Abstract
Background
Physical therapy for youth with cerebral palsy (CP) who are ambulatory includes interventions to increase functional mobility and participation in physical activity (PA). Thus, reliable and valid measures are needed to document PA in youth with CP.
Objective
The purpose of this study was to evaluate the inter-instrument reliability and concurrent validity of 3 accelerometer-based motion sensors with indirect calorimetry as the criterion for measuring PA intensity in youth with CP.
Methods
Fifty-seven youth with CP (mean age=12.5 years, SD=3.3; 51% female; 49.1% with spastic hemiplegia) participated. Inclusion criteria were: aged 6 to 20 years, ambulatory, Gross Motor Function Classification System (GMFCS) levels I through III, able to follow directions, and able to complete the full PA protocol. Protocol activities included standardized activity trials with increasing PA intensity (resting, writing, household chores, active video games, and walking at 3 self-selected speeds), as measured by weight-relative oxygen uptake (in mL/kg/min). During each trial, participants wore bilateral accelerometers on the upper arms, waist/hip, and ankle and a portable indirect calorimeter. Intraclass coefficient correlations (ICCs) were calculated to evaluate inter-instrument reliability (left-to-right accelerometer placement). Spearman correlations were used to examine concurrent validity between accelerometer output (activity and step counts) and indirect calorimetry. Friedman analyses of variance with post hoc pair-wise analyses were conducted to examine the validity of accelerometers to discriminate PA intensity across activity trials.
Results
All accelerometers exhibited excellent inter-instrument reliability (ICC=.94–.99) and good concurrent validity (rho=.70–.85). All accelerometers discriminated PA intensity across most activity trials.
Limitations
This PA protocol consisted of controlled activity trials.
Conclusions
Accelerometers provide valid and reliable measures of PA intensity among youth with CP.
Cerebral palsy (CP) is the most common physical disability of childhood.1–3 Although the neurologic insult associated with CP is nonprogressive, youth with CP are less physically active and spend more time in sedentary behaviors compared with their peers with typical development (TD), mostly because of the increased demands of growth on their compromised neuromuscular and musculoskeletal systems.4,5 Despite the motor impairments associated with this condition (ie, muscle weakness, spasticity, and decreased motor control and balance), the majority of youth with CP are ambulatory.5,6
Physical therapy interventions for youth with CP who are ambulatory often focus on activity-based strategies to promote functional mobility and participation in physical activity (PA).7–10 However, the majority of intervention studies have relied on self-report PA measures to evaluate effectiveness.11–15 Although self-report measures may be useful for describing the general PA behaviors of youth with CP, they are subject to social desirability and recall bias and thus may not be sufficiently valid or reliable for clinical outcome studies.16,17
Accelerometer-based motion sensors are objective measures of PA that have been used in youth with TD for many years.17–22 These devices provide date-time stamped information about the acceleration of the body during human motion, allowing end users to obtain real-time estimates of PA frequency, intensity, and duration with minimal burden to participants.21,23 More recently, accelerometer-based motion sensors such as the StepWatch (Modus Health LLC, Washington, District of Columbia) and ActiGraph (ActiGraph, Pensacola, Florida) have been used to measure habitual PA in youth with CP.24–35 Findings from studies using StepWatch monitors showed that youth with CP at GMFCS levels I through III took significantly fewer steps per day than their counterparts with TD.26,36 Other studies using StepWatch monitors to measure PA in youth with CP showed that there were significantly higher step counts on weekdays than on weekend days and that younger children with CP (<10 years of age) have higher step counts than older children with CP.30,34 A study using the ActiGraph accelerometer to measure PA in youth with CP showed that they accumulated approximately 90 minutes of light-intensity PA and 30 minutes of moderate- to vigorous-intensity PA (MVPA) daily.28 Results of another study with youth with CP at GMFCS levels I and II showed that, on average, youth participated in 158 minutes of light-intensity PA daily and 44 minutes of MVPA daily.27
Although ActiGraph and StepWatch monitors are being used more often as objective PA measures in youth with CP, only a few studies provide evidence on the reliability and validity of these measures. Excellent inter-instrument reliability (ICC=.96–.99) was established for the ActiGraph accelerometers to examine left versus right waist/hip placement in a feasibility study of the reliability and validity of the ActiGraph in youth with CP.24 However, this study had a small sample size (N=8), most participants were classified at GMFCS level I, and the PA protocol consisted only of the Six-Minute Walk Test (6MWT) performed at 3 self-selected speeds.24 Two studies evaluated the validity of the ActiGraph in measuring PA intensity during walking trials.24,25 One study reported good concurrent validity between ActiGraph count data and oxygen consumption (V̇o2) measured by indirect calorimetry,24 whereas the other study showed that the ActiGraph could differentiate PA intensities across walking trials.25
The SenseWear armband monitor (BodyMedia Inc, Pittsburgh, Pennsylvania) uses multiple sensors to record acceleration, skin temperature, skin galvanic response, and heat flux.37,38 Recent studies have been conducted to examine the validity and reliability of the SenseWear monitor in youth with CP.39,40 One of the studies reported validity between the SenseWear armband monitor and indirect calorimetry (V̇o2) data and inter-instrument reliability between SenseWear armband monitor left and right arm placement in youth with hemiplegic CP.39 Limitations in both studies included small sample sizes, few participants at GMFCS level III, and PA protocols consisting of overground or treadmill walking only.
