Abstract
Rationale
Group II metabotropic glutamate receptors (mGluR2 and mGluR3) have been suggested to play an important role in mediation of drug-reinforced behaviors, as well as in the mechanisms underlying relapse in abstinent subjects. The prototypical mGluR2/3 agonist, LY379268, has been shown to attenuate nicotine reinforcement and cue-induced reinstatement of drug seeking in rats, as well as reinstatement induced by drug-associated stimuli and contexts across different drugs of abuse (i.e., cocaine, heroin, and methamphetamine). However, in primates, LY379268 has been shown to produce conflicting results on abuse-related effects of cocaine, and there are no data available for nicotine.
Objectives
To explore the therapeutic potential of mGluR2/3 agonists, we compared the effects of LY379268 (0.03–1.0 mg/kg) on nicotine, cocaine, and food self-administration under a fixed-ratio (FR10) schedule in three separate groups of squirrel monkeys. Moreover, we studied the effects of LY379268 on nicotine/cocaine priming-induced and cue-induced reinstatement of drug-seeking behavior in nicotine- and cocaine-experienced groups of animals.
Results
LY379268 blocked nicotine, but not cocaine, self-administration in monkeys. There was a partial overlap between doses that affected nicotine and food self-administration. In abstinent monkeys, LY379268 dose-dependently blocked nicotine, but not cocaine, priming-induced reinstatement of drug seeking. In both cocaine-experienced and nicotine-experienced groups of animals, LY379268 potently reduced cue-induced reinstatement of drug-seeking behavior.
Conclusions
The present findings provide strong support for the potential utility of mGlu2/3 receptor agonists for the treatment of nicotine dependence and suggest their utility for prevention of relapse induced by environmental cues associated with drug taking.
Keywords: Cocaine, Glutamate, Nicotine, Reinstatement, Relapse, Self-administration, Smoking cessation, Squirrel monkey
Introduction
Alterations in glutamatergic neurotransmission that occur in response to chronic nicotine exposure may be critically involved in nicotine dependence by contributing to the motivation to maintain nicotine intake and the reinitiation of intake after a period of abstinence. Nicotine exerts its primary rewarding effects partly by activation of excitatory nicotinic acetylcholine receptors on glutamate terminals in the ventral tegmental area (VTA) that provide excitatory input to mesolimbic dopamine neurons projecting to the nucleus accumbens (NAc) shell (Mansvelder et al. 2002; Picciotto and Corrigall 2002). Presynaptic metabotropic glutamate 2/3 (mGlu2/3) autoreceptors, that negatively modulate excitatory glutamate transmission (Schoepp et al. 1999), are expressed in corticolimbic brain areas involved in reward processes, including the VTA and the NAc (Ohishi et al. 1993a, b; Richards et al. 2005; Tamaru et al. 2001). Accordingly, stimulation of mGlu2/3 receptors decrease extracellular glutamate in the NAc (Baker et al. 2002; Xi et al. 2002) and dopamine in the NAc shell, but not in the core (Greenslade and Mitchell 2004). In contrast, mGlu2/3 receptor blockade increases dopamine in the NAc shell (Karasawa et al. 2006). Drug-paired cues elicit an increase in glutamate within the NAc (Hotsenpiller et al. 2001). Similar increases in NAc glutamate are associated with drug-induced reinstatement of drug-seeking behavior (Cornish and Kalivas 2000; McFarland et al. 2003). Furthermore, microinjection of the mGlu2/3 receptor agonist LY379268 into the VTA or NAc shell, but not the NAc core, attenuates context-induced reinstatement of heroin-seeking in rats (Bossert et al. 2004, 2006).
Modulating glutamate transmission in limbic brain sites also appears to modulate drug self-administration behavior and the influence of drug-associated cues on behavior. Notably, administration of the mGluR2/3 agonist, LY379268, reduces intravenous cocaine self-administration behavior and cue-induced reinstatement of extinguished cocaine-seeking behavior under certain conditions in both rodents and non-human primates (Adewale et al. 2006; Baptista et al. 2004; Jin et al. 2010; Peters and Kalivas 2006). However, Bauzo et al. (2009) did not find a prominent effect of LY379268 on cocaine-maintained behavior in squirrel monkeys. We have recently shown that LY379268 also reduces nicotine self-administration behavior and cue-induced reinstatement of nicotine-seeking behavior in abstinent rats (Liechti et al. 2007) but has no effect on food-maintained behavior, except at high dosages. Interestingly, nicotine-induced increases in dopamine levels in the nucleus accumbens were blocked with LY379268 only when nicotine was self-administered in the presence of the cues/context previously associated with nicotine intake (D’Souza et al. 2011). The effects of this mGluR2/3 agonist on nicotine self-administration behavior and cue-induced reinstatement of nicotine-seeking behavior are likely mediated by antagonizing nicotine- and cue-induced increases in glutamate transmission. These findings in rats suggest that mGluR2/3 agonists may be useful for the treatment of nicotine dependence in humans. However, no validation has been performed in a non-human primate model of nicotine self-administration.
Nicotine maintains self-administration behavior at a high rate in squirrel monkeys, in contrast to lower rates in rats under similar conditions (Corrigall 1999; Donny et al. 2003; Goldberg et al. 1981; Le Foll et al. 2007; Markou et al. 2004; Mascia et al. 2011; Panlilio et al. 2012). This behavior is related to the effects of nicotine, because after training, lever pressing extinguished when the nicotine solution was replaced by saline solution. Furthermore, varying the dose of nicotine that the monkey could self-administer resulted in an inverted U-shaped dose-response curve (Goldberg et al. 1981; Goldberg and Spealman 1982; Le Foll et al. 2007; Sannerud et al. 1994) that is typically seen with other drugs of abuse with this self-administration procedure (Goldberg 1973; Justinova et al. 2003, 2008). The aim of the present study was to explore the effects of the mGluR2/3 agonist LY379268 on abuse-related effects of nicotine in squirrel monkeys. However, in light of conflicting results regarding the effects of LY379268 on cocaine reinforcement and reinstatement in squirrel monkeys under a second-order schedule (that is considered a model of human drug seeking), we also performed parallel experiments with cocaine. Thus, we assessed the effects of LY379268 in squirrel monkeys that had learned to intravenously self-administer nicotine or cocaine under a fixed-ratio schedule of reinforcement, an animal model that is considered reflective of human drug-taking behavior. For comparison, we assessed the effects of LY379268 in squirrel monkeys that had learned to respond for food pellets under similar conditions. Finally, we assessed the effects of LY379268 on both nicotine/cocaine priming- and cue-induced reinstatement of extinguished nicotine- and cocaine-seeking behavior in abstinent squirrel monkeys.
