Abstract
N19, a string of human universal CD4 T-cell epitopes from various pathogen-derived antigens, was shown to exert a stronger carrier effect than CRM197 for the induction of anti-group C Neisseria meningitidis capsular polysaccharide (MenC), after immunization of mice with various dosages of N19-MenC or CRM-MenC conjugate vaccines. After two immunizations, the N19-based construct induced anti-MenC antibody and protective bactericidal antibody titers higher than those induced by three doses of the CRM-MenC conjugate and required lower amounts of conjugate. N19-based conjugates are superior to CRM-based conjugates to induce protective immune responses to MenC conjugates.
In the past 2 decades, the development of conjugate vaccines, consisting of bacterial capsular polysaccharides conjugated to protein carriers, has been successful. The Haemophilus influenzae type b (Hib) conjugate vaccine has reduced the incidence of the invasive disease due to this pathogen by >95% (16). Conjugate vaccines against Streptococcus pneumoniae (19) and group C Neisseria meningitidis (MenC) (3) have been licensed. Others are under development.
The carrier proteins used in licensed vaccines are tetanus toxoid (TT), diphtheria toxoid (DT), the nontoxic mutant of diphtheria toxin, CRM197, and the outer membrane protein complex from group B N. meningitidis. Since more conjugated vaccines are being introduced into the medical practice, infants could receive multiple injections of the carrier protein, either as a vaccine itself (e.g., TT or DT) or as a protein present in the conjugate vaccine. Carrier proteins are highly immunogenic at the B- and T-cell level, and carrier overload may induce immune suppression in primed individuals (7). This phenomenon, termed carrier-induced epitopic suppression, is thought to be due to carrier-specific antibodies and intramolecular antigenic competition (9). This has been well documented in animals (4, 11), although it remains controversial in humans (15). Ideally carrier proteins should induce strong helper effect to the conjugated B-cell epitope (e.g., polysaccharide) without inducing important antibody response against itself. The use of universal epitopes, which are immunogenic in the context of most major histocompatibility complex class II molecules, is one approach towards this goal (1). Universal epitopes have been identified within TT and other proteins (see below) (8), and their use as carrier proteins has been tested in mice and in humans (13, 18).
It was previously shown that a genetically engineered protein, termed N19, expressed in Escherichia coli and consisting of several human CD4+ T-cell universal epitopes, behaves as a strong carrier when conjugated to Hib polysaccharide (10). In the present work, we evaluated the strength of the helper effect of N19 to induce protective antibodies against MenC compared to a conjugate vaccine containing CRM197, which confers protection in infants (3).
The universal CD4+ T-cell epitopes contained within the N19 recombinant protein are shown in Table 1. All epitopes are present in double copies, except for the one from the influenza matrix protein (10). N19 recombinant polyepitope was expressed in E. coli and purified by immobilized metal affinity chromatography as described previously (10). MenC oligosaccharide and CRM-MenC conjugate were prepared as previously described (6). A similar procedure was used for the conjugation of MenC to N19 protein. The saccharide content of each conjugate was quantified by sialic acid determination (17) and the protein content by micro bicinchoninic acid assay (Pierce, Rockford, Ill.). The sugar-to-protein ratio (wt/wt) was 0.4 in the N19-MenC conjugate and 0.49 in the CRM-MenC vaccine.
TABLE 1.
Universal human CD4+ T-cell epitopes contained in the N19 recombinant polyepitopea
| Pathogen | Antigen | Epitope | Amino acid position | Amino acid sequence |
|---|---|---|---|---|
| Clostridium tetani | Tetanus toxin | P23TT | 1084-1099 | VSIDKFRIFCKANPK |
| C. tetani | Tetanus toxin | P32TT | 1174-1189 | LKFIIKRYTPNNEIDS |
| C. tetani | Tetanus toxin | P21TT | 1064-1079 | IREDNNITLKLDRCNN |
| Plasmodium falciparum | Circumsporozoite protein | PfT3 | 380-398 | EKKIAAKMEKASSVFNVVN |
| C. tetani | Tetanus toxin | P30TT | 947-967 | FNNFTVSFWLRVPKVVSASHLE |
| C. tetani | Tetanus toxin | P2TT | 830-843 | QYIKANSKFIGITE |
| HBV | Nucleocapsid | 50-69 | PHHTALRQAILCWGELMTLA | |
| Influenza virus | Hemagglutinin | 307-319 | PKYVKQNTLKLAT | |
| HBV | Surface antigen | 19-33 | FFLLTRILTIPQSLD | |
| Influenza virus | Matrix protein | 17-31 | YSGPLKAEIAQRLEDV |
See reference 10 for references specific to each T-cell epitope present in the N19 protein. HBV, hepatitis B virus.
