Abstract
Objective
Cancer is one of the most common diagnoses in elderly patients. Of all types of abdominal cancer, colorectal cancer (CRC) is undoubtedly the most frequent. Median age at diagnosis is approximately 70 years old worldwide. Due to the multiple comorbidities affecting elderly people, frailty evaluation is very important in order to avoid over- or under-treatment. This pilot study was designed to investigate the variables capable of predicting the long-term risk of mortality and living situation after surgery for CRC.
Methods
Patients with 70 years old and older undergoing elective surgery for CRC were prospectively enrolled in the study. The patients were preoperatively screened using 11 internationally-validated-frailty-assessment tests. The endpoints of the study were long-term mortality and living situation. The data were analyzed using univariate Cox proportional-hazard regression analysis to verify the predictive value of score indices in order to identify possible risk factors.
Results
Forty-six patients were studied. The median follow-up time after surgery was 4.6 years (range, 2.9-5.7 years) and no patients were lost to follow-up. The overall mortality rate was 39%. Four of the patients who survived (4/28, 14%) lost their functional autonomy. The preoperative impaired Timed Up and Go (TUG), Eastern Cooperative Group Performance Status (ECOG PS), Instrumental Activities of Daily Living (IADLs), Vulnerable Elders Survey (VES-13) scoring systems were significantly associated with increased long term mortality risk.
Conclusion
Simplified frailty-assessing tools should be routinely used in elderly cancer patients before treatment in order to stratify patient risk. The TUG, ECOG-PS, IADLs and VES-13 scoring systems are potentially able to predict long-term mortality and disability. Additional studies will be needed to confirm the preliminary data in order to improve management strategies for oncogeriatric surgical patients.
KEYWORDS : Elderly, geriatric assessment, surgical oncology, risk assessment, screening tools, colorectal cancer (CRC)
Introduction
The number of cancer cases is steadily increasing, and malignancies are expected to become the first cause of death worldwide in the upcoming decades1. The number of elderly people is rapidly increasing worldwide and 50% of all cancer cases and 70% of cancer related deaths occur in this group2. This is particularly true for intra-abdominal malignancies which are often diagnosed in elderly patients3. Colorectal cancer (CRC) is the third most common neoplasm worldwide with 1.36 million new cases/year4, and half of the new diagnoses are made in people 70 years old or older. Multimodal treatment and multidisciplinary teams have been widely recognized as a fundamental approach in treating cancer patients5,6; however, despite progress in medical and radiation oncology, surgery remains the cornerstone of treatment and the only chance for cure of many CRCs.
A variety of challenges need to be faced when taking care of elderly patients, mainly related to the complexity of this particular group of patients7. Age ‘per se’ should not be considered a contraindication for accessing treatment since several studies worldwide have failed to prove its direct correlation with any risk of postoperative complications8. Elderly individuals should not be denied radical surgery due to their chronological age as studies showed that elderly people have similar cancer-free survival to their younger counterparts9,10.
Many factors, such as comorbidities, sarcopenia, polypharmacy and nutritional status11, instead, play a significant role in an elderly patient’s life expectancy and are able to predict whether patients will tolerate cancer treatment. Geriatric evaluation should be carried out before treatment decisions are made in order to assess whether the patient’s remaining life expectancy will be reduced by cancer or by the patient’s coexisting conditions. In particular, preservation of functional independence is considered one of the most important patient-reported end-point (frequently over the disease free survival) and for this reason it becomes crucial to investigate predictors for long-term status maintenance.
Comprehensive Geriatric Assessment (CGA) is recognized as the best tool for preoperatively evaluating oncogeriatric patients (OP)12,13 but, unfortunately, it is time consuming and hence difficult to use in a busy surgical clinical practice. Recent studies have sought simplified tools of rapid execution14,15 capable of assessing the surgical risk in elderly patients undergoing surgery for cancer. The preoperative assessment in elderly cancer patients study (PACE) showed that Instrumental Activities of Daily Living (IADLs) and Performance Status (PS) were associated with a 50% increase in the relative risk of postoperative complications and extended hospital stay16. The preoperative risk estimation for oncogeriatric patients (PRE-OP) study has shown that Timed Up and Go (TUG), the American Society of Anesthesiologists (ASA) score and Nutritional Risk Screening (NRS) can predict short-term postoperative complications and mortality17.
