wilson SD. The Zollinger-Ellison Syndrome: Re-Appraisal and Evaluation of 260 Registered Cases. Ann Surg. 1964;160:512–530. doi: 10.1097/00000658-196409000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roy PK, Venzon DJ, Shojamanesh H, et al. Zollinger-Ellison syndrome: clinical presentation in 261 patients. Medicine. 2000;79:379–411. doi: 10.1097/00005792-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Wilcox CM, Seay T, Arcury JT, et al. Zollinger–Ellison syndrome: Presentation, response to therapy, and outcome. Digestive and Liver Disease. 2011;43:439–443. doi: 10.1016/j.dld.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Stage JG, Stadil F. The clinical diagnosis of the Zollinger-Ellison syndrome. Scand J Gastroenterol Suppl. 1979;53:79–91. [PubMed] [Google Scholar]
- 7.Kulke MH, Anthony LB, Bushnell DL, et al. NANETS treatment guidelines: well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas. 2010;39:735–752. doi: 10.1097/MPA.0b013e3181ebb168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nieto JM, Pisegna JR. The role of proton pump inhibitors in the treatment of Zollinger-Ellison syndrome. Expert Opin Pharmacother. 2006;7:169–175. doi: 10.1517/14656566.7.2.169. [DOI] [PubMed] [Google Scholar]
- 9.Metz DC, Pisegna JR, Ringham GL, et al. Prospective study of efficacy and safety of lansoprazole in Zollinger-Ellison syndrome. Dig Dis Sci. 1993;38:245–256. doi: 10.1007/BF01307541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ito T, Igarashi H, Uehara H, et al. Pharmacotherapy of Zollinger-Ellison syndrome. Expert Opin Pharmacother. 2013;14:307–321. doi: 10.1517/14656566.2013.767332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norton JA, Fraker DL, Alexander HR, et al. Surgery to cure the Zollinger–Ellison syndrome. N Engl J Med. 1999;341:635–644. doi: 10.1056/NEJM199908263410902. [DOI] [PubMed] [Google Scholar]
- 12.Ruszniewski P, Podevin P, Cadiot G, et al. Clinical, anatomical, and evolutive features of patients with the Zollinger-Ellison syndrome combined with type I multiple endocrine neoplasia. Pancreas. 1993;8:295–304. doi: 10.1097/00006676-199305000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Richardson CT, Walsh JH. The value of a histamine H2-receptor antagonist in the management of patients with the Zollinger-Ellison syndrome. N Engl J Med. 1976;294:133–135. doi: 10.1056/NEJM197601152940304. [DOI] [PubMed] [Google Scholar]
- 14.Termanini B, Gibril F, Sutliff VE, et al. Effect of long-term gastric acid suppressive therapy on serum vitamin B12 levels in patients with Zollinger-Ellison syndrome. Am J Med. 1998;104:422–430. doi: 10.1016/s0002-9343(98)00087-4. [DOI] [PubMed] [Google Scholar]
- 15.Metz DC, Jensen RT. Endocrine tumors of the pancreas. In: Haubrich WB, Schaffner F, Berk JE, editors. Bockus Gastroenterology. 5. Vol. 4. Philadelphia, PA: WB Saunders; 1994. pp. 3002–3034. [Google Scholar]
- 16.Metz DC. Peptic ulcer disease; diagnosis and treatment. In: DiMarino AJ, Benjamin SB, editors. Gastrointestinal Disease: An Endoscopic Approach. Vol. 1. Cambridge, MA: Blackwell Science Inc; 1997. pp. 285–304. Chapter 22. [Google Scholar]
- 17.World Health Organization. Physical status: The use of and interpretation of anthropometry. Report of a WHO Expert Committee. 1995 [PubMed] [Google Scholar]
- 18.Rubino F, Gagner M, Gentileschi P, et al. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg. 2004;240:236–242. doi: 10.1097/01.sla.0000133117.12646.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mozaffarian D, Hao T, Rimm EB, et al. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber HC, Venzon DJ, Lin JT, et al. Determinants of metastatic rate and survival in patients with Zollinger-Ellison syndrome: a prospective long-term study. Gastroenterology. 1995;108:1637–1649. doi: 10.1016/0016-5085(95)90124-8. [DOI] [PubMed] [Google Scholar]
- 21.Singh MH, Fraker DL, Metz DC. Importance of surveillance for multiple endocrine neoplasia-1 and surgery in patients with sporadic Zollinger–Ellison syndrome. Clin Gastroenterol Hepatol. 2012;10:1262–1269. doi: 10.1016/j.cgh.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 22.Jensen R. Management of the Zollinger-Ellison syndrome in patients with multiple endocrine neoplasia type 1. J Intern Med. 1998;243:477–488. doi: 10.1046/j.1365-2796.1998.00281.x. [DOI] [PubMed] [Google Scholar]
- 23.Ito T, Cadiot G, Jensen RT. Diagnosis of Zollinger-Ellison syndrome: increasingly difficult. World J Gastroenterol. 2012;18:5495. doi: 10.3748/wjg.v18.i39.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu F, Venzon DJ, Serrano J, et al. Prospective study of the clinical course, prognostic factors, causes of death, and survival in patients with long-standing Zollinger-Ellison syndrome. J Clin Oncol. 1999;17:615–630. doi: 10.1200/JCO.1999.17.2.615. [DOI] [PubMed] [Google Scholar]
- 25.Fraker DL, Norton JA, Alexander HR, et al. Surgery in Zollinger-Ellison syndrome alters the natural history of gastrinoma. Ann Surg. 1994;220:320–8. doi: 10.1097/00000658-199409000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]