Abstract
Legionella pneumophila, the major causative agent of Legionnaires' disease, is a facultative intracellular pathogen that grows within human macrophages and amoebae. Intracellular growth involves the formation of a replicative phagosome that requires the Icm/Dot type IV secretion system. Part of the icm/dot region in L. pneumophila contains the icmTSRQPO genes. The proteins encoded by the icmR and icmQ genes were shown to exhibit a chaperone-substrate relationship. Analysis of this region from other pathogenic Legionella species, i.e., L. micdadei and L. longbeachae, indicated that the overall organization of this region is highly conserved, but it was found to contain a favorable site for gene variation. In the place where the icmR gene was expected to be located, other open reading frames that are nonhomologous to each other or to any entry in the GenBank database were found (migAB in L. micdadei and ligB in L. longbeachae). Examination of these unique genes revealed an outstanding phenomenon; by use of interspecies complementation, the icmR, migB, and ligB gene products were found to be functionally similar. In addition, the function of these proteins was usually dependent on the presence of the corresponding IcmQ proteins. Moreover, each of these proteins (IcmR, LigB, and MigB) was found to interact with the corresponding IcmQ proteins, and the genes encoding these proteins were found to be regulated by CpxR. This study reveals new evidence of gene variation occurring in the same genomic location within the icm/dot locus in various Legionella species. The genes found at this site were shown to be similarly regulated and to encode species-specific, nonhomologous, but functionally similar proteins.
Legionella pneumophila, the major causative agent of Legionnaires' disease, is a facultative intracellular pathogen that multiplies within and kills human macrophages and amoebae (14). Besides L. pneumophila, several other Legionella species have been found to be capable of causing pneumonia. L. pneumophila accounts for the vast majority of cases in most of the world, with L. micdadei ranking second and L. longbeachae ranking third (1, 15).
Twenty-five genes required for intracellular multiplication within human macrophages were identified for L. pneumophila and named the icm/dot genes. They were shown to be organized in two separate regions on the genome: region I contains 7 genes (dotDCB, icmWX, and icmV/dotA), and region II contains 18 genes (icmTSRQPONMLKEGCDJB and icmHF) (21, 28). Eighteen of the 25 icm/dot genes encode proteins that contain significant sequence homology to conjugation-related proteins encoded from IncI plasmid R64 (26). Therefore, the Icm/Dot system is believed to form a type IV secretion apparatus through which proteins are translocated across the macrophage plasma membrane (7, 17, 18). Of the seven proteins that are not homologous to proteins that are involved in conjugation, the IcmR and IcmQ proteins (encoded by adjacent genes located in region IIa) were shown to interact with one another (6, 9), and it was proposed that IcmR functions as a chaperone for IcmQ by preventing its aggregation and regulating its pore-forming activity (9, 10).
The whole Icm/Dot system was also found in the Q fever agent Coxiella burnetii, except for the icmR gene (30, 31). In the place where the icmR gene was expected to be located according to the genomic organization of L. pneumophila, five open reading frames (ORFs) were found in C. burnetii.
We were interested in examining whether the icmR locus in the icm/dot region of L. micdadei and L. longbeachae is different from that in L. pneumophila, as in C. burnetii. Here we present new evidence of functionally similar proteins that share no sequence homology to any other proteins (including themselves) and that are all encoded by genes located in the same position in the icm/dot region and regulated by the same response regulator. A similar example of gene variation has not been described before for any type IV secretion system.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The Legionella species and strains used were as follows: L. pneumophila JR32 (wild-type strain), LELA3463 (icmQ), LELA3473 (icmR) (20), GS3001 (icmS) (23), and OG2002 (cpxR) (12); L. longbeachae serogroup 1 ATCC 33462 and L. longbeachae serogroup 2 ATCC 33484 (both clinical isolates); and MF323, MF385, and MF395 (ATCC 33462 icmS, ligB, and icmQ, respectively). The following Legionella species were all clinical isolates, with the exception of L. gratiana: L. micdadei ATCC 33218 and ATCC 33204, L. birminghamensis ATCC 43702, L. bozemanii ATCC 33217, L. cinicinnatiensis ATCC 43753, L. dumoffii ATCC 33343 and ATCC 35850, L. feeleii ATCC 35849, L. gormanii ATCC 43769, L. gratiana ATCC 48413, L. hackeliae ATCC 35250, L. oakridgensis ATCC 700515, L. pneumophila serogroup 3 ATCC 33155, L. sainthelensi ATCC 49322, and L. tucsonensis ATCC 49180. The Escherichia coli strains used were IO7012D (4) and MC1022 (5). Bacterial media, plates, and antibiotic concentrations were used as previously described (23). The plasmids used in this study are listed in Table 1.
TABLE 1.