Groups of female 7-week-old BALB/c mice (Charles River, Calco, Italy) were immunized subcutaneously on days 0, 21, and 35 with decreasing amounts of N19-MenC or CRM-MenC conjugates (from 2.5 to 0.039 μg/dose based on polysaccharide content) in the presence of 0.5 mg of aluminium hydroxide as adjuvant. Individual sera were taken at days −1 (pre), 20 (post-1), 34 (post-2), and 45 (post-3) and frozen at −20°C until use.
Titration of MenC and carrier (N19 and CRM197)-specific immunoglobulin G (IgG) was performed on individual sera as described previously (5). Maxisorp 96-well flat-bottom plates (Nunc, Roskilde, Denmark) were coated overnight at 4°C with 5 μg of methylated human serum albumin/ml and 5 μg of purified MenC/ml in phosphate-buffered saline. Other plates were coated with 2 μg of N19 or CRM197 carrier protein/ml in phosphate-buffered saline. Antigen-specific IgG was revealed with alkaline phosphatase-conjugated goat anti-mouse IgG (Sigma Chemical Co., SA Louis, Mo.). Antibody titers were expressed as the logarithm of the enzyme-linked immunosorbent assay titers that gave an optical density (OD) higher than the mean plus five times the standard deviation (SD) of the average OD obtained in the preimmune sera. The titers were normalized with respect to the reference serum assayed in parallel. Preimmunization values consistently gave OD values below 0.1. Serum bactericidal antibodies titers were determined as previously described (12, 14), with baby rabbit as the source of complement. Titers were expressed as the lowest serum dilution resulting in at least 50% killing of bacteria. Student's t test (two tails) was used to compare antibody titers between groups and at different times. A P value of <0.05 was considered statistically significant.
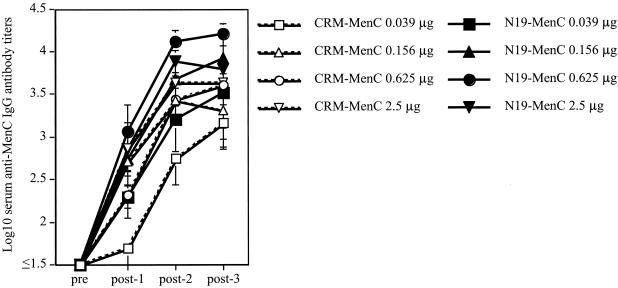
To evaluate the strength of the helper effect of N19 compared to CRM197, groups of mice were immunized with decreasing amounts of each conjugate. The conjugate containing N19 was more immunogenic than the one with CRM (Fig. 1). After two immunizations, the N19-based constructs induced serum anti-MenC IgG antibodies at titers significantly higher than those induced by three doses of the CRM-MenC conjugate (e.g., post-2 N19-MenC at 0.625 μg versus post-3 CRM-MenC at 0.625 μg [P < 0.01]; post-2 N19-MenC at 0.156 μg versus post-3 CRM-MenC at 0.156 μg [P < 0.05]). After three doses, lower amounts of N19-based conjugate induced levels of anti-MenC IgG antibodies significantly higher than those induced by the CRM-MenC conjugate (e.g., N19-MenC at 0.156 μg versus CRM-MenC at 0.625 μg [P < 0.01]).
FIG. 1.