The body of evidence is growing to support use of accelerometer-based motion sensors as objective PA measures for youth with CP. However, there are numerous gaps in the current research that we addressed in our study. First, because youth with CP often have varying degrees of movement asymmetries and atypical gait patterns,2,4 it is important to establish inter-instrument reliability for each accelerometer to identify optimal placement on the left or right side of the body. To date, only 2 studies have examined inter-instrument reliability.24,39 Second, only 4 studies have examined validity of accelerometer output for youth with CP using V̇o2 measured by indirect calorimetry as the criterion, and all studies used walking or treadmill protocols only.24,25,39,40 No previous study has evaluated concurrent validity across a broader range of activity trials comprising real-world activities such as sitting and writing, household chores, and active video games. Third, reliability and validity studies conducted thus far have included few participants at GMFCS level III.24,25,39,40 It is critical to examine reliability and validity of accelerometers in measuring PA across all ambulatory levels (GMFCS levels I, II, and III) for youth with CP. Finally, no studies have examined the reliability or validity of accelerometers placed on different areas of the body (arm, hip/waist, ankle) to identify optimal placement for youth with CP.
To address these gaps in the research literature, the purpose of this study was to evaluate inter-instrument reliability and concurrent validity of 3 accelerometer devices: the ActiGraph GT3X, the StepWatch activity monitor, and the SenseWear armband monitor. These accelerometers were chosen because each has evidence to support reliability and validity in youth with TD but limited evidence in children with CP. Also, each monitor has unique placement on the body (upper arm [SenseWear], waist/hip [ActiGraph], and ankle [StepWatch]) to provide evidence on optimal placement for PA measurement. In contrast to previous investigations, the current study included a relatively large sample of children and youth with CP with good distribution across GMFCS levels I, II, and III. Additionally, this study used a PA protocol featuring a range of real-world activities in which youth with CP may participate in daily routines because we wanted to determine the PA intensity (V̇o2) and the degree of difficulty these activities posed for participating youth with CP who may be deconditioned. Finally, we wanted to determine if accelerometers are valid against V̇o2 for measuring PA intensity because it is more feasible for clinicians to use accelerometers rather than indirect calorimeters in clinical settings. Our hypothesis was that the ActiGraph accelerometer would show the best reliability and validity in our PA protocol because existing evidence suggests excellent reliability and fair-to-good validity in walking trials.
Method
Participants
A total of 58 youth with CP, aged 6 to 20 years, were enrolled in the study. Data were collected on 57 youth because 1 youth was disqualified based on GMFCS inclusion criteria. The average age of the participants was 12.5 years (SD=3.3), with 29 girls (51%). Most youth were diagnosed with hemiplegia (n=28, 49.1%), and most were classified at GMFCS level I (n=28, 49.1%) (Tab. 1).
Table 1.
Participant Demographics (N=57)a
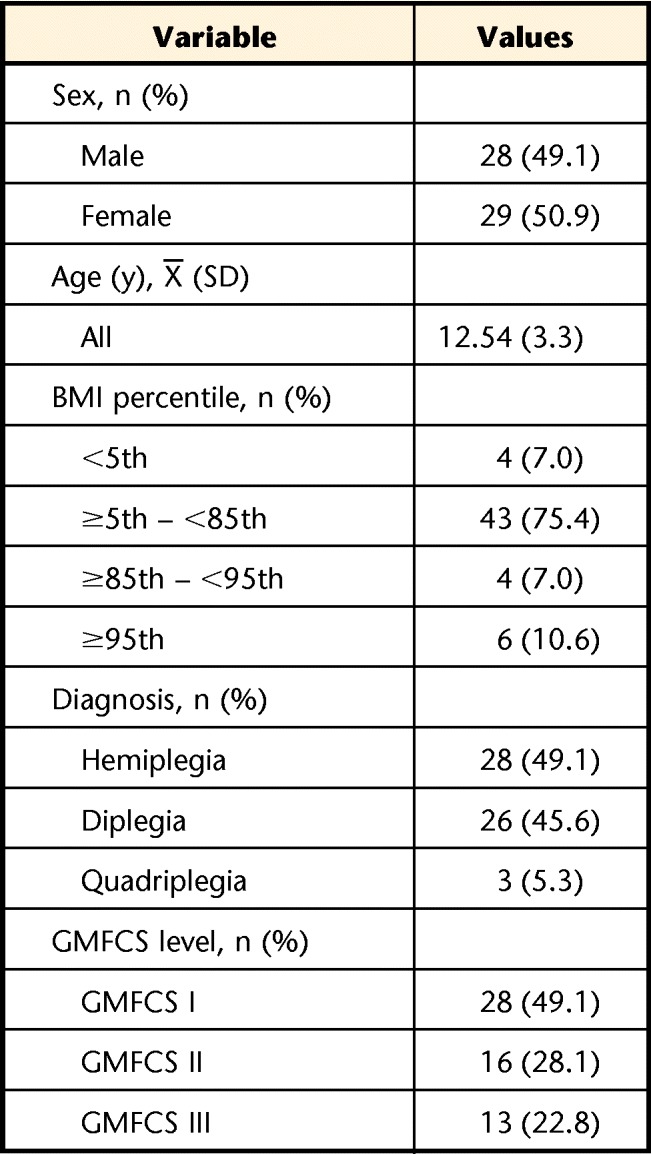
BMI=body mass index, GMFCS=Gross Motor Function Classification System.
The inclusion criteria were that youth had CP at GMFCS level I, II, or III and were ambulatory; 6 to 20 years old; and able to follow PA protocol directions and complete the PA protocol while wearing 6 accelerometers (3 pairs), a Polar heart rate (HR) monitor (Polar USA, Polar Electro Inc, Lake Success, New York), and the COSMED K4b2 indirect calorimeter (COSMED, Rome, Italy) (including face mask) and that parents and youth provided consent and assent. Youth were excluded if they had recent musculoskeletal injuries (eg, sprains, fractures, muscle strains) that limited their PA levels; orthopedic surgery within the previous 6 months; botulinum toxin or phenol injections within the previous 3 months; an unstable medical condition or a pre-existing medical condition limiting PA levels (eg, uncontrolled seizures, unstable cardiac condition, uncontrolled asthma); or unstable emotional or behavioral status. Youth who were in the process of changing medications or other interventions or were unable to complete the PA protocol and testing session also were excluded.