The aim of the current pilot study was to investigate whether some rapid execution screening tools for functional status were also able to predict long-term mortality in elderly patients undergoing surgery for CRC.
Materials and methods
Patient selection
Patients with 70 years old or older who underwent surgery for CRC were prospectively enrolled in the study. Patients requiring emergent surgery and patients who were unable to give written informed consent were excluded. Patients were recruited from the General Surgery Department of S. Orsola-Malpighi Hospital (Bologna, Italy) between September 2009 and June 2012. The study protocol was reviewed and approved by the institutional ethics committee.
Screening tools
Within 10 days prior to their surgery, the patients enrolled were evaluated in order to assess their preoperative functional status using a cluster of screening tools.
TUG14, Activity of Daily Living (ADL)18, IADLs19 and the Eastern Cooperative Oncology Group Performance Status (ECOG PS)20 were carried out to evaluate functional status. The Mini Mental State Examination (MMSE)21 was used to assess cognitive function. The Groningen Frailty Index (GFI)22 and the Vulnerable Elders Survey (VES-13)23 were used to identify possible vulnerable and frail individuals. Fatigue was analysed using Brief Fatigue Inventory (BFI)24, and depression with the Geriatric Depression Scale (GDS)25. Nutritional status was assessed using NRS26, which identifies mildly, moderately and severely impaired nutritional statuses. The ASA score determined by an anesthesiologist was also reported. The screening tools are summarized in Table 1. Data about living situation were also collected, considering it as a surrogate index of long term functional status after surgical procedure.
Table 1. Screening tools used in the study and frequencies of impaired results.
| Test | Score range | Cut-off* | Impaired** (%) |
|---|---|---|---|
| Timed Up & Go (TUG) | NA | >20 s | 11 (23.9) |
| Activities of Daily Living (ADL) | 0-6 | <6 | 11 (23.9) |
| Instrumental Activities of Daily Living (IADLs) | 0-8 | <8 | 18 (39.1) |
| ECOG Performance Status (ECOG-PS) | 0-4 | >1 | 10 (21.7) |
| Mini Mental State Examination (MMSE) | 0-30 | <25 | 23 (50.0) |
| Groeningen Frailty Index (GFI) | 0-15 | ≥4 | 24 (52.2) |
| Vulnerable Elderly Survey - 13 (VES-13) | 0-10 | ≥3 | 20 (43.5) |
| Brief Fatigue Inventory (BFI) | 0-10 | >3 | 22 (47.8) |
| Geriatric Depression Scale (GDS) | 0-15 | >5 | 12 (26.1) |
| Nutritional Risk Screening (NRS) | NA | NA | 16 (34.8) |
| American Society of Anesthesiologists score (ASA) | 1-5 | ≥3 | 32 (69.6) |
*, Cut-off for the impaired test; **, No. of impaired results for each test. NA, not available.
Data collection
After early postoperative follow-up in the outpatient clinic, long term follow up was assessed by contacting previously operated on patients or their caregivers in order to retrieve information regarding mortality and current living situation, asking whether patients were living independently, with family members/care-givers or in a nursing home.
Endpoints
The primary endpoint was to evaluate the capacity of validated preoperative screening tools to predict long term mortality.
The secondary endpoint was evaluation of long term living situation.
Statistical analysis
The categorical data were expressed as numbers (percentages), and the continuous variables as means and standard deviations. The results of the preoperative tests were divided into “normal” and “impaired”, according to the cut-offs in the literature (Table 1). Estimated long-term survival in the different scores was assessed by the Kaplan-Meier method. Univariate Cox proportional hazard regression analysis was conducted to verify the predictive value of score index, age and sex. A P value of <0.05 (2-sided) was considered significant. A multivariate analysis was generated but was not reported since it was not significant because of the small number of patients enrolled in the study. Statistical analysis was carried out using Stata Statistical Software release 14 (College Station, TX, StataCorp LP).