Plasmids used in this study
| Plasmid name | Descriptiona | Reference or source |
|---|---|---|
| pBluescript SK(+) | oriR (ColE1) MCS oriR (M13) Apr | 27 |
| pGS-lac-02 | pAB-1 with promoterless lacZ | 13 |
| pLAW344 | sacB MCS oriT (RK2) CmrloxP oriR (ColE1) AprloxP | 29 |
| pMMB207αB-Km14 | RSF1010 derivative; IncQ lacIq Cmr PtacoriT MCS containing α complementation with mobA::Km | 24 |
| pT18 | oriR (ColEI) T18 fragment of CyaA (amino acids 225-339) Apr | 16 |
| pT25 | oriR (p15A) T25 fragment of CyaA (amino acids 1-224) Cmr | 16 |
| pGS-Lc-35-14 | icmQLp gene in pMMB207αB-Km14 | 31 |
| pGS-Lc-36-14 | icmR gene in pMMB207αB-Km14 | 24 |
| pGS-Lc-37-14 | L. pneumophila icmTS genes in pMMB207αB-Km14 | 24 |
| pKP-T18R | icmR gene in pT18 | This study |
| pKP-T25R | icmR gene in pT25 | This study |
| pKP-T18Q | icmQLp gene in pT18 | This study |
| pKP-T25Q | icmQLp gene in pT25 | This study |
| pMF-SK-long110 | pBluescript SK(+) containing L. longbeachae icmT to icmQLl | This study |
| pMF-long110 | L. longbeachae icmT to icmQLl in pMMB207αB-Km14 | This study |
| pMF-long111 | ligB gene in pMMB207αB-Km14 | This study |
| pMF-long113 | ligB and icmQLl genes in pMMB207αB-Km14 | This study |
| pMF-long114 | icmQLl gene in pMMB207αB-Km14 | This study |
| pMF-ligB-lacZ | ligB regulatory region in pGS-lac-02 | This study |
| pMF-ligB::Km | pMF-SK-long110 containing the Kmr cassette inside ligB | This study |
| pMF-longQ::Km | pMF-SK-long110 containing the Kmr cassette inside icmQLl | This study |
| pMF-longS::Km | pMF-SK-long110 containing the Kmr cassette inside L. longbeachae icmS | This study |
| pMF-ligB::Km-GR | pLAW344 containing the insert of pMF-ligB::Km | This study |
| pMF-longQ::Km-GR | pLAW344 containing the insert of pMF-longQ::Km | This study |
| pMF-longS::Km-GR | pLAW344 containing the insert of pMF-longS::Km | This study |
| pMF-T18-ligB | ligB gene in pT18 | This study |
| pMF-T25-ligB | ligB gene in pT25 | This study |
| pMF-T18-longQ | icmQLl gene in pT18 | This study |
| pMF-T25-longQ | icmQLl gene in pT25 | This study |
| pMF-mic47 | pBluescript SK(+) containing L. micdadei icmT to icmP | This study |
| pMF-mic10 | L. micdadei icmT to icmQLm in pMMB207αB-Km14 | This study |
| pMF-mic18 | icmQLm gene in pMMB207αB-Km14 | This study |
| pMF-mic19 | migB gene in pMMB207αB-Km14 | This study |
| pMF-mic21 | migB and icmQLm genes in pMMB207αB-Km14 | This study |
| pMF-T18-migA | migA gene in pT18 | This study |
| pMF-T25-migA | migA gene in pT25 | This study |
| pMF-T18-migB | migB gene in pT18 | This study |
| pMF-T25-migB | migB gene in pT25 | This study |
| pMF-T18-micQ | icmQLm gene in pT18 | This study |
| pMF-T25-micQ | icmQLm gene in pT25 | This study |
MCS, multiple cloning site.
Cloning of region IIa from L. longbeachae and L. micdadei.
To clone the icmTSRQPO region from L. longbeachae and L. micdadei, a fragment containing part of their icmP and icmO genes was amplified by PCR with degenerate primers. The resulting fragment was used as a probe for Southern hybridization (22) with L. longbeachae or L. micdadei genomic DNA, and the desired fragment was cloned into pBluescript SK(+) (27). Clones harboring a 4-kb EcoRI-XhoI fragment containing the L. longbeachae icmTS, ligB, and icmQ genes (GenBank accession number AY512558) and a 3.5-kb XhoI-BglII fragment containing the L. micdadei icmTS, migAB, and icmQ genes (GenBank accession number AY512559) were named pMF-SK-long110 and pMF-mic47, respectively. These plasmids were sequenced and used for further analysis.
Plasmid construction for complementation with L. longbeachae genes.
A 4-kb EcoRI-XhoI fragment from pMF-SK-long110 was filled in and cloned into pMMB207αB-Km14 (24) that had been partially digested with SmaI to generate pMF-long110 (containing the icmTS, ligB, and icmQ genes). pMF-long111 (containing the ligB gene) was generated by cloning a 1-kb XmnI-HincII fragment from pMF-SK-long110 into the SmaI site. pMF-long113 (containing the ligB and icmQ genes) was generated by cloning a 2-kb XhoI-NruI fragment from pMF-SK-long110 (that was filled in) into the SmaI site. pMF-long114 (containing the icmQ gene) was generated by cloning a 1.5-kb BalI fragment from pMF-SK-long110 into the same SmaI site.
Plasmid construction for complementation with L. micdadei genes.
A 3.1-kb SmaI fragment from pMF-mic47 was cloned into the SmaI site of pMMB207αB-Km14 to generate pMF-mic10 (containing the icmTS, migAB, and icmQ genes). pMF-mic18 (containing the icmQ gene) was generated by cloning a 1.5-kb SnaI-SmaI fragment from pMF-mic47 into the same SmaI site. pMF-mic19 (containing the migB gene) was generated by cloning a 1-kb BalI-SspI fragment from pMF-mic47 into the SmaI site. To generate pMF-mic21 (containing the migB and icmQ genes), a 1.5-kb BalI-SwaI fragment from pMF-mic47 was cloned into the same SmaI site.