Materials and methods
Subjects
Ten adult male squirrel monkeys (Saimiri sciureus) weighing 0.8 to 1.1 kg were housed in individual cages in a temperature-and humidity-controlled room with unrestricted access to water. Each animal had a unique numeric or alphanumeric identificator (nicotine self-administering group: 441, 431, 577, 572; cocaine self-administering group: 70F7, 2F19, 39B; food self-administering group: 1549, 30A, 34A). The monkeys were trained to self-administer either nicotine or cocaine prior to the study and had self-administered nicotine, cocaine, or food for over 5 years. During this time, they typically had five self-administration sessions per week. Monkeys were fed (approximately 2 h after the session) a daily food ration consisting of biscuits of high protein monkey diet (Lab Diet 5045, PMI Nutrition International, Richmond, Indiana). The number of biscuits (8–14) was determined for each monkey individually to maintain their body weights at a constant level throughout the study. Monkeys self-administering food pellets had the number of biscuits adjusted to maintain the motivation to perform food-rewarded task. Fresh fruits, vegetables, and environmental enrichment were provided daily. Animals were maintained in facilities fully accredited by AAALAC, and experiments were conducted in accordance with guidelines of the Institutional Animal Care and Use Committee of the Intramural Research Program, NIDA, NIH, DHHS, and 2003 National Research Council Guidelines.
Monkeys were surgically prepared with chronic indwelling venous catheters (polyvinyl chloride; inside diameter 0.38 mm; outside diameter 0.76 mm) (Goldberg 1973), which were passed subcutaneously to the monkey’s back where they exited the skin. The monkeys wore nylon-mesh jackets (Lomir Biomedical, Canada) to protect their catheters.
Apparatus
Experimental chambers and other apparatus used in this study were the same as previously described (Justinova et al. 2003). Test sessions were conducted in sound-attenuating isolation chambers equipped with a Plexiglas chair, a white house light, and white noise for masking of external sound. The chair contained one response lever (Med Associates, USA) mounted on a transparent front wall; each press on the lever with a force greater than 0.2 N produced an audible click and was recorded as a response. Pairs of amber and green stimulus lights, mounted behind the transparent wall of the chair, could be illuminated and used as visual stimuli.
Self-administration procedure
Daily 1-h experimental sessions were typically conducted from Monday through Friday with each monkey. Each session, monkeys were placed into Plexiglas chairs and restrained in the seated position by waist locks. Before the start of the session, catheters were flushed with 1 ml of saline and one injection was delivered (calculated to fill the dead space of the catheter which was about 0.2 ml). The monkeys’ catheters were connected to polyethylene tubing, which passed out of the isolation chambers where they attached to motor-driven syringe pumps. The syringe pumps were calibrated so that duration of each injection was 0.2 s and injection volume was 0.2 ml. At the start of the session, a white house light was turned off and a green stimulus light was turned on. In the presence of the green light, monkeys were required to make 10 responses on the lever (10-response, fixed-ratio schedule of reinforcement (FR10)) to produce an injection of nicotine or cocaine. The completion of 10 responses on the lever turned off the green light and produced an intravenous (i.v.) injection of 30 μg/kg of nicotine in one group of animals or 30 μg/kg of cocaine in another group. Each injection was paired with a 2-s illumination of an amber stimulus light. Each injection was followed by a 60-s time-out period, during which the chamber was dark and lever presses had no programmed consequences.
First, we performed the nicotine and cocaine self-administration studies. Then, after extinction of drug-reinforced responding, we tested the monkeys for cue-induced reinstatement. We then retrained the monkeys to self-administer nicotine or cocaine over 5–10 sessions and then tested for nicotine- or cocaine priming-induced reinstatement, respectively, after extinction of the drug-reinforced responding. Subjects received all LY379268 doses that were studied in their respective group (nicotine, cocaine, or food). One exception was monkey 572 from the “nicotine group” that was not tested in nicotine priming-induced reinstatement study due to catheter failure.
Self-administration studies
This phase of the study was performed over a period of 11–14 weeks in each group of animals. When responding for the training dose of nicotine or cocaine (both 30 μg/kg/injection), was stable for at least five consecutive sessions (less than 15 % variability), testing with the mGluR2/3 receptor agonist LY379268 was then conducted. After three sessions with vehicle pretreatment, pretreatment with each LY379268 dose was tested for five consecutive sessions, followed by recovery of stable nicotine or cocaine baseline responding. The same schedule of reinforcement and experimental protocol was also used in a group of monkeys self-administering food pellets (three animals). Different doses of LY379268 were tested in the following order: 0.3, 1, and 0.1 mg/kg in “nicotine” group, 0.3 and 1 mg/kg in “cocaine” group, and 0.3, 1, 0.1, and 0.03 mg/kg in “food” group.
Reinstatement studies
This phase of the study was performed over a period of 9 to 10 weeks. In order to separately determine the reinstatement effects of a priming injection and drug-associated cues, we use two different extinction protocols (described below) that allow us to isolate the effects of these independent variables.