Procedure
Children were recruited from the outpatient CP and spasticity management clinics at Franciscan Hospital for Children (Brighton, Massachusetts) and Nemours/Alfred I. duPont Hospital for Children (Wilmington, Delaware) and through professional colleagues and parent groups. Flyers were posted in clinic areas and were disseminated via email to colleagues and parents. Participant eligibility was confirmed by telephone interview. Youth who met the inclusion and exclusion criteria were scheduled for the data collection session.
Data Collection
Each data collection session was approximately 2 to 2.5 hours and consisted of obtaining consent and assent, completion of questionnaires, recording youth anthropometric measures, and participation in 9 activity trials. Height was measured using a stadiometer (ShorrBoard 420, QuickMedical, Issaquah, Washington) in the vertical position or as a supine board, depending on the participant's ability to stand for a valid height measure (only 3 youths classified at GMFCS III were measured in a supine position). Weight was recorded using a Tanita digital scale (model UM-028, Arlington Heights, Illinois). Before measuring height and weight, the stadiometer and scale were calibrated using a standard protocol.24
The accelerometers were initialized according to manufacturers' specifications. One StepWatch monitor was placed superior to the right lateral malleolus, and the other was placed superior to the left medial malleolus. The ActiGraph accelerometers were placed on an elastic belt, and they were positioned on each side of the body superior to the iliac crest at the midaxillary line. The SenseWear armbands were placed on the dorsal side of each upper arm at the mid-belly of the triceps muscle. After the accelerometers were in place, participants were fitted with an HR monitor. Lastly, the COSMED K4b2 indirect calorimeter was fitted to the child but only after it had been calibrated to room air according to the manufacturer's standard protocol. The COSMED K4b2 chest harness with the portable data unit and battery pack and the face mask and head harness were fitted to the participant.
Next, the youth participated in the 9 trials of the PA protocol, which were performed in a standard order to build effort and minimize recovery time between high- and low-intensity level activities. A scripted protocol was used, and each participant received the same instructions. Activities were completed stepwise:
Supine rest: lying down resting but not sleeping.
Writing: using a pencil or pen and copying text from a printed sheet while sitting at a desk.
Wiping a table: spraying a countertop surface with water and then wiping the countertop with a towel. Participants walked from side to side in front of the 1.83-m-long (6-ft-long) countertop, spraying and wiping to clean the entire surface.
Folding laundry: placing 5 towels in a laundry bag, carrying the laundry across the room, emptying the laundry bag, folding the towels in half 3 times, placing the folded towels back in the bag, carrying the bag across the room.
Active video game: “River Rush” (Xbox Kinect [Microsoft Corp, Redmond, Washington])—playing an Xbox Kinect game involving jumping movements, weight shifting or stepping from side to side to keep the raft on the screen moving.
Active video game: “Space Pop” (Xbox Kinect [Microsoft Corp])—playing a second Xbox Kinect game involving moving arms up and down to make the avatar on the screen float upward and stepping side to side while reaching to pop moving bubbles on the screen.
Comfortable/slow walk: walking at a comfortable/slow, self-selected speed after receiving the following instructions: “Walk at a comfortable, slow pace—like when you are at the mall or walking in your neighborhood or at school but you are not in a hurry.”
Brisk walk: walking at a brisk self-selected speed after receiving the following instructions: “This time we want you to walk a little faster, so please walk at a faster pace—like when you are hurrying to get to class after the bell has rung.”
Fast walk: walking at a fast speed after receiving the following instructions: “This time we want you to walk as fast as you possibly can without falling or running.”
The walking protocol consisting of 3 self-selected walking speeds was validated in a previous study.24 Each walk was 6 minutes in duration and was completed on a 25-m course marked with 2 cones. For each walking speed, participants completed a practice trial first over a short distance (<25 m) and then rested briefly as instructions were repeated again for the start of the trial. For all 3 walking trials, once the participant established a pace, a physical therapist walked alongside and provided verbal encouragement to assist the participant with maintaining a consistent pace throughout the 6 minutes. A stopwatch was used to assist with pacing. The distance walked was measured at the end of the 6 minutes. The distance walked also was measured at 3 minutes for the fast walk to ensure that a steady pace was maintained because this was identified as a potential problem during the feasibility study.24
For the PA trials listed above, participants completed the activities as they would typically do them at school, home, or while playing. Participants used their dominant or less affected arm for writing, and they were encouraged to use the nondominant hand to assist by stabilizing the paper, but this was not enforced. They used their dominant hand to squeeze the trigger of the spray bottle for the wiping task. Some children automatically used the opposite hand to hold the towel and wipe the table, whereas others used the dominant hand to spray and wipe the table. They were encouraged to use both hands to fold towels for the towel folding task; however, some participants who had more upper extremity impairment completed this task primarily using their dominant arm. Participants were encouraged to move both arms and both legs during the video games.
Participants who routinely used crutches or a walker for upright activities used their usual assistive device during trials 3 through 9. Participants did not sit to rest during any part of trials 1 and 2. They did sit and rest between trials 4 through 9 until their HR reached baseline sitting resting level and they reported that they were rested and ready for the next activity.
Activities 1 through 4 had a duration of 5 minutes, activities 5 and 6 had a duration of 2.5 minutes, and activities 7 through 9 had a duration of 6 minutes. The 6-minute time was selected for the walk tests because it is standard protocol for clinical measures of functional capacity in youth with CP. During pilot testing, we determined it was not feasible to increase all other activities to 6 minutes due to the lengthy protocol lasting up to 2.5 hours. Each video game was 2.5 minutes' duration because no individual game had a duration of 5 minutes of game play. During pilot testing, we attempted to create a video game trial with 5 minutes' duration by combining 2 games. However, during the process of starting game 2, it was observed that the 30-second interval to change games resulted in a significant decrease in exercise intensity, so we decided to use 2 individual 2.5 minute video game intervals.
Measures
Demographic and health questionnaires.
