Abstract
Hyperplasia of the middle ear mucosa contributes to the sequelae of acute otitis media. Understanding the signal transduction pathways that mediate hyperplasia could lead to the development of new therapeutic interventions for this disease and its sequelae. Endotoxin derived from bacteria involved in middle ear infection can contribute to the hyperplastic response. The p38 mitogen-activated protein kinase (MAPK) is known to be activated by endotoxin as well as cytokines and other inflammatory mediators that have been documented in otitis media. We assessed the activation of p38 in the middle ear mucosa of an in vivo rat bacterial otitis media model. Strong activity of p38 was observed 1 to 6 h after bacterial inoculation. Activity continued at a lower level for at least 7 days. The effects of p38 activation were assessed using an in vitro model of rat middle ear mucosal hyperplasia in which mucosal growth is stimulated by nontypeable Haemophilus influenzae during acute otitis media. Hyperplastic mucosal explants treated with the p38α and p38β inhibitor SB203580 demonstrated significant inhibition of otitis media-stimulated mucosal growth. The results of this study suggest that intracellular signaling via p38 MAPK influences the hyperplastic response of the middle ear mucosa during bacterial otitis media.
Pathologic conditions related to otitis media include middle ear effusion, adhesive otitis, tympanic membrane perforation, tympanosclerosis, scarring of the middle ear mucosa, cholesteatoma, and atelectasis (4, 24, 37, 38, 43, 46). These sequelae can lead to permanent damage of the middle ear cavity, resulting in hearing loss or impairment (1). A contributing factor to such morbidity during otitis media is the transformation of the inflamed middle ear mucosa. Normally, the middle ear mucosa consists of a simple squamous epithelium overlying a thin lamina propria that adheres to the underlying periosteum (21). This minimal mucosa ranges from 15 to 20 μm thick. During otitis media the middle ear mucosa has the unique capacity to grow and proliferate to many times its original thickness, into a highly structured, pseudostratified, columnar epithelial complex (23, 25, 33, 42). Hyperplasia of the mucosa is associated with many of the negative sequelae of middle ear infection. Production of mucus and other elements of middle ear effusions is closely linked to the production of additional mucosal cells and their transformation into a secretory phenotype (33, 51). Expansion of the mucosa vasculature provides increased opportunity for leukocytic infiltration and tissue edema, while increases in stromal cells with their subsequent activation can lead to the generation of matrix components and fibrosis (20, 45, 46, 50). The hyperplastic response of the middle ear mucosa occurs within a few days following any number of otitis media-related stimuli, including tubal obstruction (27), bacterial infection (48, 50), endotoxin exposure (10, 26), immunologic challenge (23, 43), and exposure to inflammatory mediators such as cytokines (3). These stimuli are presumably linked to the hyperplastic response via intracellular signal transduction pathways. Understanding which intracellular mechanisms contribute to middle ear mucosal hyperplasia may better enable us to treat otitis media before irreversible damage occurs.
In other tissue systems the mitogen-activated protein kinase (MAPK) intracellular signaling pathways, including Erk, Jnk, and p38, have been shown to be linked to a variety of cellular responses, including cellular proliferation (2, 5, 6, 9, 14). This characteristic of the MAPK family makes them candidates for regulation of mucosal hyperplasia during infection. The p38 pathway in particular is a stress-induced kinase activated by various extracellular stimuli associated with otitis media, including bacterial endotoxin, osmotic shock, and inflammatory cytokines such as tumor necrosis factor and interleukin 1 (15, 19, 30, 40, 41). The presence of endotoxin, elevated levels of inflammatory cytokines, and effusion in the middle ear during bacterial otitis media provides the potential for the activation of this pathway. In middle ear epithelial cell lines, p38 activation has been linked to the expression of genes encoding cytokines and mucin (49, 53, 54).
The p38 MAPK intracellular signaling pathway is the most recently characterized and least understood MAPK pathway. The intermediate effector molecules between initial extracellular stimulation by endotoxin or cytokines and downstream p38 activity have yet to be completely identified. It is known that p38 activation is carried out by a dual phosphorylation on Thr-180 and Tyr-182 within the Thr-Gly-Tyr motif located in subdomain VIII (12, 40). It is also known that there are four p38 isoforms termed α (38,000 Da), β (39,000 Da), γ (39,000 Da), and δ (38,000 Da) (8, 18). Specificity of isoform phosphorylation is believed to be partially responsible for p38’s diverse functional influence upon cellular physiology (13, 29). The functional variability of p38 makes it a potential agent in a number of middle ear mucosal changes that occur during otitis media.
Whether the p38 intracellular signaling pathway is activated during otitis media and what role it may play in the hyperplastic response of the middle ear mucosa have not been investigated. For this reason, we explored the presence of activated p38 at different postinoculation time points in a rat in vivo otitis media model. We also developed an in vitro model of hyperplastic middle ear mucosa and explored the effects of the p38α and p38β inhibitor, SB203580, on bacterially induced mucosal growth. The combined data from this study support multiple roles for p38 MAPK signaling in the hyperplastic response of the middle ear mucosa during bacterial otitis media.
MATERIALS AND METHODS
All experiments were performed according to National Institutes of Health guidelines on the care and use of laboratory animals and were approved by the institutional committee for animal experimentation.
Assessment of p38 MAPK phosphorylation.