Cue-induced reinstatement
Before cue-induced reinstatement testing began, monkeys were allowed to self-administer the training dose of nicotine or cocaine until they reached a stable baseline (5–10 sessions). In subsequent extinction sessions, drug injections and all cues associated with drug injections were removed. This means that catheters were not connected to a pump, monkeys were not receiving infusions or visual cue presentations, and there was no time-out after completing an FR10. After 2 or 3 days of extinction, we tested for cue-induced reinstatement of extinguished drug-seeking behavior after vehicle pretreatment (i.m.) by allowing delivery of a saline injection paired with the drug-associated cues followed by a time-out after the completion of each FR10. Then, the effect of pretreatment with LY379268 (0.01–1.0 mg/kg) on cue-induced reinstatement was studied. Each reinstatement test was a single session, which was preceded and followed by extinction session(s) with vehicle pre-treatment (i.m.) 30 min before the sessions. LY379268 (0.3 or 1 mg/kg) was also given i.m. prior to an extinction session to study the effects of LY379268 on extinguished drug seeking. The cue-induced reinstatement with vehicle pretreatment was repeated after the conclusion of LY379268 testing to ascertain the reliability of the reinstatement effect over repeated tests.
Priming-induced reinstatement
After the completion of cue-induced reinstatement testing, the monkeys were returned to baseline nicotine or cocaine self-administration for 5–10 sessions. In subsequent extinction sessions, lever presses led to i.v. saline infusions plus the visual cues that had been associated previously with nicotine or cocaine. After at least two sessions of extinction, when responding had reached a low, stable level, a priming injection of nicotine or cocaine (both 0.1 mg/kg i.v.) was given immediately before the next session, during which responding (FR10) continued to produce only i.v. saline injections and the discrete visual cues. The effects of LY379268 (0.1, 0.3, or 1 mg/kg, i.m.) or vehicle were studied as pretreatments given prior to the nicotine or cocaine priming sessions. A non-contingent i.v. injection of saline was also given before each extinction session as a vehicle control for the drug priming treatment. The 1.0 mg/kg dose of LY379268 was also given i.m. prior to vehicle priming to determine whether LY379268 alone would affect extinguished drug seeking. The nicotine or cocaine priming-induced reinstatement with vehicle pretreatment was repeated after the conclusion of LY379268 testing to ascertain the reliability of the reinstatement effect over repeated tests.
Drugs
Nicotine [(−)-nicotine hydrogen tartrate] was purchased from Sigma Chemical Company (St. Louis, MO, USA). Nicotine was diluted in saline, and the pH of the solution was adjusted to 7.0 with dilute NaOH. Cocaine [(−)-cocaine hydrochloride] was obtained from NIDA, NIH (Rockville, MD) and diluted in saline. The dose of nicotine and cocaine (both 30 μg/kg/injection) for this study was selected based on our previous studies in which this dose maintained maximal rates of responding. The mGluR2/3 agonist LY379268[(1R,4R,5S, 6R)-2-oxa-4-aminobicyclo[3.1.0]hexane-4,6-dicarboxylate] was provided by Dr. Athina Markou (University of California San Diego; custom synthesized by ANAWA) and dissolved in sterile water. LY379268 or the vehicle was always injected i.m. 30 min before the session.
Statistical analysis
Cumulative-response records were obtained during all sessions to assess within-session patterns of responding. Number of lever presses and number of injections per sessions was recorded. Rates of responding during self-administration sessions are expressed as responses per second averaged over the 1-h session, with responding during time-outs not included in the calculations. Injections or pellets per session represent the total number of injections or pellets delivered per 1-h session. All data are presented as mean±S.E.M. Statistical analysis was performed using one-way or two-way repeated measures ANOVA (data met the assumptions of the test) with session and LY379268 dose as factors to assess differences between vehicle and LY379268 pretreatment conditions. In the self-administration study, the effect of treatment with each LY379268 dose was compared with the average of three sessions with vehicle (0 mg/kg) treatment that immediately preceded testing with each LY379268 dose. Post hoc comparisons were performed using the Tukey test. Statistical significance was accepted at the p<0.05 level. SigmaStat software (Systat Software Inc.) was used for all statistical analyses.
Results
Effect of LY379268 pretreatment on responding reinforced by nicotine, cocaine, or food under a fixed-ratio schedule
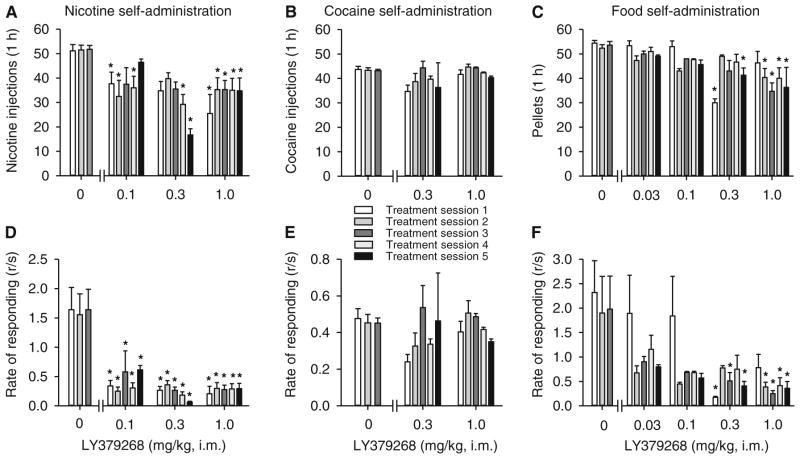
In this study, at the 30 μg/kg injection dose of nicotine, monkeys self-administered an average of 51.47±1.98 injections per 1-h session and response rate in the presence of the green light indicating drug availability averaged 1.61±0.35 responses per second (Fig. 1). Cocaine 30 μg/kg injections maintained self-administration behavior at an average of 43.39±0.89 injections per 1-h session and response rate averaged 0.46±0.03 responses per second. Under the same schedule, monkeys self-administered 52.25±0.46 food pellets over the 1-h session with an average response rate of 1.81±0.51 responses per second. Cocaine maintained lower rates of responding than nicotine or food in the current study, but the cocaine injection dose of 30 μg/kg was the peak of the cocaine dose-response curve as shown in previous studies.
Fig. 1.