Serum anti-MenC IgG antibody responses. Groups of six BALB/c mice were immunized three times with decreasing amounts of N19-MenC or CRM-MenC (2.5, 0.625, 0.156, and 0.039 μg of MenC/dose) and 0.5 mg of aluminium hydroxide. Serum samples were collected before (pre) and after (post-1, -2, and -3) each immunization and tested individually to quantitate MenC-specific IgG antibody titers. Each point represents the mean antibody titer (± 1 SD) of each group at each time point.
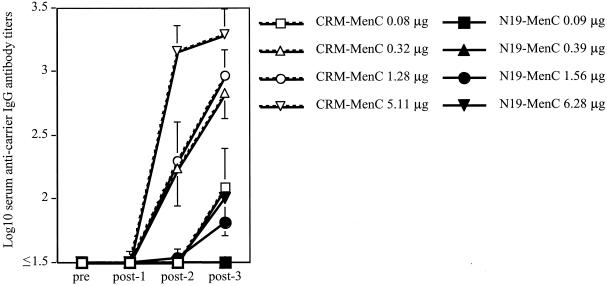
Two and three immunizations with CRM-based conjugates elicited strong antibody responses against CRM even at the lowest doses tested (i.e., 0.3 μg and lower). On the contrary, the N19-specific antibody response was always negligible and was detectable (even though at very low titers) only at the highest dose (i.e., 6 μg) (Fig. 2). These low-titer anti-N19 antibodies did not recognize TT in solid phase (data not shown). These data clearly show that the strong helper effect of the N19 polyepitope is not accompanied by the induction of significant levels of antibodies to itself nor to native proteins.
FIG. 2.
Anti-carrier IgG antibody responses in single serum samples of mice immunized as described before. Since mice were immunized with equal amounts of MenC in either conjugate, the final amount of carrier protein is slightly different in the groups receiving the CRM-MenC and those that received the N19-MenC, due to the slight difference in the sugar-to-protein ratios in the two constructs. Serum samples were collected before (pre) and after (post-1, -2, and -3) each immunization and tested individually to quantitate carrier-specific IgG antibody titers. Each point represents the mean antibody titer (± 1 SD) of each group at each time point.
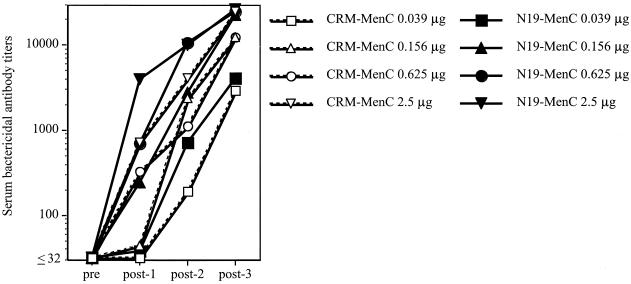
Protective immunity against MenC relies mainly on bactericidal antibodies that kill the bacteria in the presence of complement (2). Results in Fig. 3 show that N19-based conjugates induced bactericidal antibodies at immunizing doses lower than those used with CRM-based conjugates. It is noteworthy that already after one immunization at the highest dose the N19-MenC conjugate elicited bactericidal antibodies at titers similar to those induced by two doses of the CRM-MenC conjugate. Two immunizations with N19-MenC conjugate induced substantial serum bactericidal antibodies, which were much lower in mice immunized with the CRM-based conjugates. These mice required the third dose to reach bactericidal antibody titers, comparable to those induced by N19-based conjugate.
FIG. 3.
Bactericidal activity in serum samples of mice immunized three times with decreasing amounts of N19-MenC or CRM-MenC (2.5, 0.625, 0.156, and 0.039 μg of MenC/dose) and 0.5 mg of aluminium hydroxide. Bactericidal antibody titers from pooled serum samples collected before (pre) and after (post-1, -2, and -3) each immunization are shown. Results are expressed as reciprocal values of the highest serum dilution giving at least 50% bacterial killing.