Male Sprague-Dawley rats weighing between 300 and 350 g were anesthetized with a mixture of ketamine and xylazine hydrochloride (Rompun) at 100 mg/ml and acepromazine at 10 mg/ml, injected intramuscularly at a dose of 0.4 ml/100 g of body weight. Upon sedation, the animals were placed in the supine position and their bodies stabilized with masking tape. A 3-cm, vertical, midline incision was made between the animal's mandible and clavicles, and middle ear bullae exposure was obtained bilaterally with the aid of a dissecting microscope. A 25-gauge syringe needle was used to fenestrate the center of the middle ear bullae bilaterally. A solution containing 105 cells of Haemophilus influenzae strain 3655 (nontypeable, biotype II) per ml was injected bilaterally into the middle ear bullae until the solution overflowed the fenestrations, a volume of approximately 5 μl. Excess fluid was absorbed with a cotton swab. The original fascia covering the middle ear bullae were then used to re-cover the bulla holes, and the incisions were stapled closed. Each animal was then examined to guarantee that their tympanic membranes had not been ruptured. A sham operation control group received saline in the middle ear. The animals were sacrificed, and the middle ear bullae were surgically extracted bilaterally from the rats at one of seven different postinoculation time points. The time points included 1, 6, 24, 48, and 72 h and 7 days postinfection. The bullae were immediately placed in a petri dish containing phosphate-buffered saline (PBS). The middle ear mucosae were surgically removed intact from the anteriolateral part of the bullae with the aid of a dissecting microscope. For each Western blot, mucosae from two to six middle ears were pooled to obtain sufficient tissue for analysis, depending on the state of the mucosa at the time it was sampled. Six middle ear mucosae from three nonsurgical, noninfected rats were also dissected to serve as negative controls. Upon extraction all the mucosae were immediately frozen at −70°C. Three separate groups of middle ear samples were used to generate three replicate Western blots.
The frozen ear tissues for each group were homogenized in 100 μl of RIPA buffer, which contained 20 mM Tris (pH 7.5), 1 mM EDTA, 140 mM NaCl, 1% Nonidet P-40, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 50 mM sodium fluoride, and aprotinin (10 μg/ml), using a Potter-Elvehjem homogenizer. The homogenates were centrifuged at 14,000 × g at 4°C and the pellets were discarded. Protein concentrations were equalized by spectrophotometry, and equal protein fractions of the total cell lysates were mixed with 2× sodium dodecyl sulfate (SDS) sample buffer. The samples were boiled and the proteins resolved on an SDS-polyacrylamide gel. After being transferred to polyvinylidene difluoride paper, the proteins were immunoblotted with rabbit polyclonal antibody that reacted only with phosphorylated p38 kinase (Promega, Madison, Wis.). The anti-active p38 MAPK antibody was generated with a dual phosphorylated peptide derived from the catalytic core of the active form of p38 MAPK, corresponding to Thr-182 of the mammalian p38 enzyme, and used at a dilution of 1:2,000. Blots were stripped and reprobed with an antibody against total p38 (Santa Cruz Biotechnologies, Santa Cruz, Calif.). After incubation with secondary horseradish peroxidase-conjugated anti-rabbit antibodies, the proteins were visualized by enhanced chemiluminescence (Pierce, Rockford, Ill.). Because of the use of separate antibodies and stripped blots, the amounts of total and phosphorylated p38 were not quantitatively comparable.
p38 inhibition with SB203580 in infected mucosae.
To design an in vitro hyperplastic middle ear mucosa model, previous studies (39) were conducted to determine the greatest level of in vitro hyperplasia of stimulated rat middle ear mucosa. Explants of middle ear mucosae were cultured for ten days from rat middle ears inoculated with H. influenza for 6, 24, 48, 72, or 120 h. Though mucosae from all time points demonstrated a permanent hyperplastic transformation, the middle ear mucosae cultured 48 h postinfection demonstrated the highest level of hyperplasia in vitro compared to the other time points. The surface area of the bacterially exposed 48-h explant outgrowths was approximately 3.5 times that of the outgrowth from the control explants. This ratio remained approximately the same over ten days of culture. The 6-, 24-, and 120-h explants grew to approximately 1.1, 1.3, and 2.2 times the area of the control explants, respectively. Explants extracted 72 h after infection lacked sufficient structural integrity to be cultured. These experiments demonstrated that middle ear mucosae cultured 48 h after in vivo bacterial inoculation display maximal hyperplasia in vitro. This is consistent with in vivo observations that mucosal hyperplasia is maximal from 48 to 72 h after initiation of experimental otitis media (16, 33).
Inhibition of infected mucosae with the p38 inhibitor SB203580.