Effects of LY379268 on self-administration of nicotine, cocaine, or food under a fixed-ratio schedule (FR10) in monkeys. Number of nicotine 30 μg/kg injections (a), cocaine 30 μg/kg injections (b), or food pellets (c) self-administered per 1-h session and rates of responding (d, e, f) after pretreatment with vehicle (0 mg/kg) or LY379268 (0.03, 0.1, 0.3, and 1 mg/kg) are shown. Each bar represents the mean±SEM from four (nicotine) or three monkeys (cocaine, food). *p<0.05, post hoc comparisons with the average of three sessions with vehicle (0 mg/kg) treatment immediately preceding testing with each LY379268 dose, Tukey test. Note: Ordinates on d, e, f have different scales
In monkeys self-administering nicotine, pretreatment with LY379268, at all three doses (0.1, 0.3, and 1.0 mg/kg), produced consistent statistically significant decreases in the number of injections self-administered per 1-h session during the 5 days of treatment (Fig. 1a; session × LY379268 dose interaction, F(10, 29)=3.69, p<0.004), but number of injections self-administered per session remained above saline self-administration levels (5.93±0.55 injections per session). There were no significant differences between the effects of different doses of LY379268 on number of self-administered nicotine injections (Fig. 1a; effect of dose, F(2, 29)=1.97, n.s.). Rates of responding were significantly reduced by LY379268 pretreatment at all three doses (Fig. 1d; session × LY379268 dose interaction, F(10, 29)=3.33, p<0.006) but stayed above saline self-administration levels (0.02±0.01 responses per second). Self-administration behavior rapidly returned to high levels when LY379268 treatment was discontinued (data not shown). In monkeys self-administering cocaine under FR10, pretreatment with LY379268 at doses 0.3 or 1.0 mg/kg did not affect the number of injections self-administered per 1-h session during the 5 days of treatment (Fig. 1b; session × LY379268 dose interaction, F(5, 10)=0.64, n.s.). Rates of responding were not affected by pretreatment with LY379268 (Fig. 1e; session × LY379268 dose interaction, F(5, 10)=1.26, n.s.). In monkeys self-administering food, LY379268 at doses of 0.3 and 1.0 mg/kg, but not 0.03 and 0.1 mg/kg, decreased the number of pellets earned during the 1-h sessions (Fig. 1c; session × LY379268 dose interaction, F(15, 30)=3.81, p<0.001) and rate of responding (Fig. 1f; session × LY379268 dose interaction, F(15, 30)=2.08, p<0.05).
Effect of pretreatment with LY379268 on nicotine/cocaine priming-induced reinstatement of extinguished drug seeking
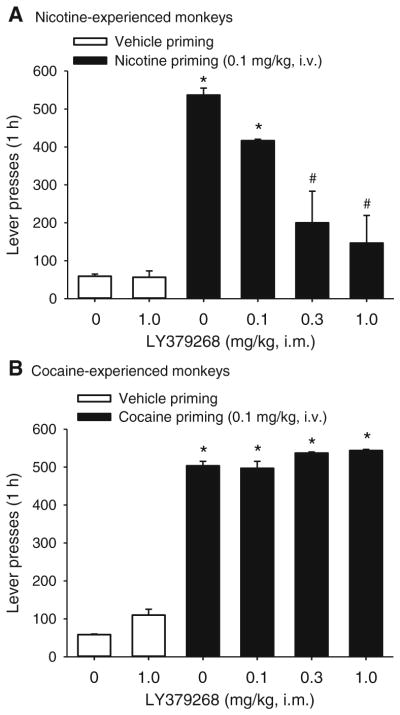
When vehicle was substituted for nicotine in the self-administration procedure (extinction), monkeys’ lever-pressing decreased to low rates (Fig. 2a; lever presses: 59.33 ±5.46; not shown, rate of responding: 0.02±0.01 responses per second). When a non-contingent priming injection of nicotine (0.1 mg/kg, i.v.) was given, lever-pressing was reinstated (Fig. 2a; lever presses: 536.67±18.56; not shown: rate of responding: 1.88±0.55 responses per second) to approximately the same levels that had been obtained during self-administration at the peak nicotine dose (as seen in Fig. 1a, d). Pretreatment with LY379268 (0.1, 0.3, or 1.0 mg/kg) significantly and dose-dependently prevented this priming effect of nicotine (Fig. 2a; effect of LY379268 dose, F(5, 10)= 23.68, p<0.001). When the 1 mg/kg dose of LY379268 was administered prior to a vehicle priming injection, LY379268 did not reinstate extinguished nicotine-seeking behavior (p>0.1).
Fig. 2.
Effects of LY379268 on priming-induced reinstatement of extinguished drug-seeking behavior in nicotine- or cocaine-experienced monkeys. LY379268 (0.1, 0.3, or 1 mg/kg) or vehicle was administered prior to the vehicle, nicotine (a) or cocaine (b) priming injections. Lever presses produced per 1-h session are shown. Bars represent means±SEM from four (a) or three (b) monkeys. “Vehicle priming + 0 mg/kg” bars represent the average of five extinction sessions prior to the test sessions. “Nicotine priming + 0 mg/kg” or “cocaine priming + 0 mg/kg” bars represent the average from two tests. *p<0.05, post hoc comparisons with “vehicle priming + 0 mg/kg” condition; #p<0.05, post hoc comparisons with “nicotine priming + 0 mg/kg” or “cocaine priming + 0 mg/kg,” Tukey test
In cocaine-abstinent monkeys (i.e., when vehicle was substituted for cocaine), a cocaine priming injection (0.1 mg/kg i.v.) reinstated (F(5, 10)=373.65, p<0.001) extinguished cocaine seeking (Fig. 2b; lever presses: 505.00 ±15.28; not shown, rate of responding: 1.02±0.18 responses per second) compared to when the priming injection consisted only of saline (lever presses: 58.33±2.03; rate of responding: 0.02±0.01). Pretreatment with LY379268 (0.1, 0.3, or 1.0 mg/kg) had no effect on cocaine priming-induced reinstatement (Fig. 2b, all p’s>0.1). When the 1 mg/kg dose of LY379268 was administered prior to a vehicle priming injection, it did not reinstate extinguished cocaine-seeking behavior (p>0.1).
