Abstract
Chagas disease, caused by Trypanosoma cruzi, is the main cause of dilated cardiomyopathy in the Americas. Antiparasitic treatment mostly relies on benznidazole (Bzl) due to Nifurtimox shortage or unavailability. Both induce adverse drug effects (ADE) of varied severity in many patients, leading to treatment discontinuation or abandonment. Since dosage may influence ADE, we aimed to assess Bzl efficacy in terms of parasiticidal and anti-inflammatory activity, using doses lower than those previously reported. BALB/c mice infected with the T. cruzi RA strain were treated with different doses of Bzl. Parasitaemia, mortality and weight change were assessed. Parasite load, tissue infiltrates and inflammatory mediators were studied in the heart. Serum creatine kinase (CK) activity was determined as a marker of heart damage. The infection-independent anti-inflammatory properties of Bzl were studied in an in vitro model of LPS-treated cardiomyocyte culture. Treatment with 25 mg/kg/day Bzl turned negative the parasitological parameters, induced a significant decrease in IL-1β, IL-6 and NOS2 in the heart and CK activity in serum, to normal levels. No mortality was observed in infected treated mice. Primary cultured cardiomyocytes treated with Bzl showed that inflammatory mediators were reduced via inhibition of the NF-κB pathway. A Bzl dose lower than that previously reported for treatment of experimental Chagas disease exerts adequate antiparasitic and anti-inflammatory effects leading to parasite clearance and tissue healing. This may be relevant to reassess the dose currently used for the treatment of human Chagas disease, aiming to minimize ADE.
Keywords: Trypanosoma cruzi, Chemotherapy, Inflammatory mediators, NF-κB
Graphical abstract
Highlights
-
•
Benznidazole is effective as an antiparasitic drug at a dose lower than the usual one.
-
•
NOS2 and pro-inflammatory cytokines are inhibited by low dose of benznidazole.
-
•
Benznidazole at low concentrations inhibits NF-κB pathway in cultured cardiomyocytes.
1. Introduction
Chagas disease is caused by infection with the obligate intracellular protozoan parasite Trypanosoma cruzi. This disease is endemic throughout Central and South America, representing a major public health problem. The disease is characterized by an acute phase with high parasitaemia and variable symptoms, including acute myocarditis, meningoencephalitis or generalized infection symptoms (hepatosplenomegaly). This is followed by a chronic phase that may remain asymptomatic during the whole life or develop into serious digestive or cardiac alterations, which are found in about 30% of infected individuals and may lead to dilated cardiomyopathy (Teixeira et al., 2002).
The disease is currently treated with benznidazole (Bzl) (N-benzyl-2-nitroimidazole acetamide), a drug known to reduce parasite burden during acute and early chronic infection (Coura, 2009). During the chronic phase, the effect of Bzl is more controversial, although some reports have shown that individuals treated with Bzl and evaluated decades after the initial infection acquire significant protection from progression of heart pathology (Viotti et al., 2006, Fabbro et al., 2007). Although Bzl has been used in clinical settings, its mechanisms of action have not been fully elucidated yet (Maya et al., 2007). However, several studies have suggested that Bzl treatment should still be recommended at late phases of Chagas disease to prevent progression, regardless of the lack of complete parasite clearance (Garcia et al., 2005, Sosa-Estani and Segura, 2006, Viotti et al., 2006). Indeed, there is a general premise that etiological treatment contributes to reducing parasite load and rearranging the host immune response, leading to a balanced inflammatory response, which is crucial to control Chagas disease morbidity (Garcia et al., 2005, Viotti et al., 2006).
Campi-Azevedo et al. (2015) have recently characterized the phagocytic capacity and cytokine profile of leukocytes from Chagas disease patients in the indeterminate and cardiac phases, both before and one year after Bzl treatment. Their findings highlighted that Bzl treatment contributes to an overall immunomodulation in the indeterminate phase and induces a broad change of the immune response in patients in the cardiac phase, eliciting an intricate phenotypic/functional network compatible with beneficial and protective immunological events. However, Bzl contains a nitro group linked to an imidazole whereby unwanted side effects are common. The most important adverse reactions observed with Bzl are cutaneous reactions (allergic dermatitis) (Pérez-Molina et al., 2009), digestive intolerance, polyneuritis, bone marrow depression, toxic hepatitis (Viotti et al., 2009), peripheral neuropathy and angioedema (Miller et al., 2015). These side effects, which force about 10% of patients to suspend the treatment, represent the main disadvantage of Bzl treatment. In addition, Hasslocher-Moreno et al. (2012) showed that up to 26% of patients treated with Bzl during the chronic phase develop skin reactions and that some show gastrointestinal (10%) and/or neurological (around 5%) disorders. Nevertheless, the incidence of adverse reactions has been insufficiently reported, making it difficult to interpret the safety profile of Bzl. Current evidence supports the treatment of adults without advanced cardiac disease or significant morbidity using either Bzl or nifurtimox, the other drug available (Jackson et al., 2010). Nifurtimox is associated with gastrointestinal and neuropsychiatric side effects in nearly all patients, only half of whom can tolerate the full treatment course (Priotto et al., 2009). Bzl is better tolerated, but due to intermittent medication shortages and drug-induced rash including Stevens–Johnson syndrome, many patients fail to complete the treatment. Although, as stated above, it has been proposed that Bzl is usually better tolerated than Nifurtimox, Rojo et al. (2014) have recently reported about the controversial toxicity of both drugs. However, Maya et al. (2007) found no significant adverse drug effects in a large series of patients treated with these drugs. Upon infection, the parasite is able to invade and multiply within diverse cell types, including macrophages. The acute phase of infection is characterized by the presence of parasites in the host bloodstream and diverse tissues. A crucial step in cardiomyopathy is the infiltration of monocytes and their differentiation into macrophages. These cells may either inhibit T. cruzi multiplication or provide a favourable environment in which it can divide and be disseminated to other sites within the body (Tanowitz et al., 1992, Penas et al., 2015). Besides, there is substantial evidence showing that cardiac tissue, an important target of T. cruzi infection, produces marked amounts of pro-inflammatory cytokines, chemokines and enzymes, including inducible nitric oxide synthase (NOS2) and metalloproteinases, resulting in inflammation and cardiac remodelling in response to parasite infection (Penas et al., 2013).
In addition to its antiparasitic activity, Bzl exerts immunomodulatory effects in macrophages stimulated with lipopolysaccharide (LPS) and treated with a high concentration of Bzl (1 mM) (Piaggio et al., 2001). These immunomodulatory effects of Bzl have also been described in LPS-challenged mice pre-treated with high doses of Bzl, showing the ability of Bzl to increase survival and decrease serum levels of IL-6 and TNF-α in C57BL/6 mice (Pascutti et al., 2004). The fact that components of T. cruzi as glycosylphosphatidylinositol-anchored mucin-type glycoproteins and glycoinositolphospholipids through the toll-like receptors TLR2/TLR6 and TLR4, respectively, induce proinflammatory cytokines (Junqueira et al., 2010) validates the use of LPS in studies that explore the mechanism of action of Bzl.
In the present study, we considered the administration of doses of Bzl lower than those usually used in experimental models of T. cruzi infection, to evaluate its parasiticidal as well as immunomodulatory effects, to minimize the adverse side reactions that usually lead to cessation of therapy.
2. Materials and methods
2.1. In vivo model: mice and infection
Mice used in this study were bred and maintained in the animal facility at the Instituto de Investigaciones en Microbiología y Parasitología Médica, Universidad de Buenos Aires – CONICET. All procedures carried out with mice were approved by the Institutional Committee for the Care and Use of Laboratory Animals (CICUAL, Facultad de Medicina de la Universidad de Buenos Aires) and are in accordance with guidelines of the Argentinean National Administration of Medicines, Food and Medical Technology (ANMAT), Argentinean National Service of Sanity and Agrifoods Quality (SENASA) and also based on the US NIH Guide for the Care and Use of Laboratory Animals. Eight-weeks old BALB/c male mice (7 per group) were infected intraperitoneally with 500 bloodstream trypomastigotes of the lethal RA (pantropic/reticulotropic) strain of T. cruzi, (DTU VI) as previously described (Celentano and González Cappa, 1993, Zingales et al., 2009). Benznidazole (Abarax®, ELEA, Argentina. PubChem Compound Database CID = 31593), suspended in corn oil, was administered orally at 10, 25 and 100 mg/kg/day, for 30 consecutive days. The groups received the following treatments: Group 1 = Corn oil, Group 2 = Bzl 10 mg/kg/day, Group 3 = Bzl 25 mg/kg/day, Group 4 = Bzl 100 mg/kg/day, Group 5 = Uninfected, untreated. Treatment started soon after the detection of parasites in blood, which occurred at day 7 post-infection (p.i.).
Parasitaemia of infected untreated mice (Group 1) peaks between day 10 and 13. Therefore, they were sacrificed at the time when they began to show signs of cachexia, since they did not survive after 15 days. Mice of Groups 2 through 5 were sacrificed at day 55 p.i. Each experiment was carried out three times.
2.2. Parasitaemia and survival
Presence of parasites in blood was evaluated by microhematocrit method (Feilij et al., 1983). Parasitaemia was analysed using Pizzi's technique modified by Brener (1962) every three to seven days, and survival was observed daily, until the end of the experiment. Parasitaemia was expressed as parasites per millilitre of blood.