Plasmid construction for allelic exchange.
In order to generate insertions in the L. longbeachae chromosome, a kanamycin resistance cassette digested with PstI was cloned into pMF-SK-long110 that had been partially digested with PstI to generate an insertion in the ligB gene. A kanamycin resistance cassette that had been digested with EcoRV was cloned into pMF-SK-long110 that had been digested with NruI to generate an insertion in the icmS gene or with SwaI to generate an insertion in the icmQ gene. The three resulting plasmids were digested with PvuII, and the inserts were cloned into pLAW344 (29) that had been digested with EcoRV to generate pMF-ligB::Km-GR, pMF-longS::Km-GR, and pMF-longQ::Km-GR. The allelic exchange procedure was performed as previously described (25).
Plasmid construction for the two-hybrid analysis.
To clone the L. pneumophila icmR and icmQ genes, the L. longbeachae ligB and icmQ genes, and the L. micdadei migA, migB, and icmQ genes into the pT18 and pT25 vectors of the Bordetella pertussis cyaA two-hybrid system (16), all of these genes were amplified by PCR with primers designed to form in-frame fusions with the cyaA gene product. Each of the seven genes was amplified with two sets of primers: one PCR product was cloned into pT18 to generate pKP-pT18R, pKP-pT18Q, pMF-T18-ligB, pMF-T18-longQ, pMF-T18-migA, pMF-T18-migB, and pMF-T18-micQ, and the second PCR product was cloned into pT25 to generate pKP-pT25R, pKP-pT25Q, pMF-T25-ligB, pMF-T25-longQ, pMF-T25-migA, pMF-T25-migB, and pMF-T25-micQ. All of these plasmids were sequenced and then introduced as pairs into E. coli IO7012D (ΔcyaA), and lacZ expression levels were measured by using the β-galactosidase assay as previously described (31).
Construction of a lacZ translational fusion.
To generate a ligB::lacZ translational fusion, the regulatory region of the ligB gene was amplified by PCR, digested with BamHI-EcoRI, cloned into pGS-lac-02 (13), and sequenced. Expression levels were measured by using the β-galactosidase assay as previously described (13).
Intracellular growth in Acanthamoeba castellanii and HL-60-derived human macrophages.
Intracellular assays for the growth of L. pneumophila, L. longbeachae, and their derived strains in A. castellanii and HL-60-derived human macrophages were performed as previously described (25).
Low-stringency Southern hybridization.
The Southern hybridization procedure was performed as described previously (22) with 20% formamide.
RESULTS
Region IIa differs among Legionella species.
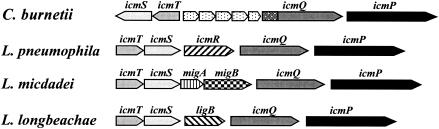
When region IIa of L. pneumophila was compared with that of C. burnetii in the place where the icmR gene is located in L. pneumophila, five ORFs were found in C. burnetii (an icmR homologue is not present in the C. burnetii genome); four of these ORFs had no homology to any entry in the GenBank database, and the fifth ORF was homologous to a transposase gene. Cloning and sequencing of region IIa from L. micdadei and L. longbeachae (see Materials and Methods) revealed that in the place where the icmR gene was expected to be located (between icmS and icmQ), new ORFs were found; in L. micdadei, two new ORFs were found and named migA and migB (L. micdadei icm gene) (Fig. 1), and in L. longbeachae, one ORF was found in the same location and named ligB (L. longbeachae icm gene) (the ligA gene that was previously described for L. pneumophila [11] is not related to the ligB gene) (Fig. 1). These three genes (migA, migB, and ligB) showed no homology to any entry in the GenBank database, while the icmT, icmS, icmQ, and icmP gene products from the three Legionella species showed a very high degree of homology to one another (the identity between the IcmTSP homologous proteins was 74 to 92%, and that between the IcmQ homologous proteins was 57 to 68%).
FIG. 1.
Schematic drawing of region IIa in C. burnetii, L. pneumophila, L. micdadei, and L. longbeachae. Homologous genes are indicated by different colors or patterns, and the gene names are indicated above the arrows. C. burnetii icmQ contains an N-terminal region that is not present in the other icmQ homologues. The map is not drawn to scale.
For the sake of convenience, the different icmQ genes were named icmQLp, for the L. pneumophila icmQ gene, icmQLl, for the L. longbeachae icmQ gene, and icmQLm, for the L. micdadei icmQ gene.
Distributions of newly discovered genes among different Legionella species.
As shown in Fig. 1, the icmR, ligB, migA, and migB genes were located in the same position (between the icmS and icmQ genes), sharing no sequence homology to one another or to any entry in GenBank, implying that these genes are species specific. In order to verify this hypothesis, we performed low-stringency Southern hybridization with genomic DNA from 18 different Legionella species and strains (described in Materials and Methods) and six different probes—for the L. pneumophila icmS, icmR and icmQLp genes, for the L. longbeachae ligB gene, and for the L. micdadei migA and migB genes. The icmS gene was found in all species examined (Fig. 2A); the same result was obtained for the icmQLp gene (data not shown), indicating the presence of the icm/dot system in all of the species examined. The migB gene was found to be present only in the two L. micdadei strains examined (Fig. 2B), as was the migA gene (data not shown). The icmR gene was found to be present only in the two L. pneumophila strains examined (Fig. 2C). These results indicate that the migA, migB, and icmR genes are indeed species-specific genes (at least in the species examined). Surprisingly, the ligB gene was found in three additional Legionella species besides the two L. longbeachae strains examined (Fig. 2D). This gene was found in L. cincinnatiensis, L. sainthelensi, and L. gratiana, all of which are very closely related to one another (19); these results indicate that the ligB gene is not a species-specific gene. This information suggests that the icmR, ligB, and migAB genes entered the Legionella evolutionary tree through separate branches, while the icmS and icmQ genes entered earlier, as they are also present in C. burnetii. Since seven of the species examined did not contain any of the genes described above, it is possible that each of them has a distinctive gene located in the same position.