Effect of pretreatment with LY379268 on cue-induced reinstatement of extinguished drug seeking
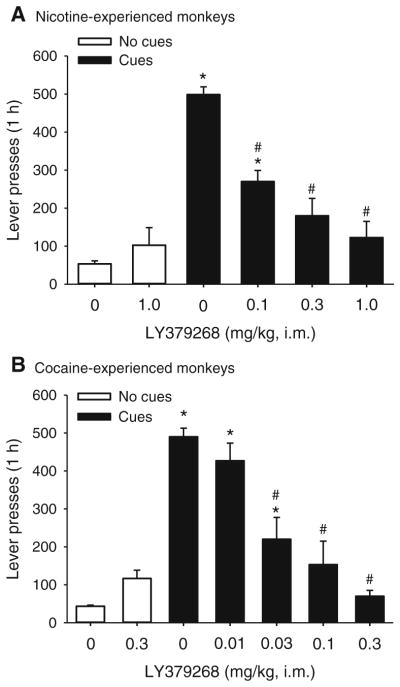
When responding no longer produced nicotine or the interoceptive cues produced by i.v. injection or the visual cues that were previously associated with nicotine, monkeys’ response rates decreased to very low levels (Fig. 3a; lever presses: 53.39±8.09; not shown, rate of responding: 0.02±0.01 responses per second). Reintroduction of all nicotine-associated cues produced significant reinstatement of nicotine-seeking behavior (Fig. 3a; lever presses: 498.50± 20.50; not shown: rate of responding: 1.34±0.26 responses per second). Pretreatment with LY379268 (0.1, 0.3, or 1.0) significantly and dose-dependently attenuated cue-induced reinstatement of nicotine-seeking behavior (Fig. 3a; effect of LY379268 dose: F(5, 15)=20.77, p<0.001). When the most effective dose (1 mg/kg) of LY379268 was administered prior to an extinction session, it did not significantly affect extinguished drug-seeking behavior (p>0.1).
Fig. 3.
Effects of LY379268 on cue-induced reinstatement of extinguished drug-seeking behavior in nicotine- or cocaine-experienced monkeys. LY379268 (0.01, 0.03, 0.1, 0.3, or 1 mg/kg) or vehicle was administered prior to the sessions, in which cue presentations and saline injections were not present or were reinstated. Lever presses produced per 1-h sessions are shown in nicotine- (a) or cocaine-experienced (b) monkeys. Bars represent means±SEM from four (a) or three (b) monkeys.”No cues + 0 mg/kg” bars represent the average of five extinction sessions prior to the test sessions. “Cues + 0 mg/kg” bars represent the average of two tests. *p<0.05, post hoc comparisons with “no cues + 0 mg/kg” condition; #p<0.05, post hoc comparisons with “cues + 0 mg/kg,” Tukey test
Similarly, in cocaine-experienced animals, removal of all cocaine-associated cues and injections lead to very low levels of responding (Fig. 3b; lever presses: 43.44±3.00; not shown, rate of responding: 0.01±0.00 responses per second). Reintroduction of all cocaine-associated cues produced significant reinstatement of cocaine-seeking behavior (Fig. 3b; lever presses: 490.00±22.55; not shown: rate of responding: 1.02 ±0.06 responses per second), which was dose-dependently blocked by pretreatment with LY379268 (0.01, 0.03, 0.1, or 0.3 mg/kg) (Fig. 3b; effect of LY379268 dose: F(6, 12)= 28.21, p<0.001). When the most effective dose (0.3 mg/kg) of LY379268 was administered prior to an extinction session, it did not significantly affect extinguished drug-seeking behavior (p>0.1).
Discussion
In this study, systemic administration of the mGlu2/3 receptor agonist LY379268 decreased intravenous nicotine self-administration under a fixed-ratio schedule in squirrel monkeys. The number of nicotine injections self-administered per session was reduced by approximately 40 %. All tested LY379268 doses had similar effects; thus, effects cannot be described as dose-dependent. The effects lasted for the duration of the treatment and nicotine self-administration behavior recovered to baseline levels upon discontinuation of LY379268 treatment. Acute systemic injections of LY379268 also blocked both nicotine priming-induced and cue-induced reinstatement of nicotine-seeking behavior, and these effects were dose dependent. Thus, mGlu2/3 receptors are promising targets for the development of medications for nicotine dependence. However, the LY379268 doses that blocked nicotine self-administration partially overlapped with doses that also affected monkeys’ responding for food under the same conditions. It is possible that LY379268 at higher doses could produce aversive experiences (e.g., emetic side effects reported by Adewale et al. in 2006 at dose 1 mg/kg and Bauzo et al. in 2009 at dose 3 mg/kg) that could contribute to the disruption of food self-administration behavior. In the current study, we did not observe emesis but cannot exclude the presence of nausea. The overlap between the effects of mGluR2/3 agonists on drug-versus food-reinforced behavior was also shown before in rodent studies (Jin et al. 2010; Liechti et al. 2007; Peters and Kalivas 2006). This indicates that stimulation of mGluR2/3 has a non-selective effect on responding for drug versus non-drug reinforcement. This potentially problematic therapeutic profile could be avoided by using receptor-selective compounds or allosteric modulators (Jin et al. 2010).
There are previous neurochemical data in rodents supporting our findings suggesting that mGluR2/3 activation may serve as a target for developing medications to reduce nicotine reward and relapse. In rats, Liechti et al. (2007) have shown that administration of LY379268, systemically or directly into the posterior VTA or the NAc shell, dose dependently decreased nicotine self-administration at doses that did not affect responding for food. These authors also showed that LY379268 blocked cue-induced reinstatement of both nicotine- and food-seeking behavior. Furthermore, nicotine-induced increases in dopamine levels in the nucleus accumbens were blocked with systemic LY379268 only when nicotine was self-administered in the presence of the cues/context previously associated with nicotine intake (D’Souza et al. 2011). These results, together with the present findings, provide evidence of a critical role for mGlu2/3 receptors in the VTA and the NAc shell in the primary reinforcing effects of nicotine and in the motivational impact of nicotine-associated environmental stimuli.