The bullae of six male Sprague-Dawley rats weighing between 300 and 350 g were injected with nontypeable H. influenzae (NTHI) at 105 cells per ml in the manner described above. After 48 hours, the animals were anesthetized and sacrificed by decapitation. After each animal was sacrificed, the middle ear bullae were surgically extracted and the middle ear mucosae, bilaterally, were dissected as described above. Each middle ear mucosal sample was immediately placed in a separate 60-mm-diameter Falcon petri dish coated with a thin layer of Sylgard from Dow Corning (Midland, Mich.). Culture medium consisting of a mixture of Dulbecco's modified Eagle's medium and Ham's F-12 medium (3:1) supplemented with fetal calf serum (5%), hydrocortisone (0.4 μg/ml), isoproterenol (10−6 M), penicillin (100 U/ml), and streptomycin (100 μg/ml) was then added (52). The middle ear mucosae were divided into roughly 0.5-mm2 tissue explants with a Fine Science Tools (Foster City, Calif.) diamond knife. The explants from each bulla were then individually transplanted, epithelium uppermost, into single wells of a 24-well Falcon cell culture plate containing 170 μl of the above culture medium. They were then placed in an incubator at 37°C with 5% CO2 for 24 h and allowed to adhere to the culture plate surface. On day one, all wells with healthy, attached explants were randomly divided into four groups. The p38 inhibitor, SB203580 (Calbiochem, San Diego, Calif.), was reconstituted in dimethyl sulfoxide (DMSO) and added to one of three groups at 1, 10, and 100 nM in 400 μl of culture medium. The last group served as a negative control, the media receiving a supplement of DMSO (1 μl/ml; Sigma-Aldrich, St. Louis, Mo.) alone, the same concentration of DMSO used for all concentrations of SB203580. Each day, the medium from each well was removed and 400 μl of fresh culture medium was added with the appropriate concentration of SB203580 and/or DMSO. The explants from each group were also treated with 5-bromo-2-deoxyuridine (BrdU) (Sigma-Aldrich) every day for the entire duration of the experiment. The explants were kept in culture for ten days.
For each individual concentration of SB203580, six to nine explants were evaluated. Each explant was photographed each day with an RT SPOT color digital camera and their surface areas calculated using SPOT computer software calibrated to the appropriate magnification. The data were entered into Statview 5.0 and an analysis of variance was run with a P value criterion of <0.05. The tissue explants were also immunohistochemically stained with an anti-BrdU primary antibody (Sigma-Aldrich) followed by a biotinylated secondary antibody (Santa Cruz Biotechnologies, Santa Cruz, Calif.). After the appropriate biotin and avidin blocks, the cultures were treated with a fluorescein isothiocyanate-labeled strepavidin (DAKO, Carpinteria, Calif.). Each well was then photographed again under various magnifications with a GFP fluorescent filter.
Inhibition of noninfected mucosae with SB203580.
The middle ear mucosae of six male Sprague-Dawley rats that had not been infected with H. influenzae were dissected, divided, and cultured in the manner described above. The explants were divided into three groups. The first group was cultured with 10 nM SB203580, the second group was cultured with 100 nM SB203580, and the last group served as a control being cultured with DMSO (1 μl/ml, the same concentration of DMSO used for all concentrations of SB203580). The cultured tissue was cared for and photographed as described above for the duration of 10 days. For each individual concentration of SB203580, 10 to 14 explants were evaluated. Explant outgrowth surface areas were measured from the photographs on days 1, 3, 7, and 10. Analysis of variance was run with a P criterion of <0.05.
Evaluation of p38 in vitro.
The presence and activation of p38 and the effects of SB203580 were determined in our in vitro culture system. Middle ear mucosal explants were prepared from healthy rat middle ears and from middle ears inoculated with NTHI 48 h previously. Explants were cultured as above for 72 h, with or without 100 nM SB203580. Explants were then harvested and assayed by Western blotting for total and phospho-p38 as described above.
RESULTS
Activation of p38 in the infected middle ear mucosa.
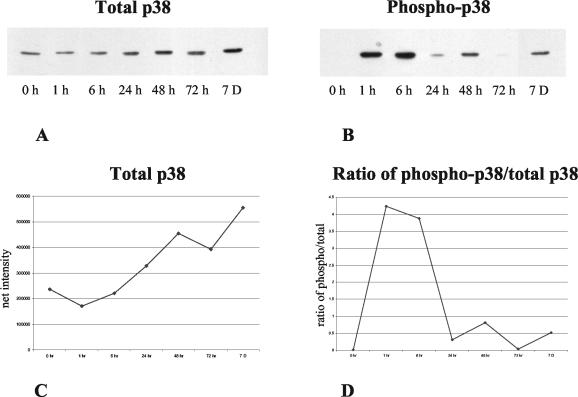
Figure 1 illustrates the levels of total and activated p38 in the middle ear mucosa at various in vivo postinoculation time points. Total p38 protein (Fig. 1A and C) showed a gradual rise over the period evaluated. No phospho-p38 was detected in untreated, control mucosae (0 h in Fig. 1B). A high level of p38 activity was observed 1 and 6 h after inoculation with NTHI. Lower levels of phospho-p38 were seen at 24 and 48 h. While very little p38 activity was present at 72 h, moderate phosphorylation was again observed at 7 days. The ratio of phospho-p38 to total p38 (Fig. 1D) showed strong phosphorylation at 1 and 6 h and weaker phosphorylation at later periods. Similar patterns of p38 activation were observed in three additional series of mucosal samples (data not shown). Injection of saline into the middle ear had no effect on either total or phospho-p38 (data not shown).
FIG. 1.
Western blot of total (A) and phosphorylated (B) p38 in middle ear mucosa at various postinoculation times. Total protein was equalized in all lanes. Quantitative analysis of total p38 (C) shows a gradual increase over time, while the ratio of phospho-p38 to total p38 (D) shows strong p38 phosphorylation 1 to 6 h after bacterial inoculation, with lower levels thereafter. Higher levels of intensity observed for phospho-p38 than for total p38 at some time points presumably reflect differential sensitivity of the antibodies used, blotting efficiency, and the fact that total p38 was measured on stripped blots.
p38 inhibition with SB203580 in infected middle ear mucosa.