FIG. 2.
Analysis of the distributions of icm genes in different Legionella species. The icmS gene is present in all Legionella species examined (A), migB is present in only two L. micdadei strains (B), icmR is present in only two L. pneumophila strains (C), and ligB is present in two L. longbeachae strains and in three additional closely related species (D). Hybridization was performed with chromosomal DNA digested with EcoRI as described in Materials and Methods.
Interspecies complementation.
Due to the high degree of homology among the majority of genes in region IIa (see above), we tested whether the L. longbeachae and L. micdadei region IIa gene products function in a manner similar to that of the L. pneumophila region IIa gene products. Therefore, we performed interspecies complementation assays with L. pneumophila icmS, icmR and icmQLp mutants and complemented each of them with plasmids containing the L. longbeachae and L. micdadei region IIa genes. As expected, full complementation of the L. pneumophila icmS and icmQLp mutants by the L. longbeachae and L. micdadei genes was observed in HL-60-derived macrophages as well as in A. castellanii (data not shown). However, to our surprise, complementation was also obtained for the L. pneumophila icmR mutant, although no icmR homologues were present on either of the complementing plasmids (data not shown). This unexpected result was studied further in order to determine which of the L. longbeachae and L. micdadei region IIa genes was responsible for complementation of the icmR mutant.
IcmR, MigB, and LigB are functionally homologous.
The interspecies complementation assays described above marked the L. longbeachae ligB and L. micdadei migB genes as possible candidates for complementation of the icmR mutant due to their genomic location. To examine whether functional homology exists among the icmR, ligB, and migB gene products, we performed interspecies complementation assays with an L. pneumophila icmR insertion mutant and complemented it with plasmids containing the relevant genes from L. micdadei and L. longbeachae. The strain containing the insertion in the L. pneumophila icmR gene was fully complemented by the plasmid containing the icmR gene alone (Fig. 3A). However, the L. longbeachae ligB gene only partially complemented the L. pneumophila icmR insertion mutant, and the L. micdadei migB gene did not complement it at all (Fig. 3A and B, respectively). Nevertheless, plasmids containing a combination of the L. longbeachae ligB and icmQLl genes or the L. micdadei migB and icmQLm genes completely restored the ability of the icmR insertion mutant to grow intracellularly in HL-60-derived macrophages (Fig. 3A and B, respectively), although no icmR homologue was present on these plasmids. Similarly, in A. castellanii, neither the ligB gene nor the migB gene complemented the icmR insertion mutant, but a combination of the L. longbeachae ligB and icmQLl genes or the L. micdadei migB and icmQLm genes fully complemented the same insertion mutant (Fig. 3C and D, respectively). The data presented in Fig. 3 indicate that the ligB, migB, and icmR genes are functionally similar and that the presence of the L. longbeachae LigB and IcmQLl proteins together or the L. micdadei MigB and IcmQLm proteins together may have a significant role in pathogenesis which is similar to the role of the L. pneumophila IcmR and IcmQLp proteins. These results clearly indicate that although no sequence homology exists among the icmR, migB, and ligB genes, they seem to function similarly and their participation in the virulence pathway is related to the corresponding icmQ genes.
FIG. 3.
Interspecies complementation of the L. pneumophila icmR insertion mutant. Experiments were performed with HL-60-derived human macrophages (A and B) or A. castellanii (C and D). Symbols: ⧫, wild-type L. pneumophila JR32; ▪, L. pneumophila icmR mutant (LELA3473) containing the vector; ✻, L. pneumophila icmR gene; ▵, L. longbeachae ligB gene; ▴, L. longbeachae ligB and icmQLl genes; ○, L. micdadei migB gene; •, L. micdadei migB and icmQLm genes. The experiments were performed at least three times, and similar results were obtained. The error bars indicate standard deviations.
Analysis of IcmQ proteins from three Legionella species.
The finding that L. longbeachae region IIa and L. micdadei region IIa complemented the icmQLp insertion mutant predicted that the icmQLl and icmQLm genes, respectively, were responsible for these results. However, the complementation results for the L. pneumophila icmR mutant predicted that the IcmQLl and IcmQLm proteins function together with the protein encoded by the gene located upstream from their genes. These two observations led us to examine whether the icmQLl and icmQLm genes by themselves can complement the icmQLp mutant. When examined in A. castellanii, the icmQLl gene only partially complemented the icmQLp mutant (Fig. 4C), while the icmQLm gene did not complement it at all (Fig. 4D). However, plasmids containing a combination of the L. longbeachae ligB and icmQLl genes or the L. micdadei migB and icmQLm genes together fully complemented the icmQLp insertion mutant in A. castellanii (Fig. 4C and D, respectively). When examined in HL-60-derived macrophages, the icmQLl gene fully complemented the ability of the icmQLp mutant to grow intracellularly, whereas the icmQLm gene only partially complemented this mutant (Fig. 4A and B, respectively). In this situation, too, a plasmid containing the L. micdadei migB and icmQLm genes was found to fully complement the icmQLp insertion mutant in HL-60-derived macrophages (Fig. 4B), implying that a combination of the L. micdadei migB and icmQLm genes was required for the complementation to occur. These results indicate that the icmQLl and icmQLm genes require the presence of the relevant gene located upstream from them for complete complementation, even though they are both homologous to the icmQLp gene.