2.3. Histopathological studies
Hearts from T. cruzi infected untreated and benznidazole-treated infected mice (25 mg/kg/day), were fixed in formalin and embedded in paraffin. Six non-contiguous sections (5 μm) were cut and stained with haematoxylin-eosin. Cellular infiltrates and the presence of amastigote nests were examined using a Nikon Eclipse E600 microscope (Nikon Inc.). Images were captured with a Spot RT digital camera. At least thirty random microscopic fields (400×) were analysed in each microscopic section, using the open source Image J software (NIH, USA).
2.4. Creatine kinase activity
The activity of CK as a marker heart injury was determined using commercially available assay kits according to manufacturer's instructions (Wiener Lab, Rosario, Argentina). The kit relies on the reduction of NADP+ and the increase of absorbance measured at 340 nm.
2.5. In vitro model: neonatal mouse primary cardiomyocytes culture
One-to 3-day old neonatal outbred CF-1 strain mice were euthanized by decapitation after CO2 exposure, and cardiomyocytes were obtained as previously described (Hovsepian et al., 2013).
Each experiment was carried out 3 times with 5 replicates per group.
2.6. Purification of bloodstream trypomastigotes of T. cruzi
Bloodstream trypomastigotes were obtained by cardiac puncture, from euthanized 21-days old male CF-1 mice infected 7 days previously by intraperitoneal route with 1.0 × 105 trypomastigotes of RA strain of T. cruzi, as previously described (González Cappa et al., 1981b, Maggini et al., 2010). Briefly, blood diluted 1:5 in culture medium was centrifuged at 400 g at room temperature and then left at 37 °C for 1 h in a water bath. Trypomastigotes in the supernatant were essentially devoid of blood cells and platelets according to microscopic examination. Quantification of parasites was performed with a Neubauer chamber.
2.7. Assay to evaluate the trypanocidal effect of benznidazole in vitro
Cardiomyocytes were cultured at 37 °C under 5% CO2 atmosphere and infected at a 5:1 parasite: cell ratio in six well polystyrene plates. After 3 h, the infected cultures were washed five times with fresh 1% FCS-DMEM: M-199 medium to remove free parasites. The infected cells were cultured for 48 h and then treated with 3, 15 or 75 μM of Bzl, for 72 h. Then, genomic DNA (gDNA) was obtained and parasitic load in cardiac cells was analysed by quantitative real-time PCR (qPCR).
2.8. Assay to evaluate benznidazole effects on inflammatory mediators
Cardiomyocytes in culture were pre-treated for 30 min with Bzl 3, 15 or 75 μM and stimulated with LPS (10 mg/L) from Escherichia coli O26:B6 (Sigma–Aldrich Co) for the indicated period of time. mRNA and protein extracts were obtained, and RT-qPCR, Western blot and immunofluorescence analysis were performed (See below).
2.9. mRNA and gDNA purification
Total RNA was obtained from heart tissue homogenates and from cultured cells using Quickzol reagent (Kalium), treated with DNAse (Life Technologies) and total RNA was reversed transcribed using Expand Reverse Transcriptase (Promega Corporation), following the manufacturer's instructions. gDNA was obtained from heart tissue or the cultured cells using phenol-chloroform extraction (Laird et al., 1991).
2.10. Quantitative real-time polymerase chain reaction (qPCR and RT-qPCR)
Both qPCR and RT-qPCR were performed using 5X HOT FIREPOL EVAGREEN qPCR (Solis BioDyne) in an Applied Biosystems 7500 sequence detector. mRNA expression was analysed by RT-qPCR. The parameters were: 52 °C for 2 min, 95 °C for 15 min, and 40 cycles of 95 °C for 15 s, 60 °C (for NOS2, IL-6 and 18S rRNA) or 57 °C (for IL-1β) for 30 s and 72 °C for 1 min. Expression of 18S rRNA was analysed in the samples in the same run for normalization. Quantification of parasite load in heart tissue or cultured cardiac cells was performed by qPCR with the primers TCZ (Cummings and Tarleton, 2003, Duffy et al., 2009). These primers amplify a 146-bp sequence of the highly repetitive (104 repeats) satellite genomic DNA. The high number of copies increases the sensitivity of the technique allowing the detection of less than 1 parasite/ml, which is defined as parasite equivalents.
Quantification was calculated using the comparative threshold cycle (Ct) method and efficiency of the RT reaction (relative quantity, 2−ΔΔCt). The replicates were then averaged and fold induction was determined, considering the value at zero time as 1 (Schmittgen and Livak, 2008).
Primer sequences are shown in Supplementary Information section.
2.11. Preparation of cytosolic, nuclear and total protein extracts for Western blot
Heart tissue (100 mg) was homogenized with 300 μl of Buffer A (See below), or cultured cells were washed with PBS and scraped off the dishes, with 50 μl of the same buffer, and NP-40 (Life Technologies) was added to reach 0.5% (v/v). After 15 min at 4 °C, the tubes were gently vortexed for 10 s, and cytosolic extracts were collected by centrifugation at 13,000 g for 90 s. The supernatants were stored at −20 °C (cytosolic extracts), and heart pellets were resuspended in 100 μl buffer A supplemented with 20% (v/v) glycerol and 0.4 M KCl, and mixed for 30 min at 4 °C. Nuclear proteins were obtained by centrifugation at 13,000 g for 5 min, and aliquots of the supernatant (nuclear extracts) were stored at −80 °C.
Total protein extracts were obtained after washing the hearts with PBS and adding 300 μl of RIPA modified lysis buffer (See below), or washing the cultured cells and scraped off the dishes with 50ul of the same buffer. Then, the tubes were kept on ice for 30 min with swirling and the samples were centrifuged at 7000 g at 4 °C for 10 min. The supernatant was stored at −20 °C. Protein concentrations of sera and protein extracts were determined by the Bradford method using the Bio-Rad Protein Assay (Bio-Rad, USA) reagent and bovine serum albumin (BSA) (Sigma–Aldrich Co.) as a standard (Kruger, 1994).
2.12. Western blot analysis
Proteins extracts were boiled in Laemmli sample buffer, and equal amounts of protein (50–100 mg) were separated by 8–12% SDS–PAGE. The gels were blotted onto a Hybond-P membrane (GE Health-care, Madrid, Spain) and incubated with the following antibodies: anti-NOS2, anti-IκB-α, anti-p65 (Santa Cruz Biotechnology, CA, USA), and anti-α-actin (Sigma–Aldrich Co). The blots were revealed by enhanced chemiluminescence (ECL) in an Image Quant 300 cabinet (GE Healthcare Biosciences, USA) following the manufacturer instructions. Band intensity was analysed using the Image J software.
2.13. NO measurement
To determine the amount of NO released into the culture medium, nitrate was reduced to nitrite and measured spectrophotometrically by the Griess reaction (Bryan and Grisham, 2007, Díaz-Guerra et al., 1996). The absorbance at 540 nm was compared with a standard curve of NaNO2.
2.14. Immunofluorescence
Myocardial cells grown on round glass coverslips were fixed with methanol and blocked with 3% BSA in PBS. The expression of IκBα and NOS2 were determined by immunofluorescence. For this purpose, rabbit polyclonal IgG anti-NOS2 or IgG anti-IκBα (Santa Cruz Biotechnology, CA, USA) and FITC-labelled goat anti-rabbit IgG (Sigma–Aldrich Co), were used at 1:200 dilutions (determined by titration). Cells nuclei were stained with DAPI (300 nM). At least 30 random microscopic fields (400×) and 1000 cells per culture were acquired using a Spot RT digital camera attached to an Eclipse 600 fluorescence microscope (Nikon Inc., USA).
2.15. Buffer composition
HBSS: (in g/L): 0.4 KCl, 0.06 KH2PO4, 8.0 NaCl and 0.05 Na2HPO4, pH 7.4.
HBSS Plus (in g/L): HBSS plus 0.14 CaCl2, 0.047 MgCl2, 0.049 MgSO4, 0.35 NaHCO3 and 1.0 d-glucose, pH 7.40.
Buffer A: 10 mmol/L HEPES pH = 7.90, 1 mmol/L EDTA, 1 mmol/L EGTA, 10 mmol/L KCl, 1 mmol/L DTT, 0.5 mmol/L PMSF, 40 mg/L leupeptin, 2 mg/L tosyl-lysyl-chloromethane, 5 mmol/L NaF, 1 mmol/L NaVO4, 10 mmol/L Na2MoO4.
RIPA modified lysis buffer: 150 mM NaCl, 50 mM Tris–HCl (pH = 7.40), 1% Triton X-100, 1 mM EDTA, 1 mM PMSF; 2.5 g/L Protease Inhibitor Cocktail (Sigma Aldrich), 1 mM Na3VO4, 1 mM NaF.
2.16. Statistical analysis
Data are expressed as mean of 3 independent experiments ± SEM for each treatment (7 mice or 5 culture replicates/group). The Kaplan–Meier method and Mantel–Cox test were used to compare survival curves of the studied groups. One-way ANOVA was used to analyse the statistical significance of the differences observed between the infected, treated or untreated groups. The Dunnet post-hoc test was performed to compare treated groups against the control group (T. cruzi or LPS according to the experimental setting). Differences were considered statistically significant when P < 0.05. All analyzes were performed using the Prism 5.01 Software (GraphPad, USA).