Results
A total of 46 CRC patients were enrolled in this study, 24 males and 22 females. The median age of our patients was 80.52±6.68 years. Before surgery, the majority of patients were living independently (n=35; 76%) while 11 (24%) were institutionalized or living with assistance from family members or care-givers, because of cognitive/functional status impairment.
For each of the performed screening tool we obtained the following frequencies of impaired results (cut offs are shown in Table 1): VES-13: 20; MMSE: 23; ADL: 11; IADLs: 18; GDS: 12; ECOG PS: 10; GFI: 24; BFI: 32; NRS: 16; TUG: 11.
The median follow-up time after surgery was 4.6 years (range, 2.9-5.7 years) and no patients were lost to follow-up.
Death occurred in 18 patients (39%) during follow up. The Kaplan Meier method was used to evaluate the ability of each test to predict mortality. The TUG, ECOG-PS, IADLs and NRS results statistically correlated with long-term mortality risk (Figures 1,2,3,4). Univariate Cox proportional hazard regression was undertaken to verify the predictive value of the proposed scores; the results are reported in Table 2. Age was associated with an increased risk per year (HR =1.10; P=0.003; 95% CI, 1.03-1.17). Gender was not associated with an increased mortality rate. There were no significant differences between genders.
Figure 1.
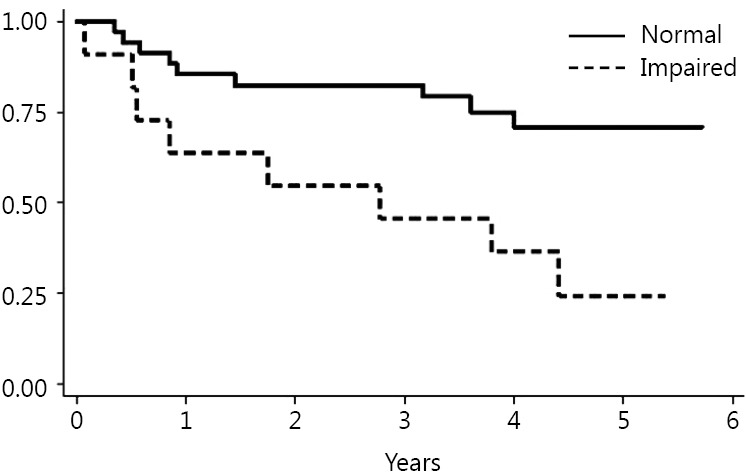
Timed Up & Go (TUG).
Figure 2.
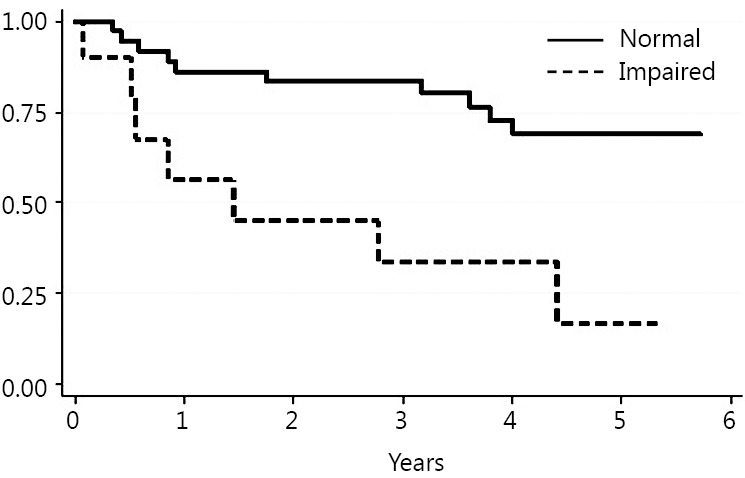
ECOG Performance Status (ECOG-PS).
