Abstract
While most mycoplasma species appear to have evolutionarily lost the ability to synthesize isoprenoid precursors, Mycoplasma penetrans has retained the nonmevalonate pathway that proceeds via the immunogenic intermediate (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP). Consequently, this pathogen is capable of stimulating human Vγ9/Vδ2 T cells.
Mycoplasmas are among the smallest self-replicating organisms known and are the only prokaryotes that lack a cell wall. Considerable genome rearrangements during evolution from low-G+C-content, gram-positive ancestors led to the loss of numerous dispensable biochemical pathways, such as amino acid, fatty acid, and vitamin biosynthesis, thereby creating a life-form with a coding capacity reduced to a certain minimum (8). In consequence, mycoplasmas depend heavily on metabolites provided exogenously and thus comprise closely adapted host- and tissue-specific commensals and parasites of higher eukaryotes, often without obvious pathological manifestation or virulence (10).
In humans, a number of mycoplasma species have been isolated from the mucous surfaces of the respiratory and urogenital tracts, the eyes, alimentary canal, and joints, whereas only a few species yet have been unmistakably established as etiological agents of human diseases (2-4, 8, 11-14). Perhaps the most thoroughly characterized species among these, Mycoplasma pneumoniae, is a leading cause of respiratory tract infections, particularly in children and adolescents, ranging from pharyngitis and bronchitis to severe pneumonia and chronic asthma. Mycoplasma genitalium has been associated with nongonococcal urethritis in men and mucopurulent cervicitis, endometritis, and tubal infertility in women and constitutes one of the most widespread sexually transmitted human pathogens. The closely related species Ureaplasma urealyticum is a common inhabitant of the urogenital tract but also plays a role in pelvic inflammatory diseases as well as postabortion and postpartum fever. Mycoplasma penetrans was first reported a decade ago as a newly emerging infectious agent and has since been implicated in the deterioration of the immune system in AIDS patients. However, M. penetrans also appears to be a primary cause of non-human immunodeficiency virus-related urethritis and respiratory disease. Other mycoplasma species of relevance for the present study include Mycoplasma pulmonis, a causative agent of respiratory disease in mice and rats; Mycoplasma arthritidis, an experimental model organism that induces in rodents acute and chronic autoimmune pathology closely resembling human rheumatoid arthritis; and finally Mycoplasma gallisepticum, a respiratory pathogen in poultry and wild songbirds that is responsible for considerable economic losses to the farming industry worldwide.
Despite the growing awareness of the previously widely neglected significance of mycoplasmas in classical but also newly emerging clinical manifestations, little is known about the interference with the host cell metabolism and the immune response during infection (7, 10). We recently identified the nonmevalonate, 2-C-methyl-d-erythritol-4-phosphate (MEP) pathway of isoprenoid biosynthesis in a wide range of microbial infectious agents and were able to correlate the presence of this pathway with the pathogen's ability to stimulate human Vγ9/Vδ2 T cells (6). The molecular basis for this phenomenon lies in the high immunogenicity of the intermediate (E)-4-hydroxy-3-methyl-but-2-enyl-pyrophosphate (HMB-PP) produced by these organisms. Therefore, we checked the available mycoplasma genome data for genes involved in either the MEP pathway that is present in most pathogenic bacteria, with the important exception of streptococci, staphylococci, and Borrelia; or the classical mevalonate pathway that is utilized by these three organisms, but also by all higher eukaryotes including humans.
Sequence homologies were analyzed by TBLASTN searches at the public servers of the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov), the Center for Genome Research (http://www.broad.mit.edu), and The Institute for Genomic Research (http://www.tigr.org). In none of the genomes of the mycoplasmas M. arthritidis, M. fermentans, M. gallisepticum, M. genitalium, M. mobile, M. mycoides, M. penetrans, M. pneumoniae, M. pulmonis, and U. urealyticum, gene homologues coding for any enzyme of the mevalonate pathway, such as HMG-coenzyme A reductase, could be identified (data not shown). Surprisingly though, most of these species were also negative for the genes of the MEP pathway, implying that many mycoplasmas depend on the host cell's supply with isoprenoid precursors (Table 1). However, both M. gallisepticum and M. penetrans contain the sequence information for most enzymes of the MEP pathway in their genome (Table 2); this finding could be corroborated by a PCR analysis with primers specific for the gcpE gene encoding HMB-PP synthase (GcpE) (Table 1). Yet, we were not able to identify the kinase YchB in either genome and neither the YgbP protein and HMB-PP reductase (LytB) in M. gallisepticum, suggesting that the function of those enzymes may be complemented by the action of compensatory proteins, as suspected recently with respect to other mycoplasma “household” genes (9).