3. Results
3.1. Benznidazole is effective as an antiparasitic drug at a dose lower than the usual one
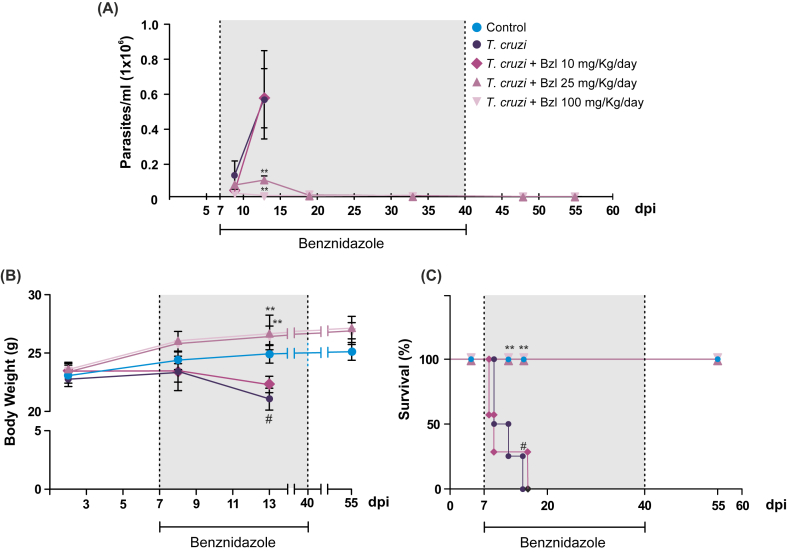
BALB/c mice were infected with 500 trypomastigotes of the virulent and lethal RA T. cruzi strain by the intraperitoneal route to assess whether Bzl has effective trypanocidal activity at concentrations lower than those usually used in experimental models (100 mg/kg/day). The presence of parasites in blood, evaluated from day 5 post-infection (p.i.) by the microhematocrit method, became apparent on day 7 p.i. in all experimental groups, reaching quantifiable values by day 9 p.i. (0.0315 × 106 ± 0.003 parasites/mL, n = 3), with a maximum on day 13 (0.561 × 106 ± 0.169 parasites/mL, n = 3). Different groups were daily treated with 10 mg/kg/day (low dose), 25 mg/kg/day (mid dose) or 100 mg/kg/day (high dose) of Bzl, respectively, from day 7 p.i. Treatment with 25 mg/kg/day of Bzl was almost as effective in its trypanocidal action as the usual dose of 100 mg/kg/day on day 13 (mid dose vs high dose, 0.094 ± 0.020 vs 0.00 ± 0.00 parasites/mL, n = 3, P < 0.001) (Fig. 1A). In contrast, the treatment with the low dose showed no antiparasitic effect on day 13 p.i. (infected vs infected treated with low dose, 0.561 × 106 ± 0.169 parasites vs 0.581 × 106 ± 0.252 parasites/mL, n = 5, P < 0.05). A decrease in weight was observed in the infected untreated mice and mice treated with low-dose Bzl in comparison with uninfected controls. Moreover, the body weight of mice treated with mid and high Bzl doses remained unchanged throughout the study (Fig. 1B). Consistently with the increase of parasitaemia, infected untreated mice did not survive longer than day 15 p.i., whereas mice treated with 25 mg/kg/day of Bzl had survival rates similar to those treated with 100 mg/kg/day (Fig. 1C).
Fig. 1.
Benznidazole is effective as antiparasitic drug at a lower dose than usual. BALB/c mice were infected with 500 trypomastigotes of the lethal RA T. cruzi strain by intraperitoneal route and treated with different doses of Bzl for 30 consecutive days. Parasitaemia (parasites/mL) (A) and body weight (grams) (B) were measured. Survival was observed daily, until the end of the experiment and analysed by the Kaplan–Meyer method (C). Results are expressed as mean of 3 independent experiments (7 mice/group) ± SEM. **P < 0.001 vs T. cruzi-infected mice. #P < 0.05 vs Control mice.
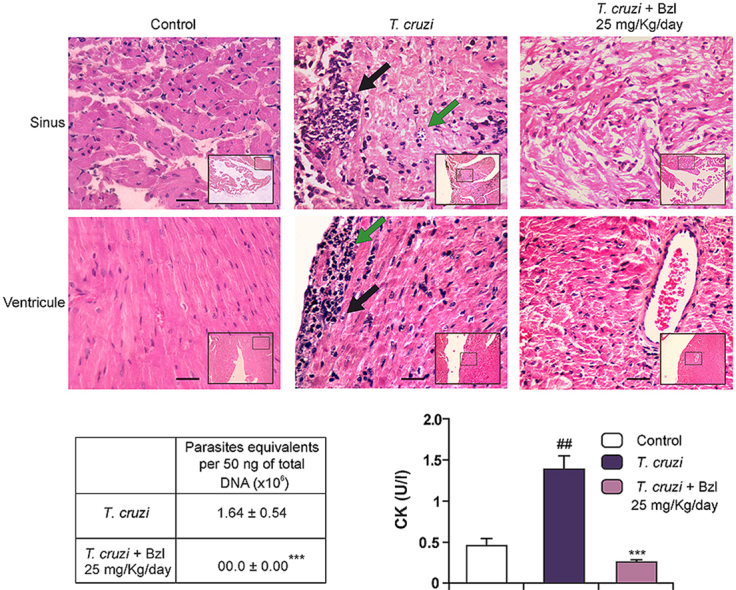
3.2. Benznidazole clears tissue parasite load, attenuates inflammatory reaction and normalizes creatine kinase activity in T. cruzi-infected mice
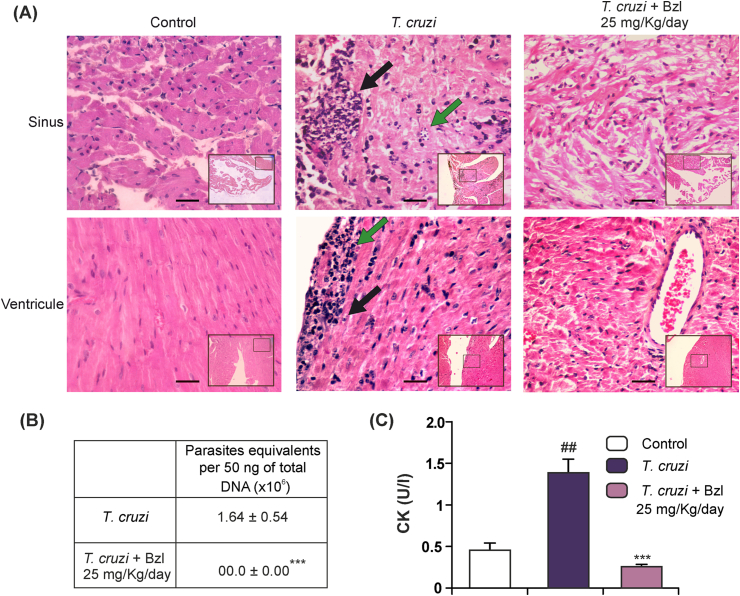
Given that the effects in reducing parasitaemia and maintaining survival of the dose of 25 mg/kg/day were similar to those of the dose of 100 mg/kg/day, we investigated the presence of parasites and inflammatory reaction in the heart. While T. cruzi-infected untreated mice showed significant numbers of amastigote nests in sinuses and ventricles, as well as inflammatory reaction associated with them, mice treated with 25 mg/kg/day showed neither inflammatory cells nor amastigote nests (Fig. 2A). To further confirm that T. cruzi-infected Bzl-treated mice were devoid of parasites in the heart, qPCR using TCZ primers was performed after gDNA extraction from this tissue. No gDNA amplification was obtained in the hearts of mice treated with 25 mg/kg/day (Fig. 2B).
Fig. 2.
Benznidazole clears tissue parasite load, attenuates inflammatory reaction and normalizes creatine kinase activity in T. cruzi-infected mice. Cardiac parasite load and inflammatory reaction was analysed in histological sections of the hearts of uninfected mice (left panels), infected mice at 15 d.p.i (centre panels) or infected and Bzl-treated mice (25 mg/kg/day, right panels) at 55 d.p.i. Inflammatory reaction in the sinus of a T. cruzi-infected mouse is shown (Black left-hand arrow). Amastigotes and necrotic myofibers can be observed. An isolate amastigote nest not surrounded by inflammatory reaction is also shown (Green right-hand arrow) (upper centre panel). Preserved architecture of the sinus of a T. cruzi-infected and Bzl-treated mouse is shown. Neither amastigote nests nor inflammatory reaction are observed (upper right panel). Inflammatory reaction in the ventricle of a T. cruzi-infected mouse is observed (black right-hand arrow). Amastigotes (green left-hand arrow) and necrotic myofibers can be observed (lower centre panel). Preserved architecture of the sinus and ventricle of a T. cruzi-infected and Bzl-treated mouse are shown. Neither amastigote nests nor inflammatory reaction is observed. Notice the preserved architecture of the vascular wall (right panel). Haematoxylin-Eosin stain. Magnification: 400×. Panoramic view of the detailed area: 100×. Bar: 100 μm. (A). Heart parasite load was detected by qPCR of infected mice at 15 d.p.i or infected and Bzl-treated mice (25 mg/kg/day) at 55 d.p.i. (B). CK activity in the sera of T. cruzi-infected mice at 15 d.p.i. or T. cruzi-infected and Bzl-treated (25 mg/kg/day) mice at 55 d.p.i, was determined as a marker of heart injury (C). Results are expressed as mean of 3 independent experiments (7 mice/group) ± SEM. ***P < 0.0001 vs T. cruzi-infected mice; ##P < 0.001 vs uninfected control mice. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
We measured changes in serum CK activity as a biochemical marker of heart injury upon infection. A significant increase in the level of CK activity on day 15 p.i. was observed in infected mice in comparison with uninfected untreated control mice (uninfected vs infected: 0.448 ± 0.116 vs 1.368 ± 0.149, n = 3, P < 0.0001). Moreover, 30 days after Bzl treatment with 25 mg/kg/day, CK levels dropped to values found in control mice, suggesting that tissue injury was precluded by Bzl (0.240 ± 0.041, n = 3, P < 0.0001) (Fig. 2C).