Prior to the activity trials, parents and youth completed questionnaires on child and family demographic characteristics and PA behaviors. Parents completed a medical information form and provided information on their children's medical status, health and rehabilitation services, and equipment and medication needs.
COSMED K4b2.
The COSMED K4b2 is a portable indirect calorimeter and a reliable and valid measure of V̇o2, energy expenditure, and HR.41 Indirect calorimetry is considered a criterion measure of PA intensity for children with CP.24,25,40 Oxygen consumption was measured during each activity using breath-by-breath data collection and was averaged every 10 seconds and expressed relative to body mass (mL/kg/min). Measured metabolic equivalents were calculated by dividing mean V̇o2 by resting V̇o2, which was estimated using the Schofield equation for children aged 3 to 10 years or 10 to 18 years.42 In this study, data were collected continuously during the PA protocol (9 activity trials). Some participants needed to remove the mask during the rest intervals between PA trials because of discomfort (skin irritation). Rest periods were marked to be sure data collected in these intervals were not included in the PA data processing and analysis.
Accelerometers.
Accelerometer data were collected at the lowest epoch intervals available for each device so that accelerometry data could be synchronized with the breath-by-breath data collected from the COSMED K4b2 unit. The lowest epoch setting is 1 second for the ActiGraph, 3 seconds for the StepWatch, and 60 seconds for the SenseWear.
ActiGraph GT3X.
The ActiGraph GT3X is a small (3.8 cm × 3.7 cm × 1.8 cm, 27 g), lightweight triaxial motion sensor16,17 designed to detect accelerations ranging from 0.05 to 2.5 g in the vertical, medial-lateral, and anterior-posterior planes.16 The vector magnitude (VM) is reported in the ActiGraph output and is calculated by combining the activity counts from all 3 axes using Pythagoras' theorem. ActiGraph data analyzed in this study were step counts, vertical axis counts, and VM output were recorded and analyzed.17
StepWatch activity monitor.
This is a small (7.5 cm × 5 cm × 2 cm), lightweight (38 g) uniaxial monitor that records step counts. Steps are summed and recorded for specified epochs, and the sampling rate is 128 Hz.26 The sensitivity of the StepWatch monitor is adjustable to improve data collection for individuals with atypical gait patterns. Sensitivity is adjusted using standard or advanced programming options. The standard program allows the user to adjust sensitivity by entering the individual's height, weight, and several gait pattern characteristics. The advanced program options are used only if standard options cannot provide accurate step counts. The StepWatch accuracy is greater than 98% in independent validation studies. Prior to data collection, calibration trials of 40 to 50 steps were conducted to compare step count data from the StepWatch monitor to a handheld step counter to ensure that the sensitivity was adjusted properly for each participant. Data were processed using the StepWatch proprietary software (version 3.1).26
BodyMedia SenseWear Pro Armband.
This monitor is a small (5.5 cm × 6.2 cm × 1.3 cm), lightweight (45.4 g) triaxial device that has a calibrated range of ±1.0 g with a minimum resolution of 0.01 g and a 2-standard deviation error of ±0.08 g on all axes. The SenseWear uses multiple sensor technology and pattern-recognition equations to estimate energy cost in PA.37,38 The SenseWear provides several types of data, including step counts, energy expenditure, galvanic skin response, and temperature. For the current study, step counts were analyzed. Data were processed using the SenseWear proprietary software (professional version 7.0).
Data Processing
Oxygen consumption and accelerometer output values were calculated by averaging data from the middle portion of each trial. For the 5-minute activity trials, data from minutes 2 through 4 were analyzed. For each of the three 6MWT trials, data from minutes 2 through 5 were analyzed. For each of the 2.5-minute active video gaming trials, data from the middle 1 minute were analyzed. Data from the middle portion of each activity trial were used to ensure that the participants were at a steady pace and were not building up speed or cadence at the beginning or slowing down on speed or cadence toward the end of each trial.
Data Analysis
Descriptive statistics were calculated for accelerometer and V̇o2 output. Differences in V̇o2 and accelerometer output across PA trials were examined using Friedman 2-way analysis of variance and post hoc pair-wise comparisons (Wilcoxon signed ranks test). These nonparametric statistics were chosen because the accelerometry data were not normally distributed. Intraclass correlation coefficients and 95% confidence intervals (CIs) were calculated to examine inter-instrument reliability between left- and right-side accelerometer placement. Spearman rho correlation coefficients were calculated to evaluate concurrent validity between accelerometer and V̇o2 output.
Role of the Funding Source
Funding for this project was provided by an NIH R24 Resource-Related Research Projects Grant awarded to Boston University, Boston Rehabilitation Outcomes Center (Boston ROC), titled “Improving Outcome Measurement for Medical Rehabilitation Clinical Trials.”
Results
Table 2 presents the descriptive statistics (medians and interquartile ranges [IQRs]) for accelerometer output (activity and step counts) for both left- and right-side devices and indirect calorimetry output (V̇o2, in mL/kg/min). Results from the Friedman analyses of variance confirmed that V̇o2 and accelerometer output increased significantly in a dose-response manner as the intensity of the activity trials increased.
Table 2.