Figure 3.
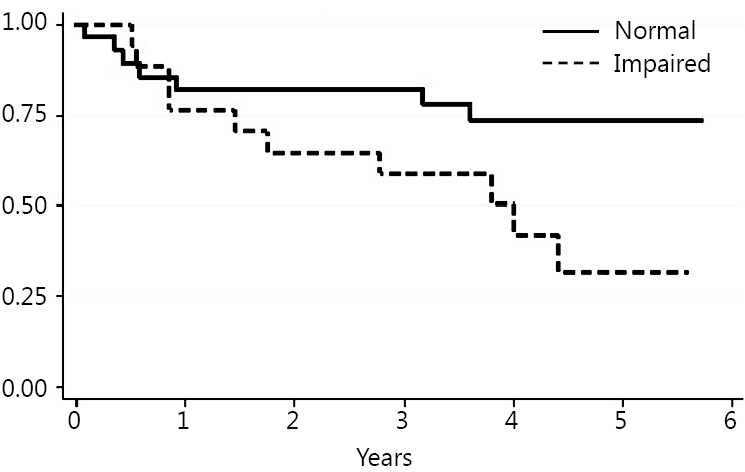
Instrumental Activities of Daily Living (IADLs).
Figure 4.
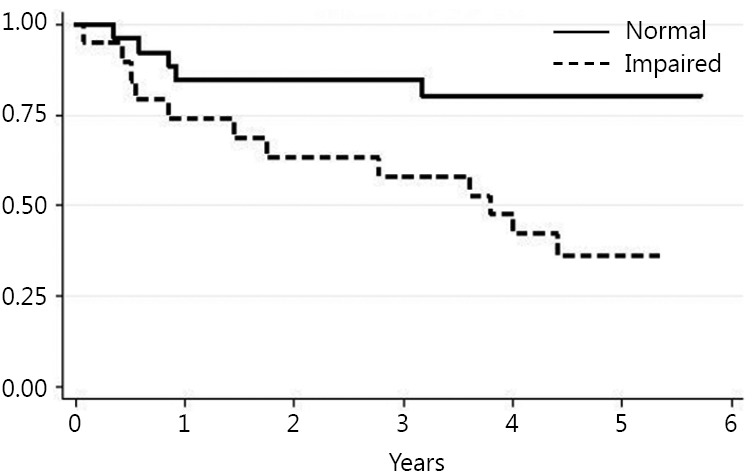
Vulnerable Elderly Survey - 13 (VES-13).
Table 2. Univariate Cox regression.
| Test | HR | P | 95% CI |
|---|---|---|---|
| Vulnerable Elderly Survey - 13 (VES-13) | 3.664 | 0.015* | 1.288-10.416* |
| Mini Mental State Examination (MMSE) | 2.680 | 0.064 | 0.943-7.615 |
| Activities of Daily Living (ADL) | 1.713 | 0.313 | 0.601-4.880 |
| Instrumental Activities of Daily Living (IADLs) | 2.716 | 0.044* | 1.028-7.177* |
| Geriatric Depression Scale (GDS) | 1.710 | 0.291 | 0.631-4.634 |
| ECOG Performance Status (ECOG-PS) | 4.139 | 0.004* | 1.564-10.951* |
| Groeningen Frailty Index (GFI) | 2.265 | 0.108 | 0.836-6.136 |
| Brief Fatigue Inventory (BFI) | 2.138 | 0.124 | 0.811-5.638 |
| American Society of Anesthesiologists score (ASA) | 2.347 | 0.180 | 0.674-8.171 |
| Nutritional Risk Screening (NRS) | 2.264 | 0.094 | 0.871-5.884 |
| Timed Up & Go (TUG) | 3.507 | 0.010* | 1.350-9.113* |
*, Significant results.