In contrast to our findings in nicotine-experienced monkeys, LY379268 treatment was ineffective in reducing cocaine self-administration or cocaine priming-induced reinstatement under the same schedule of reinforcement, even at doses that affected food-maintained behavior. Thus, the involvement of mGluR2/3s in the primary reinforcing effects of cocaine seems limited in squirrel monkeys. It is important to note that the effects of LY379268 were studied on only one dose of cocaine (or nicotine) that maintained the maximal rates of responding in our self-administration model. Our finding with a fixed-ratio schedule is in line with another report with squirrel monkeys by Bauzo et al. (2009), which used a second-order schedule of intravenous cocaine injection to study the effects of LY379268. Under a second-order schedule, responding is maintained primarily by the conditioned reinforcing effects of stimuli (cues) intermittently associated with injection of the drug (Arroyo et al. 1998; D’Souza et al. 2011; Everitt and Robbins 2000; Schindler et al. 2002). The study by Bauzo and colleagues found that the effects LY379268 on the reinforcing properties of cocaine and on cocaine priming-induced reinstatement were not robust or consistent across doses. However, our results are in contrast to the results of another study performed in squirrel monkeys (Adewale et al. 2006), where LY379268 dose dependently decreased responding under a second-order schedule of cocaine self-administration and also reduced cocaine priming-induced reinstatement. The exact reason for discrepant results between the two studies using similar second-order schedules (Adewale et al. 2006; Bauzo et al. 2009) is not clear. Regarding our study, it is possible that lower rates maintained by cocaine self-administration as compared to nicotine or food self-administration could have contributed to the different findings against these three reinforcers. However, the complete lack of effect of LY379268 on cocaine priming-induced reinstatement, where the rates of responding induced by a cocaine prime reached higher levels, supports our self-administration findings with cocaine.
The effects of LY379268 on cocaine self-administration in rodents also seem somewhat limited, with studies showing blockade of cocaine self-administration at doses that also affect food-maintained behavior or showing blockade only over a very limited dose range (Baptista et al. 2004; Hao et al. 2010; Jin et al. 2010). In addition, in rodents, LY379268 does not decrease heroin (Bossert et al. 2005) or ethanol (Backstrom and Hyytia 2005; Rodd et al. 2006) self-administration behavior under fixed-ratio schedules, except at doses that also decrease locomotor activity. Thus, glutamate transmission may be less involved in the primary reinforcing effects of cocaine, opiates, and ethanol compared to nicotine in rodents. There is, however, a study in rats that showed that intra-VTA injections of LY379268 dose-dependently decreased cocaine-induced reinstatement, and the authors suggested that activation of VTA mGluR2/3s may inhibit cocaine-induced reinstatement by decreasing the motivational effect of cocaine (Lu et al. 2012). It is important to note that LY379268 and related compounds activate mGluR3, in addition to mGluR2, and the physiological role of mGluR3 is unknown (Schoepp et al. 1999; Tamaru et al. 2001). This limitation of various compounds has led to development of new compounds selective for mGluR2 (Dhanya et al. 2011; Jin et al. 2010). Jin and colleagues (Jin et al. 2010) showed that the positive allosteric mGluR2 modulator, BINA, blocked cocaine self-administration in rats without affecting food-maintained behavior, thus suggesting a specific role of mGluR2 in abuse-related behaviors of cocaine.
The results of our study did show striking effects of LY379268 on reinstatement of cocaine-seeking behavior in abstinent, cocaine-experienced monkeys, where LY379268 dose-dependently blocked cue-induced reinstatement, but not reinstatement induced by a cocaine prime. This finding is in line with other reports from rodents showing that systemic LY379268 administration decreases cue-induced reinstatement of cocaine seeking and context-induced reinstatement of heroin seeking in rats (Baptista et al. 2004; Bossert et al. 2004; Cannella et al. 2013). mGluR2/3 agonists have also been shown to decrease cue-induced reinstatement of nicotine, heroin, and methamphetamine seeking in rats (Bossert et al. 2005; Kufahl et al. 2013; Liechti et al. 2007). Thus, decreases in glutamate transmission induced by mGluR2/3 agonists seem particularly effective in reducing drug-seeking behavior induced by drug-associated stimuli. Interestingly, LY379268 appeared to be somewhat more potent in blocking cue-induced reinstatement of cocaine seeking than nicotine seeking in our study.
In summary, the present findings show that the mGlu2/3 receptor agonist LY379268 reduces nicotine-taking, as well as nicotine-seeking, behavior in squirrel monkeys. These results extend our previous findings (Liechti et al. 2007) from rodents to non-human primates and confirm mGluR2/3 as a target for anti-smoking medications. In addition, LY379268 had no significant effects on cocaine-maintained behavior and cocaine-induced reinstatement at doses that affected these behaviors produced by nicotine. However, LY379268 was effective in blocking the cue-induced reinstatement of both nicotine and cocaine seeking. This confirms the effectiveness of mGluR2/3 stimulation in attenuating the effects of environmental stimuli associated with taking of different drugs. Thus, mGluR2/3 stimulation has promising effects in non-human primate models of nicotine reinforcement and relapse, and this mechanism should be further studied for potential use in smoking cessation and relapse prevention.
Acknowledgments
With great sadness, we dedicate this article to memory of Dr. Steven R. Goldberg (†November 25, 2014).
We thank Dr. Ira Baum and Philip White for their excellent veterinary assistance during the study. We thank Dr. Sevil Yasar for advice and administrative assistance with the study and Dr. Charles Schindler for his comments on the manuscript.