Descriptive Data From V̇o2 and Accelerometer Output for Each Activity Trial in the PA Protocola
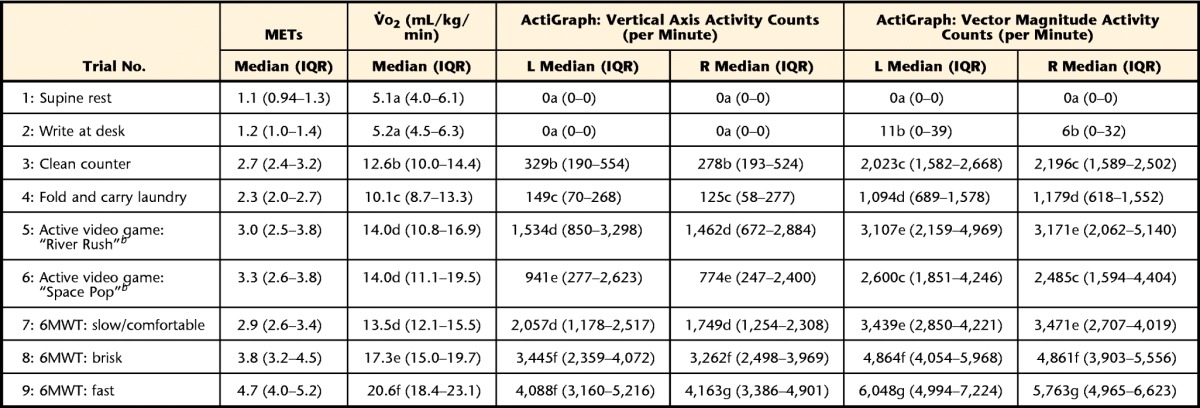
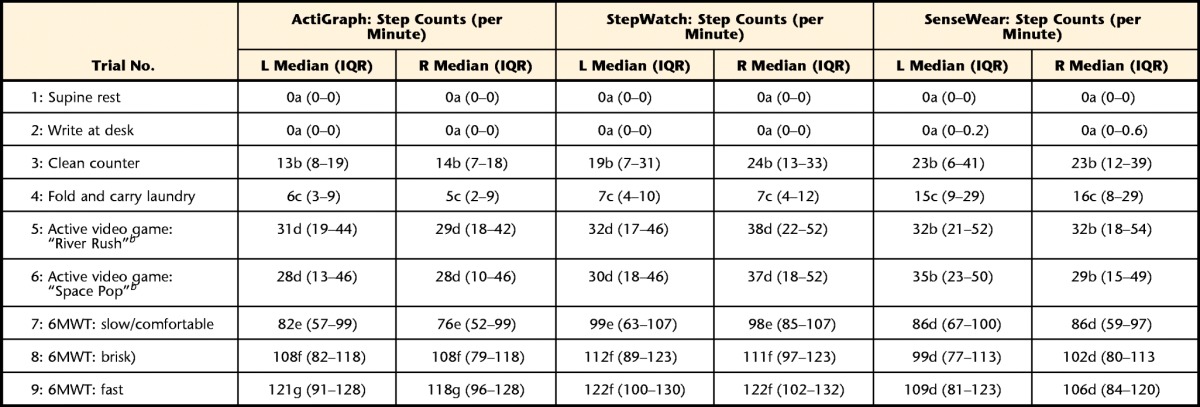
Within each column, median values with different letters are significantly different. V̇o2=oxygen consumption, METs=metabolic equivalents, 6MWT=Six-Minute Walk Test, IQR=interquartile range, L=left, R=right.
b Xbox Kinect (Microsoft Corp, Redmond, Washington).
Table 3 shows the inter-instrument reliability for left and right accelerometer placement and for step and activity count data. All 3 accelerometers demonstrated good reliability, with coefficient values above .75.43 ActiGraph and StepWatch accelerometers demonstrated the highest levels of inter-instrument reliability, with ICCs exceeding .975, whereas inter-instrument reliability for the SenseWear monitor was slightly lower (ICC=.94).
Table 3.
Inter-instrument Reliabilitya
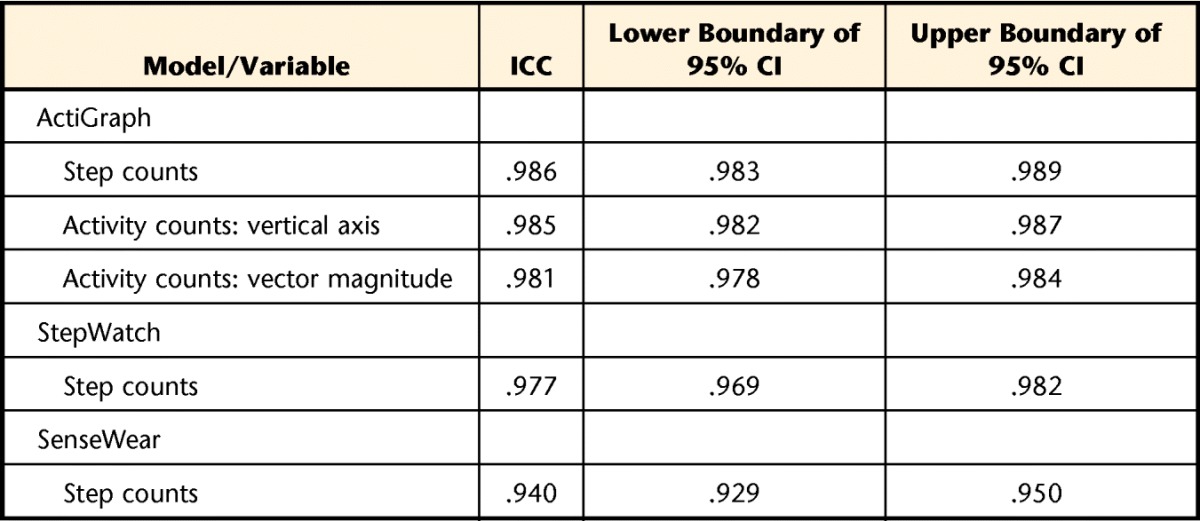
Degree of absolute agreement among measurements. ICC=intraclass correlation coefficient, CI=confidence interval.
Table 4 shows the concurrent validity between the accelerometer and V̇o2 output. The ActiGraph and StepWatch accelerometers demonstrated good concurrent validity, with correlations above .75, and the SenseWear accelerometer demonstrated fair to good concurrent validity.43
Table 4.