At univariate analysis, four screening tools were able to predict an increased risk of death in this group of patients: VES-13 (HR =3,664; P=0.015; 95% CI, 1.29-10.42), IADL (HR =2,716; P=0.044; 95% CI, 1.03-7.18), ECOG-PS (HR =4,139; P=0.004; 95% CI, 1.56-10.95) and TUG (HR =3,507; P=0.010; 95% CI, 1.35-9.11). Of the patients who survived at follow-up, only a minority (4/28, 14.2%) had changed their living situations from “independent” to “dependent” while 24 patients who were living independently before their surgery maintained their status.
Due to the small number of surviving patients who postoperatively lost independence during the study period, no correlation with preoperative assessment tools was evaluated statistically, even if the vast majority of long-term living patients conserved their status of independence in daily activities.
Discussion
Since the number of elderly patients affected by cancer is growing together with life expectancy, geriatric oncology has become an important area of research, and surgical treatment for this subset of patients still represents an open area for debate. It is nowadays accepted that chronological age is no longer a limitation for surgery, and it should no longer be considered as a surrogate for fitness for surgery.
Elderly cancer patients, if appropriately treated, can have an excellent survival rate after curative surgery9,10,27 as confirmed by this study, showing a notable long-term survival rate reported for octogenarians (61% at 4.6 years). Thus, the key to reaching optimal outcomes is to differentiate between biological and chronological age, tailoring the right treatment for the right patient.
Physical and cognitive status, together with comorbidities and frailty, should be accurately assessed prior to surgery in order to identify patients who can tolerate standard therapies.
Surgeons have historically been reluctant to incorporate time-consuming and complex screening tools in their practice, and CGA has never been widely adopted in daily clinical activity. In this pilot study, the ability of 11 simplified screening tools to predict long-term mortality was evaluated.
In the geriatric setting, TUG, VES-13, ECOG-PS and IADLs have already been widely recognized but they have never been utilized in a surgical routine in order to predict long-term results15,23,28,29. Being able to stratify patients could improve outcomes, in particular for the most vulnerable patients who could benefit from pre-habilitation30,31 or a tailored surgical strategy, considering their quality of life (QOL) as the most important endpoint. Our study highlighted that even in the surgical setting these screening tools could be useful to identify patients at higher risk of mortality. Including TUG, VES-13, ECOG-PS and IADLs in the routine practice could enhance dedicated pathways for oncogeriatric patients, putting surgical oncologists in better condition to distinguish those who could really benefit from a surgical treatment.
EUROCARE-5 study has recently provided interesting data in terms of long term survival for patients affected by colon cancer. Patients aged 65-74 years old had a 59.5% rate of cancer-related survival, while youngers (45-54 years old) had a 62.4% rate32. This trend in survival was confirmed by the rate in our cohort study where it was found to be 61%.
Significant data were provided by a recent study by Booth and colleagues that showed that elderly patients who underwent liver resection for colorectal metastasis have similar long term survival compared with their younger counterparts33.
Another recent population-based study promoted in the Netherlands showed that elderly patients who underwent surgery for pancreatic cancer who survived 90 days postoperatively exhibited an overall survival close to younger patients34.
To stratify elderly according to frailty status becomes crucial: this is because fit-for-surgery patients will be more prone to have excellent long term survival rate, while those who are at higher risk for complications may be offered alternative therapeutic strategies, avoiding overtreatment. For this reason we strongly believe that preoperative screening tools, such as TUG, VES-13, ECOG-PS and IADLs could have a pivotal role in a comprehensive evaluation of OPs candidate for surgical treatment.
Furthermore, the vast majority of those patients who survived and who were alive at the end of the follow-up, conserved their preoperative living situation. Our study did not identify surgical variables able to predict living situation impairment; for this reason, living situation needs to be deeply explored in future larger longitudinal studies since functional status preservation could represent the most important endpoint from the perspective of the elderly patient35.