FIG. 4.
Interspecies complementation of the icmQLp insertion mutant. The experiments were performed with HL-60-derived human macrophages (A and B) or A. castellanii (C and D). Symbols: ⧫, wild-type L. pneumophila JR32; ▪, icmQLp mutant (LELA3463) containing the vector; ✻, icmQLp gene; ○, icmQLl gene; •, L. longbeachae ligB and icmQLl genes; ▵, icmQLm gene; ▴, L. micdadei migB and icmQLm genes. The experiments were performed at least three times, and similar results were obtained. The error bars indicate standard deviations.
The L. longbeachae icm genes are required for intracellular growth.
Since the involvement of the icm genes in L. longbeachae pathogenesis has not been examined before, three insertion mutations in the L. longbeachae icmS, ligB, and icmQLl genes were constructed. All of the intracellular growth experiments with L. longbeachae strains were performed with HL-60-derived human macrophages, since L. longbeachae does not grow in A. castellanii (data not shown). The L. longbeachae icmS gene was found to be required for intracellular growth, and it was complemented by the L. longbeachae, L. pneumophila, and L. micdadei icmS genes (data not shown). The icmQLl insertion mutant showed a partial intracellular growth defect, which was fully complemented by the icmQLp, icmQLl, and icmQLm genes (Fig. 5A). The ligB insertion mutant also showed a partial intracellular growth phenotype, which was completely restored by complementing plasmids containing the ligB or the icmR gene (Fig. 5B) but not the migB gene or a combination of the migB and icmQLm genes (Fig. 5B). These results show that the L. longbeachae icmS, ligB, and icmQLl genes are required for intracellular growth, that the ligB and icmR genes are functionally similar, but that the migB gene functions in a manner similar to that of the icmR gene (Fig. 2B) but not that of the ligB gene (Fig. 5B).
FIG. 5.
Interspecies complementation of L. longbeachae mutants. (A and B) Complementation analysis of the icmQLl (A) and ligB (B) mutants with HL-60-derived human macrophages. Symbols for panel A: ⧫, wild-type L. longbeachae ATCC 33462; □, icmQLl mutant (MF395) containing the vector; +, icmQLl gene; ▴, icmQLp gene; ○, icmQLm gene. Symbols for panel B: ⧫, wild-type L. longbeachae ATCC 33462; ▪, L. longbeachae ligB mutant (MF385) containing the vector; ▴, L. longbeachae ligB gene; ▵, L. pneumophila icmR gene; ○, L. micdadei migB gene; •, L. micdadei migB and icmQLm genes. The experiments were performed at least three times, and similar results were obtained. The error bars indicate standard deviations.
IcmQ interacts with IcmR, MigB, and LigB.
The functional homology among the three nonhomologous proteins (IcmR, LigB, and MigB), in addition to previous reports showing that the IcmR and IcmQLp proteins interact with one another (6, 9), led us to analyze whether each of the IcmR, MigB, and LigB proteins interacts with the corresponding IcmQ protein. For this analysis, a bacterial two-hybrid system that is based on the CyaA toxin of B. pertussis was used (16). The L. pneumophila icmR and icmQLp genes, the L. longbeachae ligB and icmQLl genes, and the L. micdadei migA, migB, and icmQLm genes were fused to the T18 (N-terminal fusion) and T25 (C-terminal fusion) fragments, and interactions among them were examined. In this system, an interaction between two proteins results in cyclic AMP production, which is determined by measuring the levels of expression of the lacZ gene product.
As shown in Fig. 6A to C, each of the three proteins interacts with the corresponding IcmQ protein (the MigA protein does not interact either with the IcmQLm protein or with the MigB protein) (Fig. 6C), strongly supporting the notion that the IcmR, LigB, and MigB proteins play similar roles and that their functions involve interactions with the corresponding IcmQ protein. Moreover, the interaction between each of the three proteins and the corresponding IcmQ protein was very strong when the IcmQ protein was fused to the T18 fragment and the when the IcmR/LigB/MigB proteins were fused to the T25 fragment, while the interaction in the opposite direction was significantly weaker. These results indicate that the interactions of the proteins examined probably occur between the C-terminal domain of the IcmR/LigB/MigB proteins and the N-terminal domain of the corresponding IcmQ protein.
FIG. 6.
Two-hybrid analysis of the interactions between the L. pneumophila, L. longbeachae, and L. micdadei protein pairs. Interactions were examined for the L. pneumophila IcmR and IcmQLp proteins (A), the L. longbeachae LigB and IcmQLl proteins (B), and the L. micdadei MigB and IcmQLm proteins (C); interspecies interactions were examined for the IcmQLp protein (D), the IcmQLl protein (E), and the IcmQLm protein (F). β-Galactosidase activity was measured in Miller units [M.U.] at stationary phase. The names of the genes used in this assay are marked with a code composed of two letters and a number between them. The letter on the left represents the species: P, L. pneumophila; L, L. longbeachae; M, L. micdadei. The number indicates to which cyaA fragment the examined gene was fused: 18, T18; 25, T25. The letter on the right represents the gene examined: R, icmR; A, migA; B, ligB (when the left letter is L) or migB (when the left letter is M); Q, icmQ. The results are the averages and standard deviations of at least three independent experiments.