Taken together, our data show that the string of universal T-cell epitopes present in the N19 polypeptide exhibit a carrier effect for capsular bacterial polysaccharide with better strength than the CRM, in terms of induction of serum antibody titers and of antibodies with bactericidal activity, i.e., participating in the effector mechanisms of protection against group C meningococci (2). These data suggest that the use of the N19 polypeptide as a carrier protein may allow the reduction of the amount of conjugate vaccine used and, possibly, also the number of doses required for a fast, efficient, protective priming against group C meningococci.
Compared to most of the carrier proteins currently in use, including the CRM, the N19 polypeptide induces a very poor antibody response against itself. This would have been expected based on the fact that N19 is made of a series of sequences representing CD4 T-cell epitopes. The low titers of antibodies detected may be directed against particular sequences and/or structures created during the assembly of the protein and not necessarily present in the native proteins. Despite the fact that N19 contains 10 copies of 5 epitopes from TT, anti-N19 antibodies were unable to recognize TT in solid phase. It is very likely that N19-based conjugates will not induce epitope-specific suppression, although this is a phenomenon observed mainly in mice but is still controversial in humans.
Work is currently in progress to evaluate the carrier effect of this protein with other bacterial capsular polysaccharides and to dissect the mechanisms behind the strong and efficient carrier effect of the N19 polyepitope.
Acknowledgments
This work was partially supported by the grant QLRT-PL 1999-0429 (Improving Vaccination in Early Life) from the European Commission.
We thank Silvia Mancianti for superb technical assistance and Marco Tortoli for technical help with mice.
Editor: J. T. Barbieri
REFERENCES
- 1.Alexander, J., M. F. del Guercio, A. Maewal, L. Qiao, J. Fikes, R. W. Chesnut, J. Paulson, D. R. Bundle, S. DeFrees, and A. Sette. 2000. Linear PADRE T helper epitope and carbohydrate B cell epitope conjugates induce specific high titer IgG antibody responses. J. Immunol. 164:1625-1633. [DOI] [PubMed] [Google Scholar]
- 2.Balmer, P., and R. Borrow. 2004. Serologic correlates of protection for evaluating the response to meningococcal vaccines. Expert Rev. Vaccines 3:77-87. [DOI] [PubMed] [Google Scholar]
- 3.Balmer, P., R. Borrow, and E. Miller. 2002. Impact of meningococcal C conjugate vaccine in the UK. J. Med. Microbiol. 51:717-722. [DOI] [PubMed] [Google Scholar]
- 4.Barrios, C., A. R. Lussow, J. Van Embden, R. Van der Zee, R. Rappuoli, P. Costantino, J. A. Louis, P. H. Lambert, and G. Del Giudice. 1992. Mycobacterial heat-shock proteins as carrier molecules. II. The use of the 70-kDa mycobacterial heat-shock protein as carrier for conjugated vaccines can circumvent the need for adjuvants and Bacillus Calmette Guerin priming. Eur. J. Immunol. 22:1365-1372. [DOI] [PubMed] [Google Scholar]
- 5.Carlone, G. M., C. E. Frasch, G. R. Siber, S. Quataert, L. L. Gheesling, S. H. Turner, B. D. Plikaytis, L. O. Helsel, W. E. DeWitt, W. F. Bibb, B. Swaminathan, G. Arakere, C. Thompson, D. Phipps, D. Madore, and C. V. Broome. 1992. Multicenter comparison of levels of antibody to the Neisseria meningitidis group A capsular polysaccharide measured by using an enzyme-linked immunosorbent assay. J. Clin. Microbiol. 30:154-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costantino, P., F. Norelli, A. Giannozzi, S. D'Ascenzi, A. Bartoloni, S. Kaur, D. Tang, R. Seid, S. Viti, R. Paffetti, M. Bigio, C. Pennatini, G. Averani, V. Guarnieri, E. Gallo, N. Ravenscroft, C. Lazzeroni, R. Rappuoli, and C. Ceccarini. 1999. Size fractionation of bacterial capsular polysaccharides for their use in conjugate vaccines. Vaccine 17:1251-1263. [DOI] [PubMed] [Google Scholar]