The limitations of the study include the small number of patients enrolled and a lack of information regarding the long-term QOL in our surgical OPs. In fact, living situation is only a surrogate indicator and does not take into account many factors which contribute to determining QOL. Furthermore, in actual practice, it is not possible to rely solely on tests to determine management strategies without thoroughly considering patient perspective. When evaluating elderly patients, it is crucial to openly discuss the options, pros and cons in the presence of the caregivers in order to establish a ‘therapeutic alliance’. Future studies will be needed to establish and validate the path for obtaining a holistic evaluation of the oncogeriatric population which could be adopted in everyday practice in order to improve patient selection for surgical treatment. These steps are essential for planning personalized treatment in order to maximize benefits and reduce risks, offering better treatment to patients unfit for standard therapy.
Footnotes
No potential conflicts of interest are disclosed.
References
- 1.American Society of Clinical Oncology . The state of cancer care in America, 2014: a report by the American Society of Clinical Oncology. J Oncol Pract 2014;10:119-142. [DOI] [PubMed] [Google Scholar]
- 2.Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010;116:544-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist 2007;12:20-37. [DOI] [PubMed] [Google Scholar]
- 4.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-E386. [DOI] [PubMed] [Google Scholar]
- 5.Balducci L. Studying cancer treatment in the elderly patient population. Cancer Control 2014;21:215-220. [DOI] [PubMed] [Google Scholar]
- 6.Papamichael D, Audisio R, Horiot JC, Glimelius B, Sastre J, Mitry E, et al. Treatment of the elderly colorectal cancer patient: SIOG expert recommendations. Ann Oncol 2009;20:5-16. [DOI] [PubMed] [Google Scholar]
- 7.Balducci L, Aapro M. Complicated and complex: helping the older patient with cancer to exit the labyrinth. J Geriatr Oncol 2014;5:116-118. [DOI] [PubMed] [Google Scholar]
- 8.Aapro MS, Köhne CH, Cohen HJ, Extermann M. Never too old? Age should not be a barrier to enrollment in cancer clinical trials. Oncologist 2005;10:198-204. [DOI] [PubMed] [Google Scholar]
- 9.Surgery for colorectal cancer in elderly patients: a systematic review. Colorectal Cancer Collaborative Group. Lancet 2000;356:968-974. [PubMed] [Google Scholar]
- 10.Kowdley GC, Merchant N, Richardson JP, Somerville J, Gorospe M, Cunningham SC. Cancer surgery in the elderly. ScientificWorldJournal 2012;2012:303852. [DOI] [PMC free article] [PubMed]
- 11.Ugolini G, Ghignone F, Zattoni D, Veronese G, Montroni I. Personalized surgical management of colorectal cancer in elderly population. World J Gastroenterol 2014;20:3762-3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng MA, McMillan DT, Crowell K, Muss H, Nielsen ME, Smith AB. Geriatric assessment in surgical oncology: a systematic review. J Surg Res 2015;193:265-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zbar AP, Gravitz A, Audisio RA. Principles of surgical oncology in the elderly. Clin Geriatr Med 2012;28:51-71. [DOI] [PubMed] [Google Scholar]
- 14.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142-148. [DOI] [PubMed] [Google Scholar]
- 15.Huisman MG, van Leeuwen BL, Ugolini G, Montroni I, Spiliotis J, Stabilini C, et al. “Timed Up & Go”: a screening tool for predicting 30-day morbidity in onco-geriatric surgical patients? A multicenter cohort study. PLoS One 2014;9:e86863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.PACE participants, Audisio RA, Pope D, Ramesh HS, Gennari R, van Leeuwen BL, et al. Shall we operate? Preoperative assessment in elderly cancer patients (PACE) can help. A SIOG surgical task force prospective study. Crit Rev Oncol Hematol 2008;65:156-163. [DOI] [PubMed]