In order to reinforce these observations, we performed interspecies interaction assays to examine all of the possible combinations of the IcmR/LigB/MigB proteins and the IcmQLp, IcmQLl, and IcmQLm proteins. Interactions were observed for all of the protein combinations (Fig. 6D to F), with the exception of MigB, which did not interact with either IcmQLp (Fig. 6D) or IcmQLl (Fig. 6E). This finding correlates with our results showing that the migB gene by itself did not complement the icmR or the ligB mutant (Fig. 3B and 5B) and can be explained by the fact that L. micdadei is evolutionarily more distant from L. longbeachae than L. pneumophila (19). All of the interactions observed occurred in the same orientation as that described above (Fig. 6D to F) and were significantly weaker in the opposite orientation (data not shown), indicating that indeed a specific orientation of the IcmR/LigB/MigB proteins is required for their interaction with the corresponding IcmQ protein. Very recently, it was shown that IcmQLp binds to IcmR via its N-terminal domain (10), a finding that strongly supports our results.
icmR, ligB, and migB are regulated by CpxR.
Although no sequence homology exists among icmR, ligB, and migB, the finding that they function similarly led us to wonder whether their expression is regulated in the same manner. It has been shown that the L. pneumophila icmR gene is positively regulated by the CpxRA two-component system through a conserved CpxR binding site (12). Careful examination of the regulatory region of the ligB and migB genes revealed that a putative CpxR binding site is also found in the ligB and migB regulatory regions (Fig. 7A). It also seems, from a comparison of the sequences shown in Fig. 7A, that the Legionella consensus binding site for the CpxR response regulator (GTAAAn6GAAA) is slightly different from the one identified in E. coli: GTAAAn5GTAAA (8). In order to test whether these genes are indeed regulated by the same response regulator, we constructed a translational fusion between the L. longbeachae ligB regulatory region and the lacZ reporter gene and introduced the resulting plasmid into L. longbeachae, L. pneumophila, and an L. pneumophila strain containing an insertion mutation in the cpxR gene. β-Galactosidase activity was measured at stationary phase, and the results obtained strongly indicated that the cpxR mutant drastically reduced the level of expression of ligB (Fig. 7B). These results suggest that the icmR/ligB/migB genes are probably regulated similarly, a fact that correlates with the functional homology described above for the three proteins encoded by these genes.
FIG. 7.
The icmR, ligB, and migB genes are probably regulated by CpxR. (A) Part of the regulatory region of the L. pneumophila icmR, L. longbeachae ligB, and L. micdadei migB genes. The consensus recognition sequence of CpxR identified in E. coli is shown at the bottom. CpxR recognition sequences are shown in bold type and are underlined, and the distances to the first ATG are indicated. (B) Expression of a ligB::lacZ translational fusion in L. longbeachae (Ll), L. pneumophila (Lp), and an L. pneumophila cpxR gene insertion mutant (ΔcpxR) at stationary phase. The results, reported in Miller units (M.U.), are the averages and standard deviations of at least three independent experiments.
DISCUSSION
L. pneumophila grows intracellularly and kills the host cell due to the activity of the Icm/Dot type IV secretion system, which is believed to form a protein complex that translocates effector proteins from the bacterial cytoplasm into the host cell. The icm/dot system was recently discovered also in C. burnetii and included all of the icm/dot genes except for icmR (30, 31). The icmR gene, together with icmQLp, is located in region IIa; their gene products are predicted to be found in the bacterial cytoplasm and are not homologous to conjugation-related proteins encoded by plasmid R64. This information, together with the finding that C. burnetii contains the entire icm/dot system except for icmR, led us to focus on region IIa as a potential cause for differences between bacteria that contain the Icm/Dot virulence system.
Besides L. pneumophila, several pathogenic Legionella species have been described, and we chose to study two of them: L. micdadei which is the second most common agent of Legionnaires' disease in the world, and L. longbeachae, which is the third most common etiological agent of Legionnaires' disease in the world but the dominant agent in Australia (1, 15). Cloning and sequencing of region IIa from L. longbeachae and L. micdadei revealed an interesting discovery: the icmR gene was found to be missing in the region between icmS and icmQ in both species, and other ORFs were found in its place. In L. longbeachae, one ORF (ligB) was found in the place where icmR was expected to be located, and in L. micdadei, two ORFs (migA and migB) were found in the same position. In order to investigate the functions of these newly discovered gene products, a homology search was performed, but no homology was detected among the IcmR, LigB, and MigB proteins or to any other entry in GenBank.