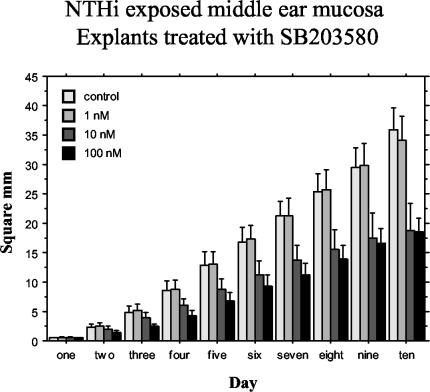
Figure 2 illustrates the effects of the p38 inhibitor, SB203580, on the outgrowth surface area of middle ear mucosal explants cultured 48 h postinoculation with NTHI. Explants from infected rats showed a high rate of growth throughout the 10-day culture period when not treated with SB203580. Such tissue explants, cultured in the presence of BrdU, demonstrated BrdU-positive cell nuclei throughout the entire outgrowth when immunohistochemically stained with a fluorescein isothiocyanate-labeled anti-BrdU antibody (data not shown). SB203580 at 1 nM (P = 0.8342) did not have a significant effect on this hyperplastic tissue outgrowth. SB203580 at 10 nM (P < 0.0001) and 100 nM (P < 0.0001) had a significant inhibitory effect compared with the negative controls. Outgrowths at SB203580 concentrations of 10 and 100 nM, however, were not significantly different compared to each other (P = 0.2497).
FIG. 2.
Average surface area (in square millimeters) of bacterially exposed middle ear mucosal explants (harvested 48 h after bacterial inoculation of the middle ear) cultured with different nanomolar concentrations of SB203580 for 10 days and measured for area of growth each day. Significant inhibition of growth was observed at 10 nM (P < 0.0001) and 100 nM (P < 0.0001). Bars represent means, and vertical lines represent 1 SEM.
p38 inhibition with SB203580 in noninfected mucosa.
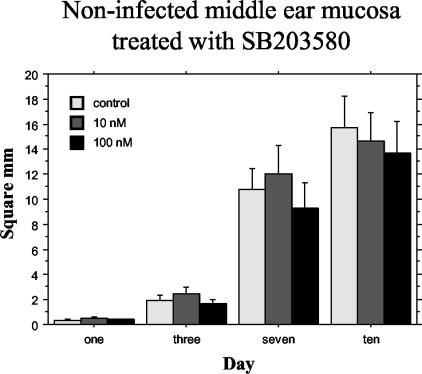
Figure 3 illustrates the effects of the p38 inhibitor on the explant outgrowth surface area of cultured, noninfected, middle ear mucosa explants. SB203580 at concentrations of 10 and 100 nM had no significant effect on mucosal growth compared to controls that were not treated with the inhibitor.
FIG. 3.
Average surface area (in square millimeters) of noninfected middle ear mucosal explants cultured with different nanomolar concentrations of SB203580 for 10 days and measured for area of growth on days 1, 3, 7, and 10. No effect of the inhibitor on explant growth was observed. Bars represent means, and vertical lines represent 1 SEM.
p38 activation in culture.
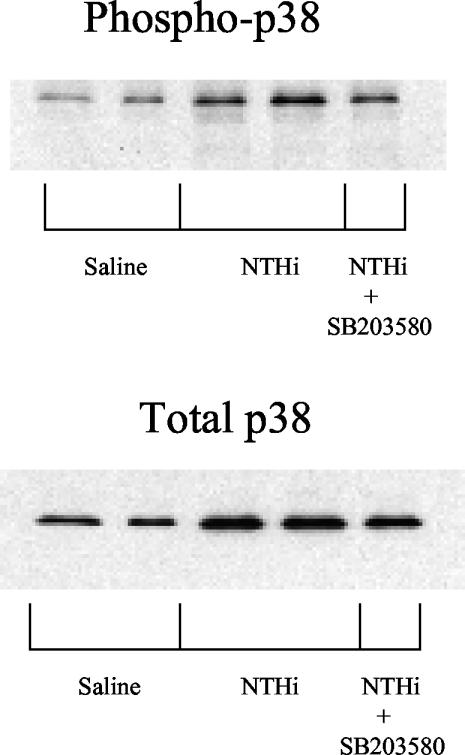
After 72 h in culture, the level of total p38 was found to be higher in explants previously exposed to bacteria than in normal, noninfected explants. Similarly, phospho-p38 was elevated in bacterially exposed explants. Treatment with SB203580 had no effect on either total or phospho-p38 for either normal or bacterially exposed mucosal explants (Fig. 4).
FIG. 4.
Western blot of total and phosphorylated p38 in middle ear mucosal explants harvested 48 h after injection of saline or bacterial inoculation and cultured for 72 hours. Total protein was equalized in all lanes.
DISCUSSION
The results of this study reveal evidence for a functional role of p38 signaling in middle ear mucosal hyperplasia during bacterial otitis media. Introduction of NTHI into the middle ear of rats produces otitis media that peaks in severity 2 to 5 days after inoculation, followed by recovery that is complete at around 2 weeks (35). We found that the mucosa of middle ears challenged in vivo with NTHI showed sharply elevated levels of activated p38 immediately after inoculation, declining to lower levels for the next few days. One week later, phospho-p38 was elevated, due partially to increasing levels of total p38 (Fig. 1). The early phospho-p38 response observed suggests that p38 is involved in the initial phases of otitis media. However, elevated levels of phospho-p38 at 7 days suggest that it may also play a role in recovery.