- 7.Del Giudice, G. 1992. New carriers and adjuvants in the development of vaccines. Curr. Opin. Immunol. 4:454-459. [DOI] [PubMed] [Google Scholar]
- 8.Diethelm-Okita, B. M., D. K. Okita, L. Banaszak, and B. M. Conti-Fine. 2000. Universal epitopes for human CD4+ cells on tetanus and diphtheria toxins. J. Infect. Dis. 181:1001-1009. [DOI] [PubMed] [Google Scholar]
- 9.Etlinger, H. M., D. Gillessen, H. W. Lahm, H. Matile, H. J. Schonfeld, and A. Trzeciak. 1990. Use of prior vaccinations for the development of new vaccines. Science 249:423-425. [DOI] [PubMed] [Google Scholar]
- 10.Falugi, F., R. Petracca, M. Mariani, E. Luzzi, S. Mancianti, V. Carinci, M. L. Melli, O. Finco, A. Wack, A. Di Tommaso, M. T. De Magistris, P. Costantino, G. Del Giudice, S. Abrignani, R. Rappuoli, and G. Grandi. 2001. Rationally designed strings of promiscuous CD4(+) T cell epitopes provide help to Haemophilus influenzae type b oligosaccharide: a model for new conjugate vaccines. Eur. J. Immunol. 31:3816-3824. [DOI] [PubMed] [Google Scholar]
- 11.Fattom, A., Y. H. Cho, C. Chu, S. Fuller, L. Fries, and R. Naso. 1999. Epitopic overload at the site of injection may result in suppression of the immune response to combined capsular polysaccharide conjugate vaccines. Vaccine 17:126-133. [DOI] [PubMed] [Google Scholar]
- 12.Granoff, D. M., S. E. Maslanka, G. M. Carlone, B. D. Plikaytis, G. F. Santos, A. Mokatrin, and H. V. Raff. 1998. A modified enzyme-linked immunosorbent assay for measurement of antibody responses to meningococcal C polysaccharide that correlate with bactericidal responses. Clin. Diagn. Lab. Immunol. 5:479-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keitel, W. A., K. E. Kester, R. L. Atmar, A. C. White, N. H. Bond, C. A. Holland, U. Krzych, D. R. Palmer, A. Egan, C. Diggs, W. R. Ballou, B. F. Hall, and D. Kaslow. 1999. Phase I trial of two recombinant vaccines containing the 19kd carboxy terminal fragment of Plasmodium falciparum merozoite surface protein 1 (msp-1(19)) and T helper epitopes of tetanus toxoid. Vaccine 18:531-539. [DOI] [PubMed] [Google Scholar]
- 14.Maslanka, S. E., L. L. Gheesling, D. E. Libutti, K. B. J. Donaldson, H. S. Harakeh, J. K. Dykes, F. F. Arhin, S. J. N. Devi, C. E. Frasch, J. C. Huang, P. Kriz-Kuzemenska, R. D. Lemmon, M. Lorange, C. C. A. M. Peeters, S. Quataert, J. Y. Tai, G. M. Carlone, and the Multilaboratory Study Group. 1997. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. Clin. Diagn. Lab. Immunol. 4:156-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McVernon, J., J. MacLennan, E. Clutterbuck, J. Buttery, and E. R. Moxon. 2003. Effect of infant immunisation with meningococcus serogroup C-CRM(197) conjugate vaccine on diphtheria immunity and reactogenicity in pre-school aged children. Vaccine 21:2573-2579. [DOI] [PubMed] [Google Scholar]
- 16.Peltola, H. 2000. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clin. Microbiol. Rev. 13:302-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Svennerholm, L. 1957. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochem. Biophys. Acta 24:604-611. [DOI] [PubMed] [Google Scholar]
- 18.Valmori, D., A. Pessi, E. Bianchi, and G. Corradin. 1992. Use of human universally antigenic tetanus toxin T cell epitopes as carriers for human vaccination. J. Immunol. 149:717-721. [PubMed] [Google Scholar]
- 19.Wuorimaa, T., and H. Kayhty. 2002. Current state of pneumococcal vaccines. Scand. J. Immunol. 56:111-129. [DOI] [PubMed] [Google Scholar]