These proteins seem to share a few properties in common with type III virulence-related chaperones. (i) The IcmR, MigB, and LigB proteins were all predicted by the PSORT program (http://psort.ims.u-tokyo.ac.jp) to be located in the bacterial cytoplasm. They were also predicted to have acidic pIs (4.75, 5.3, and 5.8, respectively), and they are all small proteins (88 to 120 amino acids). These properties are considered common features of type III secretion chaperones (2). (ii) Through interspecies complementation experiments, the IcmR, LigB, and MigB proteins were found to function similarly, although no sequence homology exists among them. Bioinformatics programs predicted that these three proteins contain an α-helical structure at their C-terminal domain, like that found for type III chaperones, which also do not share any sequence homology but which are structurally homologous (3). (iii) As was shown before for the L. pneumophila IcmR and IcmQLp proteins (6, 9), analysis of protein interactions showed that the L. longbeachae LigB and L. micdadei MigB proteins also interact with their corresponding IcmQ proteins and that the interactions occur via the C-terminal domain of the IcmR, LigB, or MigB protein and the N-terminal domain of the relevant IcmQ protein. It was shown before that type III virulence-related chaperones interact with their substrates in a specific orientation similar to the one described above (2). (iv) Type III chaperones are encoded by genes adjacent to the genes encoding their substrates, like IcmR, LigB, and MigB, which were shown to interact with the protein encoded by the gene located downstream from their genes (IcmQ).
All of the information described above strongly implies that the IcmR, LigB, and MigB proteins function similarly, probably as chaperones of the relevant IcmQ proteins. Even though the IcmR, LigB, and MigB proteins seem to share several properties in common with type III chaperones, other characteristics make these proteins quite distinct. First, all of these features of type III chaperones were found here in a type IV secretion system. Second, in a type III secretion system, each chaperone interacts with a specific substrate and not with similar substrates, as was found here. Another unique feature of these proteins is the specific location of their genes on the chromosomes of different Legionella species, a property that has not been described for type III chaperones.
As mentioned above, the C. burnetii genome contains the entire icm/dot system except for the icmR gene. However, the five ORFs that are located in its place encode large nonacidic proteins; therefore, it is highly unlikely that one of these proteins plays a role similar to that of IcmR, LigB, or MigB. In addition, it was proposed before that the C. burnetii IcmQ protein, which was shown not to complement the icmQLp mutant (30, 31), functions as its own chaperone, as it contains an extension of 47 amino acids at its N-terminal domain (Fig. 1).
Our results reveal new evidence of gene variation at a specific position in the pathogenicity region of different Legionella species; this finding has not been shown before for any type IV secretion system. The genes located at this site encode proteins that share no sequence homology but seem to function similarly and to be controlled by the same regulator. These findings lead to two main questions. (i) What is the mechanism that causes this gene variation? (ii) Why does such gene variation exist? As far as the first question, the evolutionary process that led to this intriguing phenomenon is quite mysterious due to the lack of evidence for horizontal gene transfer; this region does not contain any differences in its GC content, and no insertion sequence elements have been found in this region in the three Legionella species examined. The possible answer to the second question might be related to a recent study showing that IcmQLp functions as a pore-forming protein (10). This information leads to the hypothesis that the IcmQLp, IcmQLl, and IcmQLm proteins may be adapted to insert pores into the membrane of their specific protozoan host, a function that is mediated by the IcmR, LigB, and MigB proteins, respectively. It is possible that these proteins are part of a machinery that evolved to adapt a specific Legionella species to a certain amoeba host, thus functioning as host-specific determinants. These differences in the icm/dot systems of the three Legionella species examined may be directly or indirectly related to the fact that of these three species, L. pneumophila is the most common causative agent of Legionnaires' disease in the world.
Acknowledgments
We thank Karen Pomeranz for plasmid construction.
This work was supported by a grant from The Center for the Study of Emerging Diseases.
Editor: J. T. Barbieri
REFERENCES
- 1.Benin, A. L., R. F. Benson, and R. E. Besser. 2002. Trends in Legionnaire's disease, 1980-1998: declining mortality and new patterns of diagnosis. Clin. Infect. Dis. 35:1039-1046. [DOI] [PubMed] [Google Scholar]
- 2.Bennett, J. C., and C. Hughes. 2000. From flagellum assembly to virulence: the extended family of type III export chaperones. Trends Microbiol. 8:202-204. [DOI] [PubMed] [Google Scholar]
- 3.Birtalan, S. C., R. M. Phillips, and P. Ghosh. 2002. Three-dimensional secretion signals in chaperone-effector complexes of bacterial pathogens. Mol. Cell 9:971-980. [DOI] [PubMed] [Google Scholar]
- 4.Brickman, E., L. Soll, and J. Beckwith. 1973. Genetic characterization of mutations which affect catabolite-sensitive operons in Escherichia coli, including deletions of the gene for adenyl cyclase. J. Bacteriol. 116:582-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casadaban, M. J., and S. N. Cohen. 1980. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J. Mol. Biol. 138:179-207. [DOI] [PubMed] [Google Scholar]