The early variation in activated p38 was produced by changes in phosphorylation, since total p38 showed no increase in the first hours after inoculation. This is in contrast to our prior data on the Erk MAPKs, in which variation in mucosal phospho-Erk largely paralleled changes in total Erk (39).
A role for p38 in the mucosal response to bacteria is also supported by our in vitro data. Both total and phospho-p38 were elevated in mucosal cultures that had previously been exposed to NTHI in vivo. Moreover, inhibition of p38 signaling produced a significant, dose-dependent suppression of bacterially stimulated growth in middle ear mucosal explant cultures (Fig. 2) but had no effect on noninfected middle ear explants (Fig. 3). Since all of the cells present in the middle ear cultures were labeled by BrdU, the observed growth represents cell proliferation rather than migration from the explant. Thus inhibition of p38 markedly reduced bacterially induced mucosal proliferation. The in vitro growth of noninfected mucosal samples presumably occurred via a p38-independent mechanism, perhaps mediated by growth factors present in the media and/or produced by the samples themselves.
The function of an individual intracellular signaling pathway can be evaluated only if inhibitors that selectively suppress targeted enzymes in that pathway are available. SB203580, which operates through competition with ATP for the same docking site on the p38 kinase (55, 57), specifically inhibits members of the p38 MAPK subgroup, not the Erk or Jnk MAPKs (8). SB203580 inhibits only the α and β isoforms of p38 and has little or no effect on the γ or δ isoforms (17). This is caused by the presence of amino acids larger than threonine at position 106 of the latter isoforms, which prevent accommodation of the 4-fluorophenyl moiety of SB203580 (8). Since SB203580 does not inhibit the γ or δ isoforms, and individual isoform identity based upon molecular weight cannot be determined from Figure 1, it cannot be concluded from the data of our study whether or not the γ or δ isoforms are present or if they have significant functional involvement in mucosal hyperplasia during otitis media. However, since inhibition of the α and β isoforms via SB203580 eliminates the majority of the bacterially induced growth of middle ear mucosa in vitro, it can be argued that the γ and δ isoforms do not play a major role. Of course, our in vitro model may differ from the in vivo situation.
The sensitivity of the middle ear mucosa to SB203580 also needs to be taken into consideration. There was a potent inhibitory effect on mucosal growth at SB203580 concentrations of 10 to 100 nM, with no significant difference in inhibition between these two concentrations. Studies in most other tissue systems (6, 7) require higher concentrations of SB203580 (1 to 10 μM) to produce an inhibitory effect. Some cell types, however, such as platelets and THP-1 monocytes, display significant inhibition of p38 kinase activity at comparable concentrations of SB203580 (1 to 100 nM) (7), with bacterially exposed middle ear mucosa displaying a sensitivity similar to that of platelets, characterized by inhibition saturating at 10 nM. It should be noted that this potent inhibitory response may be related to an increased intracellular level of SB203580 due to exposure over the ten-day culture period. Long exposure time has been proposed to explain the low threshold of response to SB203580 in THP-1 monocytes (7). Of course, as with all inhibitors, it is also possible that SB203580 inhibits protein kinases or enzymes other than p38 that have not been evaluated or identified and which may relate to mucosal cell proliferation. It should also be noted that hydrocortisone was present in the culture media for both control and experimental mucosal samples. This could have influenced the growth of the mucosa, but would have affected both untreated and inhibitor-treated mucosa equally.
Total p38 and phospho-p38 were both elevated in in vitro mucosal explants previously exposed to bacteria, compared to noninfected in vitro explants. This agrees with the in vivo results and also is consistent with the effects of SB203580 on mucosal growth in bacterially exposed mucosal explants. SB203580 had no effect on total p38 levels or on p38 phosphorylation, consistent with its action downstream of p38 activation.
The p38 pathway is involved in a broad range of cellular responses in other tissue systems, including proliferation, migration, differentiation, and apoptosis (11, 22, 28, 36, 56). The divergent physiological functions of p38 may be explained in part by the existence of four different p38 isoforms. It is therefore possible that phosphorylation of different p38 isoforms at distinct times might contribute to the differential behavior of the middle ear mucosa during the time course of otitis media.
The data of this study suggest a significant role played by p38 MAPK signaling in middle ear mucosal hyperplasia. Our results complement the work of others that implicates p38 activation in the production of inflammatory cytokines and mucin (32, 49, 53, 54), which contribute to otitis media pathogenesis. These data may be clinically relevant to the treatment of bacterial otitis media. It is clear that both the middle ear mucosal hyperplastic responses and the p38 MAPK signaling pathway are highly complex and coordinated biological processes. Introducing an inhibitor in vivo might disrupt this process, blocking growth and/or proliferation of the hyperplastic middle ear mucosa and reducing the number of recurrent episodes and the severity of the sequelae of acute otitis media. However, our results suggest that timing and inhibitor specificity could play an important role if p38 inhibition is to be clinically effective. Introducing an inhibitor during the early stages of infection may cause a significant reduction in mucosal hyperplasia by inhibiting cellular proliferation and enhancing cellular apoptosis as has been demonstrated in Jurkat cells (48). However, if an inhibitor of p38 were introduced later during infection, it might possibly inhibit mucosal recovery by delaying apoptosis. Inhibition of p38 has been shown to block apoptosis in other epithelial cells (31). This could increase the duration of the mucosal hyperplasia. Additionally, even at saturated levels of SB203580, some mucosal growth still takes place in vitro. This suggests involvement of alternative pathways independent of p38 in middle ear mucosal hyperplasia, possibly indicating the need for multiple inhibitors to completely suppress the hyperplastic response. We have shown that inhibition of the Erk/MAPKs also decreases middle ear mucosal proliferation (39). A more complete understanding of which intracellular pathways are involved and the specifics of which p38 isoforms are most active during otitis media will better enable the rational design of therapeutic interventions for this disease.