- 17.Huisman MG, Audisio RA, Ugolini G, Montroni I, Vigano A, Spiliotis J, et al. Screening for predictors of adverse outcome in onco-geriatric surgical patients: A multicenter prospective cohort study. Eur J Surg Oncol 2015;41:844-851. [DOI] [PubMed] [Google Scholar]
- 18.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. the index of adl: a standardized measure of biological and psychosocial function. JAMA 1963;185:914-919. [DOI] [PubMed] [Google Scholar]
- 19.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179-186. [PubMed] [Google Scholar]
- 20.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-655. [PubMed] [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189-198. [DOI] [PubMed] [Google Scholar]
- 22.Schuurmans H, Steverink N, Lindenberg S, Frieswijk N, Slaets JP. Old or frail: what tells us more? J Gerontol A Biol Sci Med Sci 2004;59:M962-965. [DOI] [PubMed] [Google Scholar]
- 23.Saliba D, Elliott M, Rubenstein LZ, Solomon DH, Young RT, Kamberg CJ, et al. The Vulnerable Elders Survey: a tool for identifying vulnerable older people in the community. J Am Geriatr Soc 2001;49:1691-1699. [DOI] [PubMed] [Google Scholar]
- 24.Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer 1999;85:1186-1196. [DOI] [PubMed] [Google Scholar]
- 25.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res 1982-1983;17:37-49. [DOI] [PubMed] [Google Scholar]
- 26.Kondrup J, Allison SP, Elia M, Vellas B, Plauth M, Educational and Clinical Practice Committee, European Society of Parenteral and Enteral Nutrition (ESPEN) . ESPEN guidelines for nutrition screening 2002. Clin Nutr 2003;22:415-421. [DOI] [PubMed] [Google Scholar]
- 27.Chiappa A, Zbar AP, Bertani E, Biella F, Audisio RA, Staudacher C. Surgical outcomes for colorectal cancer patients including the elderly. Hepatogastroenterology 2001;48:440-444. [PubMed] [Google Scholar]
- 28.Laflamme GY, Rouleau DM, Leduc S, Roy L, Beaumont E. The Timed Up and Go test is an early predictor of functional outcome after hemiarthroplasty for femoral neck fracture. J Bone Joint Surg Am 2012;94:1175-1179. [DOI] [PubMed] [Google Scholar]
- 29.Savva GM, Donoghue OA, Horgan F, O’Regan C, Cronin H, Kenny RA. Using timed up-and-go to identify frail members of the older population. J Gerontol A Biol Sci Med Sci 2013;68:441-446. [DOI] [PubMed] [Google Scholar]
- 30.Carli F, Scheede-Bergdahl C. Prehabilitation to enhance perioperative care. Anesthesiol Clin 2015;33:17-33. [DOI] [PubMed] [Google Scholar]
- 31.Boereboom CL, Williams JP, Leighton P, Lund JN, Exercise Prehabilitation in Colorectal Cancer Delphi Study Group . Forming a consensus opinion on exercise prehabilitation in elderly colorectal cancer patients: a Delphi study. Tech Coloproctol 2015;19:347-354. [DOI] [PubMed] [Google Scholar]
- 32.Holleczek B, Rossi S, Domenic A, Innos K, Minicozzi P, Francisci S, et al. On-going improvement and persistent differences in the survival for patients with colon and rectum cancer across Europe 1999-2007 - Results from the EUROCARE-5 study. Eur J Cancer 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 33.Booth CM, Nanji S, Wei X, Mackillop WJ. Management and outcome of colorectal cancer liver metastases in elderly patients: a population-based study. JAMA Oncol 2015;1:1111-1119. [DOI] [PubMed] [Google Scholar]
- 34.van der Geest LG, Besselink MG, van Gestel YR, Busch OR, de Hingh IH, de Jong KP, et al. Pancreatic cancer surgery in elderly patients: balancing between short-term harm and long-term benefit. A population-based study in the Netherlands. Acta Oncol 2015:1-8. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 35.Rønning B, Wyller TB, Jordhøy MS, Nesbakken A, Bakka A, Seljeflot I, et al. Frailty indicators and functional status in older patients after colorectal cancer surgery. J Geriatr Oncol 2014;5:26-32. [DOI] [PubMed] [Google Scholar]