3.3. NOS2 and pro-inflammatory cytokines are inhibited by benznidazole in hearts of T. cruzi-infected mice
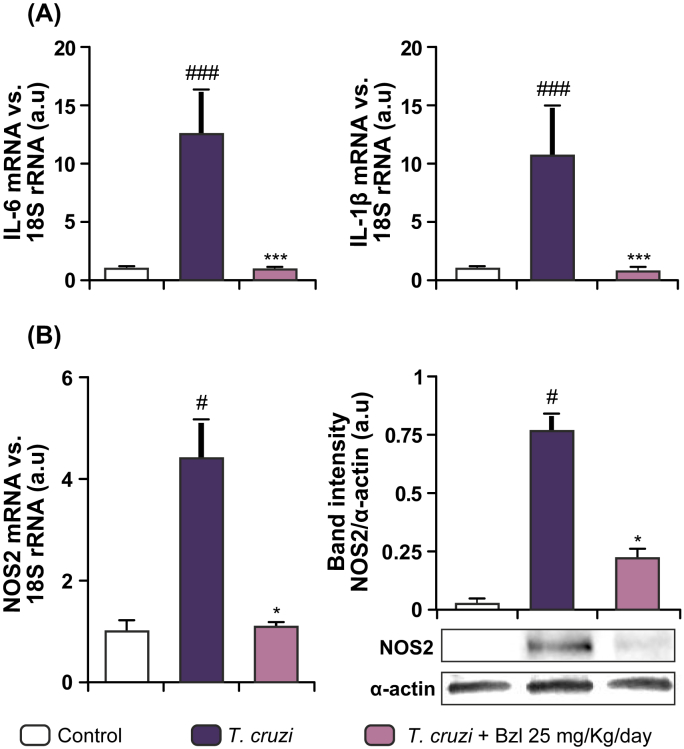
Previous studies have shown that Bzl treatment modulates pro-inflammatory cytokines in vitro models of LPS stimulation (Revelli et al., 1999, Piaggio et al., 2001) and an in vivo model of sepsis (Pascutti et al., 2004, Ronco et al., 2011). To determine whether Bzl modulates inflammatory mediators in experimental acute Chagas disease, T. cruzi-infected mice were treated with 25 mg/kg/day of Bzl, the lowest antiparasitic dose. The hearts of RA-infected mice expressed considerable amounts of IL-1β mRNA (uninfected vs. infected, 0.725 ± 0.275 vs 10.370 ± 4.502, n = 3, P < 0.001) and IL-6 mRNA (uninfected vs. infected, 0.849 ± 0.301 vs 13.260 ± 2.873 n = 3, P < 0.0001) measured by RT-qPCR on day 15 p.i., before death (Fig. 3A). Furthermore, infected mice treated with Bzl for 30 days showed a marked decrease in the cardiac levels of both IL-1β (0.690 ± 0.136, n = 3, P < 0.05) and IL-6 (0.549 ± 0.135, n = 3, P < 0.0001) mRNA expression. Fig. 3B shows that the same applied to NOS2 mRNA (uninfected vs infected: 1.107 ± 0.475 vs 4.369 ± 0.845; infected treated: 1.052 ± 0.202, n = 3, P < 0.05) and protein expression determined at the same times (uninfected vs infected: 0.394 ± 0.240 vs 1.718 ± 0.406; infected treated: 0.690 ± 0.104, n = 3, P < 0.05).
Fig. 3.
NOS2 and Pro-inflammatory cytokines are inhibited by benznidazole in hearts of T. cruzi infected mice. IL-6 and IL-1β mRNA levels were analysed by RT-qPCR in total RNA heart extracts of infected mice (15 d.p.i) or infected and Bzl-treated mice (25 mg/kg/day) at 55 d.p.i (A). NOS2 mRNA levels and NOS2 expression were determined in the same conditions as in (A), by RT-qPCR and Western blot with specific primers and antibodies, respectively (B). The RT-qPCR results were normalized against 18S rRNA. Protein levels were normalized against α-actin. Results are expressed as mean of 3 independent experiments (7 mice/group) ± SEM. *P < 0.05, ***P < 0.0001 vs T. cruzi-infected mice; #P < 0.05 vs. control mice. ###P < 0.0001 vs uninfected control mice.
3.4. Trypanocidal effect of benznidazole on primary cultures of infected cardiomyocytes
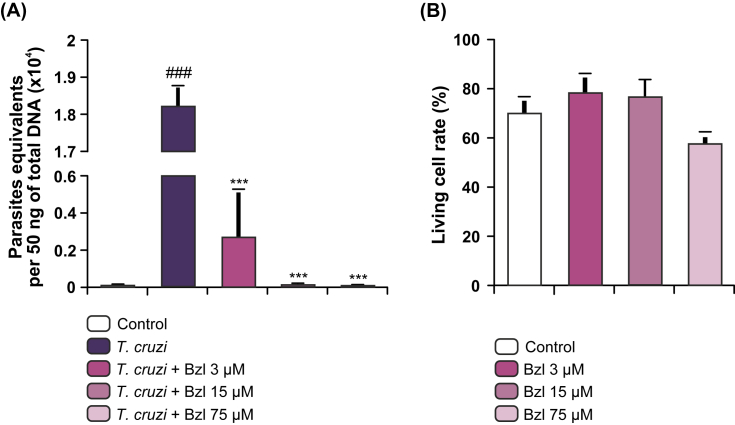
To assess the parasiticidal effect of low concentrations of Bzl in vitro, primary cardiomyocyte cultures were infected with T. cruzi at a 5:1 parasite: cell ratio and incubated with different concentrations of Bzl for 72 h. Fig. 4A shows that 75 μM Bzl significantly reduced the parasite load in comparison with untreated cells. Furthermore, treatment with 15 μM Bzl rendered the parasite load to almost negligible levels when measured as equivalent parasites per 50 ng total gDNA by qPCR, in this period of time. Cell viability was studied after treatment with different concentrations of Bzl in cardiomyocytes. We found that cell viability was not affected by any of the concentrations of Bzl tested (Fig. 4B).
Fig. 4.
Trypanocidal effect of benznidazole on primary cultures of infected cardiomyocytes. Cardiomyocytes were infected with T. cruzi for 48 h and treated with different doses of Bzl for 72 h. Parasite load was measured by qPCR. Parasite equivalents per 50 ng total gDNA are shown. (A). Cell viability upon Bzl treatment was evaluated in uninfected cardiomyocytes by Trypan blue dye exclusion assay and expressed as percentage of total cells. Bar hues represent the same Bzl concentrations as in left panel (B). Results are expressed as mean of 3 independent experiments (3 replicates/treatment) ± SEM. ***P < 0.0001 vs infected untreated cells; ###P < 0.0001 vs control cells. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
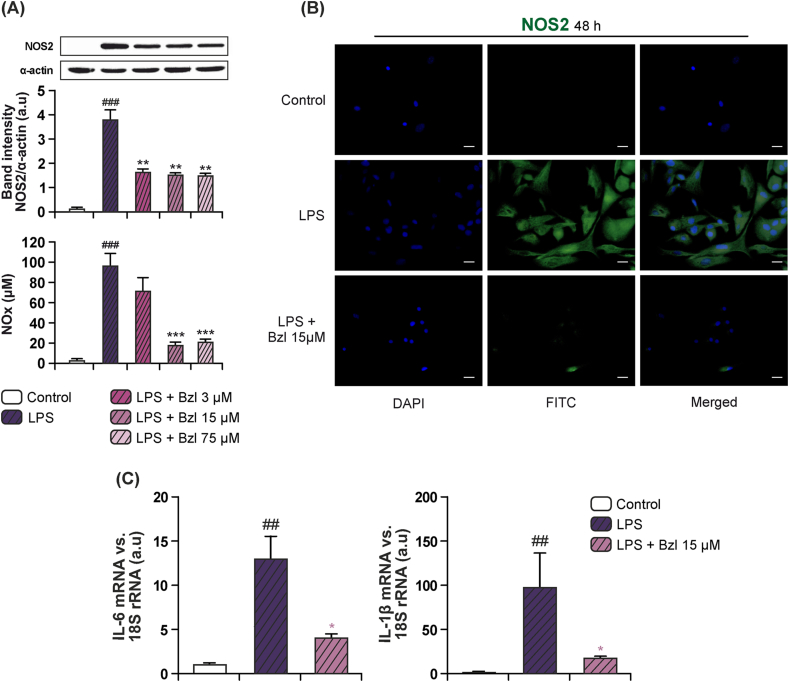
3.5. Benznidazole inhibits inflammatory mediators in cultured cardiomyocytes
We have previously described that T. cruzi infection produces an intense inflammatory response, which is critical for the control of the evolution of Chagas disease. In this regard, we determined the expression of typical inflammatory markers like NOS2 with high levels of nitric oxide (NO) or inflammatory cytokines like IL-1β, IL-6 and TNFα in infected cardiomyocytes (Hovsepian et al., 2011, Hovsepian et al., 2013). Other authors have previously reported the inhibitory effect of Bzl on the production of NOS2-dependent NO by RAW 264.7 macrophages at high concentrations ranging from 0.1 mM to 1 mM (Revelli et al., 1999). With the aim to dissociate the antiparasitic effect of Bzl from its anti-inflammatory properties, the ability of the lowest doses of Bzl to inhibit inflammatory mediators were assessed in cultured cardiomyocytes treated with Bzl and stimulated with LPS. When the cells were pre-incubated with 15 μM Bzl (a concentration rendering parasite DNA almost undetectable by qPCR) and stimulated with 10 mg/L of LPS, a significant inhibition of both NOS2 expression (Fig. 5A and B) and NOS2-dependent NO production was observed (Fig. 5A). Moreover, decreased IL-1β and IL-6 mRNA levels were found after 4 h, as shown by RT-qPCR (Fig. 5C).