ACKNOWLEDGEMENTS
This work was supported by grant DC00129 from the NIH/NIDCD and by the Research Service of the United States Department of Veteran Affairs.
Editor: D. L. Burns
REFERENCES
- 1.Brooks, D. 1979. Otitis media and child development-design factors in the identification and assessment of hearing loss. Ann. Otol. Rhinol. Laryngol. Suppl. 88:29-47. [PubMed] [Google Scholar]
- 2.Campbell, S., R. Khosravi-Far, K. Rossman, G. Clark, and C. Der. 1998. Increasing complexity of Ras signaling. Oncogene 17:1395-1413. [DOI] [PubMed] [Google Scholar]
- 3.Catanzaro, A., A. F. Ryan, and S. Batcher. 1991. The response to human rIL-1, rIL-2 and rTNF in the middle ear of guinea pigs. Laryngoscope 101:271-275. [DOI] [PubMed] [Google Scholar]
- 4.Cawthorne, T. 1956. Chronic adhesive otitis. J. Laryngol. Otol. 70:559-564. [DOI] [PubMed] [Google Scholar]
- 5.Cobb, M., and E. Goldsmith. 1995. How MAP kinases are regulated. J. Biol. Chem. 270:14843-14846. [DOI] [PubMed] [Google Scholar]
- 6.Craxton, A., G. Shu, J. Graves, J. Saklatvala, E. Krebs, and E. Clark. 1998. p38 MAPK is required for CD4-induced gene expression and proliferation in B lymphocytes. J. Immunol. 161:3225-3236. [PubMed] [Google Scholar]
- 7.Cuenda, A., J. Rouse, Y. Doza, R. Meier, P. Cohen, T. Gallagher, P. Young, and J. Lee. 1995. SB203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 364:229-233. [DOI] [PubMed] [Google Scholar]
- 8.Davies, S., H. Reddy, M. Caivano, and P. Cohen. 2000. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 351:95-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis, R. J. 1994. MAPKs: new JNK expands the group. Trends Biochem. Sci. 19:470-473. [DOI] [PubMed] [Google Scholar]
- 10.DeMaria, T. F., J. M. Billy, and D. G. Danahey. 1996. Growth factors during endotoxin-induced experimental otitis media. Acta Otolaryngol. 116:854-856. [DOI] [PubMed] [Google Scholar]
- 11.Dieckgraefe, B., D. Weems, S. Santoro, and D. Alpers. 1997. ERK and p38 MAP kinase pathways are mediators of intestinal epithelial wound-induced signal transduction. Biochem. Biophys. Res. Commun. 233:389-394. [DOI] [PubMed] [Google Scholar]
- 12.Doza, Y., A. Cuenda, G. Thomas, P. Cohen, and A. Nebreda. 1995. Activation of the MAP kinase homologue RK requires the phosphorylation of Thr-180 and Tyr-182 and both residues are phosphorylated in chemically stressed KB cells. FEBS Lett. 364:223-228. [DOI] [PubMed] [Google Scholar]
- 13.Eckert, R. L., T. Efimova, S. Balasubramanian, J. F. Crish, F. Bone, and S. Dashti. 2003. p38 Mitogen-activated protein kinases on the body surface—a function for p38 delta. J. Investig. Dermatol. 120:823-828. [DOI] [PubMed] [Google Scholar]
- 14.Ewen, M. 2000. Relationship between ras pathways and cell cycle control. Prog. Cell Cycle Res. 4:1-17. [DOI] [PubMed] [Google Scholar]
- 15.Freshney, N., L. Rawlinson, F. Guesdon, E. Jones, S. Cowley, J. Hsuan, and J. Saklatvala. 1994. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp 27. Cell 78:1039-1049. [DOI] [PubMed] [Google Scholar]
- 16.Friedmann, I. 1955. The comparative pathology of otitis media—experimental and human. II. The histopathology of experimental otitis of the guinea pig with particular reference to experimental cholesteatoma. J. Laryngol. 69:588-601. [PubMed] [Google Scholar]
- 17.Goedert, M., A. Cuenda, M. Craxton, R. Jakes, and P. Cohen. 1997. Activation of the novel stress-activated protein kinase SAPK4 by cytokines and cellular stresses is mediated by SKK3 (MKK6); comparison of its substrate specificity with that of other SAP kinases. EMBO J. 16:3563-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hale, K., D. Trollinger, M. Rihanek, and C. Manthey. 1999. Differential expression and activation of p38 mitogen-activated protein kinase α, β, γ, and δ in inflammatory cell lineages. J. Immunol. 162:4246-4252. [PubMed] [Google Scholar]
- 19.Han, J., J. Lee, L. Bibbs, and R. Ulevitch. 1994. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265:808-811. [DOI] [PubMed] [Google Scholar]
- 20.Hauptmann, S., A. Siegert, S. Berger, C. Denkert, M. Kobel, S. Ott, A. Siri, and L. Borsi. 2003. Regulation of cell growth and the expression of extracellular matrix proteins in colorectal adenocarcinoma: a fibroblast-tumor cell coculture model to study tumor-host interactions in vitro. Eur. J. Cell Biol. 82:1-8. [DOI] [PubMed] [Google Scholar]
- 21.Junqueira, L. C., J. Carneiro, and R. O. Kelley. 1995. Basic histology. Appleton & Lange, Norwalk, Conn.
