Abstract
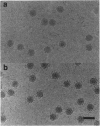
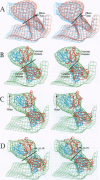
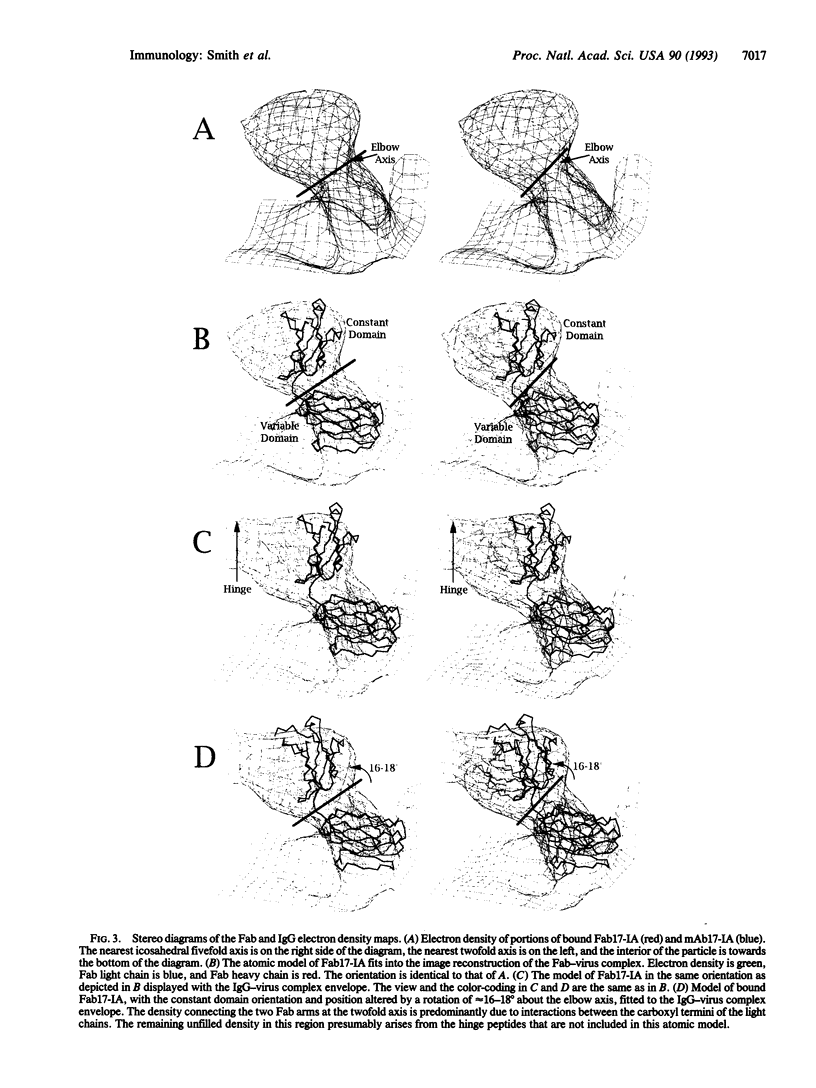
The structure of a neutralizing immunoglobulin (monoclonal antibody mAb17-IA), bound to human rhinovirus 14 (HRV14), has been determined by cryo-electron microscopy and image reconstruction. The antibody bound bivalently across icosahedral twofold axes of the virus, and there were no detectable conformational changes in the capsid. Thus, bivalently bound IgGs do not appear to cause gross deformations in the capsid. Differences between the electron density of the constant domains of the bound Fab fragment and IgG structures suggested that conformational changes occur about elbow axes upon bivalent attachment as was previously predicted. No significant density was observed for the Fc fragment, which adds further evidence for a high degree of mobility about the hinge region.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheng R. H., Olson N. H., Baker T. S. Cauliflower mosaic virus: a 420 subunit (T = 7), multilayer structure. Virology. 1992 Feb;186(2):655–668. doi: 10.1016/0042-6822(92)90032-k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonno R. J., Callahan P. L., Leippe D. M., Rueckert R. R., Tomassini J. E. Inhibition of rhinovirus attachment by neutralizing monoclonal antibodies and their Fab fragments. J Virol. 1989 Jan;63(1):36–42. doi: 10.1128/jvi.63.1.36-42.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther R. A. Procedures for three-dimensional reconstruction of spherical viruses by Fourier synthesis from electron micrographs. Philos Trans R Soc Lond B Biol Sci. 1971 May 27;261(837):221–230. doi: 10.1098/rstb.1971.0054. [DOI] [PubMed] [Google Scholar]
- Emini E. A., Ostapchuk P., Wimmer E. Bivalent attachment of antibody onto poliovirus leads to conformational alteration and neutralization. J Virol. 1983 Nov;48(2):547–550. doi: 10.1128/jvi.48.2.547-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris L. J., Larson S. B., Hasel K. W., Day J., Greenwood A., McPherson A. The three-dimensional structure of an intact monoclonal antibody for canine lymphoma. Nature. 1992 Nov 26;360(6402):369–372. doi: 10.1038/360369a0. [DOI] [PubMed] [Google Scholar]