Fig. 5.
Benznidazole inhibits inflammatory mediators in cultured cardiomyocytes. Cardiomyocytes were treated with different concentrations of Bzl for 30 min and then with 10 mg/L LPS for 48 h. NOS2 expression was determined by Western blot with a specific antibody and normalized against α-actin. NO levels were quantified by the Griess reaction in culture supernatants (A). Cardiomyocytes were pre-treated with 15 μM Bzl for 30 min and then with 10 mg/L LPS for 48 h. NOS2 expression was detected by immunofluorescence with a rabbit polyclonal antibody specific for NOS2 and a secondary FITC-labelled anti-rabbit IgG. Cells nuclei were stained with 300 nM DAPI. Representative microphotographs (400×) are shown (B). Cardiomyocytes were pre-treated with 15 μM Bzl for 30 min and then with 10 mg/L LPS for 4 h. IL-6 and IL-1β mRNA levels were analysed by RT-qPCR and normalized against 18S rRNA (C). Scale bar: 10 μm. Results are expressed as mean of 3 independent experiments (3 replicates/treatment) ± SEM. *P < 0.05, **P < 0.001, ***P < 0.0001 vs LPS-stimulated cells; #P < 0.05, ##P < 0.001 vs control cells.
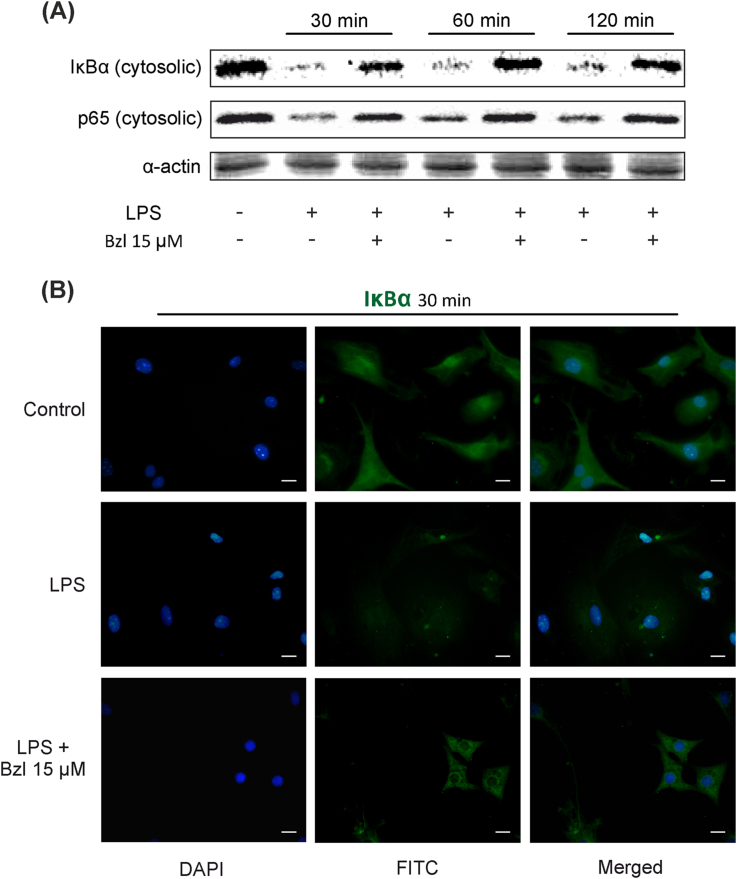
3.6. Benznidazole at low concentrations also inhibits the NF-κB pathway
To assess whether Bzl inhibits inflammatory mediators through inhibition of the NF-κB pathway, after an inflammatory stimulus, cardiomyocyte cultures were pre-treated with Bzl and incubated for 30, 60 or 120 min with LPS. The specific inhibitor of this transcription factor, IκBα, as well as the p65-NF-κB subunit, diminished in the cytoplasmic fraction after 30 min of LPS incubation as assessed by Western blot, confirming the activation of this pathway (0.111 ± 0.006 and 0.403 ± 0.029 respectively, n = 3, P < 0.0001). Significantly, pre-treatment with 15 μM Bzl impaired both IκBα and p65 disappearance from the cytosolic fraction (0.650 ± 0.028 and 0.971 ± 0.064 respectively, n = 3, P < 0.0001) (Fig. 6A). This suggests that this trypanocidal drug exerts regulation through the NF-κB pathway at a concentration lower than that previously reported (Piaggio et al., 2001). The effect of low concentrations of Bzl was confirmed by immunocytochemistry. Detection of IκBα by immunofluorescence showed that it disappeared from the cytoplasm when NF-κB was activated by LPS. Moreover, IκBα levels were restored to values similar to those in unstimulated cells when cardiomyocytes were pre-treated with Bzl before LPS stimulation (Fig. 6B).
Fig. 6.
Benznidazole at low concentrations also inhibits the NF-κB pathway. Cardiomyocytes were treated with 15 μM Bzl for 30 min and then with 10 mg/L LPS for 30, 60 or 120 min. IκBα and p65 cytosolic expression were determined by Western blot with specific antibodies. Protein levels were normalized against α-actin. A representative result out of 3 experiments performed is shown (A). IκBα expression was detected by immunofluorescence with a polyclonal rabbit anti-IκBα antibody and a secondary FITC-labelled anti-rabbit IgG. Cells nuclei were stained with 300 nM DAPI. Representative microphotographs (400×) are shown (B). Scale bar: 10 μm.
4. Discussion
The current treatment for Chagas disease with Bzl is effective to cure the infection in the acute phase, limiting the incapacity and preventing morbidity and mortality (Rassi et al., 2009, Rassi et al., 2011). However, this treatment has serious limitations, such as the side effects exhibited by some patients (Castro et al., 2006). These include organic manifestations indicative of systemic toxicity such as allergic dermatitis, fever, vomiting, gastrointestinal syndromes, bone marrow depression and polyneuropathy. All these symptoms are evident in the first days of treatment and represent a major threat of Bzl in clinical use with frequent therapy discontinuation (Rodriques Coura and de Castro, 2002, Castro et al., 2006, Rassi et al., 2009, Viotti et al., 2009, Pinazo et al., 2013). Thus, finding options to enhance the effectiveness of Bzl and to decrease its adverse effects is very desirable, if we consider that this is the only antiparasitic treatment currently available in most countries. The effectiveness of different experimental protocols aiming to modify the dose of this antiparasitic drug has not been thoroughly tested. Therefore, in the present study we proposed to test doses lower than normally used in experimental Chagas disease treatment to determine the minimal effective dose.
We report for the first time that Bzl has parasiticidal properties in vivo as well as in vitro at doses lower than those previously reported for a highly virulent T. cruzi strain. In addition, treatment with a lower dose of Bzl was also able to inhibit the expression of inflammatory mediators and tissue inflammation.
Our results show that, in mice infected with the RA strain of T. cruzi, the treatment with 25 mg/kg/day of Bzl was as effective as 100 mg/kg/day, the therapeutic dose usually used to cure experimental Chagas disease (de Andrade et al., 1996, Guedes et al., 2011, Molina-Berríos et al., 2013). This was reflected in the rate of survival after treatment, since no significant differences were observed between the experimental groups using both doses. Moreover, after treatment with low doses of Bzl, mice regained the weight they had lost due to infection. An optimal dose of Bzl (25 mg/kg/day) was associated with the absence of inflammatory reaction and tissue parasite load as measured by qPCR. In this regard, Martins et al. (2008) have previously reported for an experimental murine model, that serological and blood parasitological tests for T. cruzi are less sensitive than tissue qPCR. Thus, our results strongly suggest that this low-dose Bzl treatment is able to clear T. cruzi from the infected host. In contrast, other research groups argue that only the optimal dose of 100 mg/kg/day of Bzl confers almost complete protection against death to infected mice (Toledo et al., 2003, Assíria Fontes Martins et al., 2015)
This study was carried out using the T. cruzi RA strain that belongs to DTU VI (Zingales et al., 2009). This strain has a wide tissue tropism and is highly lethal for the mouse (Mirkin et al., 1994). Besides, susceptibility of this strain to Benznidazole has been retained upon passage in outbred CF1 mice, since its isolation from a child with acute Chagas disease, living in an endemic region of Argentina (González Cappa et al., 1981a).