- 22.Keates, S., A. Keates, M. Warny, R. Peek, P. Murray, and C. Kelly. 1999. Differential activation of mitogen-activated protein kinases in AGS gastric epithelial cells by cag+ and cag− Helicobacter pylori. J. Immunol. 163:5552-5559. [PubMed] [Google Scholar]
- 23.Keithley, E. M., T. Krekorian, P. Sharp, J. P. Harris, and A. F. Ryan. 1990. Comparison of immune-mediated models of acute and chronic otitis media. Arch. Otorhinolaryngol. 247:247-251. [DOI] [PubMed] [Google Scholar]
- 24.Kilby, D., S. Richards, and G. Hart. 1972. Grommets and glue ears: two-year results. J. Laryngol. Otol. 86:881-888. [DOI] [PubMed] [Google Scholar]
- 25.Koutnouyan, H. A., A. Baird, and A. F. Ryan. 1994. Acidic and basic FGF mRNA expression in the middle ear mucosa during experimental acute and chronic otitis media. Laryngoscope 104:350-358. [DOI] [PubMed] [Google Scholar]
- 26.Krekorian, T., E. M. Keithley, M. Takahashi, A. F. Ryan, J. Fierir, and J. P. Harris. 1990. Endotoxin induced otitis media with effusion in the mouse. Acta Otolaryngol. 109:288-299. [DOI] [PubMed] [Google Scholar]
- 27.Kuijpers, W., J. M. H. van der Beek, and E. C. T. Willart. 1979. The effect of experimental tubal obstruction on the middle ear. Acta Otolaryngol. 87:345-352. [DOI] [PubMed] [Google Scholar]
- 28.Kyriakis, J. M., and J. Avruch. 1996. Sounding the alarm: protein kinase cascades activated by stress and inflammation. J. Biol. Chem. 271:24313-24316. [DOI] [PubMed] [Google Scholar]
- 29.Kyriakis, J. M., and J. Avruch. 2001. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81:807-869. [DOI] [PubMed] [Google Scholar]
- 30.Lee, J., J. Laydon, P. McDonnell, T. Gallagher, S. Kumaar, D. Green, D. McNulty, M. Blumenthal, J. Heys, and S. Landvatter. 1994. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372:739-746. [DOI] [PubMed] [Google Scholar]
- 31.Leung, W. K., Q. Wu, P. M. Hannam, B. C. McBride, and V. J. Uitto. 2002. Treponema denticola may stimulate both epithelial proliferation and apoptosis through MAP kinase signal pathways. J. Periodont. Res. 37:445-455. [DOI] [PubMed] [Google Scholar]
- 32.Li, J. D. 2003. Exploitation of host epithelial signaling networks by respiratory bacterial pathogens. J. Pharmacol. Sci. 91:1-7. [DOI] [PubMed] [Google Scholar]
- 33.Lim, D., and H. Birck. 1971. Ultrastructural pathology of the middle ear mucosa in serous otitis media. Ann. Otol. Rhinol. Laryngol. 80:838-853. [DOI] [PubMed] [Google Scholar]
- 34.Lowell, S., S. K. Juhn, and G. S. Giebink. 1980. Experimental otitis media following middle ear inoculation of nonviable Streptococcus pneumoniae. Ann. Otol. Rhinol. Laryngol. 89:479-482. [DOI] [PubMed] [Google Scholar]
- 35.Magnuson, K., A. Hermansson, A. Melhus, and S. Hellstrom. 1997. The tympanic membrane and middle ear mucosa during non-typeable Haemophilus influenzae and Haemophilus influenzae type b acute otitis media. A study in the rat. Acta Otolaryngol. 117:396-405. [DOI] [PubMed] [Google Scholar]
- 36.Maher, P. 1999. p38 Mitogen-activated protein kinase activation is required for fibroblast growth factor-2-stimulated cell proliferation but not differentiation. J. Biol. Chem. 274:17491-17498. [DOI] [PubMed] [Google Scholar]
- 37.Mawson, S., and P. Fagan. 1972. Tympanic effusions in children: long-term results of treatment by myringotomy, aspiration, and indwelling tubes. J. Laryngol. Otol. 86:105-119. [PubMed] [Google Scholar]
- 38.Morgan, W., Jr. 1977. Tympanosclerosis. Laryngoscope 87:1821-1825. [DOI] [PubMed] [Google Scholar]
- 39.Palacios, S. D., K. Pak, A. G. Kayali, A. Z. Rivkin, C. Aletsee, A. Melhus, N. J. Webster, and A. F. Ryan. 2002. Participation of Ras and extracellular regulated kinase in the hyperplastic response of middle-ear mucosa during bacterial otitis media. J. Infect. Dis. 186:1761-1769. [DOI] [PubMed] [Google Scholar]
- 40.Raingeaud, J., S. Gupta, J. Rogers, M. Dickens, J. Han, R. Ulevitch, and R. Davis. 1995. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J. Biol. Chem. 270:7420-7426. [DOI] [PubMed] [Google Scholar]
- 41.Rouse, J., P. Cohen, S. Trigon, M. Morange, A. Alonso-Llamazares, D. Zamanillo, T. Hunt, and A. Nebreda. 1994. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell 78:1027-1037. [DOI] [PubMed] [Google Scholar]
- 42.Ryan, A. F., and A. Baird. 1993. Growth factors during proliferation of the middle ear mucosa. Acta Otolaryngol. 113:68-74. [DOI] [PubMed] [Google Scholar]
- 43.Ryan, A. F., A. Catanzaro, S. Wasserman, and J. P. Harris. 1986. Secondary immune response in the middle ear: physiological, anatomical and immunological observations. Ann. Otol. Rhinol. Laryngol. 95:242-249. [DOI] [PubMed] [Google Scholar]
- 44.Sade, J. 1977. Pathogenesis of attic cholesteatoma: the metaplasia theory, p. 212-232. In B. F. McCabe, J. Sade, and M. Abramson (ed.), Cholesteatoma: first international conference. Aesculapius Publishing Co., Birmingham, Ala.