TABLE 1.
Presence of the MEP pathway in selected mycoplasma species
| Mycoplasma species | Genome accession no. | Result by:
|
Culture mediumc | Bioactivity (μg of HMB-PP/g of protein)d | |
|---|---|---|---|---|---|
| Homology searcha | PCR analysisb | ||||
| M. arthritidis | NC_004819 | − | − | BEa | <0.006 |
| M. gallisepticum | NC_004829 | + | + | BEg | 55.1 ± 26.9 |
| M. genitalium | NC_000908 | − | − | SP4 | <0.021 |
| M. penetrans | NC_004432 | + | + | BEg | 5.2 ± 1.6 |
| SP4 | 4.1 ± 0.5 | ||||
| M. pneumoniae | NC_000912 | − | − | SP4 | <0.005 |
| M. pulmonis | NC_002771 | − | − | BEg | <0.012 |
| U. urealyticum | NC_002162 | − | − | SU | <0.015 |
Analyzed by TBLASTN, using the sequences of the seven E. coli enzymes depicted in Table 2. Other genomes negative for the MEP pathway include those of M. fermentans, M. mobile, and M. mycoides subsp. mycoides SC (data not shown).
Performed on genomic template DNA, using the degenerate gcpE-specific primers ATTATTGCWGATATTCAYTTTAATCCT and GGWCCRTTTACTGMACAACCTAA.
Culture media used to obtain cell pellets for subsequent preparation of LMW filtrates (6a).
Shown is the mean Vγ9/Vδ2 T-cell bioactivity ± standard error (n = 3) of mycoplasma LMW filtrates as determined with an HMB-PP standard and calibrated against the protein concentration of the original bacterial extract.
TABLE 2.
Gene homologues controlling the MEP pathway in M. penetrans and M. gallisepticum
| E. coli protein | Accession no. | Gene no.a
|
|
|---|---|---|---|
| M. penetrans | M. gallisepticum | ||
| Dxs | P77488 | MYPE730 | MGR_369 |
| Dxr | P45568 | MYPE1470 | MGR_092 |
| YgbP (IspD) | Q46893 | MYPE2760 | ? |
| YchB (IspE) | P24209 | ? | ? |
| YgbB (IspF) | P36663 | MYPE10270 | MGR_024 |
| GcpE (IspG) | P27433 | MYPE9400 | MGR_307 |
| LytB (IspH) | P22565 | MYPE1330 | ? |
Shown are homologues to the corresponding E. coli proteins, as encoded in the genomes of M. penetrans strain HF-2 and M. gallisepticum strain R. ?, not detected.
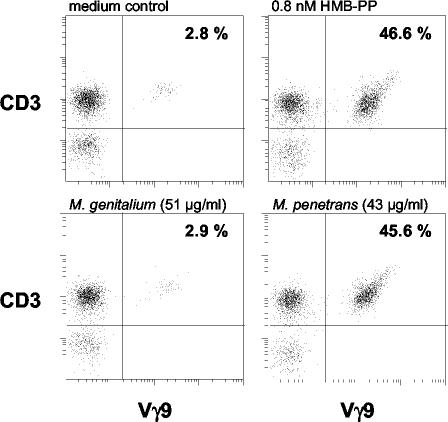
In order to confirm that the MEP pathway is actually operational in M. gallisepticum and M. penetrans, the potential of low-molecular-weight (LMW) extracts to stimulate human Vγ9/Vδ2 T cells was investigated. This method provides a sensitive bioassay for the highly immunogenic metabolite HMB-PP (1, 5). Bacteria were harvested from fresh cultures and sonicated in 10 mM NH4HCO3, pH 8.0. Insoluble debris was removed by centrifugation, the supernatant was passed through a 0.1-μm-pore-diameter sterile filter unit (Millipore), and the protein concentration was determined using the bicinchoninic acid protein assay kit (Pierce). LMW fractions were eventually obtained by using cellulose filters with a molecular mass cutoff of 3,000 Da (Millipore), and added to human peripheral blood mononuclear cells (PBMCs) at dilutions between 1 in 20 and 1 in 106 (Fig. 1). Strikingly, while preparations from M. arthritidis, M. genitalium, M. pneumoniae, M. pulmonis, and U. urealyticum did not induce detectable Vγ9/Vδ2 T-cell activation and proliferation even at high concentrations, those from M. penetrans and M. gallisepticum showed indeed a considerable bioactivity that could be estimated to approximately 5 and 50 μg of HMB-PP per g of protein, respectively, with the detection thresholds typically being 0.002 to 0.02 μg/g (Table 1).