Its high lethality was a desired characteristic, since it allowed us to test the effectiveness of the parasiticidal treatment with benznidazole at the tested doses. In this regard, as few as 500 parasites kill all mice in a short time period. Thus, in the present work we show that untreated mice or mice treated with the lowest dose, 10 mg/kg/day, succumb to infection. On the contrary, mice treated with 25 mg/kg/day for 30 days and tested 20 days after the end of the treatment, remained without detectable parasites in blood and heart, granting effective cure and leading to 100% survival, as the standard treatment scheme (100 mg/kg/day for 30 days). Thus, in the context of drug optimization we consider adequate and necessary to test the efficacy of lower doses, using a T. cruzi strain already known to be susceptible to the drug.
Molina-Berríos et al. (2013) found that treatment with 30 mg/kg/day resulted in the parasitological cure of BALB/c mice infected with 500 bloodstream trypomastigotes with the non-lethal DM28c (DTU I) after 20 days treatment, beginning on day 2 post-infection. It must be noted, however, that the advantage of our model is that the use of a lethal strain grants that, in case of inadequate treatment, mice will die. Also, the fact that the treatment was effective even when it began after patent parasitaemia was detected (day 7 post-infection), ensures that mice were actually infected which allowed the comparison in effectiveness between the different doses tested.
It may be argued that this study was carried out using only one parasite strain, belonging to a particular DTU. This may raise issues regarding the effectiveness of such treatment in view of the feasible appearance of resistance to benznidazole in different strains. It must be noted, however, that no evidence has been found so far, regarding the association between a particular DTU and resistance to benznidazole. Each DTU may include T. cruzi populations of varied resistance to this drug (Gruendling et al., 2015). Moreover, several studies have shown that resistance may arise at the clonal level if forced by selective drug pressure upon discontinuous treatment schemes in vivo (Dos Santos et al., 2008), or upon continuous drug pressure in vitro (Mejia et al., 2012). Our results strongly suggest that emergence of parasite resistance was avoided with the reduced dose scheme, since no parasites were detected in blood and heart at least 20 days after treatment cessation.
Therefore, optimization of the treatment schedule is something that should be considered in the case of human Chagas disease, as far as new trypanocidal drugs are not available (Alsford et al., 2013).
Our results show that T. cruzi infection stimulates the inflammatory response in terms of IL-1β and IL-6 mRNA expression and NOS2 production in the mouse heart. Accordingly, we have previously described the onset of inflammatory response in mice infected with T. cruzi strains of different lethality (Penas et al., 2013) and in neonatal cardiac cells stimulated with LPS or infected with T. cruzi (Hovsepian et al., 2010, Hovsepian et al., 2011). The expression of inflammatory genes in the heart plays an essential role in organ dysfunction. In this regard, it has been shown that NOS2 is involved in the pathogenesis of cardiac diseases (Qian et al., 2001, Goren et al., 2004). Interestingly, in Chagas disease, NO has been described as a trypanocidal mediator (Muñoz-Fernández et al., 1992). However, it has also been suggested that excessive release of NO by inflammatory cells would lead to heart failure (Massion et al., 2003). In this study, we report for the first time that a low dose of Bzl (25 mg/kg/day) is able to inhibit the expression of proinflammatory cytokines and NOS2 expression in a murine experimental model of T. cruzi infection. Consistently, reduction of CK activity, a typical marker of heart damage, was also observed. In this regard, our results are in agreement with those previously reported by Pereira et al. (2014).
The anti-inflammatory properties of this parasiticidal drug had been previously tested in different experimental models of sepsis or macrophages stimulated in culture, but not in an experimental model of Chagas disease. In the liver of septic mice, Bzl decreases the mRNA and protein expression of TNF-α and NOS2 by inhibition of NF-κB and MAPK (p-38 and ERK) (Piaggio et al., 2001, Ronco et al., 2011) provided evidence that Bzl is able to specifically inhibit NO production and cytokine release in RAW 264.7 macrophages stimulated with LPS and/or IFN-γ. Moreover, in RAW 264.7 macrophages stimulated with LPS, Manarin et al. (2010) determined that treatment with Bzl inhibits IκBα phosphorylation and hence its degradation, but not IκB kinase α/β phosphorylation, despite which, Bzl behaves as a broad-range specific inhibitor of NF-κB activation, independently of the stimuli tested. Since in those works high concentrations of Bzl (1 mM) were used in vitro, in the present work we decided to test the parasiticidal effect of Bzl at lower doses in isolated cardiomyocytes in culture, infected with the RA strain of T. cruzi. We determined that 15 μM Bzl was the optimal concentration to reduce the parasite load in infected cells as measured by qPCR. This finding led us to study its anti-inflammatory properties knowing the optimal parasiticidal concentration. In this case, the test system involved stimulation of the cells with LPS. Using this approach, we herein demonstrate that, in vitro, a concentration of Bzl lower than that usually used inhibits IL-1β and IL-6 mRNA levels in stimulated cardiomyocytes. Furthermore, 15 μM Bzl was able to inhibit NOS2 expression and NO release, in comparison to other studies showing that 1 mM Bzl impaired LPS induction of macrophage NOS2 gene transcription (Piaggio et al., 2001, Manarin et al., 2010). Revelli et al. (1999) have also demonstrated that a high concentration of Bzl down-regulates NO and cytokines in macrophages stimulated with LPS, suggesting that it may also alter the balance between pro- and anti-inflammatory responses. Thorough studies have been carried out in macrophages stimulated with LPS and treated with 1 mM Bzl to clarify the mechanism whereby the expression of NOS2 and pro-inflammatory cytokines is inhibited (Piaggio et al., 2001, Manarin et al., 2010). These studies have shown that inhibition of the NF-κB pathway is responsible for the down-regulation of pro-inflammatory mediators. Our present results show that, in LPS-stimulated cardiomyocytes, 15 μM Bzl also inhibited this pathway, demonstrating that the anti-inflammatory effects of this drug may be reached at a concentration lower than the one widely reported.
5. Conclusions
In conclusion, this study shows for the first time that optimal effects of Bzl, in terms of parasite clearance from blood and heart tissue and reduction of inflammatory reaction, can be achieved at doses significantly lower than those usually used for the treatment in an experimental murine model using a highly virulent benznidazole-susceptible T. cruzi strain. This finding may be a relevant for dose optimization in the treatment of acute human Chagas disease. This is especially true if one considers the number and varied severity of adverse effects generated by the use of Bzl, which lead to the abandonment of treatment by a significant proportion of patients.