Concurrent Validity: Accelerometer to Oxygen Consumption Output
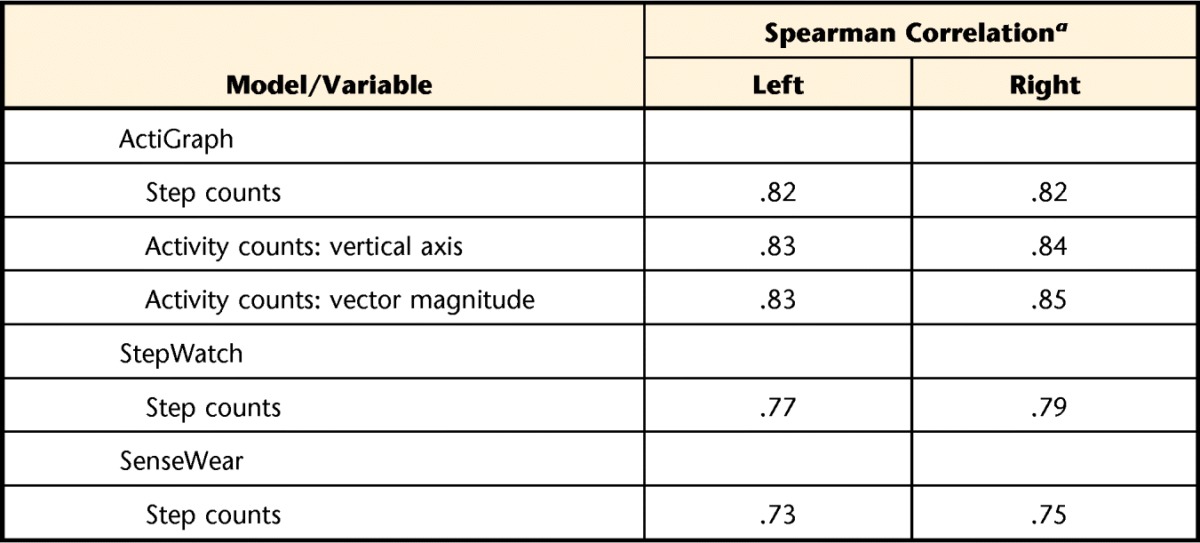
a All Spearman correlations are significant at P<.001.
Discussion
Physical therapists provide services to promote functional mobility and PA participation in youth with CP. Reliable and valid objective measures of PA are needed in research and clinical settings to evaluate changes in PA levels and intervention effectiveness. Findings indicate that the 3 accelerometers examined in this study have strong interrater reliability and fair to good concurrent validity. The ActiGraph GT3X exhibited marginally higher reliability and validity coefficients. These findings suggest that, despite the differences in data acquisition, each accelerometer is stable in data collection on the left and right sides of the body, indicating that movement asymmetries may not influence PA measures.
All 3 accelerometers provide step count data. Median values for step counts were similar for each accelerometer across PA trials, suggesting that step count data are consistent regardless of accelerometer type or placement. The ActiGraph provides activity counts on 3 axes, and the output from the vertical axis and VM are usually reported. Results indicate that the ICCs and Spearman correlation coefficients were the same for both measures, suggesting that activity count data recorded by the VM and vertical axis are strongly correlated and that the VM does not give additional information than that provided by the vertical axis.
Because all 3 accelerometer models exhibited excellent inter-instrument reliability for measuring PA in a variety of real-world activities, it may be appropriate for youth with CP to follow the standard protocol used by youth with TD and to wear accelerometers on the right side.17 Accelerometers exhibited fair (StepWatch and SenseWear) and good (ActiGraph GT3X) concurrent validity; therefore, it is recommended that accelerometers be used to measure PA intensity in youth with CP.42 Considerations when choosing an activity monitor include the outcome of interest (ie, step versus activity counts), participant tolerance, and placement that is least obtrusive for the participant and the activity. A major strength of this study is the PA protocol design, which incorporated activities of daily living across a range of PA intensities. These activities represent a wide variety of daily tasks in which youth with CP are likely to participate. The activities include upper and lower extremity movement, bilateral and one-handed activities, and full-body movements. To date, studies that examined PA intensity in youth with CP have been limited to walking protocols.24,25,38,39 Thus, this study contributes new information on reliability and validity of accelerometers in measuring PA across a wide variety of activities, including novel tasks such as active video gaming. Another strength of the study is that it provides information on inter-instrument reliability and concurrent validity for 3 different accelerometers worn in 3 different areas of the body. Although each accelerometer is reliable and valid for the full sample, it may be important to examine reliability and validity of each accelerometer by GMFCS level or clinical diagnosis.
There are several limitations to this study that warrant mention. First, the study was conducted in a clinical setting and not in the real world. This was a necessary choice due to the trials in the PA protocol and because of the measurement instrumentation needed to determine PA intensity for each trial. However, as mentioned above, the PA protocol was designed to resemble real-world activities as much as possible. Second, although the PA protocol included activity trials that, on average, ranged from sedentary to moderate-to-vigorous intensity, we did not have any activity trials that reached vigorous-intensity levels. This design decision was based on real-world activities in which youth with CP participate regularly and ensured that all participants (GMFCS levels I, II, and III) were successful in completing the full PA protocol. Also, youth with CP at GMFCS III often can run only short distances and for limited time, so we did not include a running trial in the PA protocol. A third limitation is that, due to the short duration of the video game trials, participants may not have achieved true steady state. However, there is little consensus on the length of time required to achieve steady state in children. Furthermore, V̇o2 was assessed breath by breath with the data averaged over 6 consecutive 10-second epochs and thus present a reasonable estimate of oxygen uptake for this activity trial. Finally, the number of devices the participant wore during the protocol may have interfered with the participant's ability to perform each trial. However, the light weight of the portable metabolic system and position of the monitors did not appear to impede movement.
Future studies should address the limitations mentioned above. For example, it is important to measure PA intensity in natural environments to document PA intensity in real-world activities. This information will be very useful for planning PA strategies in physical therapy interventions. Also, future studies should include vigorous-intensity activities for all participants. Because the current study was a measurement study, it did not examine responsiveness of accelerometers. Future intervention studies are needed to determine responsiveness of accelerometers as outcome measures.