FIG. 1.
γδ T-cell bioactivity of mycoplasma species. Human PBMCs were cultured with serial dilutions of LMW extracts or of synthetic HMB-PP. Outgrowth of Vγ9+ CD3+ cells within the lymphocyte gate was monitored after 6 days by flow cytometry. The plots show representative results obtained for M. genitalium and M. penetrans at dilutions corresponding to the indicated protein concentrations in comparison with those for the medium control and HMB-PP.
Thus, although most mycoplasmas appear to have lost their intrinsic isoprenoid biosynthesis during evolution, both M. gallisepticum and M. penetrans utilize the MEP pathway, as they are obviously capable of synthesizing HMB-PP. Whether in the case of M. gallisepticum this metabolite is further transformed by an HMB-PP reductase into isopentenyl pyrophosphate (IPP) (5), the common building block of all higher isoprenoids, remains open, as no lytB homologue could be identified in the genome; since the MEP pathway is restricted to bacteria and the plastids of plants and protozoa, it is unlikely that the host cell possesses an enzyme with a LytB-like activity. Yet, it is inconceivable why the avian pathogen M. gallisepticum should produce HMB-PP, whose only other known biological role is to affect primate γδ T lymphocytes, and rather choose to obtain IPP from the host.
If one presumes that the presence of the MEP pathway is essential for survival of M. gallisepticum and M. penetrans, this opens the possibility of selective therapeutic treatment, for it should render them susceptible to inhibitors of the MEP pathway. One such compound, the DOXP reductoisomerase inhibitor fosmidomycin, has already successfully passed several phase II studies in human malaria patients and proved to be both effective and safe (15). It will be interesting to test whether fosmidomycin or related substances may clear infections with M. gallisepticum in chickens and turkeys and with M. penetrans in human patients.
Does staying supposedly independent from the host metabolism provide any selective advantage during the infection process, especially if it involves having to display HMB-PP to the immune system like a beacon? The synthesis of HMB-PP by M. penetrans is likely to have an impact on the patient's immune system, leading to stimulation and possibly accumulation of Vγ9/Vδ2 T cells at the penetration site. Since to our knowledge there are no differential histopathological data available on the mucosal lymphocyte infiltrates, it is difficult to speculate about the significance of Vγ9/Vδ2 T cells in these diseases. Are Vγ9/Vδ2 T cells a potent effector population involved in the clearance of M. penetrans, or does the pathogen benefit from this stimulation as part of a sophisticated immune subversion strategy? While there is clearly a need for thorough analysis of the role of Vγ9/Vδ2 T cells in mucosal immunity, a clinical comparison between the immunopathology caused by HMB-PP-producing mycoplasmas like M. penetrans and HMB-PP-deficient species like M. genitalium will shed new light on this question.
Acknowledgments
This work was supported in part by the European Commission (ICA4-2000-10290), the Fonds der Chemischen Industrie, and the Fonden til Lægevidenskabens Fremme.
We wish to thank Shih-Feng Tsai for allowing us access to and Mark Chang for BLAST analysis of the unpublished M. fermentans genome sequence; Armin Reichenberg for providing synthetic HMB-PP; Jochen Wiesner for critical discussion; and Karin Skovgaard Sørensen and Rosel Engel for excellent technical assistance.