Acknowledgements
We are grateful to Mr. Eduardo Alejandro Giménez and Mr. Ricardo Chung for their excellent technical assistance. We would also like to thank Ms. Victoria González Eusevi for her assistance in English grammar and spelling corrections.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ijpddr.2015.12.001.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Alsford S., Kelly J.M., Baker N., Horn D. Genetic dissection of drug resistance in trypanosomes. Parasitology. 2013;140:1478–1491. doi: 10.1017/S003118201300022X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assíria Fontes Martins T., de Figueiredo Diniz L., Mazzeti A.L., da Silva do Nascimento Á.F., Caldas S., Caldas I.S., de Andrade I.M., Ribeiro I., Bahia M.T. Benznidazole/Itraconazole combination treatment enhances Anti-Trypanosoma cruzi activity in experimental Chagas disease. PLoS One. 2015;10:e0128707. doi: 10.1371/journal.pone.0128707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brener Z. Observations on immunity to superinfections in mice experimentally inoculated with Trypanosoma cruzi and subjected to treatment. Rev. Inst. Med. Trop. Sao Paulo. 1962;4:119–123. [PubMed] [Google Scholar]
- Bryan N.S., Grisham M.B. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic. Biol. Med. 2007;43:645–657. doi: 10.1016/j.freeradbiomed.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campi-Azevedo A.C., Gomes J.A.S., Teixeira-Carvalho A., Silveira-Lemos D., Vitelli-Avelar D.M., Sathler-Avelar R., Peruhype-Magalhães V., Béla S.R., Silvestre K.F., Batista M.A., Schachnik N.C.C., Correa-Oliveira R., Eloi-Santos S.M., Martins-Filho O.A. Etiological treatment of Chagas disease patients with benznidazole lead to a sustained pro-inflammatory profile counterbalanced by modulatory events. Immunobiology. 2015;220:564–574. doi: 10.1016/j.imbio.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Castro J.A., de Mecca M.M., Bartel L.C. Toxic side effects of drugs used to treat Chagas' disease (American trypanosomiasis) Hum. Exp. Toxicol. 2006;25:471–479. doi: 10.1191/0960327106het653oa. [DOI] [PubMed] [Google Scholar]
- Celentano A.M., González Cappa S.M. In vivo macrophage function in experimental infection with Trypanosoma cruzi subpopulations. Acta Trop. 1993;55:171–180. doi: 10.1016/0001-706x(93)90075-m. [DOI] [PubMed] [Google Scholar]
- Coura J.R. Present situation and new strategies for Chagas disease chemotherapy: a proposal. Mem. Inst. Oswaldo Cruz. 2009;104:549–554. doi: 10.1590/s0074-02762009000400002. [DOI] [PubMed] [Google Scholar]
- Cummings K.L., Tarleton R.L. Rapid quantitation of Trypanosoma cruzi in host tissue by real-time PCR. Mol. Biochem. Parasitol. 2003;129:53–59. doi: 10.1016/s0166-6851(03)00093-8. [DOI] [PubMed] [Google Scholar]
- de Andrade A.L., Zicker F., de Oliveira R.M., Almeida Silva S., Luquetti A., Travassos L.R., Almeida I.C., de Andrade S.S., de Andrade J.G., Martelli C.M. Randomised trial of efficacy of benznidazole in treatment of early Trypanosoma cruzi infection. Lancet (London, England) 1996;348:1407–1413. doi: 10.1016/s0140-6736(96)04128-1. [DOI] [PubMed] [Google Scholar]
- Díaz-Guerra M.J., Velasco M., Martín-Sanz P., Boscá L. Evidence for common mechanisms in the transcriptional control of type II nitric oxide synthase in isolated hepatocytes. Requirement of NF-kappaB activation after stimulation with bacterial cell wall products and phorbol esters. J. Biol. Chem. 1996;271:30114–30120. doi: 10.1074/jbc.271.47.30114. [DOI] [PubMed] [Google Scholar]
- Dos Santos F.M., Caldas S., de Assis Cáu S.B., Crepalde G.P., de Lana M., Machado-Coelho G.L.L., Veloso V.M., Bahia M.T. Trypanosoma cruzi: induction of benznidazole resistance in vivo and its modulation by in vitro culturing and mice infection. Exp. Parasitol. 2008;120:385–390. doi: 10.1016/j.exppara.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Duffy T., Bisio M., Altcheh J., Burgos J.M., Diez M., Levin M.J., Favaloro R.R., Freilij H., Schijman A.G. Accurate real-time PCR strategy for monitoring bloodstream parasitic loads in chagas disease patients. PLoS Negl. Trop. Dis. 2009;3:e419. doi: 10.1371/journal.pntd.0000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbro D.L., Streiger M.L., Arias E.D., Bizai M.L., del Barco M., Amicone N.A. Trypanocide treatment among adults with chronic Chagas disease living in Santa Fe city (Argentina), over a mean follow-up of 21 years: parasitological, serological and clinical evolution. Rev. Soc. Bras. Med. Trop. 2007;40:1–10. doi: 10.1590/s0037-86822007000100001. [DOI] [PubMed] [Google Scholar]
- Feilij H., Muller L., Gonzalez Cappa S.M. Direct micromethod for diagnosis of acute and congenital Chagas' disease. J. Clin. Microbiol. 1983;18:327–330. doi: 10.1128/jcm.18.2.327-330.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia S., Ramos C.O., Senra J.F.V., Vilas-Boas F., Rodrigues M.M., Campos-de-Carvalho A.C., Ribeiro-Dos-Santos R., Soares M.B.P. Treatment with benznidazole during the chronic phase of experimental Chagas' disease decreases cardiac alterations. Antimicrob. Agents Chemother. 2005;49:1521–1528. doi: 10.1128/AAC.49.4.1521-1528.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González Cappa S.M., Bijovsky A.T., Freilij H., Muller L., Katzin A.M. Isolation of a Trypanosoma cruzi strain of predominantly slender form in Argentina. Medicina (B. Aires) 1981;41:119–120. [PubMed] [Google Scholar]
- González Cappa S.M., Katzin A.M., Añasco N., Lajmanovich S. Comparative studies on infectivity and surface carbohydrates of several strains of Trypanosoma cruzi. Medicina (B. Aires) 1981;41:549–555. [PubMed] [Google Scholar]
- Goren N., Cuenca J., Martín-Sanz P., Boscá L. Attenuation of NF-kappaB signalling in rat cardiomyocytes at birth restricts the induction of inflammatory genes. Cardiovasc. Res. 2004;64:289–297. doi: 10.1016/j.cardiores.2004.06.029. [DOI] [PubMed] [Google Scholar]
- Gruendling A.P., Massago M., Teston A.P.M., Monteiro W.M., Kaneshima E.N., Araújo S.M., Gomes M.L., Barbosa M., das G.V., Toledo M.J.O. Impact of benznidazole on infection course in mice experimentally infected with Trypanosoma cruzi I, II, and IV. Am. J. Trop. Med. Hyg. 2015;92:1178–1189. doi: 10.4269/ajtmh.13-0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedes P.M.M., Silva G.K., Gutierrez F.R.S., Silva J.S. Current status of Chagas disease chemotherapy. Expert Rev. Anti. Infect. Ther. 2011;9:609–620. doi: 10.1586/eri.11.31. [DOI] [PubMed] [Google Scholar]
- Hasslocher-Moreno A.M., do Brasil P.E.A.A., de Sousa A.S., Xavier S.S., Chambela M.C., Sperandio da Silva G.M. Safety of benznidazole use in the treatment of chronic Chagas' disease. J. Antimicrob. Chemother. 2012;67:1261–1266. doi: 10.1093/jac/dks027. [DOI] [PubMed] [Google Scholar]
- Hovsepian E., Mirkin G.A., Penas F., Manzano A., Bartrons R., Goren N.B. Modulation of inflammatory response and parasitism by 15-Deoxy-Δ(12,14) prostaglandin J(2) in Trypanosoma cruzi-infected cardiomyocytes. Int. J. Parasitol. 2011;41:553–562. doi: 10.1016/j.ijpara.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Hovsepian E., Penas F., Goren N.B. 15-deoxy-Delta12,14 prostaglandin GJ2 but not rosiglitazone regulates metalloproteinase 9, NOS-2, and cyclooxygenase 2 expression and functions by peroxisome proliferator-activated receptor gamma-dependent and -independent mechanisms in cardiac cells. Shock. 2010;34:60–67. doi: 10.1097/SHK.0b013e3181cdc398. [DOI] [PubMed] [Google Scholar]
- Hovsepian E., Penas F., Siffo S., Mirkin G.A., Goren N.B. IL-10 inhibits the NF-κB and ERK/MAPK-mediated production of pro-inflammatory mediators by up-regulation of SOCS-3 in Trypanosoma cruzi-infected cardiomyocytes. PLoS One. 2013;8:e79445. doi: 10.1371/journal.pone.0079445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson Y., Alirol E., Getaz L., Wolff H., Combescure C., Chappuis F. Tolerance and safety of nifurtimox in patients with chronic chagas disease. Clin. Infect. Dis. 2010;51:e69–75. doi: 10.1086/656917. [DOI] [PubMed] [Google Scholar]
- Junqueira C., Caetano B., Bartholomeu D.C., Melo M.B., Ropert C., Rodrigues M.M., Gazzinelli R.T. The endless race between Trypanosoma cruzi and host immunity: lessons for and beyond Chagas disease. Expert Rev. Mol. Med. 2010;12:e29. doi: 10.1017/S1462399410001560. [DOI] [PubMed] [Google Scholar]
- Kruger N.J. The Bradford method for protein quantitation. Methods Mol. Biol. 1994;32:9–15. doi: 10.1385/0-89603-268-X:9. [DOI] [PubMed] [Google Scholar]
- Laird P.W., Zijderveld A., Linders K., Rudnicki M.A., Jaenisch R., Berns A. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 1991;19:4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggini J., Mirkin G., Bognanni I., Holmberg J., Piazzón I.M., Nepomnaschy I., Costa H., Cañones C., Raiden S., Vermeulen M., Geffner J.R. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One. 2010;5:e9252. doi: 10.1371/journal.pone.0009252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manarin R., Pascutti M.F., Ruffino J.P., De Las Heras B., Boscá L., Bottasso O., Revelli S., Serra E. Benznidazole blocks NF-kappaB activation but not AP-1 through inhibition of IKK. Mol. Immunol. 2010;47:2485–2491. doi: 10.1016/j.molimm.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Martins H.R., Figueiredo L.M., Valamiel-Silva J.C.O., Carneiro C.M., Machado-Coelho G.L.L., Vitelli-Avelar D.M., Bahia M.T., Martins-Filho O.A., Macedo A.M., Lana M. Persistence of PCR-positive tissue in benznidazole-treated mice with negative blood parasitological and serological tests in dual infections with Trypanosoma cruzi stocks from different genotypes. J. Antimicrob. Chemother. 2008;61:1319–1327. doi: 10.1093/jac/dkn092. [DOI] [PubMed] [Google Scholar]