Physical therapy interventions continue to adopt strategies to increase functional mobility and PA participation. It is extremely important that research and clinical outcomes include reliable, valid, and objective measures of PA frequency, duration, and intensity to determine intervention effectiveness. Findings from this study provide evidence that accelerometers have interrater reliability and concurrent validity and are appropriate PA measures for youth with CP.
Footnotes
Dr O'Neil, Dr Fragala-Pinkham, and Dr Trost provided concept/idea/research design. Dr O'Neil, Dr Fragala-Pinkham, Ms Lennon, and Dr Trost provided writing and project management. Dr O'Neil, Dr Fragala-Pinkham, Ms Lennon, and Ms George provided data collection. Dr O'Neil, Dr Fragala-Pinkham, Ms Lennon, Ms George, and Dr Trost provided data analysis. Dr O'Neil and Dr Fragala-Pinkham provided fund procurement. Dr Fragala-Pinkham, Ms Lennon, and Dr Forman provided participants, facilities/equipment, institutional liaisons, and consultation (including review of manuscript before submission).
Institutional review board approval was obtained from Franciscan Hospital for Children (Brighton, Massachusetts), Drexel University (Philadelphia, Pennsylvania), and Nemours/Alfred I. duPont Hospital for Children (Wilmington, Delaware).
Funding for this project was provided by an NIH R24 Resource-Related Research Projects Grant awarded to Boston University, Boston Rehabilitation Outcomes Center (Boston ROC), titled “Improving Outcome Measurement for Medical Rehabilitation Clinical Trials.”
References
- 1. Winter S, Autry A, Boyle C, Yeargin-Allsopp M. Trends in the prevalence of cerebral palsy in a population-based study. Pediatrics. 2002;110:1220–1225. [DOI] [PubMed] [Google Scholar]
- 2. Rosenbaum P, Paneth N, Leviton A, et al. A report on the definition and classification of cerebral palsy April 2006. Dev Med Child Neurol Suppl. 2007;109:8–14. [PubMed] [Google Scholar]
- 3. Yeargin-Allsopp M, Van Naarden Braun K, Doernberg NS, et al. Prevalence of cerebral palsy in 8-year-old children in three areas of the United States in 2002: a multisite collaboration. Pediatrics. 2008; 121:547–554. [DOI] [PubMed] [Google Scholar]
- 4. Hanna SE, Rosenbaum PL, Bartlett DJ, et al. Stability and decline in gross motor function among children and youth with cerebral palsy aged 2 to 21 years. Dev Med Child Neurol. 2009;51:295–302. [DOI] [PubMed] [Google Scholar]
- 5. Fowler E, Kolobe TH, Damiano DL, et al. ; Section on Pediatrics Research Summit Participants; Section on Pediatrics Research Committee Task Force. Promotion of physical fitness and prevention of secondary conditions for children with cerebral palsy: section on pediatrics research summit proceedings. Phys Ther. 2007;87:1495–1510. [DOI] [PubMed] [Google Scholar]
- 6. Odding E, Roebroeck ME, Stam HJ. The epidemiology of cerebral palsy: incidence, impairments and risk factors. Disabil Rehabil. 2006;28:183–191. [DOI] [PubMed] [Google Scholar]
- 7. O'Neil ME, Fragala-Pinkham MA, Westcott SL, et al. Physical therapy clinical management recommendations for children with cerebral palsy, spastic diplegia: achieving functional mobility outcomes. Pediatr Phys Ther. 2006;18:49–72. [DOI] [PubMed] [Google Scholar]
- 8. Fragala-Pinkham MA, O'Neil ME, Bjornson KF, Boyd RN. Fitness and physical activity in children and youth with disabilities. Int J Pediatr. 2012;2012:162648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Damiano DL. Activity, activity, activity: rethinking our physical therapy approach to cerebral palsy. Phys Ther. 2006;86:1534–1540. [DOI] [PubMed] [Google Scholar]
- 10. Verschuren O, Ketelaar M, Gorter JW, et al. Exercise training program in children and adolescents with cerebral palsy: a randomized clinical trial. Arch Pediatr Adolesc Med. 2007;161:1075–1081. [DOI] [PubMed] [Google Scholar]
- 11. Palisano RJ, Copeland WP, Galuppi BE. Performance of physical activities by adolescents with cerebral palsy. Phys Ther. 2007;87:77–87. [DOI] [PubMed] [Google Scholar]
- 12. Maher CA, Williams MT, Olds T, Lane AE. Physical and sedentary activity in adolescents with cerebral palsy. Dev Med Child Neurol. 2007;49:450–457. [DOI] [PubMed] [Google Scholar]
- 13. Capio CM, Sit CH, Abernethy B, Rotor ER. Physical activity measurement instruments for children with cerebral palsy: a systematic review. Dev Med Child Neurol. 2010;52:908–916. [DOI] [PubMed] [Google Scholar]
- 14. Clanchy KM, Tweedy SM, Boyd R. Measurement of habitual physical activity performance in adolescents with cerebral palsy: a systematic review. Dev Med Child Neurol. 2011;53:499–505. [DOI] [PubMed] [Google Scholar]
- 15. Verschuren O, Darrah J, Novak I, et al. Health-enhancing physical activity in children with cerebral palsy: more of the same is not enough. Phys Ther. 2014;94:297–305. [DOI] [PubMed] [Google Scholar]
- 16. Trost SG. State of the art reviews: Measurement of physical activity in children and adolescents. Am J Lifestyle Med. 2007;1:299–314. [Google Scholar]
- 17. Trost SG. Objective measurement of physical activity in youth: current issues, future directions. Exerc Sport Sci Rev. 2001;29:32–36. [DOI] [PubMed] [Google Scholar]
- 18. Trost SG, Ward DS, Moorehead SM, et al. Validity of the computer science and applications (CSA) activity monitor in children. Med Sci Sports Exerc. 1998;30:629–633. [DOI] [PubMed] [Google Scholar]