- Marquart M., Deisenhofer J., Huber R., Palm W. Crystallographic refinement and atomic models of the intact immunoglobulin molecule Kol and its antigen-binding fragment at 3.0 A and 1.0 A resolution. J Mol Biol. 1980 Aug 25;141(4):369–391. doi: 10.1016/0022-2836(80)90252-1. [DOI] [PubMed] [Google Scholar]
- Olson N. H., Baker T. S., Willingmann P., Incardona N. L. The three-dimensional structure of frozen-hydrated bacteriophage phi X174. J Struct Biol. 1992 Mar-Apr;108(2):168–175. doi: 10.1016/1047-8477(92)90016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmann M. G., Arnold E., Erickson J. W., Frankenberger E. A., Griffith J. P., Hecht H. J., Johnson J. E., Kamer G., Luo M., Mosser A. G. Structure of a human common cold virus and functional relationship to other picornaviruses. Nature. 1985 Sep 12;317(6033):145–153. doi: 10.1038/317145a0. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G., Johnson J. E. Icosahedral RNA virus structure. Annu Rev Biochem. 1989;58:533–573. doi: 10.1146/annurev.bi.58.070189.002533. [DOI] [PubMed] [Google Scholar]
- Sherry B., Mosser A. G., Colonno R. J., Rueckert R. R. Use of monoclonal antibodies to identify four neutralization immunogens on a common cold picornavirus, human rhinovirus 14. J Virol. 1986 Jan;57(1):246–257. doi: 10.1128/jvi.57.1.246-257.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry B., Rueckert R. Evidence for at least two dominant neutralization antigens on human rhinovirus 14. J Virol. 1985 Jan;53(1):137–143. doi: 10.1128/jvi.53.1.137-143.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T. J., Olson N. H., Cheng R. H., Liu H., Chase E. S., Lee W. M., Leippe D. M., Mosser A. G., Rueckert R. R., Baker T. S. Structure of human rhinovirus complexed with Fab fragments from a neutralizing antibody. J Virol. 1993 Mar;67(3):1148–1158. doi: 10.1128/jvi.67.3.1148-1158.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade R. H., Taveau J. C., Lamy J. N. Concerning the axial rotational flexibility of the Fab regions of immunoglobulin G. J Mol Biol. 1989 Mar 20;206(2):349–356. doi: 10.1016/0022-2836(89)90484-1. [DOI] [PubMed] [Google Scholar]
- Wang G. J., Porta C., Chen Z. G., Baker T. S., Johnson J. E. Identification of a Fab interaction footprint site on an icosahedral virus by cryoelectron microscopy and X-ray crystallography. Nature. 1992 Jan 16;355(6357):275–278. doi: 10.1038/355275a0. [DOI] [PMC free article] [PubMed] [Google Scholar]