This work was supported in part by the Intramural Research Program, National Institute on Drug Abuse, NIH, DHHS, and in part by NIDA grant DA011946 to Dr. Markou.
Footnotes
Conflict of interest Athina Markou holds a patent on the use of metabotropic glutamate receptors for the treatment of drug dependence. During the last 3 years, Athina Markou has received contract research support by Astra-Zeneca, Forest Laboratories, and Bristol-Myers Squibb and honoraria from AbbVie, Germany.
The other authors have no conflicts of interest to declare.
References
- Adewale AS, Platt DM, Spealman RD. Pharmacological stimulation of group ii metabotropic glutamate receptors reduces cocaine self-administration and cocaine-induced reinstatement of drug seeking in squirrel monkeys. J Pharmacol Exp Ther. 2006;318:922–931. doi: 10.1124/jpet.106.105387. [DOI] [PubMed] [Google Scholar]
- Arroyo M, Markou A, Robbins TW, Everitt BJ. Acquisition, maintenance and reinstatement of intravenous cocaine self-administration under a second-order schedule of reinforcement in rats: effects of conditioned cues and continuous access to cocaine. Psychopharmacology (Berlin) 1998;140:331–344. doi: 10.1007/s002130050774. [DOI] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Suppression of alcohol self-administration and cue-induced reinstatement of alcohol seeking by the mGlu2/3 receptor agonist LY379268 and the mGlu8 receptor agonist (S)-3,4-DCPG. Eur J Pharmacol. 2005;528:110–118. doi: 10.1016/j.ejphar.2005.10.051. [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista MA, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci. 2004;24:4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauzo RM, Kimmel HL, Howell LL. Interactions between the mGluR2/3 agonist, LY379268, and cocaine on in vivo neurochemistry and behavior in squirrel monkeys. Pharmacol Biochem Behav. 2009;94:204–210. doi: 10.1016/j.pbb.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Liu SY, Lu L, Shaham Y. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci. 2004;24:10726–10730. doi: 10.1523/JNEUROSCI.3207-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Busch RF, Gray SM. The novel mGluR2/3 agonist LY379268 attenuates cue-induced reinstatement of heroin seeking. Neuroreport. 2005;16:1013–1016. doi: 10.1097/00001756-200506210-00026. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Gray SM, Lu L, Shaham Y. Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology. 2006;31:2197–2209. doi: 10.1038/sj.npp.1300977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannella N, Halbout B, Uhrig S, Evrard L, Corsi M, Corti C, Deroche-Gamonet V, Hansson AC, Spanagel R. The mGluR2/3 agonist LY379268 induced anti-reinstatement effects in rats exhibiting addiction-like behavior. Neuropsychopharmacology. 2013;38:2048–2056. doi: 10.1038/npp.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA. Nicotine self-administration in animals as a dependence model. Nicotine Tob Res. 1999;1:11–20. doi: 10.1080/14622299050011121. [DOI] [PubMed] [Google Scholar]
- Dhanya RP, Sidique S, Sheffler DJ, Nickols HH, Herath A, Yang L, Dahl R, Ardecky R, Semenova S, Markou A, Conn PJ, Cosford ND. Design and synthesis of an orally active metabotropic glutamate receptor subtype-2 (mGluR2) positive allosteric modulator (PAM) that decreases cocaine self-administration in rats. J Med Chem. 2011;54:342–353. doi: 10.1021/jm1012165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berlin) 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- D’Souza MS, Liechti ME, Ramirez-Nino AM, Kuczenski R, Markou A. The metabotropic glutamate 2/3 receptor agonist LY379268 blocked nicotine-induced increases in nucleus accumbens shell dopamine only in the presence of a nicotine-associated context in rats. Neuropsychopharmacology. 2011;36:2111–2124. doi: 10.1038/npp.2011.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Second-order schedules of drug reinforcement in rats and monkeys: measurement of reinforcing efficacy and drug-seeking behaviour. Psychopharmacology (Berlin) 2000;153:17–30. doi: 10.1007/s002130000566. [DOI] [PubMed] [Google Scholar]
- Goldberg SR. Comparable behavior maintained under fixed-ratio and second-order schedules of food presentation, cocaine injection or d-amphetamine injection in the squirrel monkey. J Pharmacol Exp Ther. 1973;186:18–30. [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD. Maintenance and suppression of behavior by intravenous nicotine injections in squirrel monkeys. Fed Proc. 1982;41:216–220. [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD, Goldberg DM. Persistent behavior at high rates maintained by intravenous self-administration of nicotine. Science. 1981;214:573–575. doi: 10.1126/science.7291998. [DOI] [PubMed] [Google Scholar]
- Greenslade RG, Mitchell SN. Selective action of (−)-2-oxa-4-aminobicyclo[3.1.0]hexane-4,6-dicarboxylate (LY379268), a group II metabotropic glutamate receptor agonist, on basal and phencyclidine-induced dopamine release in the nucleus accumbens shell. Neuropharmacology. 2004;47:1–8. doi: 10.1016/j.neuropharm.2004.02.015. [DOI] [PubMed] [Google Scholar]
- Hao Y, Martin-Fardon R, Weiss F. Behavioral and functional evidence of metabotropic glutamate receptor 2/3 and metabotropic glutamate receptor 5 dysregulation in cocaine-escalated rats: factor in the transition to dependence. Biol Psychiatry. 2010;68:240–248. doi: 10.1016/j.biopsych.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotsenpiller G, Giorgetti M, Wolf ME. Alterations in behaviour and glutamate transmission following presentation of stimuli previously associated with cocaine exposure. Eur J Neurosci. 2001;14:1843–1855. doi: 10.1046/j.0953-816x.2001.01804.x. [DOI] [PubMed] [Google Scholar]