- Massion P.B., Feron O., Dessy C., Balligand J.-L. Nitric oxide and cardiac function: ten years after, and continuing. Circ. Res. 2003;93:388–398. doi: 10.1161/01.RES.0000088351.58510.21. [DOI] [PubMed] [Google Scholar]
- Maya J.D., Cassels B.K., Iturriaga-Vásquez P., Ferreira J., Faúndez M., Galanti N., Ferreira A., Morello A. Mode of action of natural and synthetic drugs against Trypanosoma cruzi and their interaction with the mammalian host. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007;146:601–620. doi: 10.1016/j.cbpa.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Mejia A.M., Hall B.S., Taylor M.C., Gómez-Palacio A., Wilkinson S.R., Triana-Chávez O., Kelly J.M. Benznidazole-resistance in Trypanosoma cruzi is a readily acquired trait that can arise independently in a single population. J. Infect. Dis. 2012;206:220–228. doi: 10.1093/infdis/jis331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D.A., Hernandez S., Rodriguez De Armas L., Eells S.J., Traina M.M., Miller L.G., Meymandi S.K. Tolerance of benznidazole in a United States Chagas disease clinic. Clin. Infect. Dis. 2015;60:1237–1240. doi: 10.1093/cid/civ005. [DOI] [PubMed] [Google Scholar]
- Mirkin G.A., Jones M., Sanz O.P., Rey R., Sica R.E., González Cappa S.M. Experimental Chagas' disease: electrophysiology and cell composition of the neuromyopathic inflammatory lesions in mice infected with a myotropic and a pantropic strain of Trypanosoma cruzi. Clin. Immunol. Immunopathol. 1994;73:69–79. doi: 10.1006/clin.1994.1171. [DOI] [PubMed] [Google Scholar]
- Molina-Berríos A., Campos-Estrada C., Lapier M., Duaso J., Kemmerling U., Galanti N., Leiva M., Ferreira J., López-Muñoz R., Maya J.D. Benznidazole prevents endothelial damage in an experimental model of Chagas disease. Acta Trop. 2013;127:6–13. doi: 10.1016/j.actatropica.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Muñoz-Fernández M.A., Fernández M.A., Fresno M. Activation of human macrophages for the killing of intracellular Trypanosoma cruzi by TNF-alpha and IFN-gamma through a nitric oxide-dependent mechanism. Immunol. Lett. 1992;33:35–40. doi: 10.1016/0165-2478(92)90090-b. [DOI] [PubMed] [Google Scholar]
- Pascutti M.F., Pitashny M., Nocito A.L., Guermonprez P., Amigorena S., Wietzerbin J., Serra E., Bottasso O., Revelli S. Benznidazole, a drug used in Chagas' disease, ameliorates LPS-induced inflammatory response in mice. Life Sci. 2004;76:685–697. doi: 10.1016/j.lfs.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Penas F., Mirkin G.A., Hovsepian E., Cevey Á., Caccuri R., Sales M.E., Goren N.B. PPARγ ligand treatment inhibits cardiac inflammatory mediators induced by infection with different lethality strains of Trypanosoma cruzi. Biochim. Biophys. Acta Mol. Basis Dis. 2013;1832:239–248. doi: 10.1016/j.bbadis.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Penas F., Mirkin G.A., Vera M., Cevey Á., González C.D., Gómez M.I., Sales M.E., Goren N.B. Treatment in vitro with PPARα and PPARγ ligands drives M1-to-M2 polarization of macrophages from T. cruzi-infected mice. Biochim. Biophys. Acta. 2015;1852:893–904. doi: 10.1016/j.bbadis.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Pereira I.R., Vilar-Pereira G., Silva A.A., Lannes-Vieira J. Severity of chronic experimental Chagas' heart disease parallels tumour necrosis factor and nitric oxide levels in the serum: models of mild and severe disease. Mem. Inst. Oswaldo Cruz. 2014;109:289–298. doi: 10.1590/0074-0276140033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Molina J.A., Pérez-Ayala A., Moreno S., Fernández-González M.C., Zamora J., López-Velez R. Use of benznidazole to treat chronic Chagas' disease: a systematic review with a meta-analysis. J. Antimicrob. Chemother. 2009;64:1139–1147. doi: 10.1093/jac/dkp357. [DOI] [PubMed] [Google Scholar]
- Piaggio E., Sancéau J., Revelli S., Bottasso O., Wietzerbin J., Serra E. Trypanocidal drug benznidazole impairs lipopolysaccharide induction of macrophage nitric oxide synthase gene transcription through inhibition of NF-kappaB activation. J. Immunol. 2001;167:3422–3426. doi: 10.4049/jimmunol.167.6.3422. [DOI] [PubMed] [Google Scholar]
- Pinazo M.-J., Guerrero L., Posada E., Rodríguez E., Soy D., Gascon J. Benznidazole-related adverse drug reactions and their relationship to serum drug concentrations in patients with chronic Chagas disease. Antimicrob. Agents Chemother. 2013;57:390–395. doi: 10.1128/AAC.01401-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priotto G., Kasparian S., Mutombo W., Ngouama D., Ghorashian S., Arnold U., Ghabri S., Baudin E., Buard V., Kazadi-Kyanza S., Ilunga M., Mutangala W., Pohlig G., Schmid C., Karunakara U., Torreele E., Kande V. Nifurtimox-eflornithine combination therapy for second-stage African Trypanosoma brucei gambiense trypanosomiasis: a multicentre, randomised, phase III, non-inferiority trial. Lancet (London, England) 2009;374:56–64. doi: 10.1016/S0140-6736(09)61117-X. [DOI] [PubMed] [Google Scholar]
- Qian Z., Gelzer-Bell R., Yang Sx S.X., Cao W., Ohnishi T., Wasowska B.A., Hruban R.H., Rodriguez E.R., Baldwin W.M., Lowenstein C.J. Inducible nitric oxide synthase inhibition of weibel-palade body release in cardiac transplant rejection. Circulation. 2001;104:2369–2375. doi: 10.1161/hc4401.098471. [DOI] [PubMed] [Google Scholar]
- Rassi A., Dias J.C.P., Marin-Neto J.A. Challenges and opportunities for primary, secondary, and tertiary prevention of Chagas' disease. Heart. 2009;95:524–534. doi: 10.1136/hrt.2008.159624. [DOI] [PubMed] [Google Scholar]
- Rassi Y., Oshaghi M.A., Azani S.M., Abaie M.R., Rafizadeh S., Mohebai M., Mohtarami F., Zeinali M., kazem Molecular detection of Leishmania infection due to Leishmania major and Leishmania turanica in the vectors and reservoir host in Iran. Vector Borne Zoonotic Dis. 2011;11:145–150. doi: 10.1089/vbz.2009.0167. [DOI] [PubMed] [Google Scholar]
- Revelli S., Le Page C., Piaggio E., Wietzerbin J., Bottasso O. Benznidazole, a drug employed in the treatment of Chagas' disease, down-regulates the synthesis of nitrite and cytokines by murine stimulated macrophages. Clin. Exp. Immunol. 1999;118:271–277. doi: 10.1046/j.1365-2249.1999.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriques Coura J., de Castro S.L. A critical review on Chagas disease chemotherapy. Mem. Inst. Oswaldo Cruz. 2002;97:3–24. doi: 10.1590/s0074-02762002000100001. [DOI] [PubMed] [Google Scholar]
- Rojo G., Castillo C., Duaso J., Liempi A., Droguett D., Galanti N., Maya J.D., López-Muñoz R., Kemmerling U. Toxic and therapeutic effects of Nifurtimox and Benznidazol on Trypanosoma cruzi ex vivo infection of human placental chorionic villi explants. Acta Trop. 2014;132:112–118. doi: 10.1016/j.actatropica.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Ronco M.T., Manarin R., Francés D., Serra E., Revelli S., Carnovale C. Benznidazole treatment attenuates liver NF-κB activity and MAPK in a cecal ligation and puncture model of sepsis. Mol. Immunol. 2011;48:867–873. doi: 10.1016/j.molimm.2010.12.021. [DOI] [PubMed] [Google Scholar]
- Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Sosa-Estani S., Segura E.L. Etiological treatment in patients infected by Trypanosoma cruzi: experiences in Argentina. Curr. Opin. Infect. Dis. 2006;19:583–587. doi: 10.1097/01.qco.0000247592.21295.a5. [DOI] [PubMed] [Google Scholar]
- Tanowitz H.B., Kirchhoff L.V., Simon D., Morris S.A., Weiss L.M., Wittner M. Chagas' disease. Clin. Microbiol. Rev. 1992;5:400–419. doi: 10.1128/cmr.5.4.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira M.M., Gazzinelli R.T., Silva J.S. Chemokines, inflammation and Trypanosoma cruzi infection. Trends Parasitol. 2002;18:262–265. doi: 10.1016/s1471-4922(02)02283-3. [DOI] [PubMed] [Google Scholar]
- Toledo M.J., de O., Bahia M.T., Carneiro C.M., Martins-Filho O.A., Tibayrenc M., Barnabé C., Tafuri W.L., de Lana M. Chemotherapy with benznidazole and itraconazole for mice infected with different Trypanosoma cruzi clonal genotypes. Antimicrob. Agents Chemother. 2003;47:223–230. doi: 10.1128/AAC.47.1.223-230.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viotti R., Vigliano C., Lococo B., Alvarez M.G., Petti M., Bertocchi G., Armenti A. Side effects of benznidazole as treatment in chronic Chagas disease: fears and realities. Expert Rev. Anti-Infect. Ther. 2009;7:157–163. doi: 10.1586/14787210.7.2.157. [DOI] [PubMed] [Google Scholar]
- Viotti R., Vigliano C., Lococo B., Bertocchi G., Petti M., Alvarez M.G., Postan M., Armenti A. Long-term cardiac outcomes of treating chronic Chagas disease with benznidazole versus no treatment: a nonrandomized trial. Ann. Intern. Med. 2006;144:724–734. doi: 10.7326/0003-4819-144-10-200605160-00006. [DOI] [PubMed] [Google Scholar]
- Zingales B., Andrade S.G., Briones M.R.S., Campbell D.A., Chiari E., Fernandes O., Guhl F., Lages-Silva E., Macedo A.M., Machado C.R., Miles M.A., Romanha A.J., Sturm N.R., Tibayrenc M., Schijman A.G. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem. Inst. Oswaldo Cruz. 2009;104:1051–1054. doi: 10.1590/s0074-02762009000700021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.