- 19. Chen KY, Bassett DR., Jr The technology of accelerometry-based activity monitors: current and future. Med Sci Sports Exerc. 2005;37:S490–S500. [DOI] [PubMed] [Google Scholar]
- 20. Chen KY, Janz KF, Zhu W, Brychta RJ. Redefining the roles of sensors in objective physical activity monitoring. Med Sci Sports Exerc. 2012; 44(suppl 1):S13–S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Trost SG, McIver KL, Pate RR. Conducting accelerometer-based activity assessments in field-based research. Med Sci Sports Exerc. 2005;37(11 suppl):S531–S543. [DOI] [PubMed] [Google Scholar]
- 22. Bjornson KF, Yung D, Jacques K, et al. StepWatch stride counting: accuracy, precision, and prediction of energy expenditure in children. J Pediatr Rehabil Med. 2012;5:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Trost SG, Loprinzi PD, Moore R, Pfeiffer KA. Comparison of accelerometer cut points for predicting activity intensity in youth. Med Sci Sports Exerc. 2011;43:1360–1368. [DOI] [PubMed] [Google Scholar]
- 24. O'Neil ME, Fragala-Pinkham MA, Forman JL, Trost SG. Measuring reliability and validity of the ActiGraph GT3X accelerometer for children with cerebral palsy: a feasibility study. J Pediatr Rehabil Med. 2014;7:233–240. [DOI] [PubMed] [Google Scholar]
- 25. Clanchy KM, Tweedy SM, Boyd RN, Trost SG. Validity of accelerometry in ambulatory children and adolescents with cerebral palsy. Eur J Appl Physiol. 2011;111:2951–2959. [DOI] [PubMed] [Google Scholar]
- 26. Bjornson KF, Belza B, Kartin D, et al. Ambulatory physical activity performance in youth with cerebral palsy and youth who are developing typically. Phys Ther. 2007;87:248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mitchell LE, Ziviani J, Boyd RN. Habitual physical activity of independently ambulant children and adolescents with cerebral palsy: are they doing enough? Phys Ther. 2015;95:202–211. [DOI] [PubMed] [Google Scholar]
- 28. Gorter JW, Noorduyn SG, Obeid J, Timmons BW. Accelerometry: a feasible method to quantify physical activity in ambulatory and nonambulatory adolescents with cerebral palsy. Int J Pediatr. 2012;2012:329284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Capio CM, Sit CH, Abernethy B. Physical activity measurement using MTI (Actigraph) among children with cerebral palsy. Arch Phys Med Rehabil. 2010;91:1283–1290. [DOI] [PubMed] [Google Scholar]
- 30. van Wely L, Becher JG, Balemans AC, Dallmeijer AJ. Ambulatory activity of children with cerebral palsy: which characteristics are important? Dev Med Child Neurol. 2012;54:436–442. [DOI] [PubMed] [Google Scholar]
- 31. Gorter JW, Timmons BW. Measurement of habitual physical activity and sedentary behavior of youth with cerebral palsy: work in progress. Dev Med Child Neurol. 2014;56:911. [DOI] [PubMed] [Google Scholar]
- 32. Shkedy Rabani A, Harries N, Namoora I, et al. Duration and patterns of habitual physical activity in adolescents and young adults with cerebral palsy. Dev Med Child Neurol. 2014;56:673–680. [DOI] [PubMed] [Google Scholar]
- 33. Carlon SL, Taylor NF, Dodd KJ, Shields N. Differences in habitual physical activity levels of young people with cerebral palsy and their typically developing peers: a systematic review. Disabil Rehabil. 2013;35:647–655. [DOI] [PubMed] [Google Scholar]
- 34. Stevens SL, Holbrook EA, Fuller DK, Morgan DW. Influence of age on step activity patterns in children with cerebral palsy and typically developing children. Arch Phys Med Rehabil. 2010;91:1891–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ishikawa S, Kang M, Bjornson KF, Song K. Reliably measuring ambulatory activity levels of children and adolescents with cerebral palsy. Arch Phys Med Rehabil. 2013;94:132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Palisano RJ, Rosenbaum P, Bartlett D, Livingston MH. Content validity of the expanded and revised Gross Motor Function Classification System. Dev Med Child Neurol. 2008;50:744–750. [DOI] [PubMed] [Google Scholar]
- 37. Andreacci JL, Dixon CB, Dube JJ, McConnell TR. Validation of SenseWear Pro2 Armband to assess energy expenditure during treadmill exercise in children 7–10 years of age. J Exerc Physiol Online. 2007;10:35–42. [Google Scholar]
- 38. Calabró MA, Welk GJ, Eisenmann JC. Validation of the SenseWear Pro Armband algorithms in children. Med Sci Sports Exerc. 2009;41:1714–1720. [DOI] [PubMed] [Google Scholar]
- 39. Koehler K, Abel T, Wallmann-Sperlich B, et al. Energy expenditure in adolescents with cerebral palsy: comparison of the SenseWear armband and indirect calorimetry. J Phys Act Health. 2015;12:540–545. [DOI] [PubMed] [Google Scholar]
- 40. Ryan JM, Walsh M, Gormley J. A comparison of three accelerometry-based devices for estimating energy expenditure in adults and children with cerebral palsy. J Neuroeng Rehabil. 2014;11:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lucía A, Fleck SJ, Gotshall RW, Kearney JT. Validity and reliability of the Cosmed K2 instrument. Int J Sports Med. 1993;14:380–386. [DOI] [PubMed] [Google Scholar]
- 42. Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Human Nutr Clin Nutr. 1985;39(suppl 1):5–41. [PubMed] [Google Scholar]
- 43. Portney LG, Watkins MP. Foundations of Clinical Research: Applications to Practice. 3rd ed Prentice Hall, Upper Saddle River, NJ; 2009. [Google Scholar]