- Jin X, Semenova S, Yang L, Ardecky R, Sheffler DJ, Dahl R, Conn PJ, Cosford ND, Markou A. The mGluR2 positive allosteric modulator BINA decreases cocaine self-administration and cue-induced cocaine-seeking and counteracts cocaine-induced enhancement of brain reward function in rats. Neuropsychopharmacology. 2010;35:2021–2036. doi: 10.1038/npp.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Tanda G, Redhi GH, Goldberg SR. Self-administration of Delta(9)-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology (Berlin) 2003;169:135–140. doi: 10.1007/s00213-003-1484-0. [DOI] [PubMed] [Google Scholar]
- Justinova Z, Mangieri RA, Bortolato M, Chefer SI, Mukhin AG, Clapper JR, King AR, Redhi GH, Yasar S, Piomelli D, Goldberg SR. Fatty acid amide hydrolase inhibition heightens anandamide signaling without producing reinforcing effects in primates. Biol Psychiatry. 2008;64:930–937. doi: 10.1016/j.biopsych.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa J, Yoshimizu T, Chaki S. A metabotropic glutamate 2/3 receptor antagonist, MGS0039, increases extracellular dopamine levels in the nucleus accumbens shell. Neurosci Lett. 2006;393:127–130. doi: 10.1016/j.neulet.2005.09.058. [DOI] [PubMed] [Google Scholar]
- Kufahl PR, Watterson LR, Nemirovsky NE, Hood LE, Villa A, Halstengard C, Zautra N, Olive MF. Attenuation of methamphetamine seeking by the mGluR2/3 agonist LY379268 in rats with histories of restricted and escalated self-administration. Neuropharmacology. 2013;66:290–301. doi: 10.1016/j.neuropharm.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Wertheim C, Goldberg SR. High reinforcing efficacy of nicotine in non-human primates. PLoS One. 2007;2:e230. doi: 10.1371/journal.pone.0000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Lhuillier L, Kaupmann K, Markou A. Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J Neurosci. 2007;27:9077–9085. doi: 10.1523/JNEUROSCI.1766-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Xue Y, Steketee JD, Rebec GV, Sun W. Regulation of cocaine-induced reinstatement by group II metabotropic glutamate receptors in the ventral tegmental area. Psychopharmacology (Berlin) 2012;220:75–85. doi: 10.1007/s00213-011-2455-5. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Markou A, Paterson NE, Semenova S. Role of gamma-aminobutyric acid (GABA) and metabotropic glutamate receptors in nicotine reinforcement: potential pharmacotherapies for smoking cessation. Ann N Y Acad Sci. 2004;1025:491–503. doi: 10.1196/annals.1316.061. [DOI] [PubMed] [Google Scholar]
- Mascia P, Pistis M, Justinova Z, Panlilio LV, Luchicchi A, Lecca S, Scherma M, Fratta W, Fadda P, Barnes C, Redhi GH, Yasar S, Le FB, Tanda G, Piomelli D, Goldberg SR. Blockade of nicotine reward and reinstatement by activation of alpha-type peroxisome proliferator-activated receptors. Biol Psychiatry. 2011;69:633–641. doi: 10.1016/j.biopsych.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the messenger RNA for a metabotropic glutamate receptor, mGluR2, in the central nervous system of the rat. Neuroscience. 1993a;53:1009–1018. doi: 10.1016/0306-4522(93)90485-x. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: an in situ hybridization study. J Comp Neurol. 1993b;335:252–266. doi: 10.1002/cne.903350209. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Justinova Z, Mascia P, Pistis M, Luchicchi A, Lecca S, Barnes C, Redhi GH, Adair J, Heishman SJ, Yasar S, Aliczki M, Haller J, Goldberg SR. Novel use of a lipid-lowering fibrate medication to prevent nicotine reward and relapse: preclinical findings. Neuropsychopharmacology. 2012;37:1838–1847. doi: 10.1038/npp.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Kalivas PW. The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology (Berlin) 2006;186:143–149. doi: 10.1007/s00213-006-0372-9. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Corrigall WA. Neuronal systems underlying behaviors related to nicotine addiction: neural circuits and molecular genetics. J Neurosci. 2002;22:3338–3341. doi: 10.1523/JNEUROSCI.22-09-03338.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards G, Messer J, Malherbe P, Pink R, Brockhaus M, Stadler H, Wichmann J, Schaffhauser H, Mutel V. Distribution and abundance of metabotropic glutamate receptor subtype 2 in rat brain revealed by [3H]LY354740 binding in vitro and quantitative radio-autography: correlation with the sites of synthesis, expression, and agonist stimulation of [35S]GTPgammas binding. J Comp Neurol. 2005;487:15–27. doi: 10.1002/cne.20538. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, McKinzie DL, Bell RL, McQueen VK, Murphy JM, Schoepp DD, McBride WJ. The metabotropic glutamate 2/3 receptor agonist LY404039 reduces alcohol-seeking but not alcohol self-administration in alcohol-preferring (P) rats. Behav Brain Res. 2006;171:207–215. doi: 10.1016/j.bbr.2006.03.032. [DOI] [PubMed] [Google Scholar]
- Sannerud CA, Prada J, Goldberg DM, Goldberg SR. The effects of sertraline on nicotine self-administration and food-maintained responding in squirrel monkeys. Eur J Pharmacol. 1994;271:461–469. doi: 10.1016/0014-2999(94)90807-9. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Panlilio LV, Goldberg SR. Second-order schedules of drug self-administration in animals. Psychopharmacology (Berlin) 2002;163:327–344. doi: 10.1007/s00213-002-1157-4. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Jane DE, Monn JA. Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology. 1999;38:1431–1476. doi: 10.1016/s0028-3908(99)00092-1. [DOI] [PubMed] [Google Scholar]
- Tamaru Y, Nomura S, Mizuno N, Shigemoto R. Distribution of metabotropic glutamate receptor mGluR3 in the mouse CNS: differential location relative to pre- and postsynaptic sites. Neuroscience. 2001;106:481–503. doi: 10.1016/s0306-4522(01)00305-0. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Baker DA, Shen H, Carson DS, Kalivas PW. Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. J Pharmacol Exp Ther. 2002;300:162–171. doi: 10.1124/jpet.300.1.162. [DOI] [PubMed] [Google Scholar]