- 6.Coers, J., J. C. Kagan, M. Matthews, H. Nagai, D. M. Zuckman, and C. R. Roy. 2000. Identification of Icm protein complexes that play distinct roles in the biogenesis of an organelle permissive for Legionella pneumophila intracellular growth. Mol. Microbiol. 38:719-736. [DOI] [PubMed] [Google Scholar]
- 7.Conover, G. M., I. Derre, J. P. Vogel, and R. R. Isberg. 2003. The Legionella pneumophila LidA protein: a translocated substrate of the Dot/Icm system associated with maintenance of bacterial integrity. Mol. Microbiol. 48:305-321. [DOI] [PubMed] [Google Scholar]
- 8.De Wulf, P., A. M. McGuire, X. Liu, and E. C. Lin. 2001. Genome-wide profiling of promoter recognition by the two-component response regulator CpxR-P in Escherichia coli. J. Biol. Chem. 277:26652-26661. [DOI] [PubMed] [Google Scholar]
- 9.Dumenil, G., and R. R. Isberg. 2001. The Legionella pneumophila IcmR protein exhibits chaperone activity for IcmQ by preventing its participation in high-molecular-weight complexes. Mol. Microbiol. 40:1113-1127. [DOI] [PubMed] [Google Scholar]
- 10.Dumenil, G., T. P. Montminy, M. Tang, and R. R. Isberg. 2004. IcmR-regulated membrane insertion and efflux by the Legionella pneumophila IcmQ protein. J. Biol. Chem. 279:4686-4695. [DOI] [PubMed] [Google Scholar]
- 11.Fettes, P. S., M. Susa, J. Hacker, and R. Marre. 2000. Characterization of the Legionella pneumophila gene ligA. Int. J. Med. Microbiol. 290:239-250. [DOI] [PubMed] [Google Scholar]
- 12.Gal-Mor, O., and G. Segal. 2003. Identification of CpxR as a positive regulator of icm and dot virulence genes of Legionella pneumophila. J. Bacteriol. 185:4908-4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gal-Mor, O., T. Zusman, and G. Segal. 2002. Analysis of DNA regulatory elements required for expression of the Legionella pneumophila icm and dot virulence genes. J. Bacteriol. 184:3823-3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horwitz, M. A., and S. C. Silverstein. 1980. Legionnaires' disease bacterium (Legionella pneumophila) multiplies intracellularly in human monocytes. J. Clin. Investig. 60:441-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joshi, A. D., and M. S. Swanson. 1999. Comparative analysis of Legionella pneumophila and Legionella micdadei virulence traits. Infect. Immun. 67:4134-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karimova, G., J. Pidoux, A. Ullmann, and D. Ladant. 1998. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc. Natl. Acad. Sci. USA 95:5752-5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo, Z. Q., and R. R. Isberg. 2004. Multiple substrates of the Legionella pneumophila Dot/Icm system identified by interbacterial protein transfer. Proc. Natl. Acad. Sci. USA 101:841-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagai, H., J. C. Kagan, X. Zhu, R. A. Kahn, and C. R. Roy. 2002. A bacterial guanine nucleotide exchange factor activates ARF on Legionella phagosomes. Science 295:679-682. [DOI] [PubMed] [Google Scholar]
- 19.Ratcliff, R. M., J. A. Lanser, P. A. Manning, and M. W. Heuzenroesder. 1998. Sequence-based classification scheme for the genus Legionella targeting the mip gene. J. Clin. Microbiol. 36:1560-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadosky, A. B., L. A. Wiater, and H. A. Shuman. 1993. Identification of Legionella pneumophila genes required for growth within and killing of human macrophages. Infect. Immun. 61:5361-5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Segal, G., M. Purcell, and H. A. Shuman. 1998. Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila genome. Proc. Natl. Acad. Sci. USA 95:1669-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segal, G., and E. Z. Ron. 1993. Heat shock transcription of the groESL operon of Agrobacterium tumefaciens may involve a hairpin-loop structure. J. Bacteriol. 175:3083-3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Segal, G., and H. A. Shuman. 1997. Characterization of a new region required for macrophage killing by Legionella pneumophila. Infect. Immun. 65:5057-5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Segal, G., and H. A. Shuman. 1998. Intracellular multiplication and human macrophage killing by Legionella pneumophila are inhibited by conjugal components on IncQ plasmid RSF1010. Mol. Microbiol. 30:197-208. [DOI] [PubMed] [Google Scholar]
- 25.Segal, G., and H. A. Shuman. 1999. Legionella pneumophila utilizes the same genes to multiply within Acanthamoeba castellanii and human macrophages. Infect. Immun. 67:2117-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Segal, G., and H. A. Shuman. 1999. Possible origin of the Legionella pneumophila virulence genes and their relation to Coxiella burnetii. Mol. Microbiol. 33:669-670. [DOI] [PubMed] [Google Scholar]
- 27.Short, J. M., J. M. Fernandez, J. A. Sorge, and W. D. Huse. 1988. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 16:7583-7600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vogel, J. P., H. L. Andrews, S. K. Wong, and R. R. Isberg. 1998. Conjugative transfer by the virulence system of Legionella pneumophila. Science 279:873-876. [DOI] [PubMed] [Google Scholar]
- 29.Wiater, L. A., A. B. Sadosky, and H. A. Shuman. 1994. Mutagenesis of Legionella pneumophila using Tn903dlllacZ: identification of a growth-phase-regulated pigmentation gene. Mol. Microbiol. 11:641-653. [DOI] [PubMed] [Google Scholar]
- 30.Zamboni, D. S., S. McGrath, M. Rabinovitch, and C. R. Roy. 2003. Coxiella burnetii express type IV secretion system proteins that function similarly to components of the Legionella pneumophila Dot/Icm system. Mol. Microbiol. 49:965-976. [DOI] [PubMed] [Google Scholar]
- 31.Zusman, T., G. Yerushalmi, and G. Segal. 2003. Functional similarities between the icm/dot pathogenesis systems of Coxiella burnetii and Legionella pneumophila. Infect. Immun. 71:3714-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]