Editor: J. D. Clements
REFERENCES
- 1.Begley, M., C. G. Gahan, A. K. Kollas, M. Hintz, C. Hill, H. Jomaa, and M. Eberl. 2004. The interplay between classical and alternative isoprenoid biosynthesis controls γδ T cell bioactivity of Listeria monocytogenes. FEBS Lett. 561:99-104. [DOI] [PubMed] [Google Scholar]
- 2.Blanchard, A. 1997. Mycoplasmas and HIV infection, a possible interaction through immune activation. Wien. Klin. Wochenschr. 109:590-593. [PubMed] [Google Scholar]
- 3.Christiansen, G., L. T. Jensen, T. Boesen, J. Emmersen, S. A. Ladefoged, L. K. Schiotz, and S. Birkelund. 1997. Molecular biology of Mycoplasma. Wien. Klin. Wochenschr. 109:557-561. [PubMed] [Google Scholar]
- 4.Clausen, H. F., J. Fedder, M. Drasbek, P. K. Nielsen, B. Toft, H. J. Ingerslev, S. Birkelund, and G. Christiansen. 2001. Serological investigation of Mycoplasma genitalium in infertile women. Hum. Reprod. 16:1866-1874. [DOI] [PubMed] [Google Scholar]
- 5.Eberl, M., B. Altincicek, A. K. Kollas, S. Sanderbrand, U. Bahr, A. Reichenberg, E. Beck, D. Foster, J. Wiesner, M. Hintz, and H. Jomaa. 2002. Accumulation of a potent γδ T cell stimulator after deletion of the lytB gene in Escherichia coli. Immunology 106:200-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eberl, M., M. Hintz, A. Reichenberg, A. K. Kollas, J. Wiesner, and H. Jomaa. 2003. Microbial isoprenoid biosynthesis and human γδ T cell activation. FEBS Lett. 544:4-10. [DOI] [PubMed] [Google Scholar]
- 6a.Freundt, E. A., H. Ernø, and R. M. Lemcke. 1979. Identification of mycoplasmas. Methods Microbiol. 13:377-434. [Google Scholar]
- 7.Nicolson, G. L., M. Nasralla, and N. L. Nicolson. 1999. The pathogenesis and treatment of mycoplasmal infections. Antimicrob. Infect. Dis. Newsl. 17:81-88. [Google Scholar]
- 8.Peterson, S. N., and C. M. Fraser. 8 February 2001, posting date. The complexity of simplicity. Genome Biol. 2:comment2002.1-2002.8. [Online.] http://www.biomedcentral.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollack, J. D., M. A. Myers, T. Dandekar, and R. Herrmann. 2002. Suspected utility of enzymes with multiple activities in the small genome Mycoplasma species: the replacement of the missing “household” nucleoside diphosphate kinase gene and activity by glycolytic kinases. OMICS J. Integr. Biol. 6:247-258. [DOI] [PubMed] [Google Scholar]
- 10.Rosengarten, R., C. Citti, M. Glew, A. Lischewski, M. Droesse, P. Much, F. Winner, M. Brank, and J. Spergser. 2000. Host-pathogen interactions in mycoplasma pathogenesis: virulence and survival strategies of minimalist prokaryotes. Int. J. Med. Microbiol. 290:15-25. [DOI] [PubMed] [Google Scholar]
- 11.Rosengarten, R., C. Citti, P. Much, J. Spergser, M. Droesse, and M. Hewicker-Trautwein. 2001. The changing image of mycoplasmas: from innocent bystanders to emerging and reemerging pathogens in human and animal diseases. Contrib. Microbiol. 8:166-185. [DOI] [PubMed] [Google Scholar]
- 12.Sasaki, Y., J. Ishikawa, A. Yamashita, K. Oshima, T. Kenri, K. Furuya, C. Yoshino, A. Horino, T. Shiba, T. Sasaki, and M. Hattori. 2002. The complete genomic sequence of Mycoplasma penetrans, an intracellular bacterial pathogen in humans. Nucleic Acids Res. 30:5293-5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor-Robinson, D. 2002. Mycoplasma genitalium—an up-date. Int. J. STD AIDS 13:145-151. [DOI] [PubMed] [Google Scholar]
- 14.Uusküla, A., and P. K. Kohl. 2002. Genital mycoplasmas, including Mycoplasma genitalium, as sexually transmitted agents. Int. J. STD AIDS 13:79-85. [DOI] [PubMed] [Google Scholar]
- 15.Wiesner, J., S. Borrmann, and H. Jomaa. 2003. Fosmidomycin for the treatment of malaria. Parasitol. Res. 90(Suppl. 2):S71-S76. [DOI] [PubMed] [Google Scholar]