- 45.Sato, M., D. Shegogue, E. A. Gore, E. A. Smith, P. J. McDermott, and M. Trojanowska. 2002. Role of p38 MAPK in transforming growth factor beta stimulation of collagen production by scleroderma and healthy dermal fibroblasts. J. Investig. Dermatol. 118:704-711. [DOI] [PubMed] [Google Scholar]
- 46.Sekine, S., K. Nitta, K. Uchida, W. Yumura, and H. Nihei. 2003. Possible involvement of mitogen-activated protein kinase in the angiotensin II-induced fibronectin synthesis in renal interstitial fibroblasts. Arch. Biochem. Biophys. 415:63-68. [DOI] [PubMed] [Google Scholar]
- 47.Sheehy, J., D. Brackmann, and M. Graham. 1977. Cholesteatoma surgery: residual and recurrent disease. A review of 1,024 cases. Ann. Otol. Rhinol. Laryngol. 86:451-462. [DOI] [PubMed] [Google Scholar]
- 48.Shino, N., X. Jialing, H. Shuang, and L. Anning. 1998. Induction of apoptosis by SB202190 through inhibition of p38β mitogen-activated protein kinase. J. Biol. Chem. 273:16415-16420. [DOI] [PubMed] [Google Scholar]
- 49.Shuto, T., H. Xu, B. Wang, J. Han, H. Kai, X. X. Gu, T. F. Murphy, D. J. Lim, and J. D. Li. 2001. Activation of NF-kappa B by nontypeable Hemophilus influenzae is mediated by toll-like receptor 2-TAK1-dependent NIK-IKK alpha/beta-I kappa B alpha and MKK3/6-p38 MAP kinase signaling pathways in epithelial cells. Proc. Natl. Acad. Sci. USA 98:8774-8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tekin, M., C. Mutlu, M. M. Paparella, P. A. Schachern, V. J. Jaisinghani, and C. T. Le. 2000. Tympanic membrane and middle ear pathologic correlates in mucoid otitis media. Otolaryngol. Head Neck Surg. 123:258-262. [DOI] [PubMed] [Google Scholar]
- 51.Tos, M., and K. Bak-Pedersen. 1977. Goblet cell population in the pathological middle ear and eustachian tube of children and adults. Ann. Otol. Rhinol. Laryngol. 86:209-218. [DOI] [PubMed] [Google Scholar]
- 52.Van Blitterswijk, C., M. Ponec, G. Van Muijen, M. Wijsman, H. Koerten, and J. Grote. 1986. Culture and characterization of rat middle-ear epithelium. Acta Otolaryngol. 101:453-466. [DOI] [PubMed] [Google Scholar]
- 53.Wang, B., P. P. Cleary, H. Xu, and J. D. Li. 2003. Up-regulation of interleukin-8 by novel small cytoplasmic molecules of nontypeable Haemophilus influenzae via p38 and extracellular signal-regulated kinase pathways. Infect. Immun. 71:5523-5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang, B., D. J. Lim, J. Han, Y. S. Kim, C. B. Basbaum, and J. D. Li. 2002. Novel cytoplasmic proteins of nontypeable Haemophilus influenzae up-regulate human MUC5AC mucin transcription via a positive p38 mitogen-activated protein kinase pathway and a negative phosphoinositide 3-kinase-Akt pathway. J. Biol. Chem. 277:949-957. [DOI] [PubMed] [Google Scholar]
- 55.Wilson, K. P., P. G. McCaffrey, K. Hsiao, S. Pazhanisamy, V. Galullo, G. W. Bemis, M. J. Fitzgibbon, P. R. Caron, M. A. Murcko, and S. Su. 1997. The structural basis for the specificity of pyridinylimidazole inhibitors of p38 MAP kinase. Chem. Biol. 4:423-431. [DOI] [PubMed] [Google Scholar]
- 56.Xia, Z., M. Dickens, J. Raingeaud, R. J. Davis, and M. E. Greenberg. 1995. Opposing effects fo ERK and JNK-p38 MAP kinase on apoptosis. Science 270:1326-1331. [DOI] [PubMed] [Google Scholar]
- 57.Young, P. R., M. M. McLaughlin, S. Kumar, S. Kassis, M. L. Doyle, D. McNulty, T. F. Gallagher, S. Fisher, P. C. McDonnell, S. A. Carr, M. J. Huddleston, G. Seibel, T. G. Porter, G. P. Livi, J. L. Adams, and J. C. Lee. 1997. Pyridinyl imidazole inhibitors of p38 mitogen activated protein kinase bind in the ATP site. J. Biol. Chem. 272:12116-12121. [DOI] [PubMed] [Google Scholar]